High-Pressure Carbon Monoxide and Oxygen Mixture is Effective for Lung Preservation
Abstract
1. Introduction
2. Results
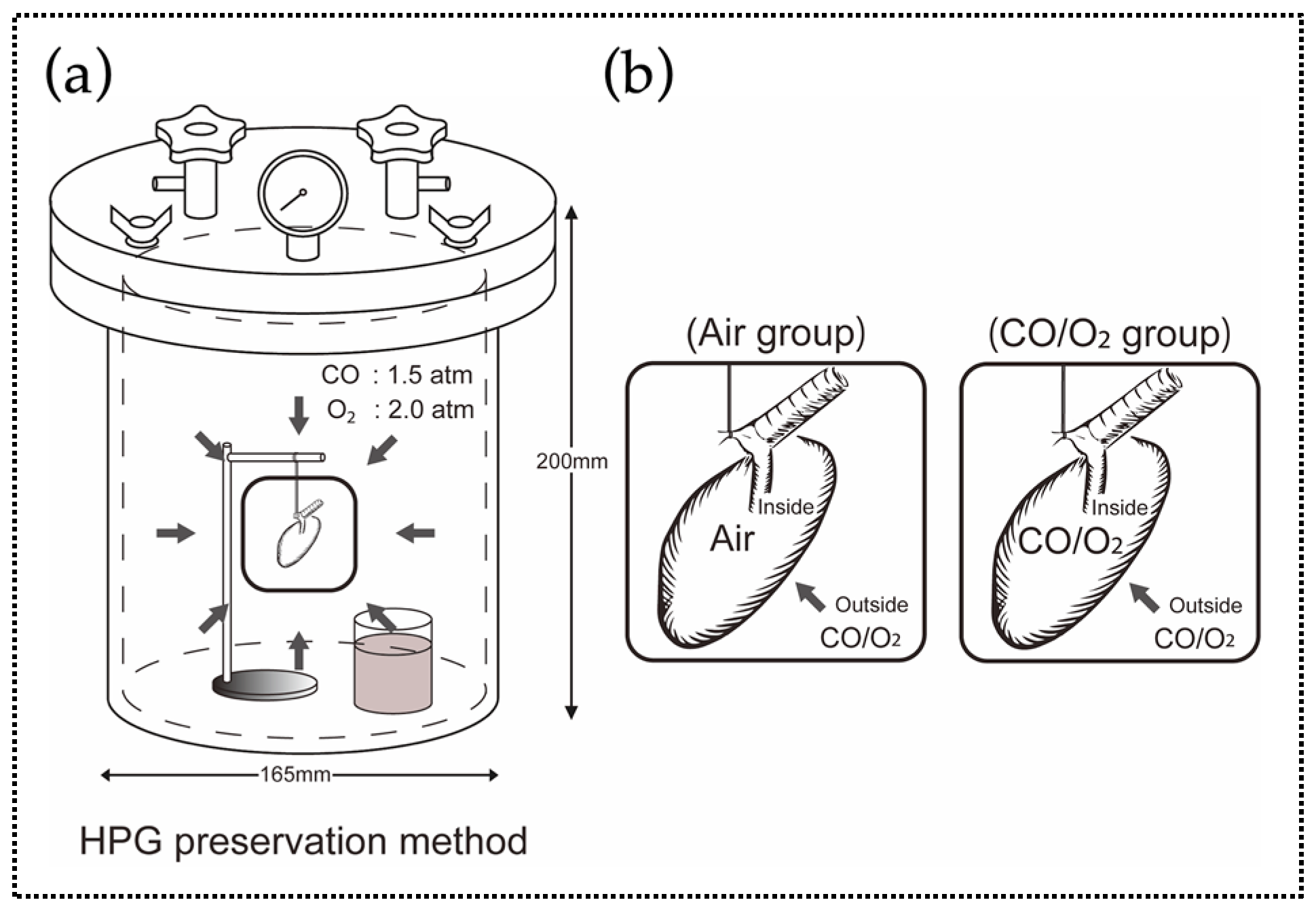
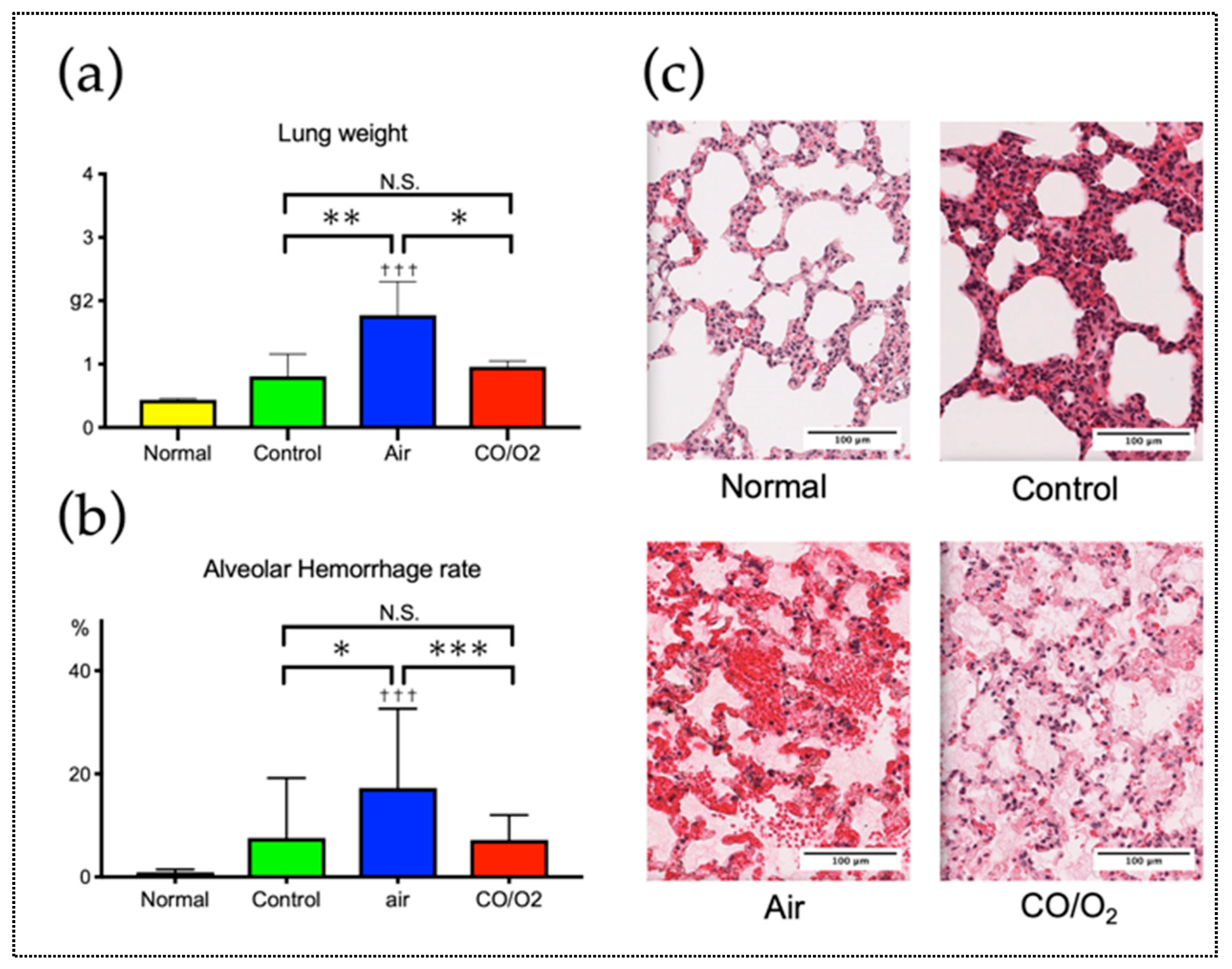
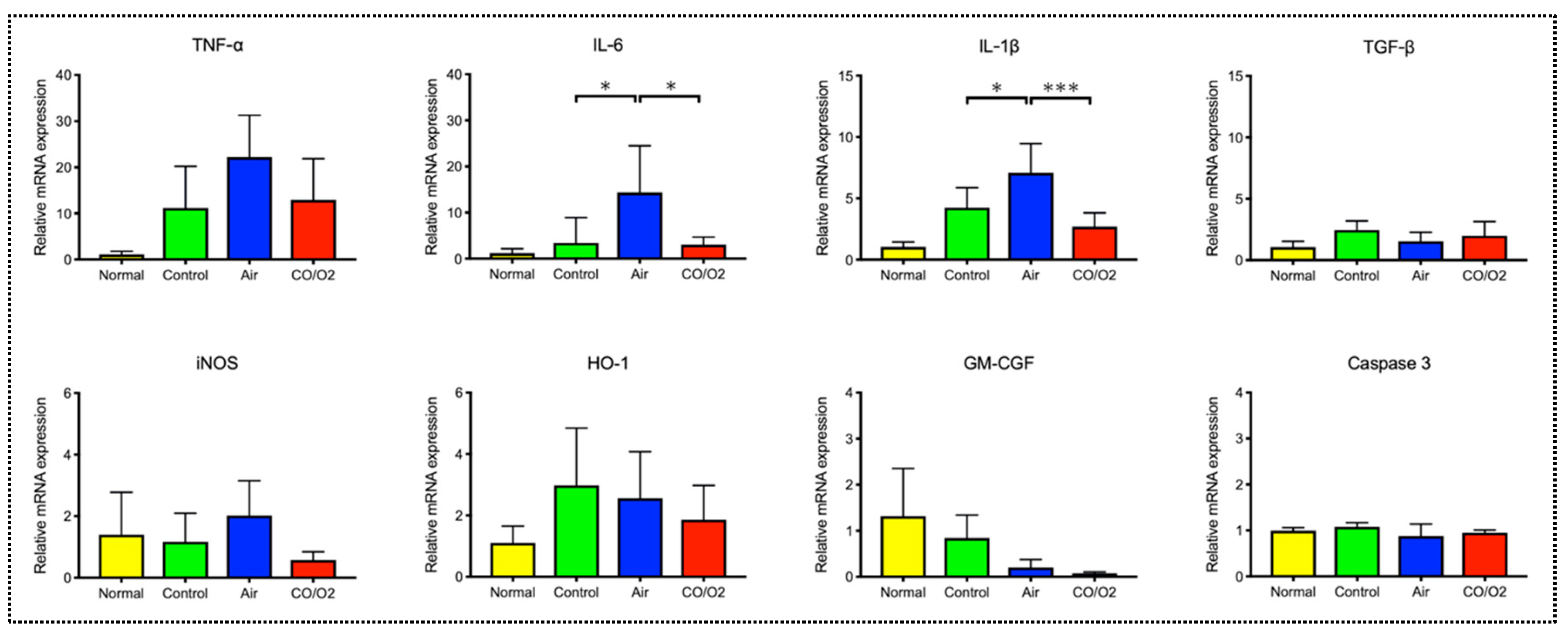
2.1. Experiment 1. Comparison of the Preservation Status of Rat Donor Lungs Filled and Preserved with Mixture of CO and O2 or Air
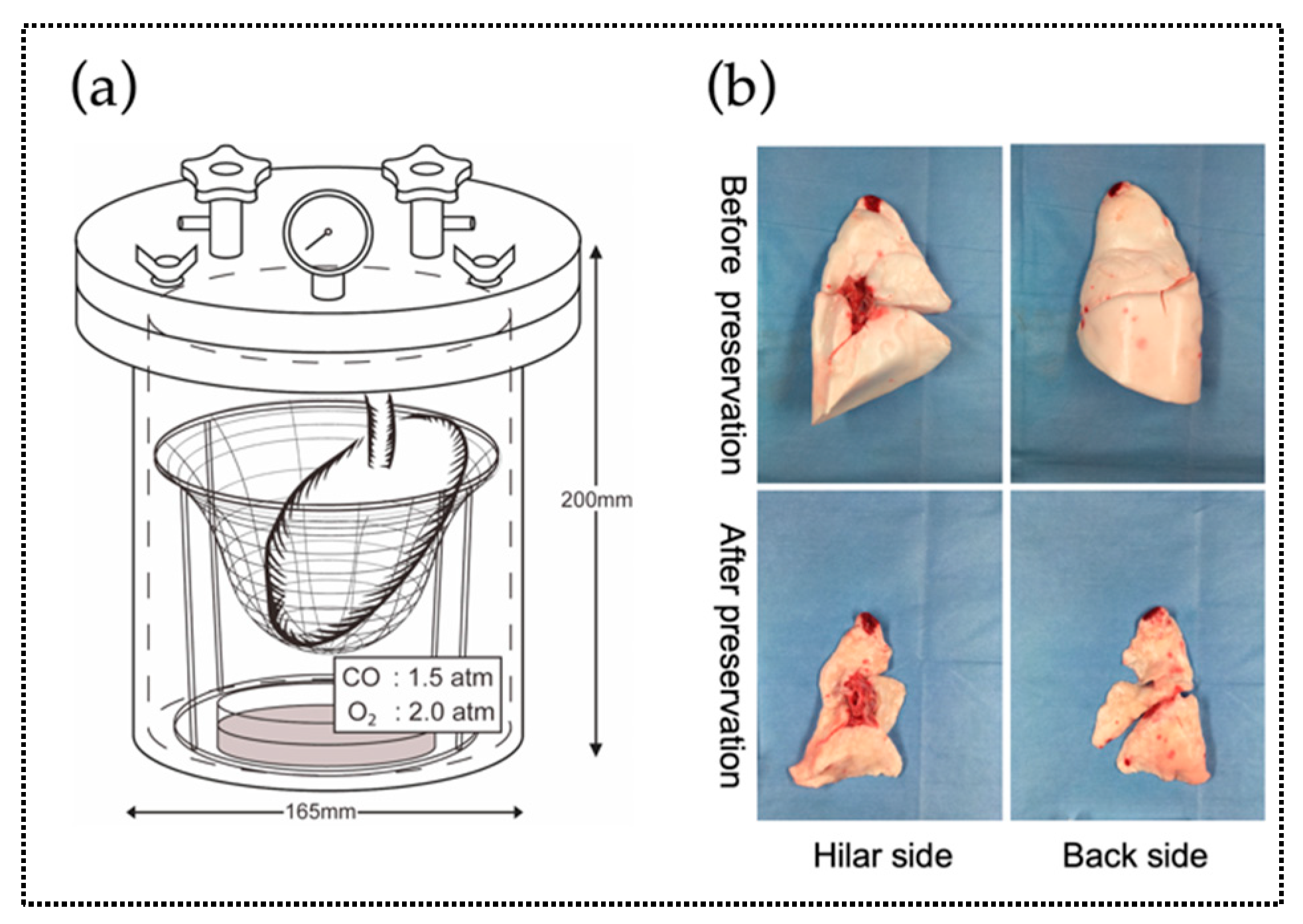
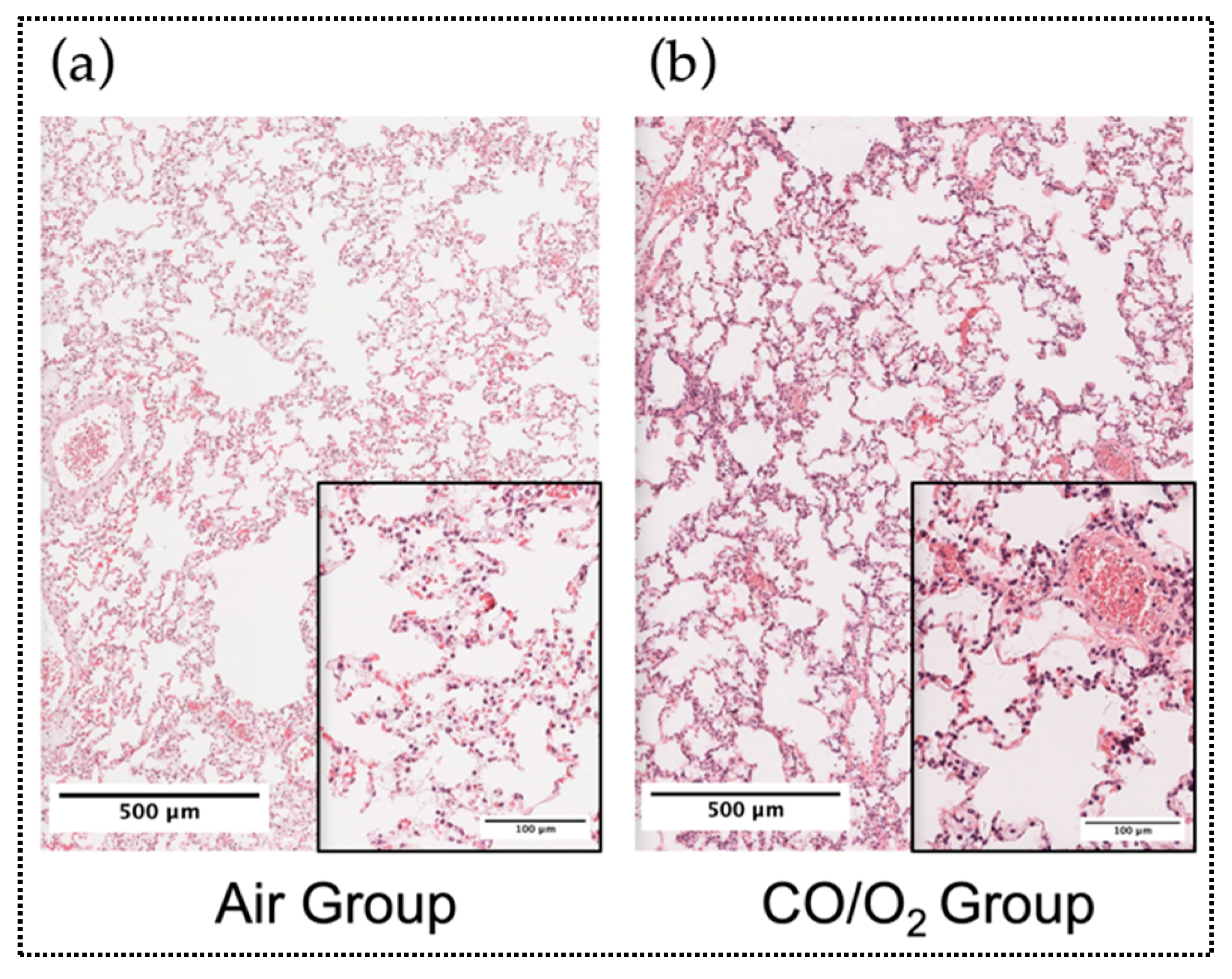
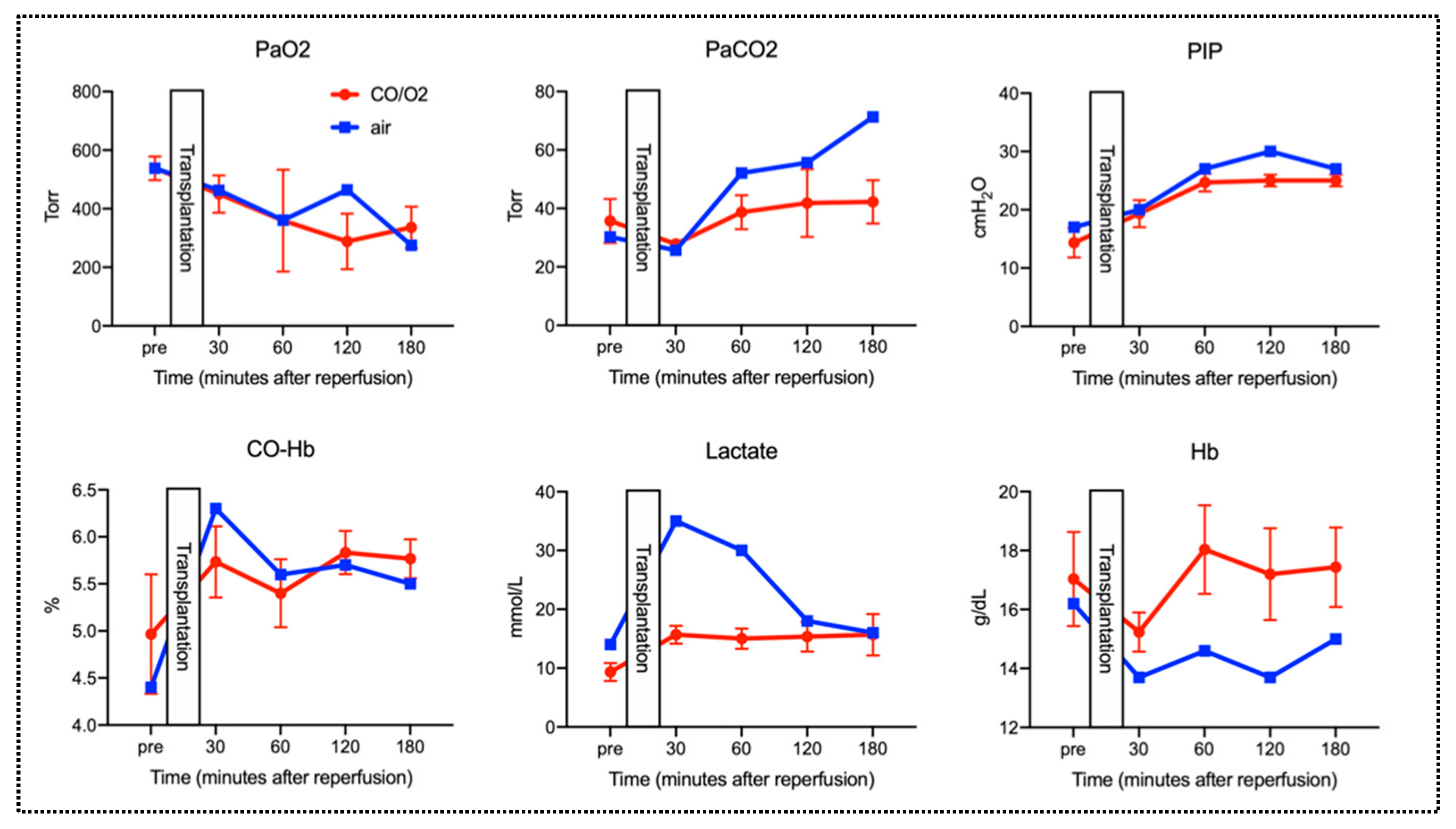
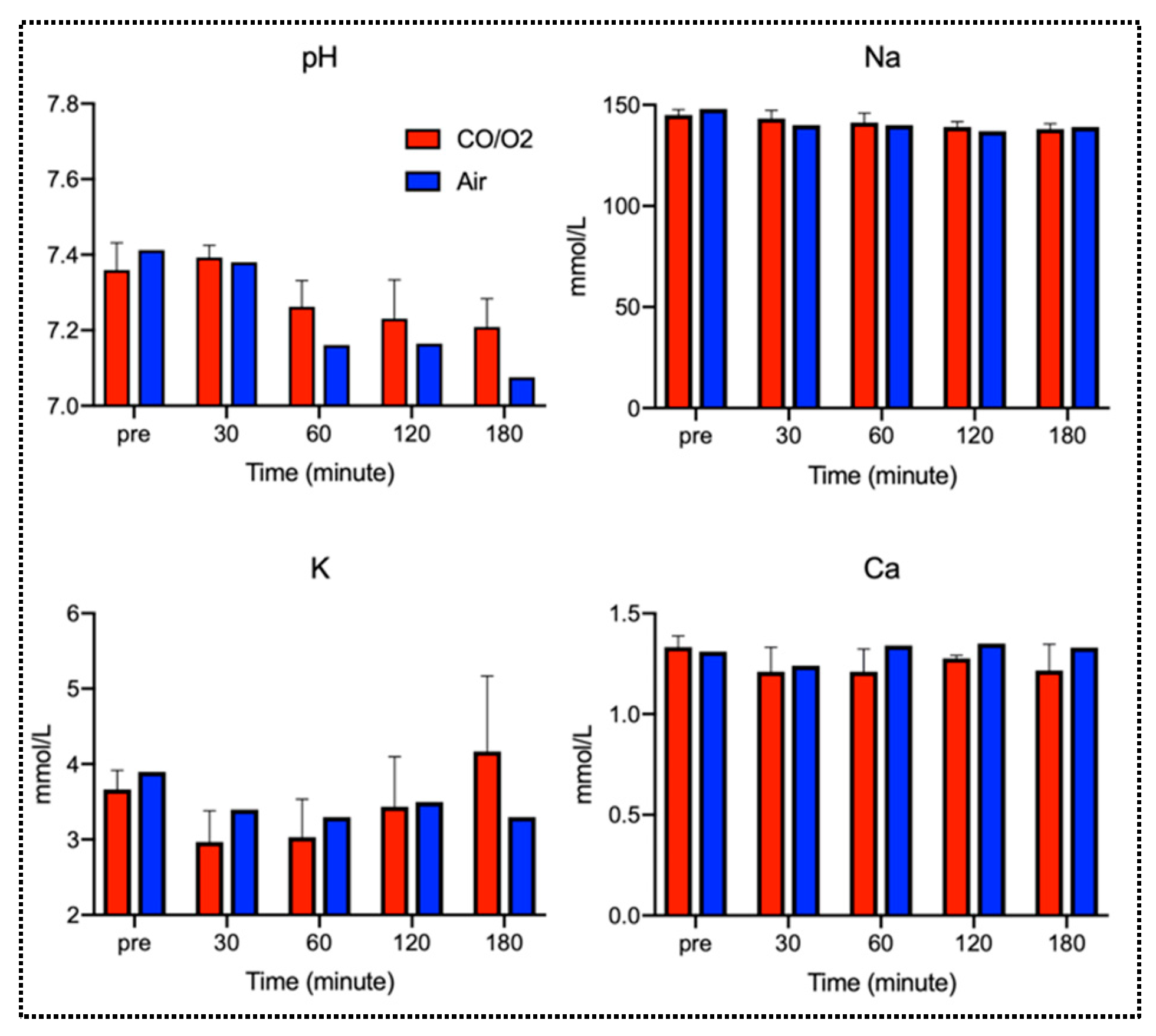
2.2. Experiment 2. Comparison of Microscopic Findings and Arterial Blood Gas (ABG) of Canine Donor Lungs Ventilated From the Inside With Different Gases and Preserved in a High-Pressure CO/O2 Gas Mixture
3. Discussion
4. Materials and Methods
4.1. Experiment 1. Rat Lung Preservation for 24 h Using the HPG Preservation Method
4.1.1. Animals
4.1.2. Anesthesia, Extraction, and Preservation of Donor Lungs, and Ectopic Lung Transplantation
4.1.3. Light Microscopy
4.1.4. Analysis for Gene Expression Levels of Mediators
4.1.5. Statistical Analysis
4.2. Experiment 2. Canine Lung Preservation for 24 h Using the HPG Preservation Method
4.2.1. Animals
4.2.2. Anesthesia
4.2.3. Extraction of the Donor Lung and Preservation
4.2.4. Allogeneic Lung Transplantation of the Preserved Lung and Functional Evaluation after Reperfusion
4.2.5. Assessment of ABG and CO-Hb Concentration
4.2.6. Light Microscopy
4.2.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CO | Carbon monoxide |
| CO-Hb | Carboxyhemoglobin |
| HO-1 | Heme oxygenase-1 |
| MAPK | Mitogen-activated protein kinase |
| IRI | Ischemia–reperfusion injury |
| HPG | High-pressure gas |
| O2 | Oxygen |
| N2 | Nitrogen |
| RT–PCR | Reverse transcription–polymerase chain reaction |
| IL | Interleukin |
| TGF | Transforming growth factor |
| iNOS | Inducible nitric oxide synthase |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| ABG | Arterial blood gas |
| PaO2 | Partial arterial oxygen pressure |
| PaCO2 | Partial arterial carbon dioxide pressure |
| PCO | Partial carbon monoxide pressure |
| PO2 | Partial oxygen pressure |
| CO2 | Carbon dioxide |
| PIP | Peak inspiratory pressure |
| Hb | Hemoglobin |
| TNF | Tumor necrosis factor |
| ANOVA | Analysis of variation |
| ABP | Arterial blood pressure |
| RIPA | Radioimmunoprecipitation assay |
| SDS-PAGE | SDS-polyacrylamide gel electrophoresis |
| PVDF | Polyvinylidene di fluoride |
References
- Bauer, I.; Pannen, B.H. Bench-to-bedside review: Carbon monoxide—From mitochondrial poisoning to therapeutic use. Crit. Care (Lond. Engl.) 2009, 13, 220. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W.; Otterbein, L.E. Carbon monoxide in biology and medicine. Bioessays News Rev. Mol. Cell. Dev. Biol. 2004, 26, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W.; Choi, A.M. Targeting heme oxygenase-1 and carbon monoxide for therapeutic modulation of inflammation. Transl. Res. J. Lab. Clin. Med. 2016, 167, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shan, P.; Otterbein, L.E.; Alam, J.; Flavell, R.A.; Davis, R.J.; Choi, A.M.; Lee, P.J. Carbon monoxide inhibition of apoptosis during ischemia-reperfusion lung injury is dependent on the p38 mitogen-activated protein kinase pathway and involves caspase 3. J. Biol. Chem. 2003, 278, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shan, P.; Alam, J.; Fu, X.Y.; Lee, P.J. Carbon monoxide differentially modulates stat1 and stat3 and inhibits apoptosis via a phosphatidylinositol 3-kinase/akt and p38 kinase-dependent stat3 pathway during anoxia-reoxygenation injury. J. Biol. Chem. 2005, 280, 8714–8721. [Google Scholar] [CrossRef]
- Kohmoto, J.; Nakao, A.; Stolz, D.B.; Kaizu, T.; Tsung, A.; Ikeda, A.; Shimizu, H.; Takahashi, T.; Tomiyama, K.; Sugimoto, R.; et al. Carbon monoxide protects rat lung transplants from ischemia-reperfusion injury via a mechanism involving p38 mapk pathway. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2007, 7, 2279–2290. [Google Scholar] [CrossRef]
- Mishra, S.; Fujita, T.; Lama, V.N.; Nam, D.; Liao, H.; Okada, M.; Minamoto, K.; Yoshikawa, Y.; Harada, H.; Pinsky, D.J. Carbon monoxide rescues ischemic lungs by interrupting mapk-driven expression of early growth response 1 gene and its downstream target genes. Proc. Natl. Acad. Sci. USA 2006, 103, 5191–5196. [Google Scholar] [CrossRef]
- Fujita, T.; Toda, K.; Karimova, A.; Yan, S.F.; Naka, Y.; Yet, S.F.; Pinsky, D.J. Paradoxical rescue from ischemic lung injury by inhaled carbon monoxide driven by derepression of fibrinolysis. Nat. Med. 2001, 7, 598–604. [Google Scholar] [CrossRef]
- Kohmoto, J.; Nakao, A.; Kaizu, T.; Tsung, A.; Ikeda, A.; Tomiyama, K.; Billiar, T.R.; Choi, A.M.; Murase, N.; McCurry, K.R. Low-dose carbon monoxide inhalation prevents ischemia/reperfusion injury of transplanted rat lung grafts. Surgery 2006, 140, 179–185. [Google Scholar] [CrossRef]
- Meng, C.; Ma, L.; Niu, L.; Cui, X.; Liu, J.; Kang, J.; Liu, R.; Xing, J.; Jiang, C.; Zhou, H. Protection of donor lung inflation in the setting of cold ischemia against ischemia-reperfusion injury with carbon monoxide, hydrogen, or both in rats. Life Sci. 2016, 151, 199–206. [Google Scholar] [CrossRef]
- Meyers, B.F.; de la Morena, M.; Sweet, S.C.; Trulock, E.P.; Guthrie, T.J.; Mendeloff, E.N.; Huddleston, C.; Cooper, J.D.; Patterson, G.A. Primary graft dysfunction and other selected complications of lung transplantation: A single-center experience of 983 patients. J. Thorac. Cardiovasc. Surg. 2005, 129, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Bando, T.; Yamada, T.; Sato, M.; Menjyu, T.; Aoyama, A.; Sato, T.; Chen, F.; Sonobe, M.; Omasa, M.; et al. Clinical application of et-kyoto solution for lung transplantation. Surg. Today 2015, 45, 439–443. [Google Scholar] [CrossRef]
- Kosaka, S.; Ueda, M.; Bando, T.; Liu, C.J.; Hitomi, S.; Wada, H. Ultrastructural damage to the preserved lung and its function after reperfusion. Jpn. J. Thorac. Cardiovasc. Surg. Off. Publ. Jpn. Assoc. Thorac. Surg. Nihon Kyobu Geka Gakkai Zasshi 2002, 50, 6–14. [Google Scholar] [CrossRef]
- Wada, H.; Liu, C.J.; Hirata, T.; Bando, T.; Kosaka, S. Effective 30-hour preservation of canine lungs with modified et-kyoto solution. Ann. Thorac. Surg. 1996, 61, 1099–1105. [Google Scholar] [CrossRef]
- Hatayama, N.; Yoshida, Y.; Seki, K. Seventy-two-hour preservation, resuscitation, and transplantation of an isolated rat heart with high partial pressure carbon monoxide gas (pCO = 400 hpa) and high partial pressure carbon dioxide (pCO2 = 100 hpa). Cell Transplant. 2012, 21, 623. [Google Scholar] [CrossRef]
- Hatayama, N.; Naito, M.; Hirai, S.; Yoshida, Y.; Kojima, T.; Seki, K.; Li, X.K.; Itoh, M. Preservation by desiccation of isolated rat hearts for 48 hours using carbon monoxide (pCO = 4,000 hpa) and oxygen (pO2 = 3,000 hpa). Cell Transplant. 2012, 21, 609–615. [Google Scholar] [CrossRef]
- Hatayama, N.; Inubushi, M.; Naito, M.; Hirai, S.; Jin, Y.N.; Tsuji, A.B.; Seki, K.; Itoh, M.; Saga, T.; Li, X.K. Functional evaluation of rat hearts transplanted after preservation in a high-pressure gaseous mixture of carbon monoxide and oxygen. Sci. Rep. 2016, 6, 32120. [Google Scholar] [CrossRef]
- Abe, T.; Yazawa, K.; Fujino, M.; Imamura, R.; Hatayama, N.; Kakuta, Y.; Tsutahara, K.; Okumi, M.; Ichimaru, N.; Kaimori, J.Y.; et al. High-pressure carbon monoxide preserves rat kidney grafts from apoptosis and inflammation. Lab. Investig. J. Tech. Methods Pathol. 2017, 97, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Hatayama, N.; Hirai, S.; Naito, M.; Terayama, H.; Araki, J.; Yokota, H.; Matsushita, M.; Li, X.K.; Itoh, M. Preservation of rat limbs by hyperbaric carbon monoxide and oxygen. Sci. Rep. 2018, 8, 6627. [Google Scholar] [CrossRef] [PubMed]
- Brouard, S.; Otterbein, L.E.; Anrather, J.; Tobiasch, E.; Bach, F.H.; Choi, A.M.; Soares, M.P. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J. Exp. Med. 2000, 192, 1015–1026. [Google Scholar] [CrossRef]
- Hayashi, S.; Takamiya, R.; Yamaguchi, T.; Matsumoto, K.; Tojo, S.J.; Tamatani, T.; Kitajima, M.; Makino, N.; Ishimura, Y.; Suematsu, M. Induction of heme oxygenase-1 suppresses venular leukocyte adhesion elicited by oxidative stress: Role of bilirubin generated by the enzyme. Circ. Res. 1999, 85, 663–671. [Google Scholar] [CrossRef]
- Sawle, P.; Foresti, R.; Mann, B.E.; Johnson, T.R.; Green, C.J.; Motterlini, R. Carbon monoxide-releasing molecules (co-rms) attenuate the inflammatory response elicited by lipopolysaccharide in raw264.7 murine macrophages. Br. J. Pharmacol. 2005, 145, 800–810. [Google Scholar] [CrossRef]
- Musameh, M.D.; Green, C.J.; Mann, B.E.; Fuller, B.J.; Motterlini, R. Improved myocardial function after cold storage with preservation solution supplemented with a carbon monoxide-releasing molecule (corm-3). J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2007, 26, 1192–1198. [Google Scholar] [CrossRef]
- Babu, D.; Motterlini, R.; Lefebvre, R.A. Co and co-releasing molecules (co-rms) in acute gastrointestinal inflammation. Br. J. Pharmacol. 2015, 172, 1557–1573. [Google Scholar] [CrossRef]
- Rodkey, F.L.; O’Neal, J.D.; Collison, H.A.; Uddin, D.E. Relative affinity of hemoglobin s and hemoglobin a for carbon monoxide and oxygen. Clin. Chem. 1974, 20, 83–84. [Google Scholar]
- Knudsen, L.; Ochs, M. The micromechanics of lung alveoli: Structure and function of surfactant and tissue components. Histochem. Cell Biol. 2018, 150, 661–676. [Google Scholar] [CrossRef]
- Livingstone, D.M.; Smith, K.A.; Lange, B. Scuba diving and otology: A systematic review with recommendations on diagnosis, treatment and post-operative care. Diving Hyperb. Med. 2017, 47, 97–109. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujiwara, A.; Hatayama, N.; Matsuura, N.; Yokota, N.; Fukushige, K.; Yakura, T.; Tarumi, S.; Go, T.; Hirai, S.; Naito, M.; et al. High-Pressure Carbon Monoxide and Oxygen Mixture is Effective for Lung Preservation. Int. J. Mol. Sci. 2019, 20, 2719. https://doi.org/10.3390/ijms20112719
Fujiwara A, Hatayama N, Matsuura N, Yokota N, Fukushige K, Yakura T, Tarumi S, Go T, Hirai S, Naito M, et al. High-Pressure Carbon Monoxide and Oxygen Mixture is Effective for Lung Preservation. International Journal of Molecular Sciences. 2019; 20(11):2719. https://doi.org/10.3390/ijms20112719
Chicago/Turabian StyleFujiwara, Atsushi, Naoyuki Hatayama, Natsumi Matsuura, Naoya Yokota, Kaori Fukushige, Tomiko Yakura, Shintaro Tarumi, Tetsuhiko Go, Shuichi Hirai, Munekazu Naito, and et al. 2019. "High-Pressure Carbon Monoxide and Oxygen Mixture is Effective for Lung Preservation" International Journal of Molecular Sciences 20, no. 11: 2719. https://doi.org/10.3390/ijms20112719
APA StyleFujiwara, A., Hatayama, N., Matsuura, N., Yokota, N., Fukushige, K., Yakura, T., Tarumi, S., Go, T., Hirai, S., Naito, M., & Yokomise, H. (2019). High-Pressure Carbon Monoxide and Oxygen Mixture is Effective for Lung Preservation. International Journal of Molecular Sciences, 20(11), 2719. https://doi.org/10.3390/ijms20112719




