Role of NF-kB RelB in Aryl Hydrocarbon Receptor-Mediated Ligand Specific Effects
Abstract
1. Introduction
2. Results and Discussion
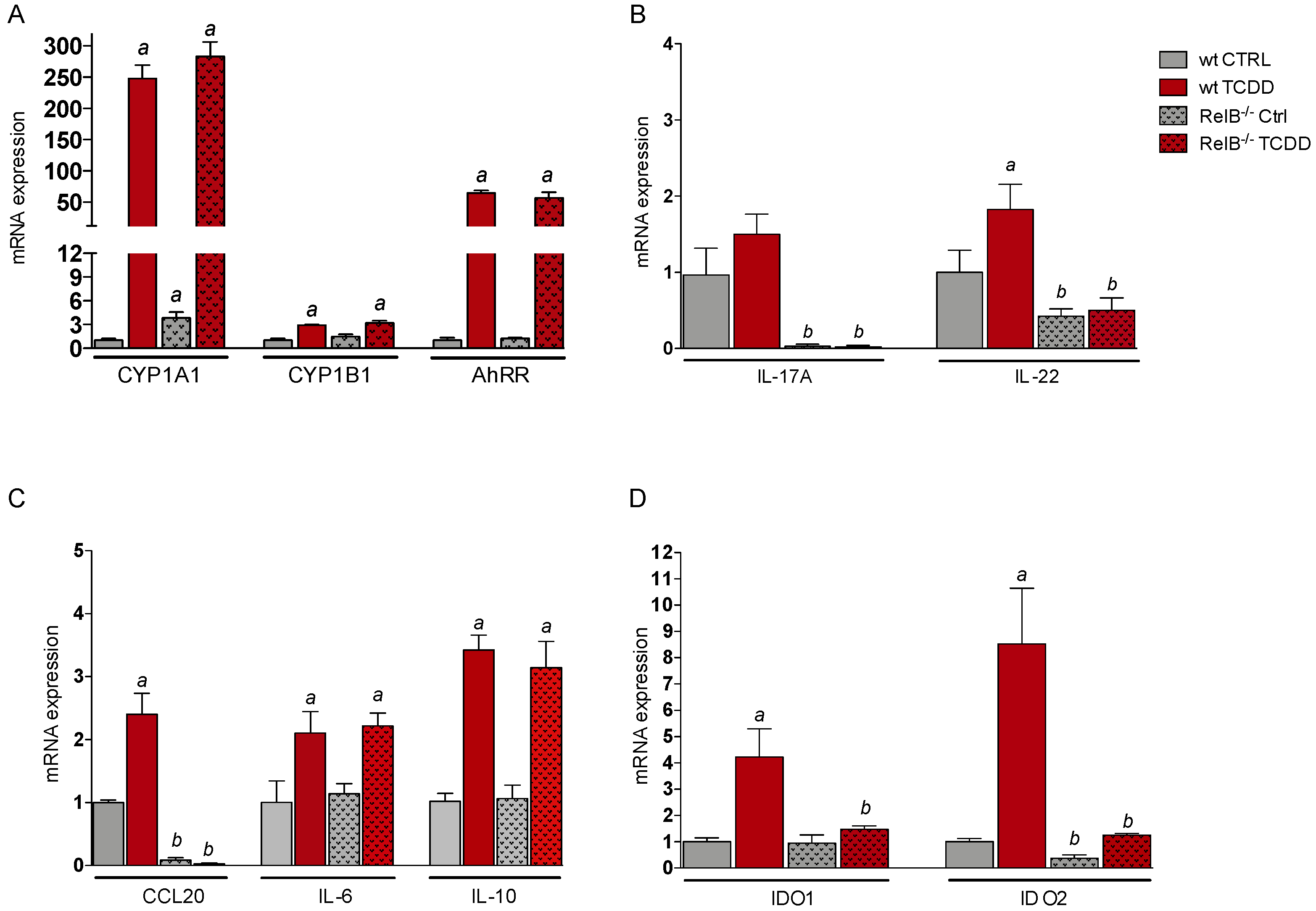
2.1. Effects of AhR Ligands on the Expression of Genes of the AhR Gene Battery, Cytokines, and Immune Regulatory Enzymes in Bone Marrow-Derived Macrophages (BMM) Derived from wt and RelB−/− Mice
2.2. Effects of TCDD on the Expression of Genes of the AhR Gene Battery, Cytokines, and Immune Regulatory Enzymes in Thymus of wt and RelB−/− Mice
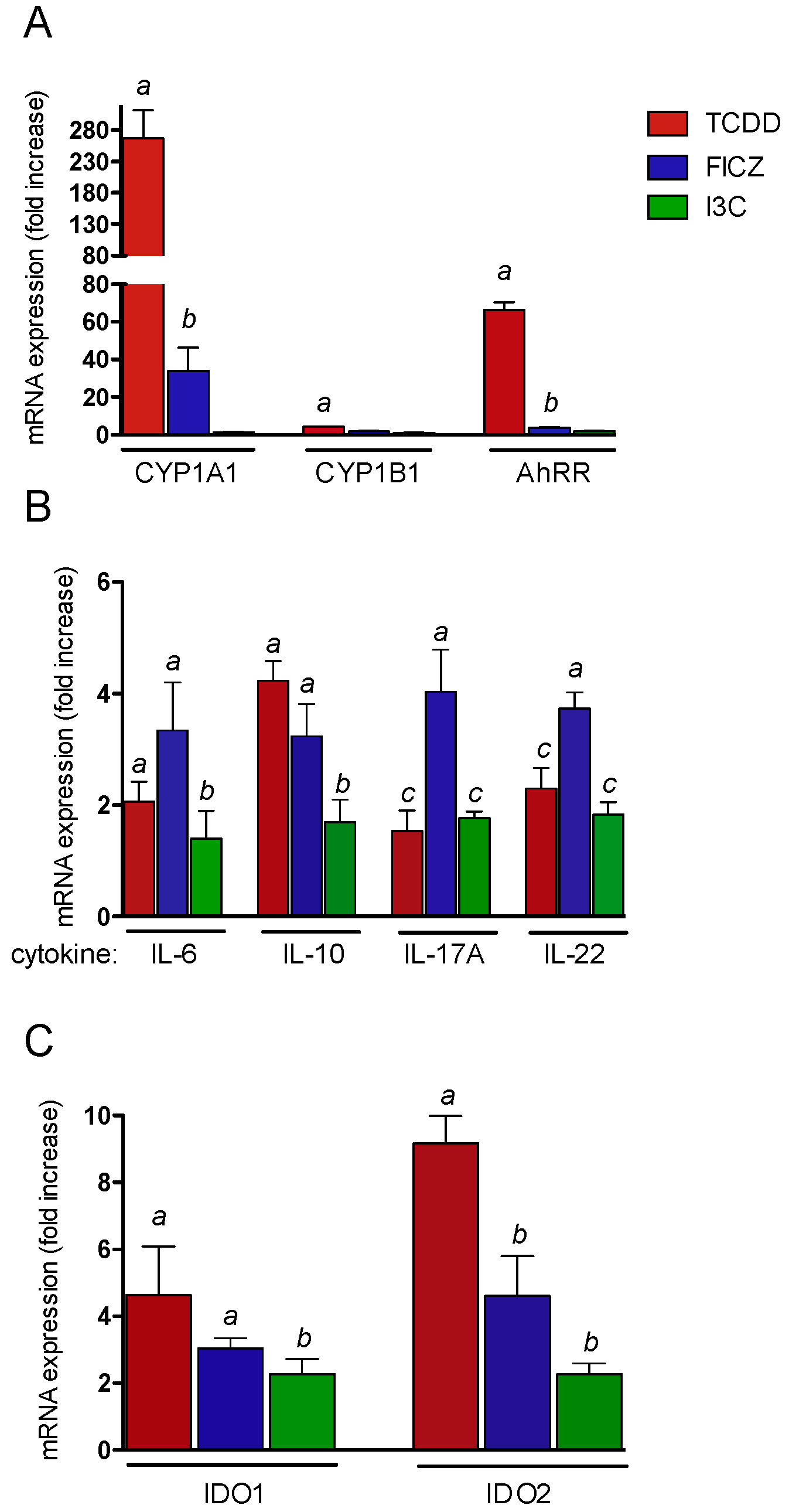
2.3. Effects of AhR Ligands on the Expression of Genes of the AhR Gene Battery, Cytokines, and Immune Regulatory Enzymes in Thymus of wt Mice
3. Materials and Methods
3.1. Reagents and Antibodies
3.2. Animals and Cell Culture
3.3. RNA Isolation and Quantitative Real-Time RT-PCR
3.4. Statistics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gu, Y.Z.; Hogenesch, J.B.; Bradfield, C.A. The PAS superfamily: Sensors of environmental and developmental signals. Annu. Rev. Pharmacol. Toxicol. 2000, 40, 519–561. [Google Scholar] [CrossRef]
- Labrecque, M.P.; Prefontaine, G.G.; Beischlag, T.V. The aryl hydrocarbon receptor nuclear translocator (ARNT) family of proteins: Transcriptional modifiers with multi-functional protein interfaces. Curr. Mol. Med. 2013, 7, 1047–1065. [Google Scholar]
- Denison, M.S.; Nagy, S.R. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 309–334. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Pei, X.; Zhang, W.; Xie, H.Q.; Zhao, B. Functional analysis of the dioxin response elements (DREs) of the murine CYP1A1 gene promoter: Beyond the core DRE sequence. Int. J. Mol. Sci. 2014, 4, 6475–6487. [Google Scholar] [CrossRef]
- Wright, E.J.; Pereira De Castro, K.; Joshi, A.D.; Elferink, C.J. Canonical and non-canonical aryl hydrocarbon receptor signaling pathways. Curr. Opinion Toxicol. 2017, 2, 87–92. [Google Scholar] [CrossRef]
- Wilson, S.R.; Joshi, A.D.; Elferink, C.J. The tumor suppressor Kruppel-like factor 6 is a novel aryl hydrocarbon receptor DNA binding partner. J. Pharmacol. Exp. Ther. 2013, 3, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Naka, T.; Nakahama, T.; Chinen, I.; Masuda, K.; Nohara, K.; Fujii-Kuriyama, Y.; Kishimoto, T. Aryl hydrocarbon receptor in combination with Stat1 regulates LPS-induced inflammatory responses. J. Exp. Med. 2009, 206, 2027–2035. [Google Scholar] [CrossRef]
- Lawrence, B.P.; Sherr, D.H. You AhR what you eat? Nat. Immunol. 2012, 2, 117–119. [Google Scholar] [CrossRef]
- Lee, J.S.; Cella, M.; McDonald, K.G.; Garlanda, C.; Kennedy, G.D.; Nukaya, M.; Mantovani, A.; Kopan, R.; Bradfield, C.A.; Newberry, R.D.; et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat. Immunol. 2011, 2, 144–151. [Google Scholar] [CrossRef]
- Yeste, A.; Mascanfroni, I.D.; Nadeau, M.; Burns, E.J.; Tukpah, A.M.; Santiago, A.; Wu, C.; Patel, B.; Kumar, D.; Quintana, F.J. IL-21 induces IL-22 production in CD4+ T cells. Nat. Commun. 2014, 5, 3753. [Google Scholar] [CrossRef] [PubMed]
- Apetoh, L.; Quintana, F.J.; Pot, C.; Joller, N.; Xiao, S.; Kumar, D.; Burns, E.J.; Sherr, D.H.; Weiner, H.L.; Kuchroo, V.K. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat. Immunol. 2010, 9, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Y.; Quintana, F.J.; da Cunha, A.P.; Dake, B.T.; Koeglsperger, T.; Starossom, S.C.; Weiner, H.L. In vivo induction of Tr1 cells via mucosal dendritic cells and AHR signaling. PLoS ONE 2011, 8, e23618. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.F.; Sciullo, E.; Li, W.; Wong, P.; Lazennec, G.; Matsumura, F. RelB, a new partner of aryl hydrocarbon receptor-mediated transcription. Mol. Endocrinol. 2007, 21, 2941–2955. [Google Scholar] [CrossRef] [PubMed]
- DiNatale, B.C.; Schroeder, J.C.; Francey, L.J.; Kusnadi, A.; Perdew, G.H. Mechanistic insights into the events that lead to synergistic induction of interleukin 6 transcription upon activation of the aryl hydrocarbon receptor and inflammatory signaling. J. Biol. Chem. 2010, 32, 24388–24397. [Google Scholar] [CrossRef]
- Vogel, C.F.; Sciullo, E.; Matsumura, F. Involvement of RelB in aryl hydrocarbon receptor-mediated induction of chemokines. Biochem. Biophys. Res. Commun. 2007, 363, 722–726. [Google Scholar] [CrossRef]
- Oesch-Bartlomowicz, B.; Oesch, F. Role of cAMP in mediating AHR signaling. Biochem. Pharmacol. 2009, 77, 627–641. [Google Scholar] [CrossRef]
- Burkly, L.; Hession, C.; Ogata, L.; Reilly, C.; Marconi, L.A.; Olson, D.; Tizard, R.; Cate, R.; Lo, D. Expression of relB is required for the development of thymic medulla and dendritic cells. Nature 1995, 373, 531–536. [Google Scholar] [CrossRef]
- Goudot, C.; Coillard, A.; Villani, A.C.; Gueguen, P.; Cros, A.; Sarkizova, S.; Tang-Huau, T.L.; Bohec, M.; Baulande, S.; Hacohen, N.; et al. Aryl Hydrocarbon Receptor Controls Monocyte Differentiation into Dendritic Cells versus Macrophages. Immunity 2017, 47, 582–596. [Google Scholar] [CrossRef]
- Vogel, C.F.; Wu, D.; Goth, S.R.; Baek, J.; Lollies, A.; Domhardt, R.; Grindel, A.; Pessah, I.N. Aryl hydrocarbon receptor signaling regulates NF-κB RelB activation during dendritic-cell differentiation. Immunol. Cell Biol. 2013, 91, 568–575. [Google Scholar] [CrossRef]
- Bankoti, J.; Rase, B.; Simones, T.; Shepherd, D.M. Functional and phenotypic effects of AhR activation in inflammatory dendritic cells. Toxicol. Appl. Pharmacol. 2010, 246, 18–28. [Google Scholar] [CrossRef]
- Stockinger, B.; Di Meglio, P.; Gialitakis, M.; Duarte, J.H. The aryl hydrocarbon receptor: Multitasking in the immune system. Annu. Rev. Immunol. 2014, 32, 403–432. [Google Scholar] [CrossRef]
- Quintana, F.J.; Sherr, D.H. Aryl hydrocarbon receptor control of adaptive immunity. Pharmacol. Rev. 2013, 65, 1148–1161. [Google Scholar] [CrossRef] [PubMed]
- Prigent, L.; Robineau, M.; Jouneau, S.; Morzadec, C.; Louarn, L.; Vernhet, L.; Fardel, O.; Sparfel, L. The aryl hydrocarbon receptor is functionally upregulated early in the course of human T-cell activation. Eur. J. Immunol. 2014, 44, 1330–1340. [Google Scholar] [CrossRef] [PubMed]
- Sharfe, N.; Merico, D.; Karanxha, A.; Macdonald, C.; Dadi, H.; Ngan, B.; Herbrick, J.A.; Roifman, C.M. The effects of RelB deficiency on lymphocyte development and function. J. Autoimmun. 2015, 15, 30040–30048. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.B.; Moore, A.J.; Head, J.L.; Neumiller, J.J.; Lawrence, B.P. Aryl hydrocarbon receptor activation reduces dendritic cell function during influenza virus infection. Toxicol. Sci. 2010, 116, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Quintana, F.J.; Basso, A.S.; Iglesias, A.H.; Korn, T.; Farez, M.F.; Bettelli, E.; Caccamo, M.; Oukka, M.; Weiner, H.L. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 2008, 453, 65–71. [Google Scholar] [CrossRef]
- Veldhoen, M.; Hirota, K.; Westendorf, A.M.; Buer, J.; Dumoutier, L.; Renauld, J.C.; Stockinger, B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 2008, 453, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Rohlman, D.; Pham, D.; Yu, Z.; Steppan, L.B.; Kerkvliet, N.I. Aryl hydrocarbon receptor-mediated perturbations in gene expression during early stages of CD4(+) T-cell differentiation. Front. Immunol. 2012, 3, 223. [Google Scholar] [CrossRef]
- Lahoti, T.S.; Boyer, J.A.; Kusnadi, A.; Muku, G.E.; Murray, I.A.; Perdew, G.H. Aryl Hydrocarbon Receptor Activation Synergistically Induces Lipopolysaccharide-Mediated Expression of Proinflammatory Chemokine (c-c motif) Ligand 20. Toxicol. Sci. 2015, 148, 229–240. [Google Scholar] [CrossRef]
- Vogel, C.F.; Goth, S.R.; Dong, B.; Pessah, I.N.; Matsumura, F. Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem. Biophys. Res. Commun. 2008, 375, 331–335. [Google Scholar] [CrossRef]
- Kado, S.; Chang, W.L.W.; Ngyuen-Chi, A.; Wolny, M.; Shepherd, D.M.; Vogel, C.F.A. Aryl hydrocarbon receptor signaling modifies Toll-like receptor-regulated responses in human dendritic cells. Arch. Toxicol. 2017, 91, 2209–2221. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, A.R.; Pinkerton, K.E.; Bein, K.J.; Magaña-Méndez, A.; Yang, H.T.; Ashwood, P.; Vogel, C.F.A. Ambient particulate matter activates the aryl hydrocarbon receptor in dendritic cells and enhances Th17 polarization. Toxicol. Lett. 2018, 292, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.F.; Khan, E.M.; Leung, P.S.; Gershwin, M.E.; Chang, W.L.W.; Wu., D.; Haarmann-Stemmann, T.; Hoffmann, A.; Denison, M.S. Cross-talk between aryl hydrocarbon receptor and the inflammatory response: A role for nuclear factor-κB. J. Biol. Chem. 2014, 289, 1866–1875. [Google Scholar] [CrossRef] [PubMed]

| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| β-actin | AGCCATGTACGTAGCCATCC | CTCTCAGCTGTGGTGGTGAA |
| AhRR | TGGACAAGCTTTCTGTCCTG | CGAAGCCATTGAGAGACTCC |
| CYP1A1 | GGCCACTTTGACCCTTACAA | CAGGTAACGGAGGACAGGAA |
| CYP1B1 | TTCTCCAGCTTTTTGCCTGT | TAATGAAGCCGTCCTTGTCC |
| IL-6 | AGTTGCCTTCTTGGGACTGA | TCCACGATTTCCCAGAGAAC |
| IL-10 | CCAAGCCTTATCGGAAATGA | TTTTCACAGGGGAGAAATCG |
| IL-17A | AGGCCCTCAGACTACCTCAACCGTT | TGGTCCAGCTTTCCCTCCGCATT |
| IL-22 | TTTCCTGACCAAACTCAGCA | TCTGGATGTTCTGGTCGTCA |
| IDO-1 | GGCTAGAAATCTGCCTGTGC | AGAGCTCGCAGTAGGGAACA |
| IDO-2 | GCTATCACCATGGGATTCGT | AGAGATCTTGGCAGCACCTT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishihara, Y.; Kado, S.Y.; Hoeper, C.; Harel, S.; Vogel, C.F.A. Role of NF-kB RelB in Aryl Hydrocarbon Receptor-Mediated Ligand Specific Effects. Int. J. Mol. Sci. 2019, 20, 2652. https://doi.org/10.3390/ijms20112652
Ishihara Y, Kado SY, Hoeper C, Harel S, Vogel CFA. Role of NF-kB RelB in Aryl Hydrocarbon Receptor-Mediated Ligand Specific Effects. International Journal of Molecular Sciences. 2019; 20(11):2652. https://doi.org/10.3390/ijms20112652
Chicago/Turabian StyleIshihara, Yasuhiro, Sarah Y. Kado, Christiane Hoeper, Shelly Harel, and Christoph F. A. Vogel. 2019. "Role of NF-kB RelB in Aryl Hydrocarbon Receptor-Mediated Ligand Specific Effects" International Journal of Molecular Sciences 20, no. 11: 2652. https://doi.org/10.3390/ijms20112652
APA StyleIshihara, Y., Kado, S. Y., Hoeper, C., Harel, S., & Vogel, C. F. A. (2019). Role of NF-kB RelB in Aryl Hydrocarbon Receptor-Mediated Ligand Specific Effects. International Journal of Molecular Sciences, 20(11), 2652. https://doi.org/10.3390/ijms20112652






