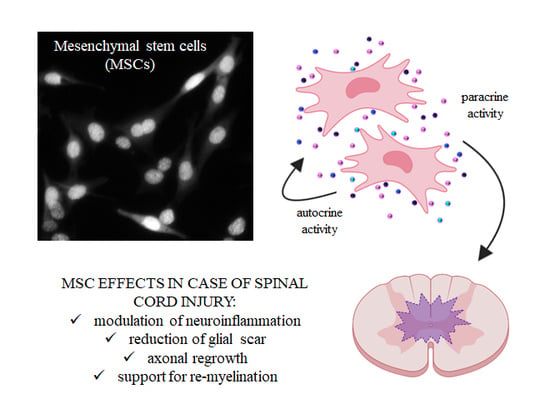
Mesenchymal Stem Cells for Spinal Cord Injury: Current Options, Limitations, and Future of Cell Therapy
Abstract
1. Introduction
2. Spinal Cord Injury
2.1. Secondary Injury
2.2. Chronic Phase and Neurodegeneration
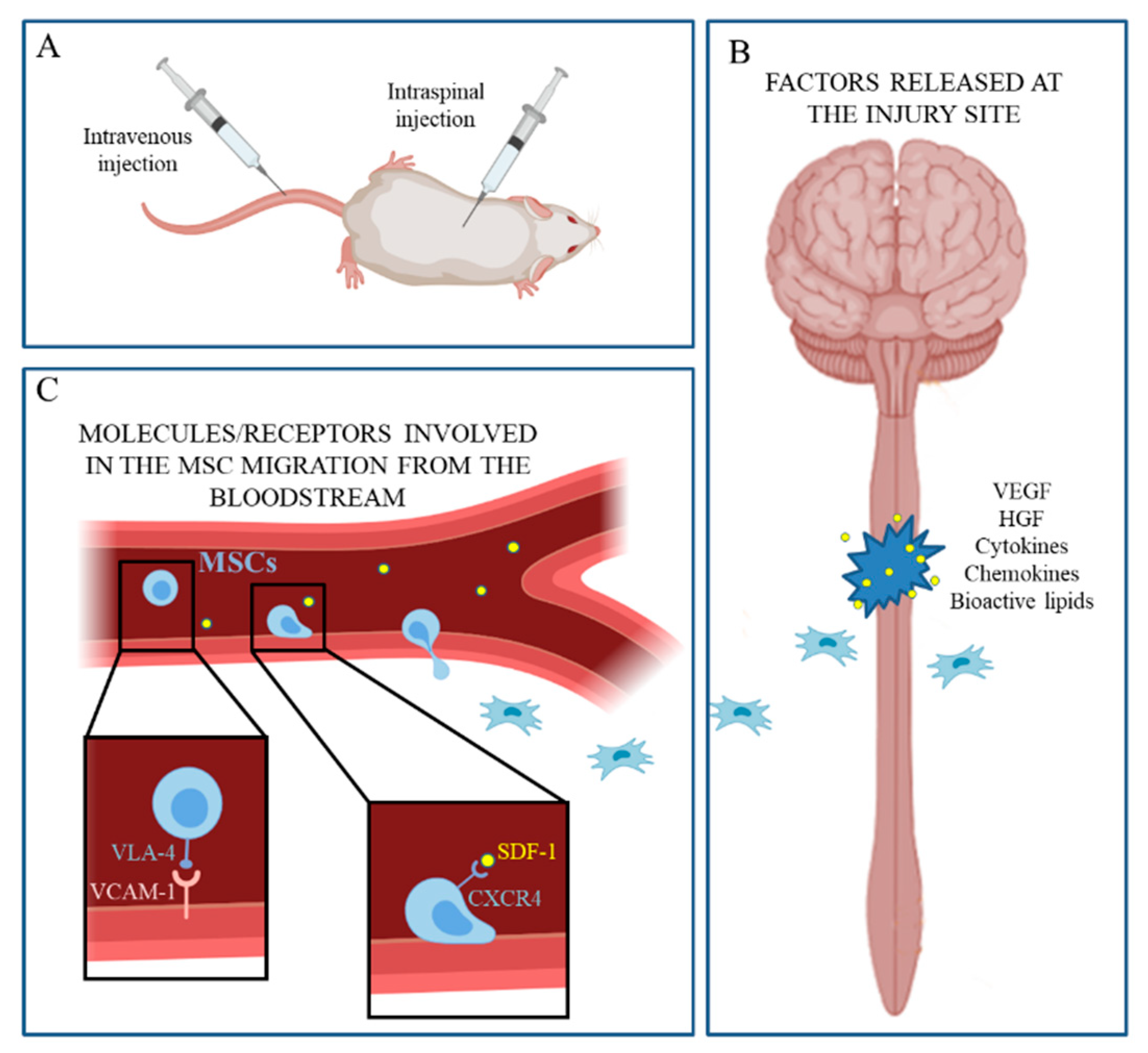
3. Stem Cell Therapy and Appeal of MSCs
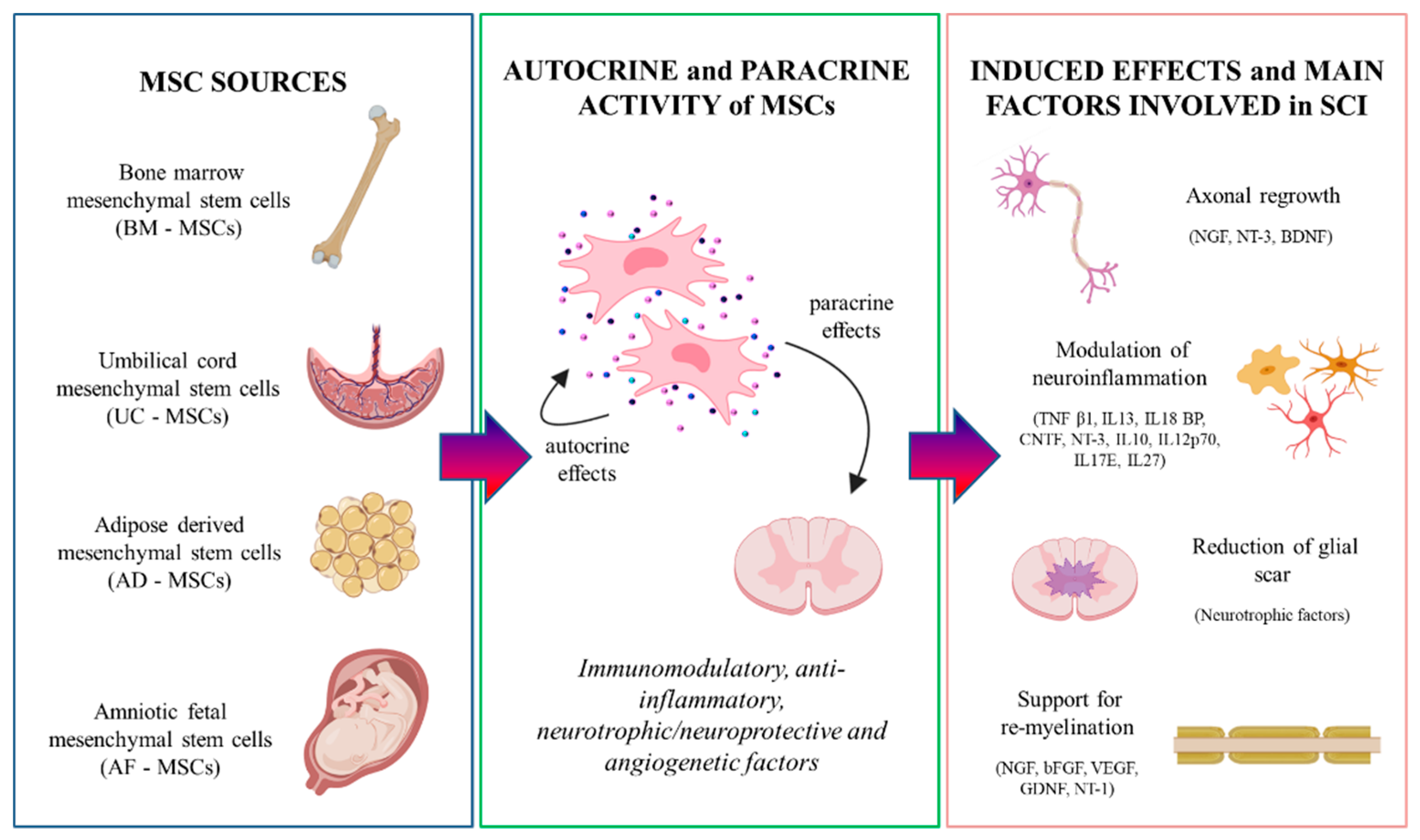
4. Secretome of MSCs
5. MSCs
5.1. Bone Marrow Mesenchymal Stem Cells (BM-MSCs)
5.2. Umbilical Cord Mesenchymal Stem Cells (UC-MSCs)
5.3. Adipose-Derived Mesenchymal Stem Cells (AD-MSCs)
5.4. Amniotic Fetal Mesenchymal Stem Cells (AF-MSCs)
6. Biomaterials and Scaffolds for Stem Cell Therapy
7. Limitations of Current Evidence and Future Directions
- The optimal therapeutic protocols regarding the preparation, type, and number of stem cells transplanted;
- The timing of transplantation and route of administration;
- The paracrine effects and their influence on functional recovery;
- The importance of biomaterials and scaffold;
- The importance of microenvironment;
- The plasticity and ability to recreate connections of neuronal cells.
- Additionally, logistics, ethical, and financial problems related to this field of research constitute a real challenge to face in order to channel basic science studies into clinical practice.
8. Conclusions
Funding
Conflicts of Interest
References
- International Perspectives on Spinal Cord Injury. Available online: http://apps.who.int/iris/bitstream/10665/94190/1/9789241564663_eng.pdf (accessed on 31 October 2014).
- Yılmaz, T.; Kaptanoğlu, E. Current and future medical therapeutic strategies for the functional repair of spinal cord injury. World J. Orthop. 2015, 6, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.R.; Forgione, N.; Fehlings, M.G. Emerging therapies for acute traumatic spinal cord injury. CMAJ 2013, 185, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.R.; Tetreault, L.A.; Kwon, B.K.; Arnold, P.M.; Mroz, T.E.; Shaffrey, C.; Harrop, J.S.; Chapman, J.R.; Casha, S.; Skelly, A.C.; et al. Timing of Decompression in Patients with Acute Spinal Cord Injury: A Systematic Review. Glob. Spine J. 2017, 7, 95S–115S. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.G.; Perrin, R.G. The role and timing of early decompression for cervical spinal cord injury: Update with a review of recent clinical evidence. Injury 2005, 36, S13–S26. [Google Scholar] [CrossRef] [PubMed]
- Rexed, B. The cytoarchitectonic organization of the spinal cord in the cat. J. Comp. Neurol. 1952, 96, 414–495. [Google Scholar] [CrossRef] [PubMed]
- Rexed, B. A cytoarchitectonic atlas of the spinal cord in the cat. J. Comp. Neurol. 1954, 100, 297–379. [Google Scholar] [CrossRef] [PubMed]
- Mai, J.K.; Paxinos, G. The Human Nervous System, 3rd ed.; Academic Press: Cambridge, MA, USA, 2011; ISBN 9780123742360. [Google Scholar]
- Ackery, A.; Tator, C.; Krassioukov, A. A global perspective on spinal cord injury epidemiology. J. Neurotrauma 2004, 21, 1355–1370. [Google Scholar] [CrossRef]
- Rowland, J.W.; Hawryluk, G.W.; Kwon, B.; Fehlings, M.G. Current status of acute spinal cord injury pathophysiology and emerging therapies: Promise on the horizon. Neurosurg. Focus 2008, 25, E2. [Google Scholar] [CrossRef]
- Zhang, N.; Yin, Y.; Xu, S.J.; Wu, Y.P.; Chen, W.S. Inflammation & apoptosis in spinal cord injury. Indian J. Med. Res. 2012, 135, 287–296. [Google Scholar]
- Beattie, M.S.; Li, Q.; Bresnahan, J.C. Cell death and plasticity after experimental spinal cord injury. Prog. Brain Res. 2000, 128, 9–21. [Google Scholar]
- Blight, A.R. Spinal cord injury models: Neurophysiology. J. Neurotrauma 1992, 9, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Grossman, S.D.; Rosenberg, L.J.; Wrathall, J.R. Relationship of altered glutamate receptor subunit mRNA expression to acute cell loss after spinal cord contusion. Exp. Neurol. 2001, 168, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.A.; Sousa, N.; Reis, R.L.; Salgado, A.J. From basics to clinical: A comprehensive review on spinal cord injury. Prog. Neurobiol. 2014, 114, 25–57. [Google Scholar] [CrossRef] [PubMed]
- Vismara, I.; Papa, S.; Rossi, F.; Forloni, G.; Veglianese, P. Current Options for Cell Therapy in Spinal Cord Injury. Trends Mol. Med. 2017, 23, 831–849. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Nakashima, H.; Nagoshi, N.; Chow, D.S.; Grossman, R.G.; Kopjar, B. Rationale, design and critical end points for the Riluzole in Acute Spinal Cord Injury Study (RISCIS): A randomized, double-blinded, placebo-controlled parallel multi-center trial. Spinal Cord 2016, 54, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Gazdic, M.; Volarevic, V.; Harrell, C.R.; Fellabaum, C.; Jovicic, N.; Arsenijević, N.; Stojkovic, M. Stem Cells Therapy for Spinal Cord Injury. Int. J. Mol. Sci. 2018, 19, 1039. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E.; Aguilar-Cevallos, J.; Silva-Garcia, R.; Ibarra, A. Cytokine and Growth Factor Activation In Vivo and In Vitro after Spinal Cord Injury. Mediat. Inflamm. 2016, 2016, 9476020. [Google Scholar] [CrossRef] [PubMed]
- Hayta, E.; Elden, H. Acute spinal cord injury: A review of pathophysiology and potential of non-steroidal anti-inflammatory drugs for pharmacological intervention. J. Chem. Neuroanat. 2017, 87, 25–31. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Lopez Vales, R.; Wee Yong, V. Harmful and beneficial effects of inflammation after spinal cord injury: Potential therapeutic implications. Handb. Clin. Neurol. 2012, 109, 485–502. [Google Scholar] [PubMed]
- Papa, S.; Caron, I.; Erba, E.; Panini, N.; De Paola, M.; Mariani, A.; Colombo, C.; Ferrari, R.; Pozzer, D.; Zanier, E.R.; et al. Early modulation of pro-inflammatory microglia by minocycline loaded nanoparticles confers long lasting protection after spinal cord injury. Biomaterials 2016, 75, 13–24. [Google Scholar] [CrossRef]
- Papa, S.; Caron, I.; Rossi, F.; Veglianese, P. Modulators of microglia: A patent review. Expert Opin. Ther. Pat. 2016, 26, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Shechter, R.; Schwartz, M. Harnessing monocyte-derived macrophages to control central nervous system pathol-ogies: No longer ‘if’ but ‘how’. J. Pathol. 2013, 229, 332–346. [Google Scholar] [CrossRef] [PubMed]
- Fournier, A.E.; GrandPre, T.; Strittmatter, S.M. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature 2001, 409, 341–346. [Google Scholar] [CrossRef] [PubMed]
- GrandPre, T.; Nakamura, F.; Vartanian, T.; Strittmatter, S.M. Identification of the Nogo inhibitor of axon regeneration as a reticulon protein. Nature 2000, 403, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Schwab, M.E.; Strittmatter, S.M. Nogo limits neural plasticity and recovery from injury. Curr. Opin. Neurobiol. 2014, 27, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Schwab, M.E. Nogo and axon regeneration. Curr. Opin. Neurobiol. 2004, 14, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Beller, J.A.; Snow, D.M. Proteoglycans: Road signs for neurite outgrowth. Neural Regen. Res. 2014, 9, 343–355. [Google Scholar]
- Yuan, Y.-M.; He, C. The glial scar in spinal cord injury and repair. Neurosci. Bull. 2013, 29, 421–435. [Google Scholar] [CrossRef]
- Sabin, K.Z.; Jiang, P.; Gearhart, M.D.; Stewart, R.; Echeverri, K. AP-1(cFos/JunB)/miR-200a regulate the pro-regenerative glial cell response during axolotl spinal cord regeneration. Commun. Biol. 2019, 2, 91. [Google Scholar] [CrossRef]
- Van Niekerk, E.A.; Tuszynski, M.H.; Lu, P.; Dulin, J.N. Molecular and Cellular Mechanisms of Axonal Regeneration After Spinal Cord Injury. Mol. Cell Proteom. 2016, 15, 394–408. [Google Scholar] [CrossRef]
- Tran, A.P.; Warren, P.M.; Silver, J. The Biology of Regeneration Failure and Success After Spinal Cord Injury. Physiol. Rev. 2018, 98, 881–917. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, T.M.; Burda, J.E.; Sofroniew, M.V. Cell biology of spinal cord injury and repair. J. Clin. Investig 2017, 127, 3259–3270. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Wang, Y.; Graham, L.; McHale, K.; Gao, M.; Wu, D.; Brock, J.; Blesch, A.; Rosenzweig, E.S.; Havton, L.A.; et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell 2012, 150, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Keirstead, H.S.; Nistor, G.; Bernal, G.; Totoiu, M.; Cloutier, F.; Sharp, K.; Steward, O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J. Neurosci. 2005, 25, 4694–4705. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, D.M.; Kim, Y.S.; Martinez, T.; Carmen, J.; Dike, S.; Shats, I.; Rubin, L.L.; Drummond, J.; Krishnan, C.; Hoke, A.; et al. Recovery from paralysis in adult rats using embryonic stem cells. Ann. Neurol. 2006, 60, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Pera, M.F.; Andrade, J.; Houssami, S.; Reubinoff, B.; Trounson, A.; Stanley, E.G.; Oostwaard, D.W.-v.; Mummery, C. Regulation of human embryonic stem cell differentiation by BMP-2 and its antagonist noggin. J. Cell Sci. 2004, 117, 1269–1280. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.W.; Liu, X.Z.; Qu, Y.; Liu, S.; Mickey, S.K.; Turetsky, D.; Gottlieb, D.I.; Choi, D.W. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat. Med. 1999, 5, 1410–1412. [Google Scholar] [CrossRef]
- Jin, M.C.; Medress, Z.A.; Azad, T.D.; Doulames, V.M.; Veeravagu, A. Stem cell therapies for acute spinal cord injury in humans: A review. Neurosurg. Focus 2019, 46, E10. [Google Scholar] [CrossRef]
- Filippi, M.; Boido, M.; Terreno, E. Imaging of MSC transplantation in neuroscience. Oncotarget 2017, 8, 10781–10782. [Google Scholar] [CrossRef]
- Filippi, M.; Boido, M.; Pasquino, C.; Garello, F.; Boffa, C.; Terreno, E. Successful in vivo MRI tracking of MSCs labeled with Gadoteridol in a Spinal Cord Injury experimental model. Exp. Neurol. 2016, 282, 66–77. [Google Scholar] [CrossRef]
- Frantz, S. Embryonic stem cell pioneer Geron exits field, cuts losses. Nat. Biotechnol. 2012, 30, 12–13. [Google Scholar] [CrossRef]
- Boido, M.; Piras, A.; Valsecchi, V.; Spigolon, G.; Mareschi, K.; Ferrero, I.; Vizzini, A.; Temi, S.; Mazzini, L.; Fagioli, F.; et al. Human mesenchymal stromal cell transplantation modulates neuroinflammatory milieu in a mouse model of amyotrophic lateral sclerosis. Cytotherapy 2014, 16, 1059–1072. [Google Scholar] [CrossRef]
- Mazzini, L.; Vercelli, A.; Ferrero, I.; Boido, M.; Cantello, R.; Fagioli, F. Transplantation of mesenchymal stem cells in ALS. Prog. Brain Res. 2012, 201, 333–359. [Google Scholar] [PubMed]
- Gunetti, M.; Tomasi, S.; Giammò, A.; Boido, M.; Rustichelli, D.; Mareschi, K.; Errichiello, E.; Parola, M.; Ferrero, I.; Fagioli, F.; et al. Myogenic potential of whole bone marrow mesenchymal stem cells in vitro and in vivo for usage in urinary incontinence. PLoS ONE 2012, 7, e45538. [Google Scholar] [CrossRef]
- Lee, M.W.; Yang, M.S.; Park, J.S.; Kim, H.C.; Kim, Y.J.; Choi, J. Isolation of mesenchymal stem cells from cryopreserved human umbilical cord blood. Int. J. Hematol. 2005, 81, 126–130. [Google Scholar] [CrossRef]
- Dasari, V.R.; Veeravalli, K.K.; Dinh, D.H. Mesenchymal stem cells in the treatment of spinal cord injuries. World J. Stem Cells 2014, 6, 120–133. [Google Scholar] [CrossRef]
- Lu, L.L.; Liu, Y.J.; Yang, S.G.; Zhao, Q.J.; Wang, X.; Gong, W.; Han, Z.B.; Xu, Z.S.; Lu, Y.X.; Liu, D.; et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica 2006, 91, 1017–1026. [Google Scholar]
- Lo, B.; Parham, L. Ethical Issues in Stem Cell Research. Endocr. Rev. 2009, 30, 204–213. [Google Scholar] [CrossRef]
- Kotobuki, N.; Hirose, M.; Takakura, Y.; Ohgushi, H. Cultured autologous human cells for hard tissue regeneration: Prepa-ration and characterization of mesenchymal stem cells from bone marrow. Artif. Organs 2004, 28, 33–39. [Google Scholar] [CrossRef]
- Boido, M.; Rupa, R.; Garbossa, D.; Fontanella, M.; Ducati, A.; Vercelli, A. Embryonic and adult stem cells promote raphespinal axon outgrowth and improve functional outcome following spinal hemisection in mice. Eur. J. Neurosci. 2009, 30, 833–846. [Google Scholar] [CrossRef]
- Zachar, L.; Bačenková, D.; Rosocha, J. Activation, homing, and role of the mesenchymal stem cells in the inflammatory environment. J. Inflamm. Res. 2016, 9, 231–240. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, H. Roles of Mesenchymal Stem Cells in Spinal Cord Injury. Stem Cells Int. 2017, 2017, 5251313. [Google Scholar] [CrossRef]
- Pelagalli, A.; Nardelli, A.; Lucarelli, E.; Zannetti, A.; Brunetti, A. Autocrine signals increase ovine mesenchymal stem cells migration through Aquaporin-1 and CXCR4 overexpression. J. Cell. Physiol. 2018, 233, 6241–6249. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, J.; He, J.; Zhou, M.; Adi, J.; Webster, K.A.; Yu, H. Impaired CXCR4 expression and cell engraftment of bone marrow-derived cells from aged atherogenic mice. Atherosclerosis 2011, 219, 92–99. [Google Scholar] [CrossRef][Green Version]
- Marquez-Curtis, L.A.; Gul-Uludag, H.; Xu, P.; Chen, J.; Janowska-Wieczorek, A. CXCR4 transfection of cord blood mesenchymal stromal cells with the use of cationic liposome enhances their migration toward stromal cell-derived factor-1. Cytotherapy 2013, 15, 840–849. [Google Scholar] [CrossRef]
- Hong, H.S.; Lee, J.; Lee, E.; Kwon, Y.S.; Lee, E.; Ahn, W.; Jiang, M.H.; Kim, J.C.; Son, Y. A new role of substance P as an injury-inducible messenger for mobilization of CD29(+) stromal-like cells. Nat. Med. 2009, 15, 425–435. [Google Scholar] [CrossRef]
- Petit, I.; Szyper-Kravitz, M.; Nagler, A.; Lahav, M.; Peled, A.; Habler, L.; Ponomaryov, T.; Taichman, R.S.; Arenzana-Seisdedos, F.; Fujii, N.; et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat. Immunol. 2002, 3, 687. [Google Scholar] [CrossRef]
- Sobacchi, C.; Palagano, E.; Villa, A.; Menale, C. Soluble Factors on Stage to Direct Mesenchymal Stem Cells Fate. Front. Bioeng. Biotechnol. 2017, 5, 32. [Google Scholar] [CrossRef]
- Baez-Jurado, E.; Hidalgo-Lanussa, O.; Barrera-Bailón, B.; Sahebkar, A.; Ashraf, G.M.; Echeverria, V.; Barreto, G.E. Secretome of Mesenchymal Stem Cells and Its Potential Protective Effects on Brain Pathologies. Mol. Neurobiol. 2019, 1–26. [Google Scholar] [CrossRef]
- Ryan, J.M.; Barry, F.P.; Murphy, J.M.; Mahon, B.P. Mesenchymal stem cells avoid allogeneic rejection. J. Inflamm. 2005, 2, 8. [Google Scholar] [CrossRef]
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Matteucci, P.; Grisanti, S.; Gianni, A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002, 99, 3838–3843. [Google Scholar] [CrossRef]
- Liang, X.; Ding, Y.; Zhang, Y.; Tse, H.F.; Lian, Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: Current status and perspectives. Cell Transplant. 2014, 23, 1045–1059. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 25, 1852. [Google Scholar] [CrossRef]
- Mead, B.; Logan, A.; Berry, M.; Leadbeater, W.; Scheven, B.A. Paracrine-mediated neuroprotection and neuritogenesis of axotomised retinal ganglion cells by human dental pulp stem cells: Comparison with human bone marrow and adipose-derived mesenchymal stem cells. PLoS ONE 2014, 9, e109305. [Google Scholar] [CrossRef]
- Kolar, M.K.; Itte, V.N.; Kingham, P.J.; Novikov, L.N.; Wiberg, M.; Kelk, P. The neurotrophic effects of different human dental mesenchymal stem cells. Sci. Rep. 2017, 7, 12605. [Google Scholar] [CrossRef]
- Teixeira, F.G.; Carvalho, M.M.; Neves-Carvalho, A.; Panchalingam, K.M.; Behie, L.A.; Pinto, L.; Sousa, N.; Salgado, A.J. Secretome of mesenchymal progenitors from the umbilical cord acts as modulator of neural/glial proliferation and differentiation. Stem Cell Rev. 2015, 11, 288–297. [Google Scholar] [CrossRef]
- Potapova, I.A.; Gaudette, G.R.; Brink, P.R.; Robinson, R.B.; Rosen, M.R.; Cohen, I.S.; Doronin, S.V. Mesenchymal stem cells support migration, extracellular matrix invasion, proliferation, and survival of endothelial cells in vitro. Stem Cells 2007, 25, 1761–1768. [Google Scholar] [CrossRef]
- Hofer, H.R.; Tuan, R.S. Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res. Ther. 2016, 7, 131. [Google Scholar] [CrossRef]
- Razavi, S.; Ghasemi, N.; Mardani, M.; Salehi, H. Remyelination improvement after neurotrophic factors secreting cells transplantation in rat spinal cord injury. Iran. J. Basic Med. Sci. 2017, 20, 392–398. [Google Scholar]
- Lu, P.; Jones, L.L.; Tuszynski, M.H. BDNF-expressing marrow stromal cells support extensive axonal growth at sites of spinal cord injury. Exp. Neurol. 2005, 191, 344–360. [Google Scholar] [CrossRef]
- Neuhuber, B.; Timothy Hilmes, B.; Shumsky, J.S.; Gallo, G.; Fischer, I. Axon growth and recovery of function supported by human bone marrow stromal cells in the injured spinal cord exhibit donor variations. Brain Res. 2005, 1035, 73–85. [Google Scholar] [CrossRef]
- Crigler, L.; Robey, R.C.; Asawachaicharn, A.; Gaupp, D.; Phinney, D.G. Human mesenchymal stem cell subpopulations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp. Neurol. 2006, 198, 54–64. [Google Scholar] [CrossRef]
- Wright, K.T.; Masri, W.E.; Osman, A.; Roberts, S.; Chamberlain, G.; Ashton, B.A.; Johnson, W.E. Bone marrow stromal cells stimulate neurite outgrowth over neural proteoglycans (CSPG), myelin associated glycoprotein and Nogo-A. Biochem. Biophys. Res. Commun. 2007, 354, 559–566. [Google Scholar] [CrossRef]
- Teixeira, F.G.; Carvalho, M.M.; Sousa, N.; Salgado, A.J. Mesenchymal stem cells secretome: A new paradigm for central nervous system regeneration? Cell. Mol. Life Sci. 2013, 70, 3871–3882. [Google Scholar] [CrossRef]
- Zanotti, L.; Angioni, R.; Calì, B.; Soldani, C.; Ploia, C.; Moalli, F.; Gargesha, M.; D’Amico, G.; Elliman, S.; Tedeschi, G.; et al. Mouse mesenchymal stem cells inhibit high endothelial cell activation and lymphocyte homing to lymph nodes by releasing TIMP-1. Leukemia 2016, 30, 1143–1154. [Google Scholar] [CrossRef]
- De Luca, A.; Gallo, M.; Aldinucci, D.; Ribatti, D.; Lamura, L.; D’Alessio, A.; De Filippi, R.; Pinto, A.; Normanno, N. Role of the EGFR ligand/receptor system in the secretion of angiogenic factors in mesenchymal stem cells. J. Cell. Physiol. 2011, 226, 2131–2138. [Google Scholar] [CrossRef]
- Arutyunyan, I.; Elchaninov, A.; Makarov, A.; Fatkhudinov, T. Umbilical Cord as Prospective Source for Mesenchymal Stem Cell-Based Therapy. Stem Cells Int. 2016, 2016, 6901286. [Google Scholar] [CrossRef]
- Boido, M.; Garbossa, D.; Fontanella, M.; Ducati, A.; Vercelli, A. Mesenchymal stem cell transplantation reduces glial cyst and improves functional outcome after spinal cord compression. World Neurosurg. 2014, 81, 183–190. [Google Scholar] [CrossRef]
- Choudhery, M.S.; Badowski, M.; Muise, A.; Harris, D.T. Comparison of human mesenchymal stem cells derived from adipose and cord tissue. Cytotherapy 2013, 15, 330–343. [Google Scholar] [CrossRef]
- Giampà, C.; Alvino, A.; Magatti, M.; Silini, A.R.; Cardinale, A.; Paldino, E.; Fusco, F.R.; Parolini, O. Conditioned medium from amniotic cells protects striatal degeneration and ameliorates motor deficits in the R6/2 mouse model of Huntington’s disease. J. Cell. Mol. Med. 2019, 23, 1581–1592. [Google Scholar] [CrossRef]
- Hass, R.; Kasper, C.; Böhm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 2011, 9, 12. [Google Scholar] [CrossRef]
- Leto Barone, A.A.; Khalifian, S.; Lee, W.P.; Brandacher, G. Immunomodulatory effects of adipose-derived stem cells: Fact or fiction? BioMed Res. Int. 2013, 2013, 383685. [Google Scholar] [CrossRef]
- Ma, J.; Wu, J.; Han, L.; Jiang, X.; Yan, L.; Hao, J.; Wang, H. Comparative analysis of mesenchymal stem cells derived from amniotic membrane, umbilical cord, and chorionic plate under serum-free condition. Stem Cell Res. Ther. 2019, 10, 19. [Google Scholar] [CrossRef]
- Menezes, K.; Nascimento, M.A.; Gonçalves, J.P.; Cruz, A.S.; Lopes, D.V.; Curzio, B.; Bonamino, M.; de Menezes, J.R.; Borojevic, R.; Rossi, M.I.; et al. Human mesenchymal cells from adipose tissue deposit laminin and promote regeneration of injured spinal cord in rats. PLoS ONE 2014, 9, e96020. [Google Scholar] [CrossRef]
- Pischiutta, F.; Brunelli, L.; Romele, P.; Silini, A.; Sammali, E.; Paracchini, L.; Marchini, S.; Talamini, L.; Bigini, P.; Boncoraglio, G.B.; et al. Protection of brain injury by amniotic mesenchymal stromal cell-secreted metabolites. Crit. Care Med. 2016, 44, e1118–e1131. [Google Scholar] [CrossRef]
- Ryu, H.H.; Kang, B.J.; Park, S.S.; Kim, Y.; Sung, G.J.; Woo, H.M.; Kim, W.H.; Kweon, O.K. Comparison of mesenchymal stem cells derived from fat, bone marrow, Wharton’s jelly, and umbilical cord blood for treating spinal cord injuries in dogs. J. Vet. Med. Sci. 2012, 74, 1617–1630. [Google Scholar] [CrossRef]
- Takahashi, A.; Nakajima, H.; Uchida, K.; Takeura, N.; Honjoh, K.; Watanabe, S.; Kitade, M.; Kokubo, Y.; Johnson, W.E.B.; Matsumine, A. Comparison of Mesenchymal Stromal Cells Isolated from Murine Adipose Tissue and Bone Marrow in the Treatment of Spinal Cord Injury. Cell Transplant. 2018, 27, 1126–1139. [Google Scholar] [CrossRef]
- Yang, C.; Wang, G.; Ma, F.; Yu, B.; Chen, F.; Yang, J.; Feng, J.; Wang, Q. Repeated injections of human umbilical cord blood-derived mesenchymal stem cells significantly promotes functional recovery in rabbits with spinal cord injury of two noncontinuous segments. Stem Cell Res. Ther. 2018, 9, 136. [Google Scholar] [CrossRef]
- Zhou, H.L.; Zhang, X.J.; Zhang, M.Y.; Yan, Z.J.; Xu, Z.M.; Xu, R.X. Transplantation of Human Amniotic Mesenchymal Stem Cells Promotes Functional Recovery in a Rat Model of Traumatic Spinal Cord Injury. Neurochem. Res. 2016, 41, 2708–2718. [Google Scholar] [CrossRef]
- Khan, I.U.; Yoon, Y.; Kim, A.; Jo, K.R.; Choi, K.U.; Jung, T.; Kim, N.; Son, Y.; Kim, W.H.; Kweon, O.K. Improved Healing after the Co-Transplantation of HO-1 and BDNF Overexpressed Mesenchymal Stem Cells in the Subacute Spinal Cord Injury of Dogs. Cell Transplant. 2018, 27, 1140–1153. [Google Scholar] [CrossRef]
- Penha, E.M.; Meira, C.S.; Guimaraes, E.T.; Mendonca, M.V.P.; Gravely, F.A.; Pinheiro, M.B.; Pinheiro, T.M.B.; Barrouin-Melo, S.M.; Ribeiro dos Santos, R.; Pereira Soares, M.B. Use of Autologous Mesenchymal Stem Cells Derived from Bone Marrow for the Treatment of Naturally Injured Spinal Cord in Dogs. Stem Cells Int. Vol. 2014, 2014, 437521. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, S.H.; Kim, W.H.; Kweon, O.K. Transplantation of adipose derived mesenchymal stem cells for acute thoracolumbar disc disease with no deep pain perception in dogs. J. Vet. Sci. 2016, 17, 123–126. [Google Scholar] [CrossRef]
- Kim, Y.; Jo, S.H.; Kim, W.H.; Kweon, O.K. Antioxidant and anti-inflammatory effects of intravenously injected adipose derived mesenchymal stem cells in dogs with acute spinal cord injury. Stem Cell Res. Ther. 2015, 6, 229. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Z.; Deng, W.; Zhang, Z.; Liu, Y.; Wei, L.; Zhang, Y.; Zhou, L.; Wang, Y. Derivation and characterization of sheep bone marrow-derived mesenchymal stem cells induced with telomerase reverse transcriptase. Saudi J. Biol. Sci. 2017, 24, 519–525. [Google Scholar] [CrossRef]
- Martin, D.R.; Cox, N.R.; Hathcock, T.L.; Niemeyer, G.P.; Baker, H.J. Isolation and characterization of multipotential mesenchymal stem cells from feline bone marrow. Exp. Hematol. 2002, 30, 879–886. [Google Scholar] [CrossRef]
- Bosnakovski, D.; Mizuno, M.; Kim, G.; Takagi, S.; Okumura, M.; Fujinaga, T. Isolation and multilineage differentiation of bovine bone marrow mesenchymal stem cells. Cell Tissue Res. 2005, 319, 243–253. [Google Scholar] [CrossRef]
- Carrade, D.D.; Affolter, V.K.; Outerbridge, C.A.; Watson, J.L.; Ga-luppo, L.D.; Buerchler, S.; Kumar, V.; Walker, N.J.; Borjesson, D.L. Intradermal injections of equine allogeneic umbilical cord-derived mesenchymal stem cells are well tolerated and do not elicit immediate or delayed hypersensitivity reactions. Cytotherapy 2011, 13, 1180–1192. [Google Scholar] [CrossRef]
- Wislet-Gendebien, S.; Hans, G.; Leprince, P.; Rigo, J.M.; Moonen, G.; Rogister, B. Plasticity of cultured mesenchymal stem cells: Switch from nestin-positive to excitable neuron-like phenotype. Stem Cells 2005, 23, 392–402. [Google Scholar] [CrossRef]
- Mishra, P.J.; Banerjee, D. Activation and Differentiation of Mesenchymal Stem Cells. Methods Mol. Biol. 2017, 1554, 201–209. [Google Scholar]
- Mortada, I.; Mortada, R. Epigenetic changes in mesenchymal stem cells differentiation. Eur. J. Med. Genet. 2018, 61, 114–118. [Google Scholar] [CrossRef]
- Kozorovitskiy, Y.; Gould, E. Stem cell fusion in the brain. Nat. Cell Biol. 2003, 5, 952–954. [Google Scholar] [CrossRef]
- Deng, Y.B.; Liu, X.G.; Liu, Z.G.; Liu, X.L.; Liu, Y.; Zhou, G.Q. Implantation of BM mesenchymal stem cells into injured spinal cord elicits de novo neurogenesis and functional recovery. Cytotherapy 2006, 8, 210–214. [Google Scholar] [CrossRef]
- Zurita, M.; Vaquero, J.; Bonilla, C.; Santos, M.; De Haro, J.; Oya, S.; Aguayo, C. Functional recovery of chronic paraplegic pigs after autologous transplantation of bone marrow stromal cells. Transplantation 2008, 86, 845–853. [Google Scholar] [CrossRef]
- Tetzlaff, W.; Okon, E.B.; Karimi-Abdolrezaee, S.; Hill, C.E.; Sparling, J.S.; Plemel, J.R.; Plunet, W.T.; Tsai, E.C.; Baptiste, D.; Smithson, L.J.; et al. A systematic review of cellular transplantation therapies for spinal cord injury. J. Neurotrauma 2011, 28, 1611–1682. [Google Scholar] [CrossRef]
- Hofstetter, C.P.; Schwarz, E.J.; Hess, D.; Widenfalk, J.; El Manira, A.; Prockop, D.J.; Olson, L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc. Natl. Acad. Sci. USA 2002, 99, 2199–2204. [Google Scholar] [CrossRef]
- Nishio, Y.; Koda, M.; Kamada, T.; Someya, Y.; Yoshinaga, K.; Okada, S.; Harada, H.; Okawa, A.; Moriya, H.; Yamazaki, M. The use of hemopoietic stem cells derived from human umbilical cord blood to promote restoration of spinal cord tissue and recovery of hindlimb function in adult rats. J. Neurosurg. Spine 2006, 5, 424–433. [Google Scholar] [CrossRef]
- Pal, R.; Chaitanya, G.; Rao, N.M.; Banerjee, P.; Krishnamororth, V.; Venkataramana, N.K. Functional recovery after transplantation of bone marrow-derived human mesenchymal stromal cells in a rat model of spinal cord injury. Cytotherapy 2010, 12, 792–806. [Google Scholar] [CrossRef]
- Nemati, S.N.; Jabbari, R.; Hajinasrollah, M.; Mehrjerdi, N.Z.; Azizi, H.; Hemmesi, K.; Moghiminasr, R.; Azhdari, Z.; Talebi, A.; Mohitmafi, S.; et al. Transplantation of Adult Monkey Neural Stem Cells into A Contusion Spinal Cord Injury Model in Rhesus Macaque Monkeys. Cell J. Yakhteh 2014, 16, 117–130. [Google Scholar]
- Gutierrez, J.; Lamanna, J.J.; Grin, N.; Hurtig, C.V.; Miller, J.H.; Riley, J.; Urquia, L.; Avalos, P.; Svendsen, C.N.; Federici, T.; Boulis, N.M. Preclinical Validation of Multilevel Intraparenchymal Stem Cell Therapy in the Porcine Spinal Cord. Neurosurgery 2015, 77, 604–612. [Google Scholar] [CrossRef]
- Hakim, R.; Covacu, R.; Zachariadis, V.; Frostell, A.; Sankavaram, S.R.; Brundin, L.; Svensson, M. Mesenchymal stem cells transplanted into spinal cord injury adopt immune cell-like characteristics. Stem Cell Res. Ther. 2019, 10, 115. [Google Scholar] [CrossRef]
- Cao, Q.; Zhang, P.; Howard, R.M.; Walters, W.M.; Tsoulfas, P.; Whittermore, S.R. Pluripotent stem cells engrafted into the normal or lesioned adulti rat spinal cord are restricted to glial lineage. Exp. Neurol. 2001, 167, 48–58. [Google Scholar] [CrossRef]
- Dasari, V.R.; Spomar, D.G.; Gondi, C.S.; Sloffer, C.A.; Saving, K.L.; Gujrati, M.; Rao, J.S.; Dinh, D.H. Axonal remyelination by cord blood stem cells after spinal cord injury. J. Neurotrauma 2007, 24, 391–410. [Google Scholar] [CrossRef]
- Cho, S.R.; Yang, M.S.; Yim, S.H.; Park, J.H.; Lee, J.E.; Eom, Y.W.; Jang, I.K.; Kim, H.E.; Park, J.S.; Kim, H.O.; et al. Neurally induced umbilical cord blood cells modesty repair injured spinal cords. Neuroreport 2008, 19, 1259–1263. [Google Scholar] [CrossRef]
- Jeon, S.R.; Park, J.H.; Lee, J.H.; Kim, D.Y.; Kim, H.S.; Sung, I.Y.; Choi, G.H.; Geon, M.H.; Kim, G.G. Treatment of spinal cord injury with bone marrow-derived, cultured autologous mesenchymal stem cells. Tissue Eng. Regen. Med. 2010, 7, 316–322. [Google Scholar]
- Park, J.H.; Kim, D.Y.; Sung, I.Y.; Choi, G.H.; Jeon, M.H.; Kim, K.K.; Jean, S.R. Long-term results of spinal cord injury therapy using mesenchymal stem cells derived from bone marrow in humans. Neurosurgery 2012, 70, 1238–1247. [Google Scholar] [CrossRef]
- Dai, G.; Liu, X.; Zhang, Z.; Yang, Z.; Dai, Y.; Xu, R. Transplantation of autologous bone marrow mesenchymal stem cells in the treatment of complete and chronic cervical spinal cord injury. Brain Res. 2013, 1533, 73–79. [Google Scholar] [CrossRef]
- El-Kheir, W.A.; Gabr, H.; Awad, M.R.; Ghannam, O.; Barakat, Y.; Farghali, H.A.; El Maadawi, Z.M.; Ewes, I.; Sabaawy, H.E. Autologous bone marrow-derived cell therapy combined with physical therapy induces functional improvement in chronic spinal cord injury patients. Cell Trans.-Plant. 2014, 23, 729–745. [Google Scholar] [CrossRef]
- Geffner, L.F.; Santacruz, P.; Izurieta, M.; Flor, L.; Maldonado, B.; Auad, A.H.; Montenegro, X.; Gonzalez, R.; Silva, F. Administration of autologous bone marrow stem cells into spinal cord injury patients via multiple routes is safe and improves their quality of life. Cell Transpl. 2008, 17, 1277–1293. [Google Scholar] [CrossRef]
- Karamouzian, S.; Nematollahi-Mahani, S.N.; Nakhaee, N.; Eskandary, H. Clinical safety and primary efficacy of bone marrow mesenchymal cell transplantation in subacute spinal cord injured patients. Clin. Neurol. Neurosurg. 2012, 114, 935–939. [Google Scholar] [CrossRef]
- Mendonça, M.V.; Larocca, T.F.; de Freitas Souza, B.S.; Villarreal, C.F.; Silva, L.F.; Matos, A.C.; Novaes, M.A.; Bahia, C.M.; de Oliveira Melo Martinez, A.C.; Kaneto, C.M.; et al. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res. Ther. 2014, 5, 126. [Google Scholar] [CrossRef]
- Park, H.C.; Shim, Y.S.; Ha, Y.; Yoon, S.H.; Park, S.R.; Choi, B.H.; Park, H.S. Treatment of complete spinal cord injury patients by autologous bone marrow cell transplantation and administration of granulocyte-macrophage colony stimulating factor. Tissue Eng. 2005, 11, 913–922. [Google Scholar] [CrossRef]
- Syková, E.; Homola, A.; Mazanec, R.; Lachmann, H.; Konrádová, S.L.; Kobylka, P.; Pádr, R.; Neuwirth, J.; Komrska, V.; Vávra, V.; et al. Autologous bone marrow transplantation in patients with subacute and chronic spinal cord injury. Cell Transpl. 2006, 15, 675–687. [Google Scholar] [CrossRef]
- Pal, R.; Venkataramana, N.K.; Bansal, A.; Balaraju, S.; Jan, M.; Chandra, R.; Dixit, A.; Rauthan, A.; Murgod, U.; Totey, S. Ex vivo-expanded autologous bone marrow-derived mesenchymal stromal cells in human spinal cord injury/paraplegia: A pilot clinical study. Cytotherapy 2009, 11, 897–911. [Google Scholar] [CrossRef]
- Moviglia, G.A.; Fernandez Viña, R.; Brizuela, J.A.; Saslavsky, J.; Vrsalovic, F.; Varela, G.; Bastos, F.; Farina, P.; Etchegaray, G.; Barbieri, M.; et al. Combined protocol of cell therapy for chronic spinal cord injury. Report on the electrical and functional recovery of two patients. Cytotherapy 2006, 8, 202–209. [Google Scholar]
- Stem Cell Spinal Cord Injury Exoskeleton and Virtual Reality Treatment Study. Identification No. NCT03225625. Available online: https://clinicaltrials.gov/ct2/showNCT03225625term=NCT03225625andrank=1 (accessed on 21 July 2014).
- Neirinckx, V.; Cantinieaux, D.; Coste, C.; Rogister, B.; Franzen, R.; Wislet-Gendebien, S. Concise review. Spinal cord injuries: How could adult mesenchymal and neural crest stem cells take up the challenge? Stem Cells 2014, 32, 829–843. [Google Scholar]
- Corcione, A.; Benvenuto, F.; Ferretti, E.; Giunti, D.; Cappiello, V.; Cazzanti, F.; Risso, M.; Gualandi, F.; Mancardi, G.L.; Pistoia, V.; et al. Human mesenchymal stem cells modulate B-cell functions. Blood 2006, 107, 367–372. [Google Scholar] [CrossRef]
- Nakajima, H.; Uchida, K.; Guerrero, A.R.; Watanabe, S.; Sugita, D.; Takeura, N.; Yoshida, A.; Long, G.; Wright, K.T.; Johnson, W.E.; et al. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J. Neurotrauma 2012, 29, 1614–1625. [Google Scholar] [CrossRef]
- Kanekiyo, K.; Nakano, N.; Homma, T.; Yamada, Y.; Tamachi, M.; Suzuki, Y.; Fukushima, M.; Saito, F.; Ide, C. Effects of multiple injection of bone marrow mononuclear cells on spinal cord injury of rats. J. Neuro-Trauma 2017, 34, 3003–3011. [Google Scholar] [CrossRef]
- Vaquero, J.; Zurita, M. Functional recovery after severe CNS trauma: Current perspectives for cell therapy with bone marrow stromal cells. Prog. Neurobiol. 2011, 93, 341–349. [Google Scholar] [CrossRef]
- Sasaki, M.; Radtke, C.; Tan, A.M.; Zhao, P.; Hamada, H.; Houkin, K.; Honmou, O.; Kocsis, J.D. BDNF-hypersecreting human mesen-chymal stem cells promote functional recovery, axonal sprout-ing, and protection of corticospinal neurons after spinal cord injury. J. Neurosci. 2009, 29, 14932–14941. [Google Scholar]
- Wang, L.J.; Zhang, R.P.; Li, J.D. Transplantation of neurotrophin-3-expressing bone mesenchymal stem cells improves recovery in a rat model of spinal cord injury. Acta Neurochir. 2014, 156, 1409–1418. [Google Scholar] [CrossRef]
- Chua, S.J.; Bielecki, R.; Yamanaka, N.; Fehlings, M.G.; Rogers, I.M.; Casper, R.F. The effect of umbilical cord blood cells on outcomes after experimental traumatic spinal cord injury. Spine 2010, 35, 1520–1526. [Google Scholar] [CrossRef]
- Caron, I.; Rossi, F.; Papa, S.; Aloe, R.; Sculco, M.; Mauri, E.; Sacchetti, A.; Erba, E.; Panini, N.; Parazzi, V.; et al. A new three dimensional biomimetic hydrogel to deliver factors secreted by human mesenchymal stem cells in spinal cord injury. Biomaterials 2016, 75, 135–147. [Google Scholar] [CrossRef]
- Dasari, V.R.; Veeravalli, K.K.; Tsung, A.J.; Gondi, C.S.; Gujrati, M.; Dinh, D.H.; Rao, J.S. Neuronal apoptosis is inhibited by cord blood stem cells after spinal cord injury. J. Neurotrauma 2009, 26, 2057–2069. [Google Scholar] [CrossRef]
- Kao, C.H.; Chen, S.H.; Chio, C.C.; Lin, M.T. Human umbilical cord blood-derived CD34 cells may attenuate spinal cord injury by stimulating vascular endothelial and neurotrophic factors. Shock 2008, 29, 49–55. [Google Scholar]
- Kuh, S.U.; Cho, Y.E.; Yoon, D.H.; Kim, K.N.; Ha, Y. Functional recovery after human umbilical cord blood cells transplanta-tion with brain-derived neutrophic factor into the spinal cord injured rat. Acta Neurochir. Wien 2005, 147, 985–992. [Google Scholar] [CrossRef]
- Kang, K.S.; Kim, S.W.; Oh, Y.H.; Yu, J.W.; Kim, K.Y.; Park, H.K.; Song, C.H.; Han, H. A 37-year-old spinal cord-injured female patient, transplanted of multipotent stem cells from human UC blood, with improved sensory perception and mobility, both functionally and morphologically. Cytotherapy 2005, 7, 368–373. [Google Scholar]
- Yao, L.; He, C.; Zhao, Y.; Wang, J.; Tang, M.; Li, J.; Wu, Y.; Ao, L.; Hu, X. Human umbilical cord blood stem cell transplantation for the treatment of chronic spinal cord injury. Neural Regen. Res. 2013, 8, 397–403. [Google Scholar]
- Zhu, H.; Poon, W.; Liu, Y.; Leung, G.K.; Wong, Y.; Feng, Y.; Stephanie, C.P.N.g.; Tsang, K.S.; Sun, D.T.F.; Yeung, D.K.; et al. Phase I–II clinical trial assessing safety and efficacy of umbilical cord blood mononuclear cell transplant therapy of chronic complete spinal cord injury. Cell Transpl. 2016, 25, 1925–1943. [Google Scholar] [CrossRef]
- De Ugarte, D.A.; Morizono, K.; Elbarbary, A.; Alfonso, Z.; Zuk, P.A.; Zhu, M.; Dragoo, J.L.; Ashjian, P.; Thomas, B.; Benhaim, P.; et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs 2003, 174, 101–109. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Ohta, Y.; Hamaguchi, A.; Ootaki, M.; Watanabe, M.; Takeba, Y.; Iiri, T.; Matsumoto, N.; Takenaga, M. Intravenous infusion of adipose-derived stem/stromal cells improves functional recovery of rats with spinal cord injury. Cytotherapy 2017, 19, 839–848. [Google Scholar] [CrossRef]
- Kolar, M.K.; Kingham, P.J.; Novikova, L.N.; Wiberg, M.; Novikov, L.N. The therapeutic effects of human adipose-derived stem cells in a rat cervical spinal cord injury model. Stem Cells Dev. 2014, 23, 1659–1674. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, Y.; Rhew, D.; Kuk, M.; Kim, M.; Kim, W.H.; Kweon, O.K. Effect of the combination of mesenchymal stromal cells and chondroitinase ABC on chronic spinal cord injury. Cytotherapy 2015, 17, 1374–1383. [Google Scholar] [CrossRef]
- Kim, E.Y.; Lee, K.B.; Kim, M.K. The potential of mesenchymal stem cells derived from amniotic membrane and amniotic fluid for neuronal regenerative therapy. BMB Rep. 2014, 47, 135–140. [Google Scholar] [CrossRef]
- Bottai, D.; Scesa, G.; Cigognini, D.; Adami, R.; Nicora, E.; Abrignani, S.; Di Giulio, A.M.; Gorio, A. Third trimester NG2-positive amniotic fluid cells are effective in improving repair in spinal cord injury. Exp. Neurol. 2014, 254, 121–133. [Google Scholar] [CrossRef]
- Gao, S.; Ding, J.; Xiao, H.J.; Li, Z.Q.; Chen, Y.; Zhou, X.S.; Wang, J.E.; Wu, J.; Shi, W.Z. Anti-inflammatory and anti-apoptotic effect of combined treatment with methylprednisolone and amniotic membrane mesenchymal stem cells after spinal cord injury in rats. Neurochem. Res. 2014, 39, 1544–1552. [Google Scholar] [CrossRef]
- Sankar, V.; Muthusamy, R. Role of human amniotic epithelial cell transplantation in spinal cord injury repair research. Neuroscience 2003, 118, 11–17. [Google Scholar] [CrossRef]
- Rossi, F.; Perale, G.; Papa, S.; Forloni, G.; Veglianese, P. Current options for drug delivery to the spinal cord. Expert Opin. Drug Deliv. 2013, 10, 385–396. [Google Scholar] [CrossRef]
- Perale, G.; Rossi, F.; Santoro, M.; Peviani, M.; Papa, S.; Llupi, D.; Torriani, P.; Micotti, E.; Previdi, S.; Cervo, L.; et al. Multiple drug delivery hydrogel system for spinal cord injury repair strategies. J. Control. Release 2012, 159, 271–280. [Google Scholar] [CrossRef]
- Lee, J.M.; Yeong, W.Y. Design and printing strategies in 3D bioprinting of cell-hydrogels. Adv. Healthc. Mater. 2016, 5, 2856–2865. [Google Scholar] [CrossRef]
- Chen, B.K.; Knight, A.M.; de Ruiter, G.C.; Spinner, R.J.; Yaszemski, M.J.; Currier, B.L.; Windebank, A.J. Axon regeneration through scaffold into distal spinal cord after transection. J. Neurotrauma 2009, 26, 1759–1771. [Google Scholar] [CrossRef]
- Lin, X.Y.; Lai, B.Q.; Zeng, X.; Che, M.T.; Ling, E.A.; Wu, W.; Zeng, S.Y. Cell transplantation and neuroengineering approach for spinal cord injury treatment: A summary of current laboratory findings and review of literature. Cell Transplant. 2016, 25, 1425–1438. [Google Scholar] [CrossRef]
- Führmann, T.; Tam, R.Y.; Ballarin, B.; Coles, B.; Elliott Donaghue, I.; van der Kooy, D.; Nagy, A.; Tator, C.H.; Morshead, C.M.; Shoichet, M.S. Injectable hydrogel promotes early survival of induced pluripotent stem cell-derived oligoden-drocytes and attenuates longterm teratoma formation in a spinal cord injury model. Biomaterials 2016, 83, 23–36. [Google Scholar] [CrossRef]
- Garbossa, D.; Boido, M.; Fontanella, M.; Fronda, C.; Ducati, A.; Vercelli, A. Recent therapeutic strategies for spinal cord injury treatment: Possible role of stem cells. Neurosurg. Rev. 2012, 35, 293–311. [Google Scholar] [CrossRef]
- Garbossa, D.; Fontanella, M.; Fronda, C.; Benevello, C.; Muraca, G.; Ducati, A.; Vercelli, A. New strategies for repairing the injured spinal cord: The role of stem cells. Neurol. Res. 2006, 28, 500–504. [Google Scholar] [CrossRef]
- Boido, M.; Garbossa, D.; Vercelli, A. Early graft of neural precursors in spinal cord compression reduces glial cyst and improves function. J. Neurosurg. Spine 2011, 15, 97–106. [Google Scholar] [CrossRef]
- Satti, H.S.; Waheed, A.; Ahmed, P.; Ahmed, K.; Akram, Z.; Aziz, T.; Satti, T.M.; Shahbaz, N.; Khan, M.A.; Malik, S.A. Autologous mesenchymal stromal cell transplantation for spinal cord injury: A Phase I pilot study. Cytotherapy 2016, 18, 518–522. [Google Scholar] [CrossRef]
- Oh, S.K.; Choi, K.H.; Yoo, J.Y.; Kim, D.Y.; Kim, S.J.; Jeon, S.R. A phase III clinical trial showing limited ef cacy of autologous mesenchymal stem cell therapy for spinal cord injury. Neurosurgery 2016, 78, 436–447. [Google Scholar] [CrossRef]
- Deng, J.; Petersen, B.E.; Steindler, D.A.; Jorgensen, M.L.; Laywell, E.D. Mesenchymal stem cells spontaneously express neural proteins in culture and are neurogenic after transplantation. Stem Cells 2006, 4, 1054–1064. [Google Scholar] [CrossRef]
- Wurmser, A.E. Gage FH Stem cells: Cell fusion causes confusion. Nature 2002, 416, 485–487. [Google Scholar] [CrossRef]
- Lu, P.; Blesch, A.; Tuszynski, M.H. Induction of bone marrow stromal cells to neurons: Differentiation, transdifferentiation, or artifact? J. Neurosci. Res. 2004, 77, 174–191. [Google Scholar] [CrossRef]
- Takeda, Y.S.; Xu, Q. Neuronal Differentiation of Human Mesenchymal Stem Cells Using Exosomes Derived from Differentiating Neuronal Cells. PLoS ONE 2015, 10, e0135111. [Google Scholar] [CrossRef]
- Cortés-Medina, L.V.; Pasantes-Morales, H.; Aguilera-Castrejon, A.; Picones, A.; Lara-Figueroa, C.O.; Luis, E.; Montesinos, J.J.; Cortés-Morales, V.A.; De la Rosa Ruiz, M.P.; Hernández-Estévez, E.; et al. Neuronal Transdifferentiation Potential of Human Mesenchymal Stem Cells from Neonatal and Adult Sources by a Small Molecule Cocktail. Stem Cells Int. 2019, 2019, 7627148. [Google Scholar] [CrossRef]
- Park, W.S.; Ahn, S.Y.; Sung, S.I.; Ahn, J.Y.; Chang, Y.S. Strategies to enhance paracrine potency of transplanted mesenchymal stem cells in intractable neonatal disorders. Pediatr. Res. 2018, 83, 214–222. [Google Scholar] [CrossRef]
| MSC Type | Availability [83] | Invasive Procedure of Collection [83] | Cell Proliferation In Vitro [81,85] | Secretome * [79,82,87] | MSC Survival at the Injury Site After Graft [88,89] | Low Immunogenicity in the Host Tissue [84,85] | Anti-Inflammatory Effect in Injured Spinal Cord ** [84] | Glial Scar Reduction [52,80,86,90] | Axonal Regrowth/Sprouting Support [52,80,86,90,91] | Use in Pre-Clinical Studies (This Review) | Use in Clinical Trials (This Review) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BM-MSCs | +++ | +++ | ++ | +++ | ++ | ++ | ++ | ++ | +++ | +++ | +++ |
| UC-MSCs | + | not invasive | +++ | +++ | +++ | +++ | +++ | ++ | +++ | ++ | ++ |
| AD-MSCs | +++ | ++ | ++ | +++ | +++ | ++ | ++ | ++ | +++ | ++ | ++ |
| AF-MSCs | + | not invasive | +++ | +++ | +++ | +++ | not reported | not reported | +++ | + | not reported |
| Study | Type of Stem Cell Transplanted | Type of SCI | Animal | Administration | Scores | Adverse Reactions | Results | Cells Analysis/Findings in the Scar |
|---|---|---|---|---|---|---|---|---|
| Wislet-Gendebien et al. [100] | BM-MSC | In vitro study | N/A | N/A | Anti-glial fibrillary acidic protein (GFAP); anti-GLAST; anti-Tuj1; anti-NeuN; anti-SMI31; anti-MAP2ab; anti synaptophysi; anti-M2; anti-M6; RT-PCR, electrical conductivity | N/A | Neuron-like cells differentiated from Nestin + cells without the mature neuron electrical features; No differentiation in oligodendrocyte-like cells | Nestin + cells; GFAP + cells |
| Deng et al. [104] | BM-MSC | Transplantation of BM MSC 2 weeks after dorsal SCI | Macaco rhesus | Intralesional | Motor and sensitive improvement (Tarlov behavior assessment), SEP, MEP | None | Motor and sensitive functions improvement (Tarlov 2-3 achieved) in treated monkeys after 3 months follow up; Improvement of SEP and MEP | NSE +, NF +, GFAP + cells |
| Zurita et al. [105] | BM-MSC | BM MSC transplanted 3 months after dorsal SCI | Pigs | Intralesional | Clinical improvement (from 0 to 10 scale where 0 means paraplegia and 10 constantly useful hike), SEP, MRI | None | 3 months after transplantation improvement of motor functions (mean score of 6.20) and SEP; reduction of the centromedullary cavity | GFAP +, NF +, S100 + cells |
| Hofstetter et al. [107] | BM-MSC | Iperacute (immediately after trauma) and acute (transplantation 1 week after dorsal trauma) | Lewis rat | Intralesional | Fibronectin, vimentin, laminin cells positivity; GFAP, electrical conduction | None | Markers of neuron-like cells, but no depolarization their membrane like mature neurons; No clinical benefit in the iper acute SCI group. In the acute SCI group Ab anti Nestin and GFAP of host astrocytes around and in the scar in the MSC treated population. Immature astrocytes Nestin + GFAP + with the possibility to differentiate into neuron-like cells | Neuron-like cells, host astrocytes closely connected with transplanted MSC cells, astrocyte-like cells |
| Nishio et al. [108] | HUCB stem cells | Acute (1 week after dorsal trauma) | Wistar rats | Intralesional | Basso, Beattie, Bresnahan locomotor scale (BBB), MRI | None | Hindlimb recovery, reduction of cystic cavity, no detection of any double-positive cells for human mitochondria and CD34, of CD4 positive cells, no significant differences between the two groups in the number of OX-42–positive or CD8-positive cells; GAP-43–positive fibers at the epicenter significantly higher than that of the control group | CD45 and human CD14, OX-42, CD4, CD8, GAP-43, 5-HT fibers, T-positive fibers |
| Pal et al. [109] | BM-MSC | Acute (1 week after dorsal trauma) | Wistar rats | Intrathecal | BBB locomotor scale, grid walk, plantar test, inclined plane; cells were tested for CD34, CD44, CD45, CD73, CD90, CD105 and HLA-DR. | None | Improved locomotor and sensory behavioral scores. Negative astroglial markers. No graft versus host immune reaction evoked by BM MSC, with the capacity to escape the immune system and be effective in wound healing | Negative astroglial markers, BM MSC |
| Nemati et al. [110] | Monkeys NSC | Acute (10 days after dorsal trauma) | Macaco rhesus monkeys | Intralesional | Spontaneous motor activity, Tarlov’s scale, limb pinch test, tail pinch test, sensory test, MRI, evaluation of neural specific markers Tuj1, MAP2, GFAP, Pax6, Sox1 | None | Improvement in the sensory and motor activity, improvement in MRI | Isolated mNSCs express NSC markers such as nestin, Sox1, and Pax6 and could differentiate into mature neurons positive for MAP2 and GFAP |
| Gutierrez et al. [111] | Human fetal cortex-derived neural progenitor cells (hNPCs) | Iperacute (immediately after cervical trauma) | Göttingen minipig | Intralesional | Tarlov scale, sensory evaluation in the form of a tactile stimulus to the interdigital space | None | Improvement in motor and sensitive functions, no significant decrease in neuronal density between groups; cell engraftment ranged from 12% to 31% | |
| Hakim et al. [112] | BM-MSC | Acute (24 h after dorsal trauma) | Mice | Intralesional | Cells were evaluated by flow cytometry, immunohistochemistry, immunocytochemistry, proliferation assay differentiation assay, confocal microscopy and automatic cell quantification | None | MSCs transplanted downregulate genes related to cell-cycle and DNA metabolic/biosynthetic processes and upregulate genes related to immune system response, cytokine production, and phagocytosis/endocytosis; Sca1 and CD29, MHC I maintained expression; upregulated expression of CD45 and MHC II; Transplanted MSCs survived and proliferated to a low extent, no expression of Caspase-3, no differentiation into neurons or astrocytes | Transplanted MSCs express CD29, Sca1, and CD45 MHC-I and MHC-II; transplanted MSCs survive and proliferate but do not undergo apoptosis or neural differentiation |
| Cao et al. [113] | NSC | Acute (10 days after dorsal trauma) | Fischer rats | Intralesional, intrathecal | Cells were evaluated by immunohistochemistry, confocal microscopy and automatic cell quantification | None | The majority of transplanted cells either differentiated into GFAP + cells or remained nestin +. No Brd-U-positive neurons or oligodendrocytes detected | GFAP+ cells, nestin+ cells, Brd-U+ cells |
| Dasari et al. [114] | HUCB stem cells | Acute (1 week after dorsal trauma) | Lewis rat | Intralesional | BBB locomotor scale, cells were tested for CD44, NF200, CNPase, O1, beta III tubulin, APC, myelin basic protein caspase 3, MAP-2A&2B, confocal/fluorescence microscope, automatic cell quantification, immune blot | None | Improved locomotor and sensory behavioral scores, downregulation HUCB cellsmediated Fas and caspase | NF-200+ cells, CNPase+ cells, CD 44+ cells, co-localization of hUCB with neurons and oligodendrocytes |
| Cho et al. [115] | HUCB stem cells | Acute (1 week after dorsal trauma) | Sprague-Dawley rats | Intralesional | BBB locomotor scale, SSEPs, cells were evaluated by immunoistochemistry | None | Improved locomotor and sensory behavioral scores, shortened SSEPs latencies in treated rats | HuNu and GFAP + cells, MBP + cells, beta III tubular + cells |
| Khan et.al. [92] | AD-MSCs + BDNF | Acute (1 week after lumbar trauma) | Beagle dogs | Intralesional | BBB locomotor scale, cells were tested for Tuj-1, NF, GAP-43, GFAP, Nestin, COX2, TNFa, IL6, STAT3, IL-10, HO-1, BDNF | None | Significant improvement in hindlimb functions, with a higher BBB score | Increase in neuroregeneration, higher expression of Tuj-1, NF-M, and GAP-43, decreased expression of the inflammatory markers interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), and an increased expression of interleukin-10 (IL-10). H&E staining showed more reduced intraparenchymal fibrosis |
| Ryu et. al. [88] | BM-MSC, AD-MSC, UCB MSC, Wharton’s jelly-derived MSC | Acute (1 week after lumbar trauma) | Beagle dogs | Intralesional | Olby score and Revised Modified Talov scale, BBB locomotor scale, confocal/fluorescence microscope. Immunoistochemistry | None | Significant differences of neurologic recovery in MSCs groups at 2 weeks following MSC transplantation. Purposeful hind limb motion of all dogs in the MSCs groups. No significant differences observed among the MSCs groups. UCB-derived MSCs (UCSCs) induced more nerve regeneration and anti-inflammation activity | Some MSCs expressed markers for neurons (NF160), neuronal nuclei (NeuN) and astrocytes (GFAP). NF160- and NeuN-positive neurons were found, GFAP-positive reactive astrocytes were observed more often in the control group than in MSCs groups. Lesion sizes were smaller, and fewer microglia and reactive astrocytes were found in the spinal cord epicenter of all MSC groups |
| Penha et.al. [93] | BM-MSC | Acute (10 days after dorsal or lumbar trauma) | Dogs | Intralesional | Clinical evaluation, MRI images | None | No changes at the MSC administration site into the spinal cord. Progressive recovery of the panniculus reflex and diminished superficial and deep pain response. Conscious reflex recovery occurred simultaneously with moderate improvement in intestine and urinary bladder functions | N/A |
| Kim et.al. [94] | AD-MSCs | Acute (1 week after dorsal or lumbar trauma) | Dogs | Intralesional | Clinical improvement: full recovery (normal neurologic state; grade 0), improved (regained deep pain perception (DPP) and recovery of ambulation, but still had mild ataxia; grade 1–2) and unsuccessful (did not regain DPP or the ability to walk without support; grade 3–5) | None | Clinical improvement (55.6% of the dogs were in full recovery, 22.2% showed improved outcomes and 22.2% had unsuccessful recovery) | N/A |
| Kim et.al. [95] | AD-MSCs | Iperacute (immediately after lumbar trauma) | Beagle dogs | Intravenous | Revised Tarlov scale, gait analysis, cells were evaluated by western blot | None | Significant enhanced motor function in AD-MSCs group compared with those in the control group at 7 days post treatment | The levels of GFAP, and GalCa were increased in the AD-MSC group, β3-tubulin levels were increased, COX-2, IL-6, and TNFα levels were significantly decreased; 3-NT level was significantly decreased, the level of 4-HNE was significantly decreased; the level of PC was significantly decreased |
| Study | Type of SCI | Administration | n of Transplanted Cells | Transplanted Cells Type | Scores | Adverse Reactions | Results |
|---|---|---|---|---|---|---|---|
| Jeon et al. [116] | 10 acute SCI patients | Intrathecal | 8 × 106 cells | Autologous MSCs | ASIA, Frankel score, EMG, SEP, MRI | None | Improvement in ASIA score, EMG, and SEP; improvement in MRI imaging |
| Dai et al. [118] | 40 human patients; chronic and complete cervical SCI (AIS A) | Perilesional | Suspension with 8 × 105 cells/microl | Autologous BM-MSCs expanded in culture | AIS, ASIA, residual urinary volume, EMG, MRI | None | 45% AIS A to B; ASIA total scores were 31.6 prior and 43.1 after treatment (p = 0.01); preoperative urinary volume 235 mL to postop volume 173 mL (p = 0.01), improvement also in EMG and MRI |
| El Kheir et al. [119] | 70 human patients; chronic complete cervical or thoracic SCI | Intrathecal | 2 × 106 cells/kg | Autologous BM-MSCs | AIS, ASIA, MRI, SEP | None | AIS conversion from AIS A to AIS B or C and from AIS B to AIC C; Improvement in ASIA score, SEP and in MRI. Higher improvement in the thoracic than in the cervical SCI group |
| Geffner 2008 [120] | 8 human patients (4 acute SCI, 4 chronic SCI) | Directly into the spinal cord, directly into the spinal canal, and intravenous | / | Autologous BM-MSCs | ASIA, Barthel, Frankel Ashworth score, residual urinary volume, MRI | None | Improvement in all of the parameters |
| Karamouzian et al. [121] | 11 human patients with acute or subacute (2-8 weeks after trauma) SCI | Intrathecal | 7 × 105 to 1.2 × 106 cells | Autologous BM-MSCs | ASIA (12-33 months follow up) | None | Improvement in the ASIA score but the score was not statistically significant (p = 0.095) |
| Mendonca et al. [122] | 14 human patients with chronic thoraco -lumbar SCI | Intralesional | 5 × 106 cells/cm3 | BM-derived MSCs expanded in culture | ASIA, SEP, MRI, urodinamic, AIS | None | AIS A to B or C; incomplete injury; urinary function improved in 9 subjects, SEP improved in 1 subject |
| Park et al. [123] | 6 human patients with cervical SCI treated at 72 h after trauma | Intralesional | 2 × 108 cells | Autologous BM-MSCs | Frankel, AIS, MRI | None | AIS A to B/C; improved MRI |
| Sykova et al. [124] | 20 human patients with complete SCI transplanted from 10 to 467 days after trauma | Intra arterial vs. intra venous | 89.7 +/− 70.7 × 106 cells | Autologous BM-MSCs | Frankel, AIS, ASIA, SEP, MRI | None | Not significant results at 3–6–12 months follow-up; however, there was a positive trend |
| Pal et al. [125] | 30 human patients with complete cervical or thoracic SCI | Intrathecal | 1 × 106 cells | Autologous BM-MSCs expanded in culture | ASIA, Barthel, SSEP, MEP, NCV, MRI | None | No significant results in ASIA score; variable patterns of recovery (especially in bladder functions), no significant variations in SSEP, MEP, NCV. Improved MRI |
| Moviglia et al. [126] | 2 human patients with cervical and thoracic chronic SCI | Intra arterial | 5 × 108 to 1 × 109 cells | Autologous BM-MSCs | SSEP, MEP, MRI, clinical examination | None | Improvement in all of the parameters |
| ClinicalTrials.Gov Identifier | Title | MSC Type | Enrolled Subjects | Phase(s) | I End Point | II End Point | Date of Completion | Site of Administration | Intervention | Status |
|---|---|---|---|---|---|---|---|---|---|---|
| NCT03521336 | Intrathecal transplantation of UC-MSC in patients with sub-acute spinal cord injury | UC-MSCs | 130 | II | ASIA score | IANR-SCIRFS score; EMG; residual urine | Dec 2022 | Intrathecal | Allogeneic UC-MSCs | Recruiting |
| NCT03308565 | Adipose stem cells for traumatic spinal cord injury | AD-MSCs | 10 | I | Acute adverse event | ASIA; MEPs; SSEPs; MRI; functional changes | Nov 2023 | Intrathecal | Autologous AD-MSCs | Recruiting |
| NCT03225625 | Stem cell spinal cord injury exoskeleton and virtual reality treatment study | BM-MSCs | 40 | N/A | ASIA score | ANS function; general well-being | Jul 2022 | Paraspinal; intravenous; intranasal | Autologous BM-MSCs | Recruiting |
| NCT02917291 | Safety and preliminary efficacy of FAB117-HC in patients with acute traumatic spinal cord injury | AD- MSCs | 46 | I/II | Safety | ISNC-SCI; SCIM III; SSEPs; MEPs | Jan 2020 | Intramedullary | Autologous AD-MSCs | Recruiting |
| NCT01676441 | Safety and efficacy of autologous mesenchymal stem cells in chronic spinal cord injury | BM-MSCs | 32 | II/III | Treatment | / | Dec 2020 | Intramedullary | Autologous BM-MSC | Recruiting |
| NCT03505034 | Intrathecal transplantation of UC-MSC in patients with late stage of chronic spinal cord injury | UC-MSCs | 43 | II | ASIA score | IANR-SCIRFS score; EMG; residual urine | Dec 2021 | Intrathecal | Umbilical cord mesenchymal stem cells | Recruiting |
| NCT02574572 | Autologous mesenchymal stem cell transplantation in cervical chronic and complete spinal cord injury | BM-MSCs | 10 | I | N of participants with treatment-related adverse events as assessed by MRI | ASIA score, ASIA impairment scale, improvement in sensorial mapping and neuropathic pain | Jun 2020 | Intralesional | Autologous BM-MSC | Recruiting |
| NCT03521323 | Intrathecal transplantation of UC-MSC in patients with early stage of chronic spinal cord injury | UC-MSCs | 66 | I/II | ASIA score | IANR-SCIRFS score; EMG; residual urine | Dec 2021 | Intrathecal | Umbilical cord mesenchymal stem cells | Recruiting |
| NCT02574585 | Autologous mesenchymal stem cell transplantation in thoracolumbar chronic and complete spinal cord injury | BM-MSCs | 40 | II | N of participants with treatment-related adverse events as assessed by MRI SCI | ASIA score, AIS score, improving in sensorial mapping and neuropathic pain | Jan 2022 | Percutaneous | Autologous BM-MSC | Not yet recruiting |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cofano, F.; Boido, M.; Monticelli, M.; Zenga, F.; Ducati, A.; Vercelli, A.; Garbossa, D. Mesenchymal Stem Cells for Spinal Cord Injury: Current Options, Limitations, and Future of Cell Therapy. Int. J. Mol. Sci. 2019, 20, 2698. https://doi.org/10.3390/ijms20112698
Cofano F, Boido M, Monticelli M, Zenga F, Ducati A, Vercelli A, Garbossa D. Mesenchymal Stem Cells for Spinal Cord Injury: Current Options, Limitations, and Future of Cell Therapy. International Journal of Molecular Sciences. 2019; 20(11):2698. https://doi.org/10.3390/ijms20112698
Chicago/Turabian StyleCofano, Fabio, Marina Boido, Matteo Monticelli, Francesco Zenga, Alessandro Ducati, Alessandro Vercelli, and Diego Garbossa. 2019. "Mesenchymal Stem Cells for Spinal Cord Injury: Current Options, Limitations, and Future of Cell Therapy" International Journal of Molecular Sciences 20, no. 11: 2698. https://doi.org/10.3390/ijms20112698
APA StyleCofano, F., Boido, M., Monticelli, M., Zenga, F., Ducati, A., Vercelli, A., & Garbossa, D. (2019). Mesenchymal Stem Cells for Spinal Cord Injury: Current Options, Limitations, and Future of Cell Therapy. International Journal of Molecular Sciences, 20(11), 2698. https://doi.org/10.3390/ijms20112698