Enhanced Protection of Biological Membranes during Lipid Peroxidation: Study of the Interactions between Flavonoid Loaded Mesoporous Silica Nanoparticles and Model Cell Membranes
Abstract
1. Introduction
2. Results and Discussion
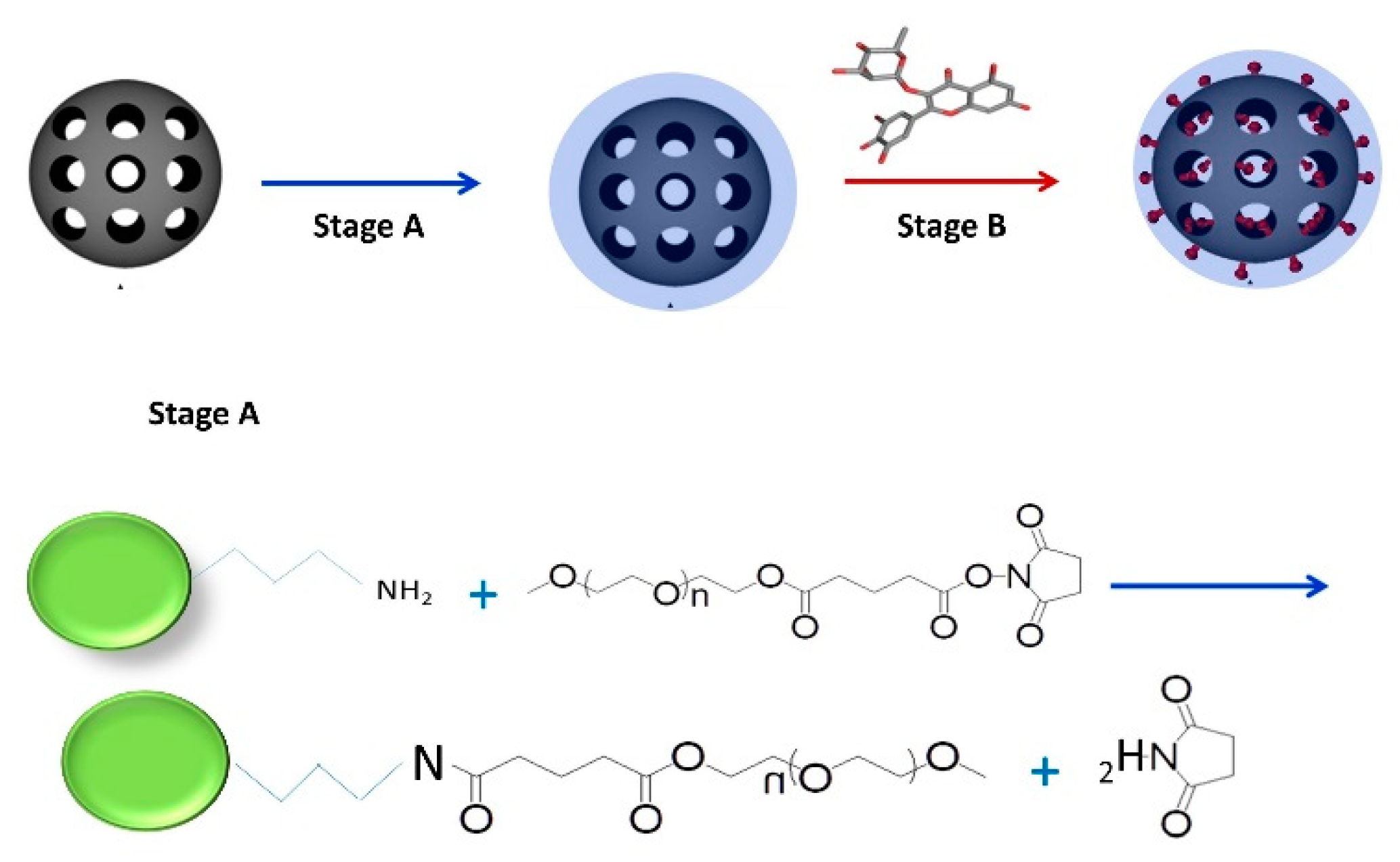
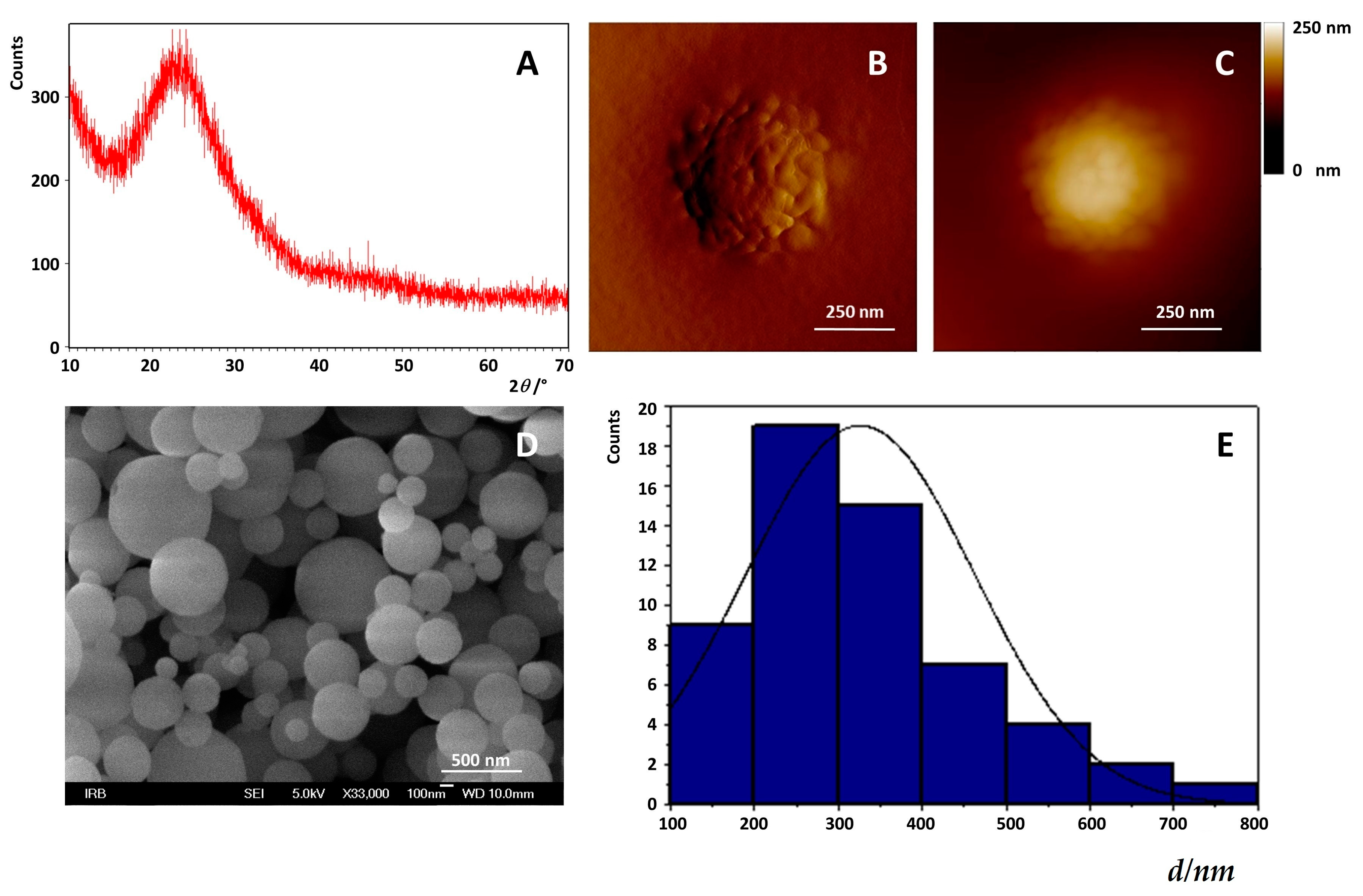
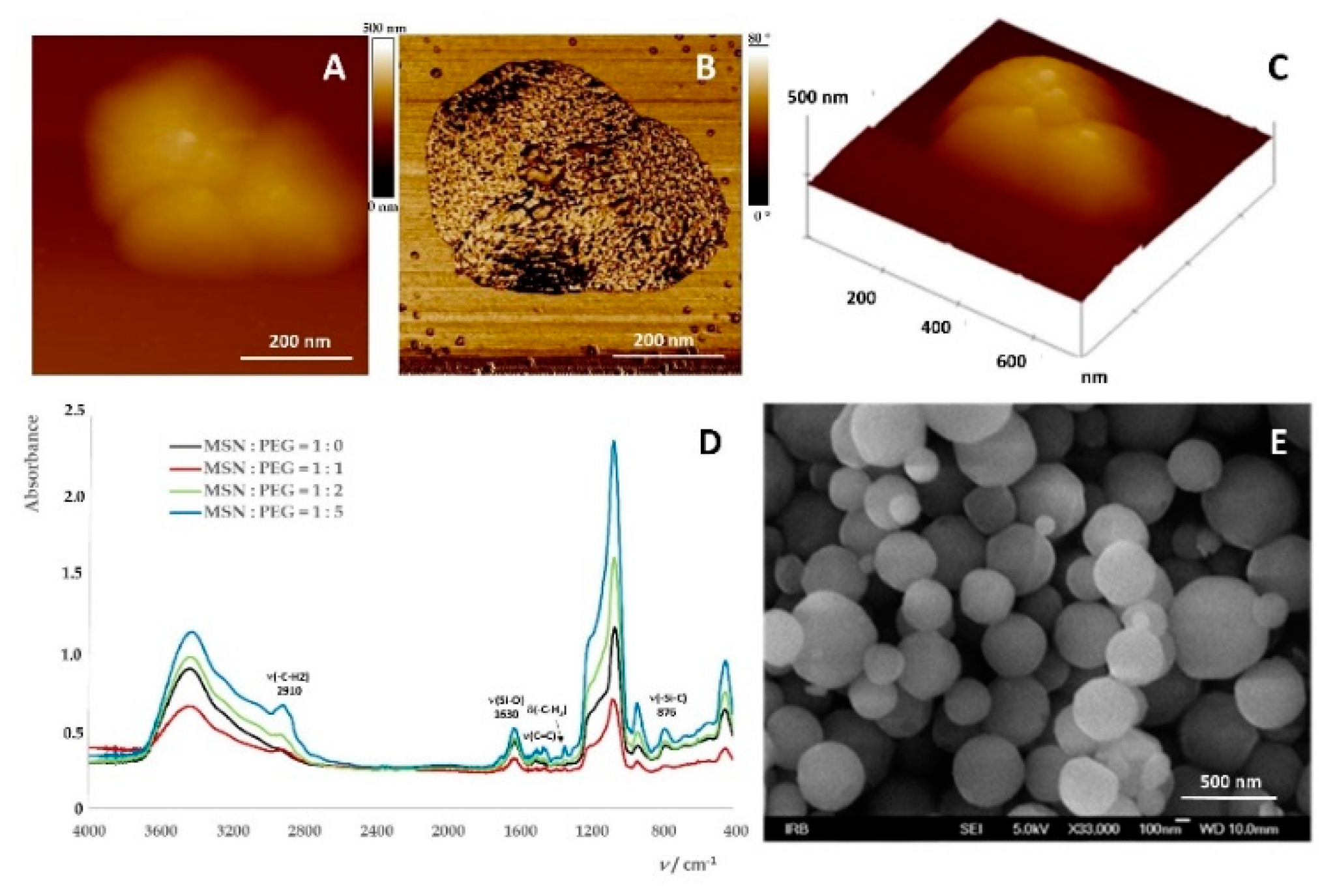
2.1. Preparation of Mesoporous Silica-PEG Nanoparticles (MSNs)
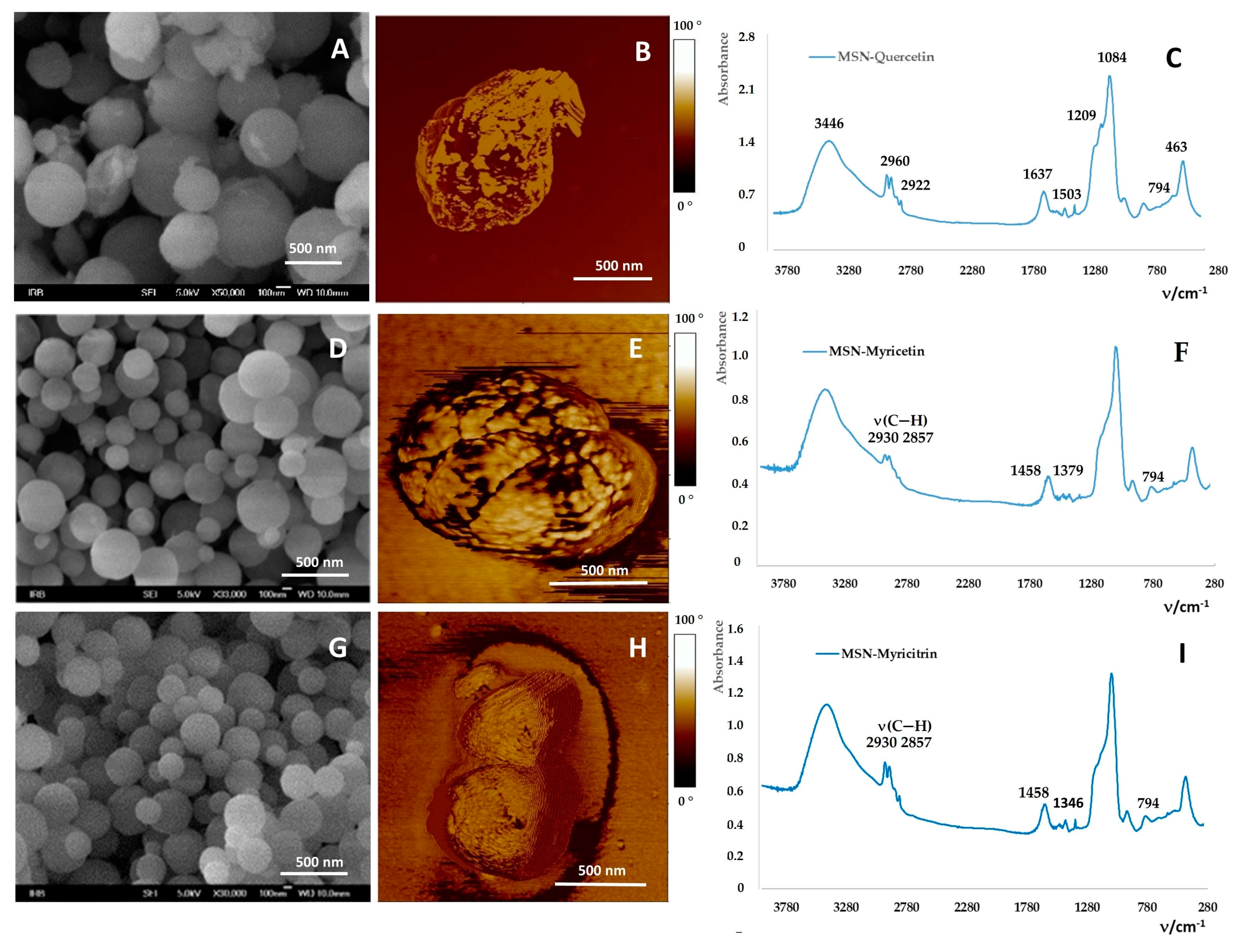
2.2. Loading of Flavonoids into MSNs
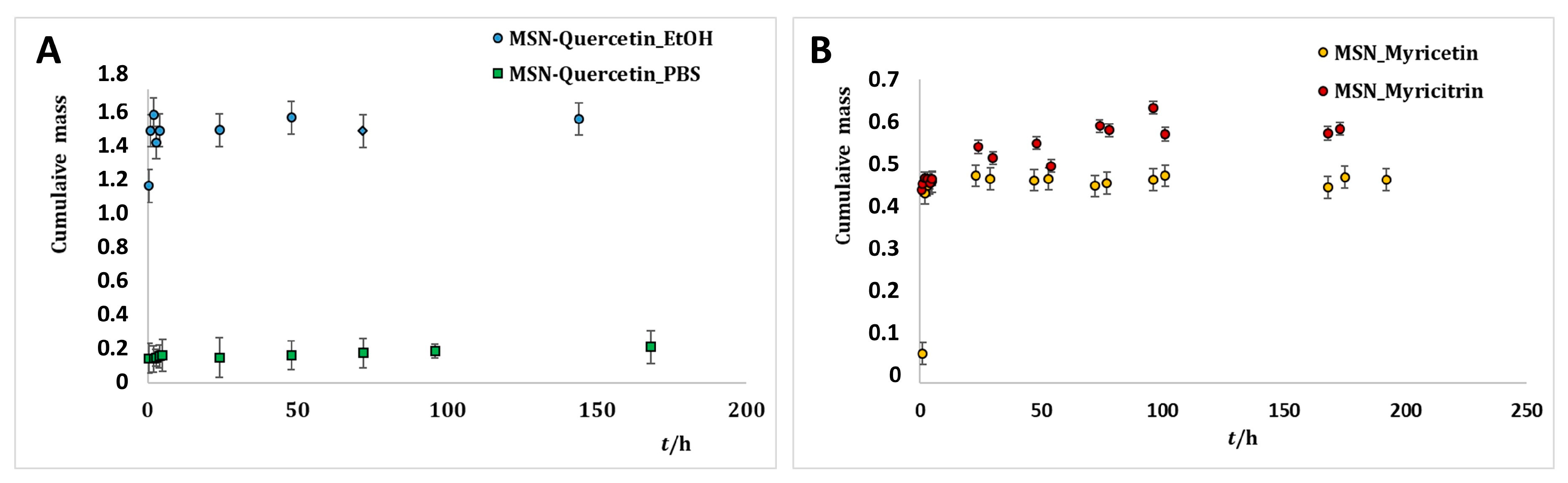
2.3. Release of Flavonoids from MSNs
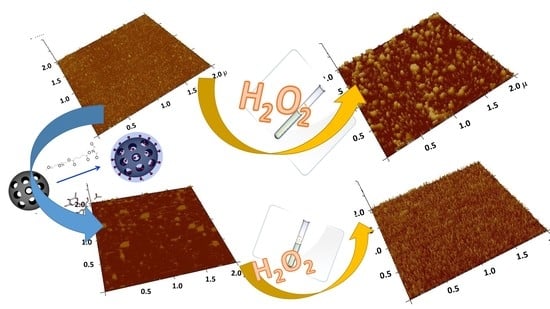
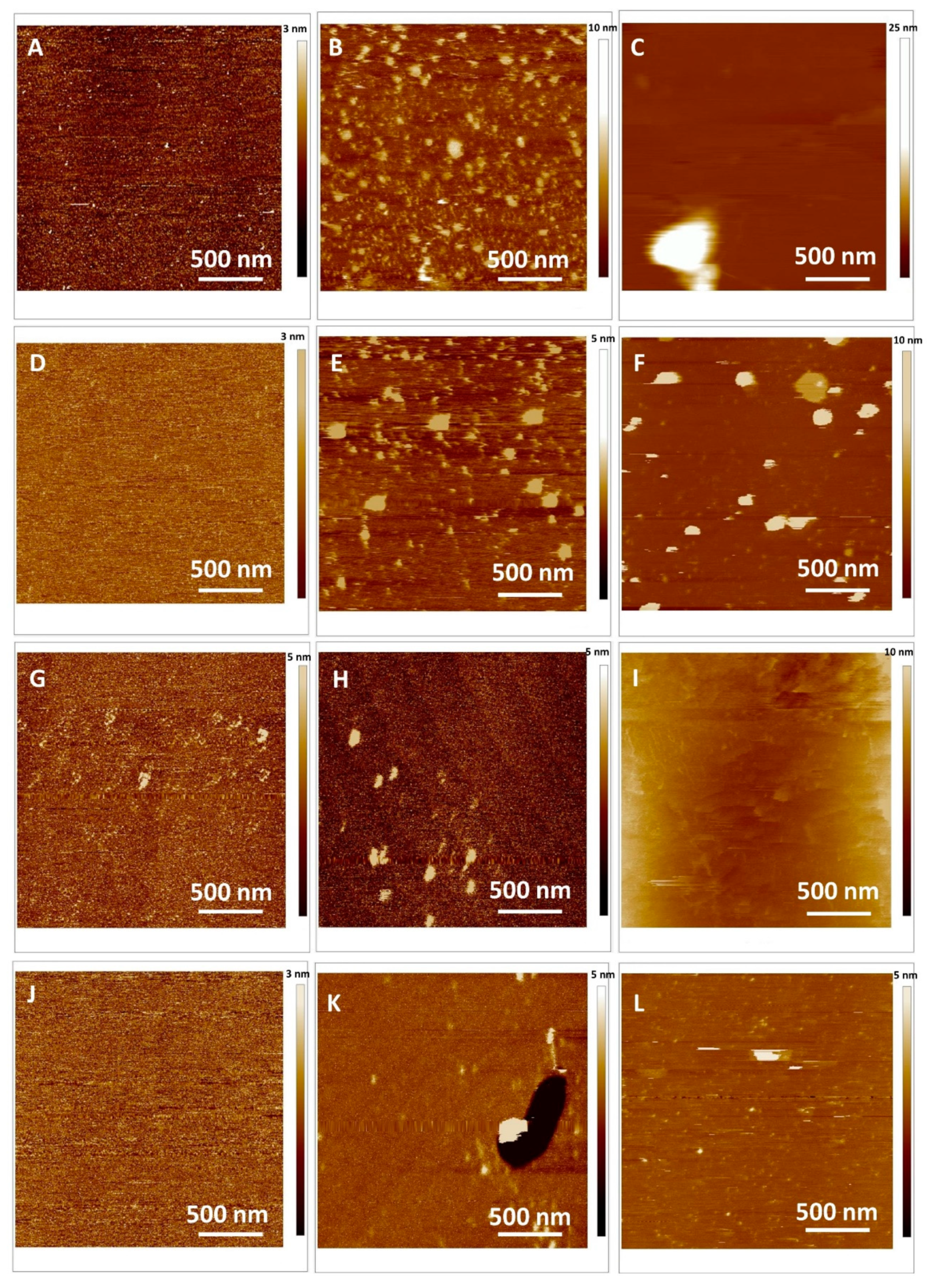
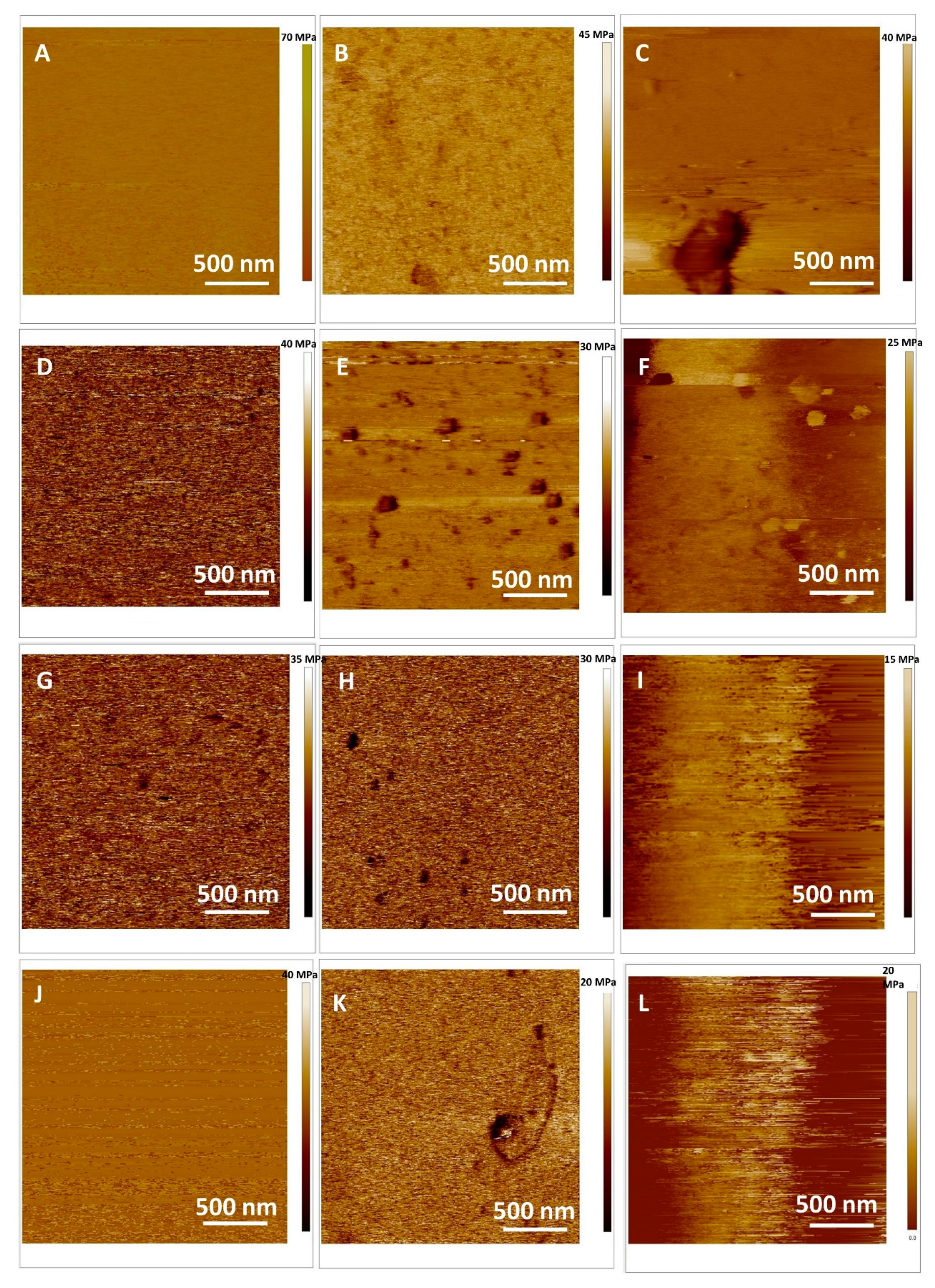
2.4. Interaction of MSNs with Model Cell Membranes
The Protective Role of Released Flavonoids into Model Membranes and Their Protective Role during H2O2 Induced Lipid Peroxidation
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation and Characterization of MSNs
Field Emission Scanning Electron Microscope (FE-SEM)
X-ray Powder Diffraction X-ray Diffraction (XRPD)
Fourier-Transform Infrared Spectroscopy (FTIR Spectroscopy)
Atomic Force Microscopy
3.2.2. Loading and Release Kinetics of Flavonoids from MSNs
UV/VIS Spectroscopy
Zeta Potential Measurements
Brunauer–Emmet–Teller (BET) Analysis for MSNs Porosity Determination
UV/VIS Spectroscopy
3.2.3. Protective Role of Flavonoids during Lipid Peroxidation Induced by Addition of H2O2
Preparation of DOPC Liposome and Supported Lipid Bilayer (SLB) With and Without Inserted Flavonoids
Atomic Force Microscopy Imaging of SLB in Fluid and Force Spectroscopy Before and After Induced Oxidative Stress
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| dH | Hydrodynamic diameter |
| DLS | Dynamic light scattering |
| DOPC | 1,2-dioleoyl-sn-glycero-3-phosphocholine |
| ELS | Electrophoretic light scattering |
| LE | Loading efficiency |
| MLV | Multilamellar vesicles |
| MSNs | Mesoporous silica nanoparticles |
| NPs | Nanoparticles |
| PBS | Phosphate buffer solution |
| SLB | Supported lipid bilayer |
References
- Federico, A.; Cardaioli, E.; Da Pozzo, P.; Formichi, P.; Gallus, G.N.; Radi, E. Mitochondria, oxidative stress and neurodegeneration. J. Neurol. Sci. 2012, 322, 2542–2562. [Google Scholar] [CrossRef] [PubMed]
- Ossola, B.; Kääriäinen, T.M.; Männistö, P.T. The multiple faces of quercetin in neuroprotection. Expert Opin. Drug Saf. 2009, 8, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Slemmer, J.E.; Shacka, J.J.; Sweeney, M.I.; Weber, J.T. Antioxidants and free radical scavengers for the treatment of stroke, traumatic brain injury and aging. Curr. Med. Chem. 2008, 15, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Kelsey, N.A.; Wilkins, H.M.; Linseman, D.I.A. Nutraceutical Antioxidants as Novel Neuroprotective Agents. Molecules 2010, 15, 7779–7814. [Google Scholar] [CrossRef] [PubMed]
- Martins, I.L.; Charneira, C.; Gandin, V.; Ferreira da Silva, J.L.; Justino, G.C.; Telo, J.P.; Vieira, A.J.; Marzano, C.; Antunes, A.M. Selenium-containing chrysin and quercetin derivatives: Attractive scaffolds for cancer therapy. Mdic. Chem. 2015, 58, 4250–4265. [Google Scholar] [CrossRef] [PubMed]
- Li, M.M.; Su, X.Q.; Sun, J.; Gu, Y.F.; Huang, Z.; Zeng, K.W. Anti-inflammatory ursane- and oleanane-type triterpenoids from Vitex negundo var. cannabifolia. J. Nat. Prod. 2014, 77, 2248–2254. [Google Scholar] [CrossRef] [PubMed]
- Hendra, R.; Ahmad, S.; Sukari, A.; Shukor, M.Y.; Oskoueian, E. Flavonoid Analyses and Antimicrobial Activity of Various Parts of Phaleria macrocarpa (Scheff.) Boerl Fruit. Int. J. Mol. Sci. 2011, 12, 3422–3431. [Google Scholar] [CrossRef] [PubMed]
- Baptista, F.I.; Henriques, A.G.; Silva, A.M.S.; Wiltfang, J.; da Cruz e Silva, O.A.B. Flavonoids as Therapeutic Compounds Targeting Key Proteins Involved in Alzheimer’s Disease. ACS Chem. Neurosci. 2014, 5, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.; Tian, J.; Liu, W.; Li, Z.; Li, Y. Poly(Lactic-co-glycolic Acid) Microspheres for the Controlled Release of Huperzine A: In Vitro and in Vivo Studies and the Application in the Treatment of the Impaired Memory of Mice. Chem. Pharm. Bull. 2007, 55, 625–628. [Google Scholar] [CrossRef]
- Pool, H.; Quintanar, D.; de Dios Figueroa, J.; Mano, C.M.; Bechara, J.E.; Godínez, L.A.; Mendoza, S. Antioxidant Effects of Quercetin and Catechin Encapsulated into PLGA Nanoparticles. J. Nanomater. 2012, 2012, 86. [Google Scholar] [CrossRef]
- Baeza, A.; Colilla, M.; Vallet-Regí, M. Advances in mesoporous silica nanoparticles for targeted stimuli-responsive drug delivery. Expert Opin. Drug Deliv. 2015, 12, 319–337. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Kierys, A.; Rawski, M.; Goworek, J. Polymer–silica composite as a carrier of an active pharmaceutical ingredient. Microporous Mesoporous Mater. 2014, 193, 40–46. [Google Scholar] [CrossRef]
- Mahapatro, A.; Singh, D.K. Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J. Nanobiotechnol. 2011, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Authimoolam, S.P.; Hilt, J.Z.; Dziubla, T.D. Quercetin conjugated poly(β-amino esters) nanogels for the treatment of cellular oxidative stress. Acta Biomater. 2015, 27, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Wu, Y.; Liu, Y.; Wu, D. High drug-loading nanomedicines: Progress, current status, and prospects. Int. J. Nanomed. 2017, 31, 4085–4109. [Google Scholar] [CrossRef]
- Das, D.K.; Chakraborty, A.; Bhattacharjee, S.; Dey, S. Biosynthesis of stabilised gold nanoparticle using an aglycone flavonoid, quercetin. J. Exp. Nanosci. 2013, 8, 649–655. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A.; Angulo, Y. Biofabrication of copper oxide nanoparticles using Andean blackberry (Rubus glaucus Benth.) fruit and leaf. J. Saudi Chem. Soc. 2017, 21, S475–S480. [Google Scholar] [CrossRef]
- Kurepa, J.; Nakabayashi, R.; Paunesku, T.; Suzuki, M.; Saito, K.; Woloschak, G.E.; Smalle, J.A. Direct isolation of flavonoids from plants using ultra-small anatase TiO2 nanoparticles. Plant J. 2014, 77, 443–453. [Google Scholar] [CrossRef]
- Bennet, D.; Kim, S. Polymer Nanoparticles for Smart Drug Delivery. In Application of Nanotechnology in Drug Delivery; InTech: London, UK, 2014. [Google Scholar]
- Sapino, S.; Ugazio, E.; Gastaldi, L.; Miletto, I.; Berlier, G.; Zonari, D.; Oliaro-Bosso, S. Mesoporous silica as topical nanocarriers for quercetin: Characterization and in vitro studies. Eur. J. Pharm. Biopharm. 2015, 89, 116–125. [Google Scholar] [CrossRef]
- AbouAitah, K.E.A.; Farghali, A.A.; Swiderska-Sroda, A.; Lojkowski, W.; Razin, A.M.; Khedr, M.H. Mesoporous Silica Materials in Drug Delivery System: pH/Glutathione-Responsive Release of Poorly Water-Soluble Pro-drug Quercetin from Two and Three-dimensional Pore-Structure Nanoparticles. J. Nanomed. Nanotechnol. 2016, 7, 2–12. [Google Scholar] [CrossRef]
- Huang, X.; Young, N.P.; Townley, H.E. Characterization and Comparison of Mesoporous Silica Particles for Optimized Drug Delivery. Nanomater. Nanotechnol. 2014, 4, 1–15. [Google Scholar] [CrossRef]
- Dave, P.N.; Chopda, L.V. A Review on Application of Multifunctional Mesoporous Nanoparticles in Controlled Release of Drug Delivery. Mater. Sci. Forum 2014, 781, 17–24. [Google Scholar] [CrossRef]
- Han, N.; Wang, Y.; Bai, J.; Liu, J.; Wang, Y.; Gao, Y.; Jiang, T.; Kang, W.; Wang, S. Facile synthesis of the lipid bilayer coated mesoporous silica nanocomposites and their application in drug delivery. Microporous Mesoporous Mater. 2016, 219, 209–218. [Google Scholar] [CrossRef]
- Nday, C.M.; Halevas, E.; Jackson, G.E.; Salifoglou, A. Quercetin encapsulation in modified silica nanoparticles: Potential use against Cu(II)-induced oxidative stress in neurodegeneration. J. Inorg. Biochem. 2015, 145, 51–56. [Google Scholar] [CrossRef]
- Jazvinšćak Jembrek, M.; Vuković, L.; Puhović, J.; Erhardt, J.; Oršolić, N. Neuroprotective Effect of Quercetin Against Hydrogen Peroxide-induced Oxidative Injury in P19 Neurons. J. Mol. Neurosci. 2012, 47, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Jazvinšćak Jembrek, M.; Vlainić, J.; Čadež, V.; Šegota, S. Atomic force microscopy reveals new biophysical markers for monitoring subcellular changes in oxidative injury: Neuroprotective effects of quercetin at the nanoscale. PLoS ONE 2018, 13, e0200119. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-M.; Kim, B.C.; Cho, Y.-H.; Choi, K.-H.; Chang, J.; Park, M.-S.; Kim, M.-K.; Cho, K.-H.; Kim, J.-K. Effects of Flavonoid Compounds on β-amyloid-peptide-induced Neuronal Death in Cultured Mouse Cortical Neurons. Chonnam Med. J. 2014, 50, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Domitrović, R.; Rashed, K.; Cvijanović, O.; Vladimir-Knežević, S.; Škoda, M.; Višnić, A. Myricitrin exhibits antioxidant, anti-inflammatory and antifibrotic activity in carbon tetrachloride-intoxicated mice. Chem. Biol. Interact. 2015, 230, 21–29. [Google Scholar] [CrossRef]
- Knekt, P.; Kumpulainen, J.; Järvinen, R.; Rissanen, H.; Heliövaara, M.; Reunanen, A.; Hakulinen, T.; Aromaa, A. Flavonoid intake and risk of chronic diseases. Am. J. Clin. Nutr. 2002, 76, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Shimosaki, S.; Tsurunaga, Y.; Itamura, H.; Nakamura, M. Anti-allergic effect of the flavonoid myricitrin from Myrica rubra leaf extracts in vitro and in vivo. Nat. Prod. Res. 2011, 25, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Meotti, F.C. Analysis of the Antinociceptive Effect of the Flavonoid Myricitrin: Evidence for a Role of the L-Arginine-Nitric Oxide and Protein Kinase C Pathways. J. Pharmacol. Exp. Ther. 2005, 316, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Margina, D.; Ilie, M.; Manda, G.; Neagoe, I.; Mocanu, M.; Ionescu, D.; Gradinaru, D.; Ganea, G. Quercetin and epigallocatechin gallate effects on the cell membranes biophysical properties correlate with their antioxidant potential. Gen. Physiol. Biophys. 2012, 31, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Pawlikowska-Pawlęga, B.; Dziubińska, H.; Król, E.; Trębacz, K.; Jarosz-Wilkołazka, A.; Paduch, R.; Gawron, A.; Gruszecki, W.I. Characteristics of quercetin interactions with liposomal and vacuolar membranes. Biochim. Biophys. Acta 2014, 1838, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Londoño-Londoño, J.; De Lima, V.R.; Jaramillo, C.; Creczynski-pasa, T. Hesperidin and hesperetin membrane interaction: Understanding the role of 7-O-glycoside moiety in flavonoids. Arch. Biochem. Biophys. 2010, 499, 6–16. [Google Scholar] [CrossRef]
- Arczewska, M.; Kamiński, D.M.; Górecka, E.; Pociecha, D.; Rój, E.; Sławińska-Brych, A.; Gagoś, M. The molecular organization of prenylated flavonoid xanthohumol in DPPC multibilayers: X-ray diffraction and FTIR spectroscopic studies. Biochim. Biophys. Acta 2013, 1828, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Freudenthal, O.; Quilès, F.; Francius, G.; Wojszko, K.; Gorczyc, M.; Korchowiec, B.; Rogalska, E. Nanoscale investigation of the interaction of colistin with model phospholipid membranes by Langmuir technique, and combined infrared and force spectroscopies. Biochim. Biophys. Acta 2016, 1858, 2592–2602. [Google Scholar] [CrossRef]
- Selvaraj, S.; Krishnaswamy, S.; Devashya, V.; Sethuraman, S.; Krishnan, U.M. Influence of membrane lipid composition on flavonoid-membrane interactions: Implications on their biological activity. Prog. Lipid Res. 2015, 58, 1–13. [Google Scholar] [CrossRef]
- Attwood, S.J.; Choi, Y.; Leonenko, Z. Preparation of DOPC and DPPC Supported Planar Lipid Bilayers for Atomic Force Microscopy and Atomic Force Spectroscopy. Int. J. Mol. Sci. 2013, 14, 3514–3539. [Google Scholar] [CrossRef]
- Jazvinšćak Jembrek, M.; Simic, G.; Hof, P.; Šegota, S. Atomic force microscopy as an advanced tool in neuroscience. Transl. Neurosci. 2015, 6, 117–130. [Google Scholar] [CrossRef]
- Ollila, F.; Halling, K.; Vuorela, P.; Vuorela, H.; Slotte, J.P. Characterization of Flavonoid–Biomembrane Interactions. Arch. Biochem. Biophys. 2002, 399, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Tushiya, H. Membrane Interactions of Phytochemicals as Their Molecular Mechanism Applicable to the Discovery of Drug Leads from Plants. Molecules 2015, 20, 18923–18966. [Google Scholar] [CrossRef]
- Arora, A.; Byrem, T.M.; Nair, M.G.; Strasburg, G.M. Modulation of liposomal membrane fluidity by flavonoids and isoflavonoids. Arch. Biochem. Biophys. 2000, 373, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Soto-Cantu, E.; Cueto, R.; Koch, J.; Russo, P.S. Synthesis and Rapid Characterization of Amine-Functionalized Silica. Langmuir 2012, 28, 5562–5569. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qi, X.R.; Maitani, Y.; Nagai, T. PEG-PLA diblock copolymer micelle-like nanoparticles as all-trans-retinoic acid carrier: In vitro and in vivo characterizations. Nanotechnology 2009, 20. [Google Scholar] [CrossRef]
- Rio-Echevarria, I.M.; Selvestrel, F.; Segat, D.; Guarino, G.; Tavano, R.; Causin, V.; Reddi, E.; Papini, E.; Mancin, F. Highly PEGylated silica nanoparticles: “ready to use” stealth functional nanocarriers. J. Mater. Chem. 2010, 20, 2780–2787. [Google Scholar] [CrossRef]
- Allen, T.M.; Hansen, C.; Martin, F.; Redemann, C.; Yau-Young, A. Liposomes containing synthetic lipid derivatives of poly(ethylene glycol) show prolonged circulation half-lives in vivo. Biochim. Biophys. Acta 1991, 1066, 29–36. [Google Scholar] [CrossRef]
- Lasic, D.D.; Woodle, M.C.; Martin, F.J.; Valentincic, T. Phase behavior of “stealth-lipid”-lecithin mixtures. Period. Biol. 1991, 93, 287–290. [Google Scholar] [CrossRef]
- Jeon, S.I.; Lee, J.H.; Andrade, J.D.; De Gennes, P.G. Protein—surface interactions in the presence of polyethylene oxide: I. Simplified theory. J. Colloid Interface Sci. 1991, 142, 149–158. [Google Scholar] [CrossRef]
- Catauro, M.; Papale, F.; Bollino, F.; Piccolella, S.; Marciano, S.; Nocera, P.; Pacifico, S. Silica/quercetin sol–gel hybrids as antioxidant dental implant materials. Sci. Technol. Adv. Mater. 2015, 16, 035001–035012. [Google Scholar] [CrossRef]
- Li, Y.; Jao, J.; Han, C.; Yang, J.; Tabassum Chaudhry, M.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Franklin, S.J.; Myrdal, P.B. Solid-State and Solution Characterization of Myricetin, AAPS. Pharm. Sci. Tech. 2015, 16, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://comptox.epa.gov/dashboard/dsstoxdb/results?search=DCYOADKBABEMIQ#toxval (accessed on 14 April 2018)Myricitrin 17912-87-7 | DTXSID40170771.
- Maity, P.; Saha, B.; Suresh Kumar, G.; Karmakar, S. Binding of monovalent alkali metal ions with negatively chargedphospholipid membranes. Biochim. Biophys. Acta 2016, 1858, 706–714. [Google Scholar] [CrossRef]
- Harris, C.S.; Mo, F.; Migahed, L.; Chepelev, L.; Haddad, P.S.; Wright, J.S.; Willmore, W.G.; Arnason, J.T.; Bennett, S.A.L. Plant phenolics regulate neoplastic cell growth and survival: A quantitative structure–activity and biochemical analysis. Can. J. Physiol. Pharmacol. 2007, 85, 1124–1138. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Diduk, R.; Ramirez-Silva, M.T.; Galano, A.; Merkoci, A. Deprotonation mechanism and acidity constants in aqueous solution of flavonols. A combined experimental and theoretical study. J. Phys. Chem. B 2013, 117, 12347–12359. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Wang, T.; Zhao, T.; Wang, Y.; Pang, B. Study of the interaction of salmon sperm DNA with myricitrin–CPB based on the enhanced resonance light scattering signal and its potential application, Spectrochimica Acta Part A: Molecular and Biomolecular. Spectroscopy 2013, 112, 397–402. [Google Scholar] [CrossRef]
- Engel, A.; Schoenenberger, C.-A.; Müller, D.J. High Resolution Imaging of Native Biological Sample Surfaces Using Scanning Probe Microscopy. Curr. Opin. Struct. Biol. 1997, 7, 279–284. [Google Scholar] [CrossRef]
- Santoro, A.L.; Carrilho, E.; Lancas, F.M.; Montanari, C.A. Quantitative structure–retention relationships of flavonoids unraveled by immobilized artificial membrane chromatography. Eur. J. Pharm. Sci. 2016, 88, 147–157. [Google Scholar] [CrossRef]
- Serra, H.; Mendes, T.; Bronze, M.R.; Simplicio, A.L. Prediction of intestinal absorption and metabolism of pharmacologically active flavones and flavanones. Bioorg. Med. Chem. 2008, 16, 4009–4018. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.S.; Lee, N.; Kim, T.; Kim, H.; Yu, T.; Song, I.C.; Moon, W.K.; Hyeon, T. Multifunctional Uniform Nanoparticles Composed of a Magnetite Nanocrystal Core and a Mesoporous Silica Shell for Magnetic Resonance and Fluorescence Imaging and for Drug Delivery. Angew. Chem. Int. Ed. 2008, 47, 8438–8441. [Google Scholar] [CrossRef]
- Šegota, S.; Vojta, D.; Pletikapić, G.; Baranović, G. Ionic strength and composition govern the elasticity of biological membranes. Chem. Phys. Lipids 2015, 186, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Šegota, S.; Vojta, D.; Kendziora, D.; Ahmed, I.; Fruk, L.; Baranović, G. Ligand-Dependent Nanoparticle Clustering within Lipid Membranes Induced by Surrounding Medium. J. Phys. Chem. B 2015, 119, 5208–5219. [Google Scholar] [CrossRef] [PubMed]
- Roa, J.J.; Oncins, G.; Diaz, J.; Sanz, F.; Segarra, M. Calculation of Young’s modulus value by means of AFM. Recent Pat. Nanotechnol. 2011, 5, 27–36. [Google Scholar] [CrossRef] [PubMed]
| Powder MSN | |
|---|---|
| Specific surface/m2 g−1 | 693.78 |
| Pore volume/cm3 g−1 | 0.84 |
| Pore size/nm | 4.82 |
| Zeta potential/mV | +26 ± 2 |
| dH/nm | 913 ± 180 |
| 1d/nm | 326 ± 137 |
| MSN_PEG5000 | |
|---|---|
| Zeta potential/mV | +27 ± 1 |
| dH/nm | 932 ± 91 |
| Quercetin | Myricitrin | Myricetin | |
|---|---|---|---|
| Specific surface area/m2 g−1 | 544.58 | 546.01 | 562.71 |
| Pore volume/cm3 g−1 | 0.6404 | 0.6527 | 0.73 |
| Pore size/nm LE (%) | 4.70 27 ± 9 (n = 6) | 4.78 8.6 ± 0.6 (n = 3) | 3.12 4 ± 2 (n = 5) |
| Sample | DOPC | DOPC/Quercetin Loaded MSNs | DOPC/Myricetin Loaded MSNs | DOPC/Myricitrin Loaded MSNs |
|---|---|---|---|---|
| 1ζ/mV | −6.1 ± 1.1 | −14.4 ± 4.7 | −6.7 ± 1.3 | −11.8 ± 3.2 |
| 2ζ/mV | −17.8 ± 6 | −8.1 ± 1.4 | −10.4 ± 2.2 | −6.3 ± 2 |
| Sample | Ra/nm | ΔRa/nm | E/MPa | ΔE/MPa | d/nm |
|---|---|---|---|---|---|
| Control DOPC | 0.08 ± 0.01 | 63.7 ± 5.2 | 7.1 ± 0.3 (n = 256) | ||
| Control DOPC/H2O2 | 0.33 ± 0.05 | +0.25 ± 0.06 | 41.5 ± 3.9 | −22.2 ± 9.1 | 6.9 ± 0.2 (n = 201) |
| Control DOPC/H2O2 + Cu2+ | 0.84 ± 0.02 | +0.76 ± 0.01 | 38.2 ± 4.1 | −25.5 ± 9.3 | 6.7 ± 0.5 (n = 322) |
| DOPC/Quercetin | 0.11 ± 0.05 | 40.6 ± 2.7 | 7.2 ± 0.4 (n = 276) | ||
| DOPC/Quercetin/H2O2 | 0.18 ± 0.06 | +0.07 ± 0.11 | 35.5 ± 1.6 | −5.1 ± 4.3 | 7.0 ± 0.3 (n = 151) |
| DOPC/Quercetin/H2O2+ Cu2+ | 0.81 ± 0.17 | +0.70 ± 0.22 | 16.6 ± 7.4 | −9.2 ± 9.0 | 6.8 ± 0.5 (n = 123) |
| DOPC/Myricetin | 0.12 ± 0.06 | 31.4 ± 2.9 | 7.2 ± 0.6 (n = 99) | ||
| DOPC/Myricetin/H2O2 | 0.22 ± 0.02 | +0.10 ± 0.08 | 25.3 ± 2.8 | −6.1 ± 5.7 | 7.0 ± 0.2 (n = 143) |
| DOPC/Myricetin/H2O2+ Cu2+ | 1.02 ± 0.05 | +0.80 ± 0.07 | 17.6 ± 2.1 | −13.8 ± 5.0 | 6.9 ± 0.5 (n = 101) |
| DOPC/Myricitrin | 0.19 ± 0.09 | 37.6 ± 4.8 | 7.3 ± 0.2 (n = 175) | ||
| DOPC/Myricitrin/H2O2 | 0.30 ± 0.04 | +0.11 ± 0.13 | 18.8 ± 4.3 | −18.8 ± 9.1 | 7.1 ± 0.3 (n = 198) |
| DOPC/Myricitrin/H2O2+ Cu2+ | 0.81 ± 0.04 | +0.62 ± 0.13 | 14.7 ± 3.4 | −22.9 ± 8.2 | 7.0 ± 0.4 (n = 125) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandić, L.; Sadžak, A.; Strasser, V.; Baranović, G.; Domazet Jurašin, D.; Dutour Sikirić, M.; Šegota, S. Enhanced Protection of Biological Membranes during Lipid Peroxidation: Study of the Interactions between Flavonoid Loaded Mesoporous Silica Nanoparticles and Model Cell Membranes. Int. J. Mol. Sci. 2019, 20, 2709. https://doi.org/10.3390/ijms20112709
Mandić L, Sadžak A, Strasser V, Baranović G, Domazet Jurašin D, Dutour Sikirić M, Šegota S. Enhanced Protection of Biological Membranes during Lipid Peroxidation: Study of the Interactions between Flavonoid Loaded Mesoporous Silica Nanoparticles and Model Cell Membranes. International Journal of Molecular Sciences. 2019; 20(11):2709. https://doi.org/10.3390/ijms20112709
Chicago/Turabian StyleMandić, Lucija, Anja Sadžak, Vida Strasser, Goran Baranović, Darija Domazet Jurašin, Maja Dutour Sikirić, and Suzana Šegota. 2019. "Enhanced Protection of Biological Membranes during Lipid Peroxidation: Study of the Interactions between Flavonoid Loaded Mesoporous Silica Nanoparticles and Model Cell Membranes" International Journal of Molecular Sciences 20, no. 11: 2709. https://doi.org/10.3390/ijms20112709
APA StyleMandić, L., Sadžak, A., Strasser, V., Baranović, G., Domazet Jurašin, D., Dutour Sikirić, M., & Šegota, S. (2019). Enhanced Protection of Biological Membranes during Lipid Peroxidation: Study of the Interactions between Flavonoid Loaded Mesoporous Silica Nanoparticles and Model Cell Membranes. International Journal of Molecular Sciences, 20(11), 2709. https://doi.org/10.3390/ijms20112709