Serum Levels of Interleukin-6 and Titers of Antibodies against Porphyromonas gingivalis Could Be Potential Biomarkers for the Diagnosis of Oral Squamous Cell Carcinoma
Abstract
1. Introduction
2. Results
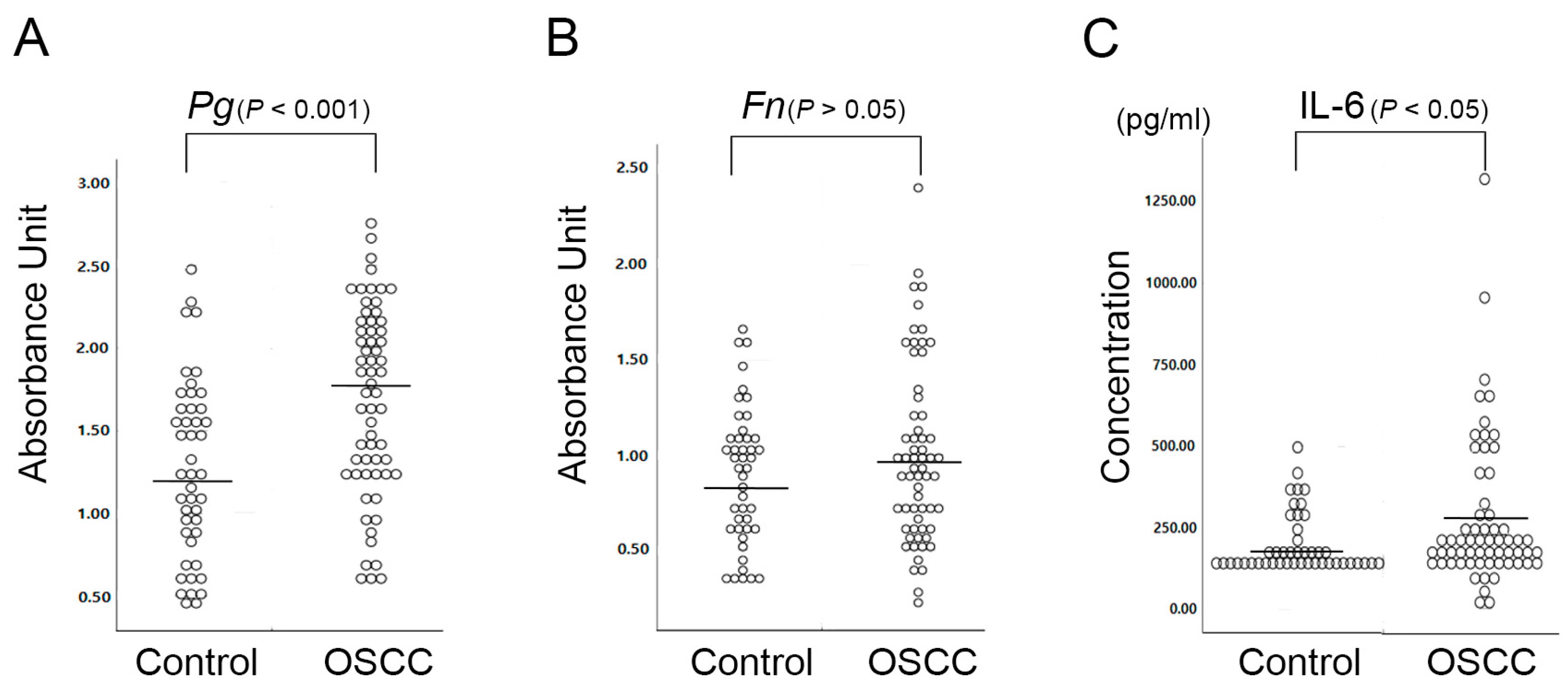
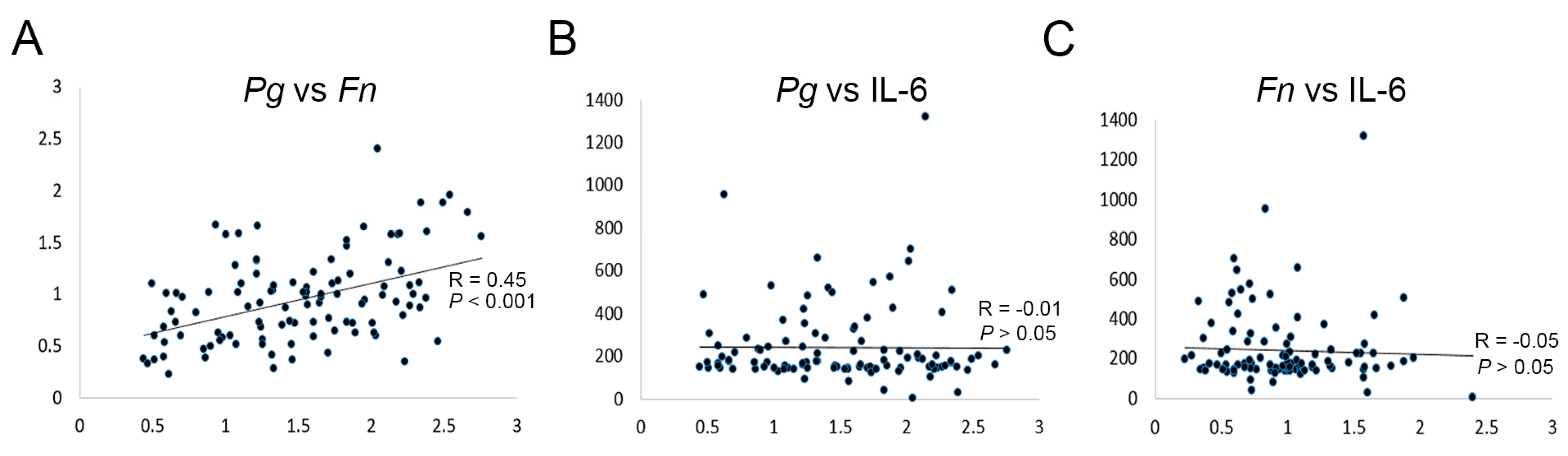
2.1. P. gingivalis Is More Closely Associated with OSCC than F. nucleatum
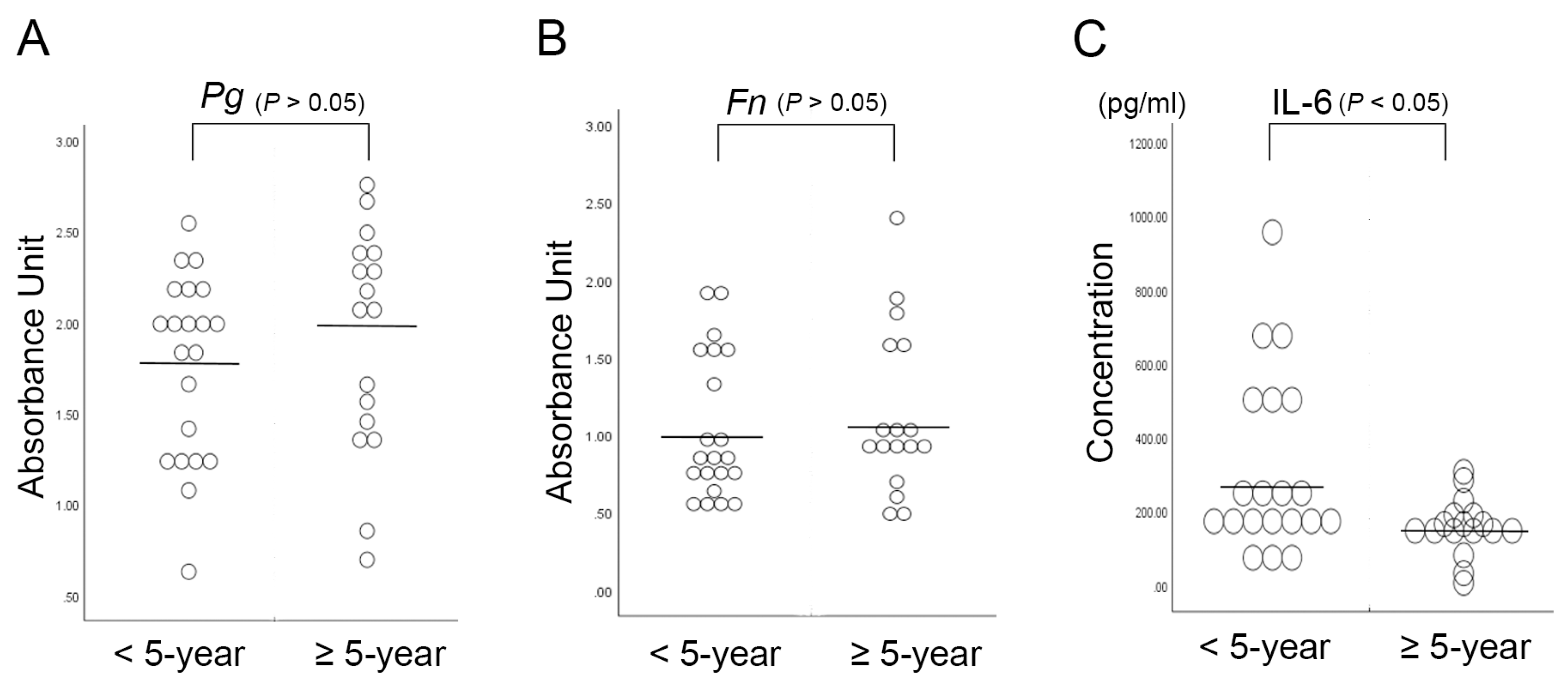
2.2. Serum Interleukin-6 Is Associated with the 5-Year Survival Rate in OSCC Patients
3. Discussion
4. Materials and Methods
4.1. Study Subjects
4.2. Enzyme-Linked Immunosorbent Assay
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| OSCC | Oral Squamous Cell Carcinoma |
| ESCC | Esophageal Squamous Cell Carcinoma |
| IL-6 | Interleukin-6 |
| ROC | Receiver Operating Characteristic |
| AUC | Area Under the ROC Curve |
References
- Devine, J.C.; Rogers, S.N.; McNally, D.; Brown, J.S.; Vaughan, E.D. A comparison of aesthetic, functional and patient subjective outcomes following lip-split mandibulotomy and mandibular lingual releasing access procedures. Int. J. Oral Maxillofac. Surg. 2001, 30, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.C.; Yazar, S.; Lin, C.H.; Cheng, M.H.; Tsao, C.K.; Chiang, Y.C. Double free flaps in head and neck reconstruction. Clin. Plast. Surg. 2005, 32, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Arruebo, M.; Vilaboa, N.; Saez-Gutierrez, B.; Lambea, J.; Tres, A.; Valladares, M.; Gonzalez-Fernandez, A. Assessment of the evolution of cancer treatment therapies. Cancers (Basel) 2011, 3, 3279–3330. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.M.; Melo, J.V. Chronic myeloid leukemia--advances in biology and new approaches to treatment. N. Engl. J. Med. 2003, 349, 1451–1464. [Google Scholar] [CrossRef] [PubMed]
- Kolokythas, A. Long-Term Surgical Complications in the Oral Cancer Patient: A Comprehensive Review. Part II. J. Oral Maxillofac Res. 2010, 1, e2. [Google Scholar] [CrossRef] [PubMed]
- Kolokythas, A. Long-term surgical complications in the oral cancer patient: A comprehensive review. Part I. J. Oral Maxillofac Res. 2010, 1, e1. [Google Scholar] [CrossRef]
- Nathan, M.R.; Schmid, P. The emerging world of breast cancer immunotherapy. Breast 2018, 37, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.D.; Ferreira, C.B.; Leite, G.B.; de Menezes Pontes, J.R.; Antunes, H.S. Oral manifestations of lymphoma: A systematic review. Ecancermedicalscience 2016, 10, 665. [Google Scholar] [CrossRef] [PubMed]
- Skoetz, N.; Trelle, S.; Rancea, M.; Haverkamp, H.; Diehl, V.; Engert, A.; Borchmann, P. Effect of initial treatment strategy on survival of patients with advanced-stage Hodgkin’s lymphoma: A systematic review and network meta-analysis. Lancet Oncol. 2013, 14, 943–952. [Google Scholar] [CrossRef]
- Bulsara, V.M.; Worthington, H.V.; Glenny, A.M.; Clarkson, J.E.; Conway, D.I.; Macluskey, M. Interventions for the treatment of oral and oropharyngeal cancers: Surgical treatment. Cochrane Database Syst. Rev. 2018, 12, CD006205. [Google Scholar] [CrossRef]
- Rivera, C.; Venegas, B. Histological and molecular aspects of oral squamous cell carcinoma (Review). Oncol. Lett. 2014, 8, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Axley, P.; Ahmed, Z.; Ravi, S.; Singal, A.K. Hepatitis C Virus and Hepatocellular Carcinoma: A Narrative Review. J. Clin Transl Hepatol 2018, 6, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Jess, T.; Rungoe, C.; Peyrin-Biroulet, L. Risk of colorectal cancer in patients with ulcerative colitis: A meta-analysis of population-based cohort studies. Clin. Gastroenterol. Hepatol. 2012, 10, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Van Der Kraak, L.; Gros, P.; Beauchemin, N. Colitis-associated colon cancer: Is it in your genes? World J. Gastroenterol. 2015, 21, 11688–11699. [Google Scholar] [CrossRef] [PubMed]
- Snider, A.J.; Bialkowska, A.B.; Ghaleb, A.M.; Yang, V.W.; Obeid, L.M.; Hannun, Y.A. Murine Model for Colitis-Associated Cancer of the Colon. Methods Mol. Biol. 2016, 1438, 245–254. [Google Scholar] [PubMed]
- Huang, P.; Liu, M.; Zang, F.; Yao, Y.; Yue, M.; Wang, J.; Fan, H.; Zhuo, L.; Wu, J.; Xia, X.; et al. The development of hepatocellular carcinoma in HCV-infected patients treated with DAA: A comprehensive analysis. Carcinogenesis 2018, 39, 1497–1505. [Google Scholar] [CrossRef]
- Yamashita, T.; Honda, M.; Kaneko, S. Molecular mechanisms of hepatocarcinogenesis in chronic hepatitis C virus infection. J. Gastroenterol. Hepatol. 2011, 26, 960–964. [Google Scholar] [CrossRef]
- Heikkila, P.; But, A.; Sorsa, T.; Haukka, J. Periodontitis and cancer mortality: Register-based cohort study of 68,273 adults in 10-year follow-up. Int. J. Cancer 2018, 142, 2244–2253. [Google Scholar] [CrossRef]
- Sfreddo, C.S.; Maier, J.; De David, S.C.; Susin, C.; Moreira, C.H.C. Periodontitis and breast cancer: A case-control study. Community Dent. Oral Epidemiol. 2017, 45, 545–551. [Google Scholar] [CrossRef]
- Chung, S.D.; Tsai, M.C.; Huang, C.C.; Kao, L.T.; Chen, C.H. A population-based study on the associations between chronic periodontitis and the risk of cancer. Int. J. Clin. Oncol. 2016, 21, 219–223. [Google Scholar] [CrossRef]
- Tezal, M.; Sullivan, M.A.; Hyland, A.; Marshall, J.R.; Stoler, D.; Reid, M.E.; Loree, T.R.; Rigual, N.R.; Merzianu, M.; Hauck, L.; et al. Chronic periodontitis and the incidence of head and neck squamous cell carcinoma. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 2406–2412. [Google Scholar] [CrossRef] [PubMed]
- Ha, N.H.; Woo, B.H.; Kim, D.J.; Ha, E.S.; Choi, J.I.; Kim, S.J.; Park, B.S.; Lee, J.H.; Park, H.R. Prolonged and repetitive exposure to Porphyromonas gingivalis increases aggressiveness of oral cancer cells by promoting acquisition of cancer stem cell properties. Tumour Biol. 2015, 36, 9947–9960. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Roberts, J.S.; Atanasova, K.R.; Chowdhury, N.; Han, K.; Yilmaz, O. Human Primary Epithelial Cells Acquire an Epithelial-Mesenchymal-Transition Phenotype during Long-Term Infection by the Oral Opportunistic Pathogen, Porphyromonas gingivalis. Front. Cell Infect. Microbiol. 2017, 7, 493. [Google Scholar] [CrossRef]
- Kim, H.J.; Cha, G.S.; Kim, H.J.; Kwon, E.Y.; Lee, J.Y.; Choi, J.; Joo, J.Y. Porphyromonas gingivalis accelerates atherosclerosis through oxidation of high-density lipoprotein. J. Periodontal Implan. 2018, 48, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Ren, H.; Guo, H.; Xing, W.; Liu, C.; Ji, Y.; Jiang, H.; Zhang, P.; Du, M. Periodontal infection with Porphyromonas gingivalis induces preterm birth and lower birth weight in rats. Mol. Oral Microbiol. 2018, 33, 312–321. [Google Scholar] [CrossRef]
- Geng, F.X.; Liu, J.C.; Guo, Y.; Li, C.; Wang, H.Y.; Wang, H.Y.; Zhao, H.J.; Pan, Y.P. Persistent Exposure to Porphyromonas gingivalis Promotes Proliferative and Invasion Capabilities, and Tumorigenic Properties of Human Immortalized Oral Epithelial Cells. Front. Cell Infect. Microbiol. 2017, 7. [Google Scholar] [CrossRef]
- Woo, B.H.; Kim, D.J.; Choi, J.I.; Kim, S.J.; Park, B.S.; Song, J.M.; Lee, J.H.; Park, H.R. Oral cancer cells sustainedly infected with Porphyromonas gingivalis exhibit resistance to Taxol and have higher metastatic potential. Oncotarget 2017, 8, 46981–46992. [Google Scholar] [CrossRef]
- Bradshaw, D.J.; Marsh, P.D.; Watson, G.K.; Allison, C. Role of Fusobacterium nucleatum and coaggregation in anaerobe survival in planktonic and biofilm oral microbial communities during aeration. Infect. Immun. 1998, 66, 4729–4732. [Google Scholar]
- Schiegnitz, E.; Kammerer, P.W.; Schon, H.; Blatt, S.; Berres, M.; Sagheb, K.; Al-Nawas, B. Proinflammatory cytokines as serum biomarker in oral carcinoma-A prospective multi-biomarker approach. J. Oral Pathol. Med. 2018, 47, 268–274. [Google Scholar] [CrossRef]
- Zanotti, L.; Paderno, A.; Piazza, C.; Pagan, E.; Bignotti, E.; Romani, C.; Bandiera, E.; Calza, S.; Del Bon, F.; Nicolai, P.; et al. Epidermal growth factor receptor detection in serum and saliva as a diagnostic and prognostic tool in oral cancer. Laryngoscope 2017, 127, E408–E414. [Google Scholar] [CrossRef]
- Rajkumar, K.; Ramya, R.; Nandhini, G.; Rajashree, P.; Ramesh Kumar, A.; Nirmala Anandan, S. Salivary and serum level of CYFRA 21-1 in oral precancer and oral squamous cell carcinoma. Oral Dis. 2015, 21, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Ha, N.H.; Park, D.G.; Woo, B.H.; Kim, D.J.; Choi, J.I.; Park, B.S.; Kim, Y.D.; Lee, J.H.; Park, H.R. Porphyromonas gingivalis increases the invasiveness of oral cancer cells by upregulating IL-8 and MMPs. Cytokine 2016, 86, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Inaba, H.; Sugita, H.; Kuboniwa, M.; Iwai, S.; Hamada, M.; Noda, T.; Morisaki, I.; Lamont, R.J.; Amano, A. Porphyromonas gingivalis promotes invasion of oral squamous cell carcinoma through induction of proMMP9 and its activation. Cell Microbiol. 2014, 16, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Michaud, D.S.; Izard, J.; Wilhelm-Benartzi, C.S.; You, D.H.; Grote, V.A.; Tjonneland, A.; Dahm, C.C.; Overvad, K.; Jenab, M.; Fedirko, V.; et al. Plasma antibodies to oral bacteria and risk of pancreatic cancer in a large European prospective cohort study. Gut 2013, 62, 1764–1770. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.G.; Yang, J.Q.; Ma, Z.K.; Yuan, X.; Zhao, C.; Wang, G.C.; Wei, H.; Feng, X.S.; Qi, Y.J. Preoperative serum immunoglobulin G and A antibodies to Porphyromonas gingivalis are potential serum biomarkers for the diagnosis and prognosis of esophageal squamous cell carcinoma. BMC Cancer 2018, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Segers, S.; Hayes, R.B. Periodontal disease, Porphyromonas gingivalis serum antibody levels and orodigestive cancer mortality. Carcinogenesis 2012, 33, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Karin, M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin. Immunol. 2014, 26, 54–74. [Google Scholar] [CrossRef]
- Brailo, V.; Vucicevic-Boras, V.; Lukac, J.; Biocina-Lukenda, D.; Zilic-Alajbeg, I.; Milenovic, A.; Balija, M. Salivary and serum interleukin 1 beta, interleukin 6 and tumor necrosis factor alpha in patients with leukoplakia and oral cancer. Med. Oral Patol. Oral Cir. Bucal. 2012, 17, e10. [Google Scholar] [CrossRef]
- Vinocha, A.; Grover, R.K.; Deepak, R. Clinical significance of interleukin-6 in diagnosis of lung, oral, esophageal, and gall bladder carcinomas. J. Cancer Res. Ther. 2018, 14, S758–S760. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]

| Variables | lgG Titer (Mean ± SD) | p | |
|---|---|---|---|
| Age | ≤60 (24 (38.7%)) | 1.61 ± 0.51 | 0.34 |
| >60 (38 (61.3%)) | 1.74 ± 0.60 | ||
| Gender | males (45 (72.6%)) | 1.70 ± 0.54 | 0.77 |
| Females (17 (27.4%)) | 1.65 ± 0.64 | ||
| Tobacco Use | No (39 (65.0%)) | 1.67 ± 0.58 | 0.74 |
| Yes (21 (35.0%)) | 1.73 ± 0.59 | ||
| Alcohol Use | No (37 (59.7%)) | 1.70 ± 0.61 | 0.70 |
| Yes (25 (40.3%)) | 1.68 ± 0.52 | ||
| Histopathologic Grade | I (21 (35.6%)) | 1.78 ± 0.44 | 0.16 |
| II (26 (44.1%)) | 1.53 ± 0.59 | ||
| III (6 (10.2%)) | 1.55 ± 0.73 | ||
| IV (6 (10.2%)) | 2.03 ± 0.39 | ||
| Lymph Node Metastasis | No (33 (59.7%)) | 1.63 ± 0.63 | 0.38 |
| Yes (29 (40.3%)) | 1.76 ± 0.49 | ||
| Tumor Size | T1 (25 (42.4%)) | 1.62 ± 0.58 | 0.74 |
| T2 (16 (27.1%)) | 1.61 ± 0.44 | ||
| T3 (5 (8.5%)) | 1.89 ± 0.67 | ||
| T4 (13 (22.0)) | 1.74 ± 0.68 | ||
| TNM Stage | I (19 (30.6%)) | 1.59 ± 0.63 | 0.53 |
| II (9 (14.5%)) | 1.54 ± 0.52 | ||
| III (11 (17.2%)) | 1.74 ± 0.45 | ||
| IV (23 (37.1%)) | 1.81 ± 0.59 | ||
| Variables | lgG Titer (Mean ± SD) | p | |
|---|---|---|---|
| Age | ≤60 (24 (38.7%)) | 1.00 ± 0.52 | 0.78 |
| >60 (38 (61.3%)) | 0.99 ± 0.43 | ||
| Gender | males (45 (72.6%)) | 1.00 ± 0.42 | 0.45 |
| Females (17 (27.4%)) | 0.99 ± 0.58 | ||
| Tobacco Use | No (39 (65.0%)) | 1.03 ± 0.49 | 0.76 |
| Yes (21 (35.0%)) | 0.96 ± 0.44 | ||
| Alcohol Use | No (37 (59.7%)) | 0.96 ± 0.45 | 0.33 |
| Yes (25 (40.3%)) | 1.05 ± 0.49 | ||
| Histopathologic Grade | I (21 (35.6%)) | 1.04 ± 0.50 | 0.64 |
| II (26 (44.1%)) | 0.91 ± 0.43 | ||
| III (6 (10.2%)) | 1.05 ± 0.52 | ||
| IV (6 (10.2%)) | 1.11 ± 0.45 | ||
| Lymph Node Metastasis | No (33 (59.7%)) | 1.02 ± 0.49 | 0.99 |
| Yes (29 (40.3%)) | 0.98 ± 0.45 | ||
| Tumor Size | T1 (25 (42.4%)) | 1.04 ± 0.50 | 0.69 |
| T2 (16 (27.1%)) | 0.92 ± 0.48 | ||
| T3 (5 (8.5%)) | 0.98 ± 0.58 | ||
| T4 (13 (22.0)) | 1.06 ± 0.42 | ||
| TNM Stage | I (19 (30.6%)) | 1.08 ± 0.54 | 0.12 |
| II (9 (14.5%)) | 0.87 ± 0.46 | ||
| III (11 (17.2%)) | 0.77 ± 0.35 | ||
| IV (23 (37.1%)) | 1.09 ± 0.43 | ||
| Variables | IL-6 (pg/mL) (Mean ± SD) | p | |
|---|---|---|---|
| Age | ≤60 (24 (38.7%)) | 303.9 ± 230.9 | 0.30 |
| >60 (38 (61.3%)) | 256.6 ± 228.2 | ||
| Gender | males (45 (72.6%)) | 260.2 ± 170.3 | 0.94 |
| Females (17 (27.4%)) | 314.0 ± 342.3 | ||
| Tobacco Use | No (39 (65.0%)) | 297.4 ± 262.5 | 0.38 |
| Yes (21 (35.0%)) | 229.7 ± 147.2 | ||
| Alcohol Use | No (37 (59.7%)) | 304.9 ± 265.3 | 0.23 |
| Yes (25 (40.3%)) | 230.6 ± 154.1 | ||
| Histopathologic Grade | I (21 (35.6%)) | 277.5 ± 288.3 | 0.59 |
| II (26 (44.1%)) | 266.4 ± 200.5 | ||
| III (6 (10.2%)) | 315.5 ± 189.8 | ||
| IV (6 (10.2%)) | 317.3 ± 236.4 | ||
| Lymph Node Metastasis | No (33 (59.7%)) | 291.1 ± 273.7 | 0.92 |
| Yes (29 (40.3%)) | 256.5 ± 165.9 | ||
| Tumor Size | T1 (25 (42.4%)) | 237.9 ± 144.4 | 0.85 |
| T2 (16 (27.1%)) | 338.3 ± 330.2 | ||
| T3 (5 (8.5%)) | 226.0 ± 145.1 | ||
| T4 (13 (22.0)) | 293.1 ± 258.5 | ||
| TNM Stage | I (19 (30.6%)) | 232.9 ± 150.7 | 0.82 |
| II (9 (14.5%)) | 396.8 ± 404.9 | ||
| III (11 (17.2%)) | 239.1 ± 180.5 | ||
| IV (23 (37.1%)) | 279.1 ± 208.8 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, D.-G.; Woo, B.H.; Lee, B.-J.; Yoon, S.; Cho, Y.; Kim, Y.-D.; Park, H.R.; Song, J.M. Serum Levels of Interleukin-6 and Titers of Antibodies against Porphyromonas gingivalis Could Be Potential Biomarkers for the Diagnosis of Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2019, 20, 2749. https://doi.org/10.3390/ijms20112749
Park D-G, Woo BH, Lee B-J, Yoon S, Cho Y, Kim Y-D, Park HR, Song JM. Serum Levels of Interleukin-6 and Titers of Antibodies against Porphyromonas gingivalis Could Be Potential Biomarkers for the Diagnosis of Oral Squamous Cell Carcinoma. International Journal of Molecular Sciences. 2019; 20(11):2749. https://doi.org/10.3390/ijms20112749
Chicago/Turabian StylePark, Dae-Gun, Bok Hee Woo, Byung-Joo Lee, Sanggyeong Yoon, Youngseuk Cho, Yong-Deok Kim, Hae Ryoun Park, and Jae Min Song. 2019. "Serum Levels of Interleukin-6 and Titers of Antibodies against Porphyromonas gingivalis Could Be Potential Biomarkers for the Diagnosis of Oral Squamous Cell Carcinoma" International Journal of Molecular Sciences 20, no. 11: 2749. https://doi.org/10.3390/ijms20112749
APA StylePark, D.-G., Woo, B. H., Lee, B.-J., Yoon, S., Cho, Y., Kim, Y.-D., Park, H. R., & Song, J. M. (2019). Serum Levels of Interleukin-6 and Titers of Antibodies against Porphyromonas gingivalis Could Be Potential Biomarkers for the Diagnosis of Oral Squamous Cell Carcinoma. International Journal of Molecular Sciences, 20(11), 2749. https://doi.org/10.3390/ijms20112749






