Abstract
Both the calcium-dependent protein kinases (CDPKs) and CDPK-related kinases (CRKs) play numerous roles in plant growth, development, and stress response. Despite genome-wide identification of both families in Cucumis, comparative evolutionary and functional analysis of both CDPKs and CRKs in Cucurbitaceae remain unclear. In this study, we identified 128 CDPK and 56 CRK genes in total in six Cucurbitaceae species (C. lanatus, C. sativus, C. moschata, C. maxima, C. pepo, and L. siceraria). Dot plot analysis indicated that self-duplication of conserved domains contributed to the structural variations of two CDPKs (CpCDPK19 and CpCDPK27) in C. pepo. Using watermelon genome as reference, an integrated map containing 25 loci (16 CDPK and nine CRK loci) was obtained, 16 of which (12 CDPK and four CRK) were shared by all seven Cucurbitaceae species. Combined with exon-intron organizations, topological analyses indicated an ancient origination of groups CDPK IV and CRK. Moreover, the evolutionary scenario of seven modern Cucurbitaceae species could also be reflected on the phylogenetic trees. Expression patterns of ClCDPKs and ClCRKs were studied under different abiotic stresses. Some valuable genes were uncovered for future gene function exploration. For instance, both ClCDPK6 and its ortholog CsCDPK14 in cucumber could be induced by salinity, while ClCDPK6 and ClCDPK16, as well as their orthologs in Cucumis, maintained high expression levels in male flowers. Collectively, these results provide insights into the evolutionary history of two gene families in Cucurbitaceae, and indicate a subset of candidate genes for functional characterizations in the future.
1. Introduction
As a ubiquitous second messenger, Calcium (Ca2+) plays an important role in sophisticated signal transduction pathways to survive frequently occurring environmental stresses during plant growth and development [1,2]. Transient changes of the Ca2+ concentration in the cytoplasm can be sensed by four types of calcium sensors: calmodulins (CaM), calmodulin-like proteins (CaML), calcineurin B-like proteins (CBL), and the calcium-dependent protein kinase (CDPK) [3,4]. Among these sensors, CDPKs not only sense but also directly translate Ca2+ signals into a downstream phosphorylation pathway, thus functioning both as Ca2+ sensors and effectors [2,5,6].
The CDPKs (also referred to as CPKs) are ser/thr protein kinases, which consist of four typical domains: a variable N-terminal domain (containing the myristoylation and palmitoylation sites), a catalytic ser/thr protein kinase domain, an auto-inhibitory domain (acting as a pseudosubstrate combined with the kinase domain to inhibit activity), and a C-terminal regulatory calmodulin-like domain that contains one to four EF-hand motifs for Ca2+ binding [7,8,9]. CDPK-related kinases (CRKs) have similar domain structures than CDPKs (such as the ser/thr kinase domain); however, they do not have EF-hand domains [10,11]. To date, genome-wide identification of CDPKs has been widely performed in a large number of plants, e.g., 34 CDPKs have been identified in Arabidopsis [7], 31 in rice [12], 29 in tomato [10], 18 in melon [11], and 19 in cucumber [9]. Similarly, CRKs have also been identified in genomes, e.g., eight CRKs in Arabidopsis [13], five in rice [14], six in tomato [10], five in pepper [15], and seven in melon [11].
Accumulating evidence indicates that CDPK and CRK genes are not only involved in plant growth and development, but also in the plant response to abiotic and hormone stresses. For example, AtCPK32 in Arabidopsis has been reported to interact with the calcium channel protein CNGC18, thus controlling the polar growth of pollen tubes [16]. In addition, AtCPK4 and its homolog AtCPK11 have been reported to function in seed germination and growth, stomatal movement, and response to salt stress [17]. The gene AtCPK23 acts as a negative regulator and plays important roles in response to drought and salt stresses via controlling K+ channels; its overexpression can increase stomatal apertures [18]. In grape plants, a large number of CDPKs have been reported to be induced in response to various abiotic and biotic stresses, as well as to hormone treatments [19]. A previous study showed that the CDPK genes in cucumber are extensively regulated by various stimuli, including salt, cold, heat, waterlogging, and abscisic acid stresses, possibly following different mechanisms [9]. Furthermore, CmCDPKs and CmCRKs in melon were also shown to be differentially expressed in response to exogenous stresses, such as biotic stress (Podosphaera xanthii inoculation), abiotic stresses (salt and cold), and hormone (abscisic acid) treatment [11]. In general, CDPK genes are ubiquitously expressed in most of the different plant organs. However, several genes show organ- or tissue-specific expression patterns. For instance, VvCDPK5 in grape plants can only be detected in pollen [20]. Similarly, AtCPK17 and AtCPK34 are both preferentially expressed in mature pollen, regulating the growth of pollen tubes [21].
The Cucurbitaceae family contains several economically important species with already published genomes, including watermelon (Citrullus lanatus), melon (Cucumis melon), cucumber (Cucumis sativus), and bottle gourd (Lagenaria siceraria), which belong to the Benincaseae tribe and three Cucurbita species (Cucurbita maxima, Cucurbita moschata, and Cucurbita pepo) of the Cucurbiteae tribe [22,23,24,25,26,27]. Although genome-wide identifications of CDPKs and CRKs have been performed in the genus Cucumis [9,11], comparative evolutionary analysis of both gene families in Cucurbitaceae is lacking. In this study, we identified a total of 128 CDPK and 56 CRK genes in six Cucurbitaceae species (C. lanatus, C. sativus, C. moschata, C. maxima, C. pepo, and L. siceraria). After mapping these identified genes onto chromosomes of watermelon, we obtained an integrated map including 25 loci (16 CDPK and nine CRK loci). Evolutionary analyses revealed that four CDPK groups and one CRK group in phylogentic trees could be further divided into loci, consistent with the integrated map. In addition, expression patterns of ClCDPKs and ClCRKs under different abiotic stresses were also analyzed. Our results provide insights into the evolutionary history of both gene families in Cucurbitaceae, and indicate a subset of candidate genes for future functional analysis.
2. Results
2.1. Genome-Wide Identification of CDPK and CRK Genes
Our previous study verified 18 CmCDPK and seven CmCRK genes in melon [11], while only 19 CDPK homologs were identified genome-wide in cucumber [9]. Hence, to comparatively analyze the CRK gene family in Cucurbitaceae, identification of CRK genes in cucumber genome was also performed in this study. As a result, a total of 128 CDPK (typically containing both STKs_CAMK protein kinase and EF-hand domains) and 56 CRK (solely harboring STKs_CAMK protein kinase domain) genes were identified in six Cucurbitaceae species (C. lanatus, C. sativus, C. moschata, C. maxima, C. pepo, and L. siceraria). All the identified genes were designated based on their chromosomal locations (Table S2). It is worth noting that two CDPKs, referred to as CsCDPK18 (Csa018149) and CsCDPK15 (Csa008536) in the previous study [9], were renamed to CsCRK6 and CsCRK7 due to their lack of EF-hand domains. Compared to the similar copy numbers of CDPK and CRK genes in four species (C. lanatus, C. melon, C. sativus, and L. siceraria) of the Benincaseae tribe (Table 1), many more homologs were identified in three Cucurbiteae tribe genomes (C. moschata, C. maxima, and C. pepo). This may have been caused by the whole-genome duplication (WGD) that only occurred in the progenitor of the Cucurbita genus [25,26].

Table 1.
Numbers and characteristic properties of CDPKs and CRKs in Cucurbitaceae species.
Physico-chemical properties of CDPKs and CRKs, including predicted amino acids, molecular weight (MW), and isoelectric point (pI), showed similar ranges in watermelon, melon, and cucumber; however, these characteristics exhibited broader intervals in the remaining four species (Table 1). In this study, six CDPKs were predicted to have long (>1000) amino acid sequences, possibly due to their long CT domains [28]. Compared to CRKs with no EF-hand domain, the majority of CDPKs were predicted to contain four EF-hands, with few exceptions harboring two or three EF-hand motifs (Table S2). Strikingly, two CDPKs (CpCDPK19 and CpCDPK27) in C. pepo were confirmed to have more than five EF-hand domains via both online tools ScanProsite and SMART. More detailed information, for example for myristoylation and palmitoylation sites, is also provided in Table S2.
2.2. Structure Variation Analysis of CpCDPK19 and CpCDPK27
As mentioned above, CpCDPK19 and CpCDPK27 in C. pepo harbored nine and eight EF-hand motifs, respectively. Previous research indicates that Cucurbita, containing two sub-genomes A and B, originated from two progenitors that diverged from one another approximately 30.75 million years ago (Mya) [26]. Sequence analysis showed that CpCDPK5 and CpCDPK13, sharing the highest similarity with CpCDPK19 (amino acid identity: 93.74%) and CpCDPK27 (amino acid identity: 95.84%), contained four and three EF-domains, respectively (Table S2). Dot plot analysis indicated that the EF-hand domains were duplicated in CpCDPK19 compared to CpCDPK5, while both STKs_CAMK protein kinase and EF-hand domains were repeated in CpCDPK27 in contrast to CpCDPK13 (Figure S1). Based on the synteny analysis (Figure 1a), we inferred that a fragment containing four EF-hand motifs in CpCDPK19 was entirely duplicated and inserted into the STKs_CAMK protein kinase domain, and the fifth EF-hand motif resulted from another duplication event. Compared to ClCDPK13, ClCDPK27 harbored three copies of STKs_CAMK protein kinase domain. Moreover, the first EF-hand domain located in the second STKs_CAMK protein kinase, as well as the second and fourth EF-hand domains, were originated from the sixth EF-hand domain, while the third (existed in the third STKs_CAMK protein kinase) and the fifth EF-hand domains were two copies of the seventh motif (Figure 1b). Using the Cucurbit Genomics Database (CuGenDB) [29], both two genes CpCDPK19 and CpCDPK27 could be detected in the fruits of two different materials at transcriptional level (Figure S2). In summary, self-duplication of conserved domains may contribute to the gene structure variations, and result in sub-functionalization or neo-functionalization.
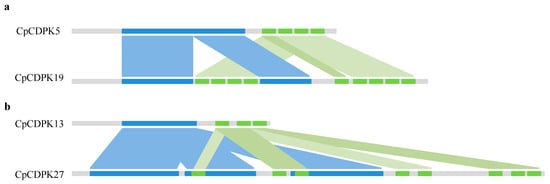
Figure 1.
Gene structure variation analysis of CpCDPK19 and CpCDPK27. Syntenic analysis of CpCDPK5 and CpCDPK19 (a), as well as CpCDPK12 and CpCDPK27 (b). Blue and green rectangles represent STKs_CAMK protein kinase and EF-hand domain, respectively.
2.3. Construction of an Integrated Map for CDPK and CRK Genes
As the genomic distribution of CDPKs and CRKs in Cucumis [9,11], all identified genes in this study were also unevenly distributed on chromosomes in five species, with a few chromosomes harboring none of them (Figure S3). A recent study has confirmed that there are twenty chromosomes in Cucurbita species and nineteen of them could be divided into two sub-genomes A and B, except for chromosome 04, consisting of two segments from sub-genome A and one from sub-genome B [26]. Interestingly, almost equal numbers of CDPKs and CRKs were found to be retained in two sub-genomes (Table S3), consistent with the similar evolved ratios of gene loss or gain of two sub-genomes after polyploidization [26].
The evolutionary scenario of Cucurbitaceae paleohistory hypothesizes that the modern chromosomal structures in cucurbits were derived from an ancestral Cucurbitaceae karyotype (ACK) that consisted of 12 protochromosomes and experienced different times of chromosomal fission and fusion events [27]. Hence, to investigate the conserved loci of CDPK and CRK in Cucurbitaceae, an integrated map was constructed using watermelon chromosomes as reference, including 16 CDPK and nine CRK loci (Figure 2). Of these, nine loci (four CDPK and five CRK) exhibited Presence/Absence polymorphism among genomes, while the remaining 16 loci (12 CDPK and four CRK) were conserved and shared by all seven species (Figure 2 and Table S4). For example, the first locus on chromosome 01 was lost in L. siceraria, while the second locus on chromosome 02 was only absent in watermelon genome.
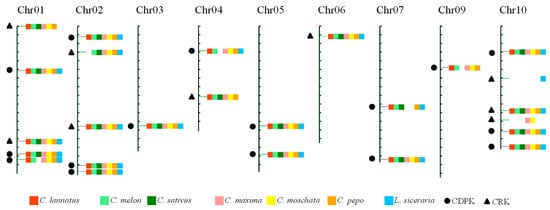
Figure 2.
An integrated map of CDPK and CRK loci in Cucurbitaceae. All CDPK and CRK genes in Cucurbitaceae were mapped onto 11 chromosomes of watermelon 97103. Loci from C. lannatus (red), C. melon (light green), C. sativus (dark green), C. maxima (pink), C. moschata (yellow), C. pepo (dark yellow), and L. siceraria (blue) have been marked with different colors, as indicated in brackets. Black dots and triangles represent CDPK and CRK loci, respectively.
2.4. Phylogenetic Analysis of CDPK and CRK Gene Families
2.4.1. In the Benincaseae Tribe
In the Benincaseae tribe, watermelon was predicted to diverge from its sister lineage bottle gourd around 10.4–14.6 million years ago (Mya) and from Cucumis 17.3–24.3 Mya [27]. To deeply analyze the phylogentic relationships of CDPK and CRK gene families in these four species, all full-length protein sequences of watermelon (18 CDPKs and six CRKs), melon (18 CDPKs and seven CRKs), cucumber (17 CDPKs and seven CRKs), and bottle gourd (17 CDPKs and seven CRKs) were aligned using the software MUSCLE, and were then used to construct an evolutionary tree using MEGA6.0 (Figure S4). Four CDPK groups (CDPK I, CDPK II, CDPK III, and CDPK IV) and one CRK group (CRK I) were observed in the distance tree. Combined with results from the integrated map, all groups (except for CDPK IV) were found to constitute homologs from at least four loci. Furthermore, most loci contained only one homolog from each individual species. Apparently, homologs from watermelon and bottle gourd were preferentially clustered together, compared to those from Cucumis species.
2.4.2. In the Cucurbiteae Tribe
It has been reported that a whole genome duplication (WGD) event has occurred in the progenitor of the Cucurbita genus [26,27]. As shown in the phylogenetic tree constructed with all identified full-length protein sequences from C. moschata (30 CDPKs and 12 CRKs), C. maxima (31 CDPKs and 12 CRKs), and C. pepo (32 CDPKs and 12 CRKs), the majority of loci in five groups contained two copies of the homolog gene from each species (Figure S5). Generally, CDPKs or CRKs from the same sub-genome (A or B) of C. moschata and C. maxima grouped together in the distance tree. For example, both CmoCDPK7 and CmaCDPK8 from sub-genome B of two species clustered together in CDPK IV, while CmoCDPK14 and CmaCDPK15 from sub-genome A formed another sister clade. Based on these observations, origins of CDPKs on chromosome 4 in two Cucurbita species could be uncovered, although the boundary sites of three segments (two from sub-genome A and one from B) of chromosome 04 were ambiguous [26], e.g., CmoCDPK26 and CmaCDPK27 in Locus 15 (group CDPK I) were from sub-genome A (Table S3 and Figure S5), suggesting that the members (CmoCDPK9 and CmaCDPK10) in its sister clade originated from sub-genome B. Similarly, CmoCDPK8 and CmaCDPK9 were inferred to originate form sub-genome A, which formed the Locus 20 in group CDPK II. It is worthy to note that both loci 06 and 24 formed two independent clades in the phylogenetic tree, due to having far more CDPK members.
2.4.3. In the Cucurbitaceae Family
To deeply analyze the evolutionary history of CDPK and CRK gene families in Cucurbitaceae, a total of 226 protein sequences were used to construct a phylogenetic tree (Figure 3). Similar to topological structures mentioned above, four CDPK and one CRK group could be further divided into 25 loci. Notably, six homologs (LsiCRK1, CsCDPK3, CsCDPK19, CpCRK1, CpCDPK1, and CpCDPK2), which failed to be mapped on the integrated map due to lack of enough flanking sequences, were clustered with their orthologs in the phylogenetic trees. CDPKs or CRKs from watermelon and bottle gourd still cluster together in most loci. Compared to sub-genome A, members from sub-genome B usually gathered together with those from the Benincaseae tribe, except for two loci in group CRK I (Figure S6), which is consistent with the evolutionary scenario of modern Cucurbitaceae genomes [27]. CDPK IV, as the smallest group in CDPK lineage, was clustered with CRK I rather than the other three CDPK groups in phylogenetic trees, indicating that groups CDPK IV and CRK I may have originated from a common ancestor [10,11,30].
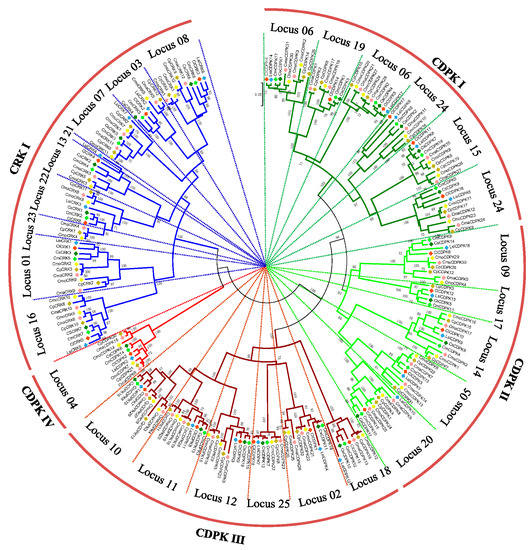
Figure 3.
Phylogenetic tree of CDPK and CRK genes from Cucurbitaceae species. Four CDPK groups and one CRK group can be found in the tree, which were further divided into 25 loci. Numbers on nodes represent bootstrap values, and values <65 are not shown.
For the comparative evolutionary analysis of two gene families in plants, a phylogenetic tree was constructed using the 226 protein sequences in Cucurbitaceae, as well as that from Arabidopsis (34 CDPKs and eight CRKs, Cruciferae), tomato (29 CDPKs and six CRKs, Solanaceae), pepper (31 CDPKs and five CRKs, Solanaceae), and rice (31 CDPKs and five CRKs, Poaceae) (Figure S7). There is no doubt that all groups contained homologs from these four families, suggesting that both gene families have conserved basal architectures in their evolutionary process.
2.5. Exon-Intron Organization in CDPK and CRK Groups
The exon-intron organization, as well as the intron numbers, can also provide important evidence to analyze the evolutionary history within gene families [9,11,30]. To obtain further insight into the phylogenetic relationships of two gene families in Cucurbitaceae, gene structures of all 226 CDPKs and CRKs were comparatively depicted, dependent on their gene annotation profiles and genomic sequences (Figure S8). The majority of members in group CDPK I contained six introns with a distinct intron phase pattern 111000, while most CDPKs in group CDPK II had seven introns, sharing a similar intron pattern of 1110020. CDPK III, as a peripheral sister clade of groups CDPK I and II, contained two major intron phases. For instance, 13 out of 17 CDPKs in loci 02 and 25 shared an intron phase 111000, which is identical to that of group CDPK I, while 28 of 37 CDPKs in the other four loci had an intron pattern of 0111000 (Figure S8). CDPK IV, as the smallest group, contained only 10 members with 11 or 12 introns. Among of them, eight CDPKs were constituted of 11 introns with a phase pattern of 02201010000, while the remaining two genes (CsCDPK6 and CmoCDPK7) had 12 introns with an extra intron gain at the 5’ or 3’ end. In group CRK I, 41 out of 63 members contained 10 introns with a phase pattern 0220110000, showing high similarity with that in group CDPK IV. Notably, members in the same loci usually had similar exon or intron lengths, such as Locus 25 in group CDPK III.
2.6. Duplication and Syntenic Analysis of CDPK and CRK Gene Families
In addition to whole genome duplication (WGD) events, both tandem and segmental duplications have also been reported to play vital roles in the expansion and function of a gene family [11,31,32]. To further explore the possible evolutionary relationships of CDPK and CRK gene families in Cucurbitaceae, duplication events were investigated in six species (including watermelon, cucumber, bottle gourd, and three Cucurbita species). Similar to the melon genome [11], only one or two segmental duplication events were detected in three Benincaceae genomes, with syntenic regions no more than 3.0 Mb (Table S5). However, many more segmental duplication events were observed in Cucurbita genus, most of which occurred between sub-genomes.
In the Benincaseae tribe, watermelon, melon, and cucumber are important cucurbit crops widely cultivated throughout the world. Synteny analyses revealed that similar numbers of syntenic regions were detected among three genomes, with average fragment lengths not exceeding 2.5 Mb (Figure 4 and Table S6). As a close sister lineage of Citrullus, approximately 15 collinear regions were found between watermelon and bottle gourd, with the largest one spanning about 16.6 Mb. In the Cucurbiteae tribe, many more collinear regions were detected, with the largest one (21.3 Mb) existing between C. moschata and C. pepo (Table S6).
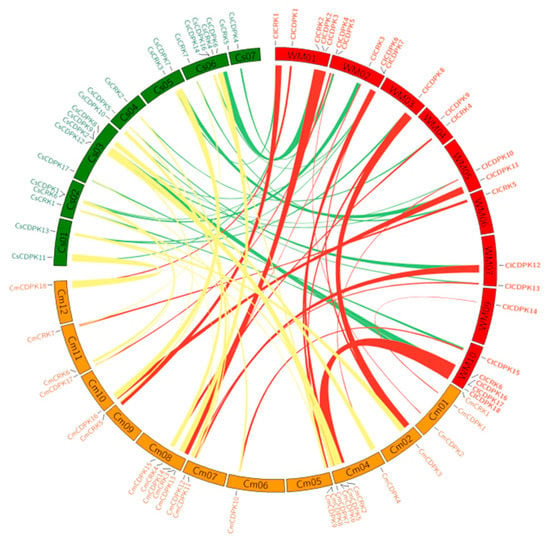
Figure 4.
Synteny analysis of CDPK and CRK genes among watermelon, cucumber, and melon. Chromosomes of three species (watermelon, cucumber, and melon) were depicted in different colors (red, green, and yellow) and in circle form. The approximate distributions of each CDPK and CRK are presented by short black lines on the circle. Colored curves denote the details of syntenic regions containing CDPK and CRK genes among genomes.
2.7. Expression Profiles of ClCDPK and ClCRK Genes in Different Tissues
To assess the potential functions of ClCDPK and ClCRK genes, their expression patterns were investigated in six different tissues, including roots, stems, leaves, tendrils, male flowers, and female flowers. As shown in Figure 5, all identified ClCDPKs and ClCRKs could be detected in at least one tissue. Some ClCDPKs and ClCRKs, such as ClCDPK2, ClCDPK3, ClCDPK9, ClCDPK15, and ClCRK1, showed significantly elevated expression levels in the root, while ClCDPK6, ClCDPK16, and ClCDPK17 were strongly expressed in the male flower. Interestingly, CmCDPK6 in melon, the ortholog of ClCDPK17 in locus 24, was also reported to have a high transcriptional abundance in the male flower [11]. Moreover, orthologs of ClCDPK6 and ClCDPK16 in melon (CmCDPK9 and CmCDPK5) and cucumber (CsCDPK14 and CsCDPK9) were also confirmed to retain high accumulations in male flowers [9,11].
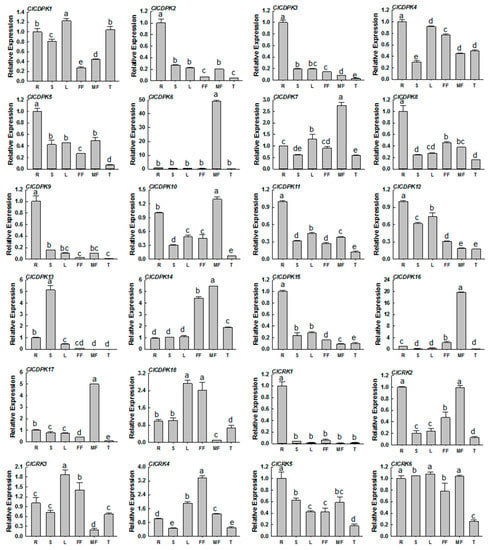
Figure 5.
Expression profiles of ClCDPK and ClCRK genes in different tissues. The transcript levels of the respective genes in roots were used as references and set to a value of 1. The data were showed as means value ± SD. All experiments were performed with three independent replicates. R = roots; S = stems; L = leave; FF = female flowers; MF = male flowers; T = tendrils.
2.8. Expression Patterns of ClCDPK and ClCRK Genes under Abiotic Stresses
Accumulation studies showed that CDPK and CRK genes are widely involved in the adaptations to environmental stimuli, and that their expression levels are affected by drought, salt, and cold [2,9,11]. To investigate the potential roles of ClCDPKs and ClCRKs in response to abiotic stresses, their dynamic expressions were analyzed under drought, salt, and cold treatments (Figure 6). Compared to cold stimuli, far more ClCDPKs and ClCRKs could be induced by drought and NaCl treatments, and seven genes could be up-regulated by both drought and NaCl treatments, including ClCDPK1, ClCDPK5, ClCDPK6, ClCDPK9, ClCDPK10, ClCDPK12, and ClCDPK14. Following cold treatment, the transcription levels of four genes (ClCDPK1, ClCDPK5, ClCDPK16, and ClCDPK17) were down-regulated, while gene ClCRK2 was continuously up-regulated at all treatment times. Compared to ClCDPK3 and ClCDPK18 down-regulated by drought stress, many more genes were obviously up-regulated, such as ClCDPK1, ClCDPK2, ClCDPK8, ClCDPK9, ClCDPK12, and ClCDPK14. In response to NaCl stress, the majority of ClCDPK and ClCRK genes were up-regulated, with few exceptions (Figure 6).
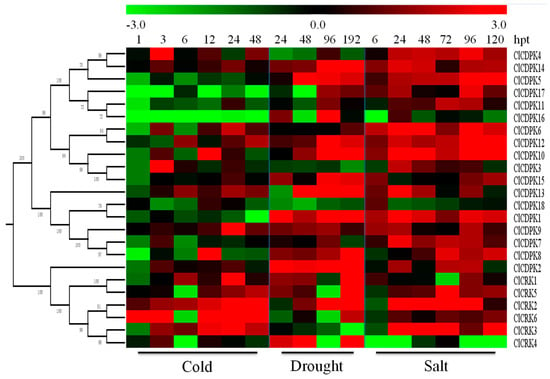
Figure 6.
Expression patterns of ClCDPK and ClCRK genes under abiotic stresses. The abiotic stresses are displayed at the low end. The relative transcript level was log2 transformed and visualized as a heat map via Mev4.8.1, using red to indicate increased expression level and green to indicate decreased expression level.
2.9. Expression Patterns of ClCDPK and ClCRK Genes under Hormone Treatments
Previous studies have indicated that CDPKs and CRKs are involved in the signaling pathways of various plant hormones [19,33]. Here, expression profiles of ClCDPKs and ClCRKs were investigated in response to four plant hormones ABA, SA, MeJA, and ETH.
Increasing evidence has shown that CDPK and CRK genes could participate in ABA-mediated signal transduction in plants [17,34,35]. In the present study, the majority of ClCDPKs were found to be induced by ABA treatment, with transcript abundance retaining at higher levels from 6 to 24 hpt (Figure 7a). Interestingly, all ClCRKs except for ClCRK1, were sharply down-regulated at 6 hpt, which is similar to observations in melon [11]. Following SA treatment, expression levels of most ClCDPKs and ClCRKs remained either unchanged or slightly changed at 0.5 and 1 hpt (Figure 7b). However, SA application significantly induced or reduced their transcript levels at 6 or 12 hpt. Notably, ClCRKs were also found to have decreased at 6 hpt, which is similar to the tendency mentioned above. In contrast to the responses to ABA and SA treatments, almost all ClCDPKs and ClCRKs showed continuous over-expression in response to ETH and MeJA stimuli, except for genes ClCDPK16 and ClCRK3 (Figure 8). Moreover, transcript abundances of most ClCDPKs and ClCRKs had either sharply increased or decreased at 0.5 hpt, implying their rapid response to ETH and MeJA stimuli. In summary, these expression analyses indicated that ClCDPKs and ClCRKs could be involved in the regulatory pathways of plant hormones, and thus participate in the plant defense against environmental stresses.
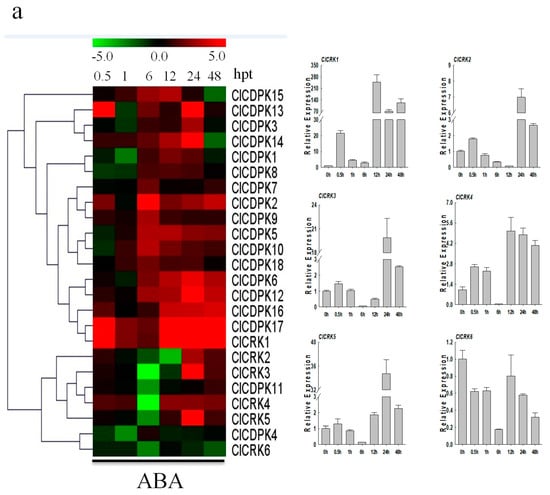
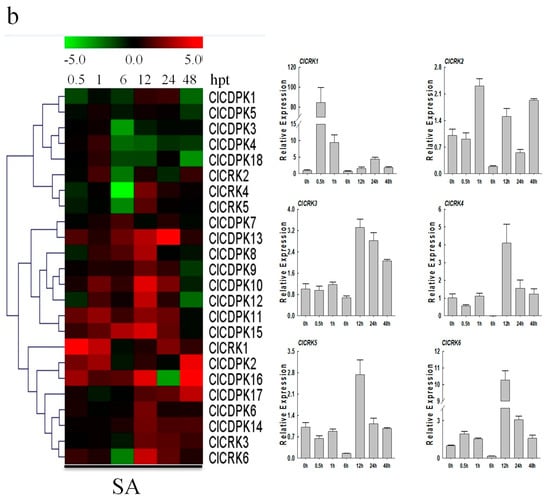
Figure 7.
Expression patterns of ClCDPK and ClCRK genes under ABA and SA hormone treatments. (a) Expression levels of ClCDPKs and ClCRKs under ABA stress visualized as a heat map (Left). Detailed expression patterns of ClCRKs under ABA stress (Right). (b) Expressions of ClCDPKs and ClCRKs under SA stress visualized as a heat map (Left). Detailed expression patterns of ClCRKs under SA stress (Right). The relative transcript level was log2 transformed and visualized as a heat map via Mev4.8.1, using red to indicate increased expression level and green to indicate decreased expression level.
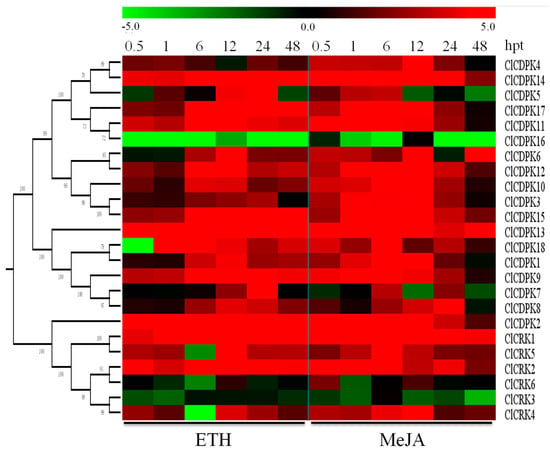
Figure 8.
Expression patterns of ClCDPK and ClCRK genes under ETH and MeJA hormone stresses. The abiotic stresses are indicated at the low end. The relative transcript level was log2 transformed and visualized as a heat map by Mev4.8.1, using red to indicate increased expression level and green to indicate decreased expression level.
3. Discussion
3.1. Characteristic Features of CDPK and CRK Genes in Cucurbitaceae
Functioning as both Ca2+ sensors and effectors, the CDPK gene family has been identified throughout the plant kingdom, as well as in several protozoa, but are absent in animals [4,28]. For instance, 34 CDPKs and eight CRKs were identified genome-wide in Arabidopsis [13], 29 CDPKs and six CRKs in tomato [10], 31 CDPKs and five CRKs in pepper [15], 30 CDPKs and nine CRKs in poplar [36], and 31 CDPKs and five CRKs in rice [12,14]. Moreover, approximately 19, 41, and 40 CDKPs have been detected in the genomes of grape [19], cotton [30], and maize [37], respectively. In Cucurbitaceae, 18 CDPKs and seven CRKs have been found in the melon genome [11], while 19 CDPKs were identified in cucumber by Xu et al. [9]. In this study, a total of 128 CDPK and 56 CRK genes were identified in six Cucurbitaceae species, including C. lanatus, C. sativus, C. moschata, C. maxima, C. pepo, and L. siceraria (Table 1). The numbers of CDPKs and CRKs are much higher in three Cucurbita species than in four Benincaseae tribe species (C. lanatus, C. melon, C. sativus, and L. siceraria), which may be due to a WGD event during the origin of this genus [25,26]. Moreover, the numbers of CRKs in three Cucurbita genomes are much higher than in other species, such as tomato, pepper, and rice, although these species contain similar copies of CDPKs.
Tandem, segmental, and whole genome duplication events are confirmed to play important roles in the expansion of gene families. Approximately 12, 13, and seven segmental duplications were reported to exist in poplar, cotton, and rice genomes [14,30,36], while many more events were found in three Cucurbita species that mainly occurred between two sub-genomes (Table S5). However, only a few segmental duplication events (one or two) were detected in genomes of watermelon, cucumber, bottle gourd, and melon [11], which may cause the low copy numbers of CDPK and CRK genes. In the present study, the majority of CDPKs contained four EF-hand motifs (Table S2), which is consistent with observations found in other species [7,11,19]. However, two CDPKs (CpCDPK19 and CpCDPK27) in C. pepo with detectable transcriptional levels had been confirmed to contain nine and eight EF-hands, respectively. Dot plot analysis showed that self-duplications of the STKs_CAMK protein kinase or EF-hand domains resulted in the gene structure variations (Figure 1), which may affect their functional specificity.
3.2. Conserved Evolution of CDPK and CRK Genes in Cucurbitaceae
Generally, CDPK and CRK genes are randomly distributed in genomes [10,11,15,36], which has also been validated in Cucurbitaceae species (Figure S3). Using watermelon chromosomes as reference, an integrated map was obtained and contained 16 CDPK and nine CRK loci, harboring almost all CDPK and CRK genes identified in Cucurbitaceae (Figure 2 and Table S4). Of these, nine loci (four CDPK and five CRK) exhibited Presence/Absence polymorphisms, while the remaining 16 loci (12 CDPK and four CRK) were shared by all seven species, implying that the flanking regions of most CDPK and CRK genes were conserved in these species during the evolutionary process.
Increasing evidence indicates that topological structures of CDPK and CRK gene families are conserved, with four CDPK and one CRK groups in phylogenetic trees [10,11,28,36]. In the present study, group CDPK IV was found to be close to CRK I rather than the other three CDPK groups in distance trees (Figure 3 and Figures S4, S5 and S7), confirming that CDPK IV and CRK I may originate from a common ancestor [10,11,30]. Moreover, the five groups can be further divided into 25 loci, according to the integrated map. The evolutionary scenario of seven modern Cucurbitaceae species revealed that watermelon diverged from bottle gourd around 10.4–14.6 Mya and from Cucumis 17.3–24.3 Mya; however, the progenitor B of Cucurbita diverged from Benincaseae around 25.5–27.0 Mya, and progenitor A diverged from the common ancestor of progenitor B and Benincaseae around 29.9–31.6 Mya [26,27]. In agreement with this evolutionary scenario, orthologs from watermelon and bottle gourd usually gathered together in phylogenetic trees, and genes from sub-genome B were preferentially clustered with those from Benincaseae tribe genomes (Figure 3 and Figure S7).
Both exon-intron structures and intron numbers can reflect the evolution, expansion, and functional relationships within a gene family, which were caused by three main types of mechanisms, including exon/intron gain/loss, exonization/pseudoexonization, and insertion/deletion [11,19,38]. Exon-intron organization analyses revealed that each group contained one or two major intron phase patterns (Figure S8). For instance, the majority of homologs in group CDPK I contained six introns with a distinct intron phase pattern of 111000, while most members in CDPK II had seven introns sharing a similar intron phase 1110020. As a peripheral sister clade of CDPK I and II, CDPK III contained two major intron phases and one of them was identical to that of group CDPK I. Moreover, the major intron pattern (02201010000) of eight members in CDPK IV is similar to that (0220110000) of most CRK homologs in CRK I (Figure S8). Combined with the topological structures of CDPK IV and CRK I, we infer that group CDPK IV is the ancient lineage of CDPK gene family, which may have diverged from the last common ancestor with CRK I before the divergence of monocots and dicots [28].
3.3. Functional Comparison of CDPK and CRK Genes
CDPKs and CRKs have been confirmed to play crucial roles in the signal pathways in response to various environmental stresses [4,5,28]. The systemic expression profiles of CDPK and CRK genes in Cucumis species under different stimuli have been reported recently [9,11]. Consequently, dynamic expression levels of CDPKs and CRKs under different stimuli were investigated in detail in watermelon. Compared to cold stimuli, far more ClCDPKs and ClCRKs were induced under drought and NaCl treatments (Figure 6), which differed from the expression trends of most homologs that were up-regulated by cold stress in cucumber and melon [9,11]. In watermelon, expression levels of ClCDPK14 were up-regulated by all three stresses (cold, drought, and salt); its ortholog CmCDPK1 in melon, could be also induced by cold and salinity treatments [11]. Completely different expression trends were also observed among species. For example, both genes ClCDPK6 and ClCDPK12, with a close relationship in group CDPK II, could be induced by salt stress (Figure S4 and Figure 6), while expression levels of their orthologs in melon (CmCDPK9 and CmCDPK11) and cucumber (CsCDPK14 and CsCDPK5) exhibited contrary responses to salinity stimuli [9,11]. Similarly, transcriptional levels of ClCRK2 and its paralog ClCRK3, as well as their orthologs CmCRK3 and CmCRK6 in melon, were increased under low temperature; however, these two groups of genes showed different expression trends under salt stress between watermelon and melon. Taken together, we inferred that the functional fates of some orthologs may be diversified during species evolution. The phytohormone ABA has been reported to be widely involved in the response of plants to biotic and abiotic stresses [17,34,35]. For instance, AtCPK4 and AtCPK11 in Arabidopsis can positively mediate the CDPK/calcium-mediated ABA signaling pathways via phosphorylation of two ABA-responsive transcription factors, ABF1 and ABF4, and loss-of-function mutations decrease the tolerance of seedlings to salt stress [17]. As their closest homolog in the phylogenetic tree (Figure S7), transcript abundance of ClCDPK14 was also up-regulated under ABA and salt stimuli (Figure 6 and Figure 7). The biosynthesis of ABA could be induced by drought, leading to stomatal closure [17,39]. In Arabidopsis, the AtCPK4 and AtCPK11 are partially involved in ABA-induced stomatal closure, and the double mutant lost more water from leaves compared to single mutants [17]; gene ClCDPK14, as their closed homolog in watermelon, was also activated by drought (Figure 6). Additionally, gene AtCPK6 has been proven to be involve in the response to drought and salt stress as a positive regulator [40]. Intriguingly, ClCDPK5 in watermelon, sharing the highest sequence similarity with AtCPK6, was also up-regulated by drought (or salt) and ABA stresses, indicating that they might function in similar pathways. In this study, the transcription levels of most ClCDPKs were increased by exogenous ABA (Figure 7), similar to the expression trend in grape but different to that in Cucumis species [9,11,19].
In watermelon, transcription levels of four genes (ClCDPK1, ClCDPK5, ClCDPK16, and ClCDPK17) were down-regulated by cold treatment, while only one gene (ClCRK2) was continuously up-regulated at all treatment times (Figure 6). Moreover, complex expression patterns were also observed under cold stimuli, such as ClCRK5 and ClCRK6. Three up-regulated genes (ClCDPK1, ClCDPK2, and ClCDPK5) and one down-regulated gene (ClCDPK18) under continuous drought treatment in this study have also been detected and regarded as different expression genes in our previous study [41]. Following salt treatment, gene ClCDPK6 was significantly up-regulated at all treatment times, which is similar to its ortholog CsCDPK14 in cucumber but in contrast with its ortholog CmCDPK9 in melon [9,11]. Additionally, two pairs of segmental duplications were detected in watermelon: ClCDPK7/ClCDPK8 and ClCDPK6/ClCDPK16 (Table S5). Interestingly, ClCDPK7 and ClCDPK8 had similar expression patterns under most treatments, while expression tendency of ClCDPK6 was usually opposite to ClCDPK16 (Figure 6, Figure 7 and Figure 8), inferring that genes ClCDPK6 and ClCDPK16 may have undergone sub-functionalization after duplication.
Generally, CDPK genes are ubiquitously expressed in plant organs, with some showing organ- or tissue-specific expression [19,21]. In the present study, most identified ClCDPKs and ClCRKs could be detected in at least one tissue, with at least five genes showing extremely high expression levels in specific organs (Figure 5). For instance, ClCDPK17 in locus 24 showed a high expression level in the male flower, similar to its ortholog CmCDPK6 in melon, which preferentially accumulated in male flowers [11]. In Arabidopsis, both genes AtCPK17 and AtCPK20 are reported to be preferentially expressed in mature pollen to regulate the growth of pollen tubes [21,42]. Interestingly, their phylogenetically-close homologs ClCDPK6 and ClCDPK16 in watermelon, as well as that in melon (CmCDPK9 and CmCDPK5) and cucumber (CsCDPK14 and CsCDPK9) (Figure S7), were also detected with high transcriptional abundances in male organs [9,11], indicating their conserved and important roles in the development of male flowers.
4. Materials and Methods
4.1. Identification and Biochemical Characterization of CDPKs and CRKs
For genome-wide identification of CDPK and CRK genes in Cucurbitaceae species, protein sequences of 18 CmCDPKs, seven CmCRKs, and 19 CsCDPKs were obtained according to recently published studies [9,11]. Then, using BLASTp program with an E-value setting of 1.0 × 10−5, these protein sequences were used as queries to search against the predicted protein files of watermelon (C. lanatus, v1), cucumber (C. sativus, v1), Cucurbita genus (C. moschata, v1; C. maxima, v1.1; C. pepo, v4.1), and bottle gourd (L. siceraria, v1), which were downloaded from the Cucurbit Genomics Database (http://cucurbitgenomics.org/). Additionally, Hidden Markov Model (HMM) profiles of the core protein kinase domain (PF00069) and EF-hand_7 domain (PF13499) were downloaded from the Pfam database (http://pfam.xfam.org/), and were also subjected to searches for CDPKs and CRKs with software HMMER 3.0 (default parameters). The reliability of candidates was verified through searching against the NCBI nr database, and all non-redundant putative genes were tested for the presence of core domains using ScanProsite (http://prosite.expasy.org/scanprosite/). Finally, all candidate genes were further characterized with the following online tools: ProtParam (http://web.expasy.org/protparam/), ScanProsite (http://prosite.expasy.org/scanprosite/), SMART (http://smart.embl-heidelberg.de/), ExPASy (http://web.expasy.org/myristoylator/), and CSS-plam 4.0 (http://csspalm.biocuckoo.org/). Dot plot analysis was performed using the software Geneious (http://www.geneious.com).
4.2. Chromosomal Location of CDPK and CRK Genes
The genomic distributions of CDPKs and CRKs on chromosomes were drawn using the software TBtools (http://cj-chen.github.io/tbtools/). To construct an integrated map, all identified CDPKs and CRKs, as well as those reported in recent studies [9,11], were mapped onto watermelon chromosomes based on the syntenic relationships of their flanking genes using BLASTp method, with a stricter E-value setting to 1.0 × 10−10. Then, in-house Perl scripts were used to parse the resulting files and to visualize the chromosomal locations of CDPK and CRK loci.
4.3. Phylogenetic, Gene Structure, and Syntenic Analyses of CDPKs and CRKs
Full-length CDPK and CRK protein sequences were aligned using the software Muscle [43], and then were used to construct phylogenetic trees via MEGA 6.0 using the neighbor-joining method with 1000 bootstrap replicates [11]. To perform gene structure analyses, genomic and cDNA sequences of CDPKs and CRKs were obtained from their corresponding genomes. Then, the exon-intron organization was displayed via the online tool GSDS 2.0 (http://gsds.cbi.pku.edu.cn/). The destination tabular (-m 8) files of the BLASTp program (with an E-value setting of 1.0 × 10−10) and GFF profiles served as input documents for MCScanX to analyze the synteny relationships [44], which were then visualized using software CIRCOS (http://circos.ca/).
4.4. Plant Material and Treatments
The watermelon inbred line “Y34” provided by the Cucurbits Germplasm Resource Research Group at the Northwest A&F University in China was used in this study. For tissue-specific analysis, germinated seeds of watermelon “Y34” were directly sown in the experimental base, and six organs (roots, stems, leaves, tendrils, and both male and female flowers) were independently sampled during the fruit maturation period (approximately 60–70 days after sowing). For stress experiments, seedlings were cultured in plastic pots (8 cm × 7 cm × 7 cm) filled with commercial peat-based compost (Shaanxi Yufeng Seed Industry Co., Ltd., Yangling, China). All plants were grown under springtime natural light with temperatures of 28–35 °C/16–20 °C (day/night) in a greenhouse, which were uniformly watered and nourished weekly with half-strength Hoagland’s solution before treatments. Four weeks after sowing, seedlings were used for the following treatments.
For the salinity treatment, seedlings irrigated with 300 mM NaCl solution (80 mL per plant) were sampled at 6, 24, 48, 72, 96, and 120 h post-treatment (hpt), while plants irrigated with distilled water were used as control. Compared to seedlings planted in a growth chamber at 27 ± 1 °C and 80% humidity under a light intensity of 300 mmol·m−2·s−1 PPFD, leaves of plants kept at 4 °C were sampled at 1, 3, 6, 12, 24, and 48 hpt for cold treatment. To simulate a national drought treatment [45], all seedlings were uniformly well-watered to 70 ± 5% field capacity based on their weight. Then, leaves of drought-treated (unwatered) and control plants were sampled at 24, 48, 96, and 192 hpt. Leaves sprayed with 100 μM abscisic acid (ABA) [46], 1 mM salicylic acid (SA) [47], 100 μM methyljasmonate (MeJA), and 10 mM ethephon (ETH) [48] were collected at 0.5, 1, 6, 12, 24, and 48 hpt for hormone treatments, while control seedlings were only sprayed with equal volumes of the corresponding solution without hormones. In this study, leaves of four plants were pooled at each time point for each treatment with three biological replicates, which were immediately frozen in liquid nitrogen and stored at −80 °C until further analysis.
4.5. RNA Isolation and qRT-PCR
The total RNA of samples was extracted using the RNASimple Total RNA Kit (TIANGEN, China) following the manufacturer’s instructions. Then, approximately 1 μg of total RNA was used to synthesize the first strand of cDNA using the FastKing RT Kit with gDNase (TIANGEN, China). Gene-specific primers for ClCDPKs and ClCRKs are listed in Table S1. Amplification was conducted in a 20 μL reaction volume, containing 10.0 μLSYBR Green Premix, 0.8 μL of each primer (10 μM), and 1.0 μL cDNA template (80 ng/μL), which was diluted with ddH2O to 20 μL. The PCR conditions consisted of pre-denaturing at 95 °C for 5 min, followed by 40 cycles of 95 °C for 10 s and 60 °C for 30 s. The watermelon β-actin gene (Cla007792) was used as the internal control gene [49]. Each treatment was repeated thrice, and all data were calculated for relative expressions following the 2−ΔΔCt method, as described by Livak and Schmittgen [50]. The relative expressions were then log2 transformed and visualized in a heat map using Mev 4.8.1 (http://www.mybiosoftware.com/). All data were analyzed via IBM SPSS Statistics 21 and values were presented as the means ± SD of three biological and three technical replicates (Table S7). The significance of expression between treatments and controls was evaluated by one-way ANOVA and Duncan’s multiple range tests.
5. Conclusions
In the present study, a total of 128 CDPK and 56 CRK genes were identified in six Cucurbitaceae species. Using the watermelon genome as reference, an integrated map containing 25 loci (16 CDPK and nine CRK loci) was obtained, 16 of which (12 CDPK and four CRK) were shared by all seven Cucurbitaceae species. Combined with exon-intron organizations, topological analyses indicated an ancient origination of groups CDPK IV and CRK, which will contribute to elucidating the evolutionary history of these two gene families in Cucurbitaceae. Moreover, expression patterns of ClCDPKs and ClCRKs under different abiotic stresses were also performed in this study. Importantly, comparative analyses indicated a subset of valuable orthologous genes for future functional characterizations. For instance, both ClCDPK6 and its ortholog CsCDPK14 in cucumber could be induced by salinity, while ClCDPK6 and ClCDPK16, as well as their orthologs in Cucumis, maintained high expression levels in male flowers, which may play important roles in plant organ development and response to environmental stresses.
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/20/10/2527/s1.
Author Contributions
C.W. and X.Z. designed the study. C.W., R.Z., X.Y., and C.Z. contributed to the experiments and data analysis. J.M. provide the seed for the experiment. Y.Z. and J.Y. provided guidance throughout the study. C.W. wrote, and H.L. revised the manuscript. All authors reviewed and approved the final manuscript.
Funding
This work was supported by National Key R&D Program of China (2018YFD0100704), Scientific Startup Foundation for Doctors of Northwest A&F University (Z109021604), National Natural Science Foundation of China Grant No. 31701939, and the Modern Agro-industry Technology Research System of China (No. CARS-26-18).
Acknowledgments
We wish to thank all the reviewers and editors for their careful reading and helpful comments on this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CDPK | Calcium-dependent protein kinase |
| CRK | CDPK-related kinases |
| hpt | hours post-treatment |
| ABA | abscisic acid |
| SA | salicylic acid |
| MeJA | methyljasmonate |
| ETH | ethephon |
References
- Hepler, P.K. Calcium: A central regulator of plant growth and development. Plant Cell 2005, 17, 2142–2155. [Google Scholar] [CrossRef]
- Boudsocq, M.; Sheen, J. CDPKs in immune and stress signaling. Trends Plant Sci. 2013, 18, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Ranty, B.; Aldon, D.; Galaud, J.P. Plant calmodulins and calmodulin-related proteins: Multifaceted relays to decode calcium signals. Plant Signal. Behav. 2006, 1, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Hamel, L.P.; Sheen, J.; Seguin, A. Ancient signals: Comparative genomics of green plant CDPKs. Trends Plant Sci. 2014, 19, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, A.A.; Romeis, T.; Jones, J.D. CDPK-mediated signalling pathways: Specificity and cross-talk. J. Exp. Bot. 2004, 55, 181–188. [Google Scholar] [CrossRef]
- Boudsocq, M.; Willmann, M.R.; McCormack, M.; Lee, H.; Shan, L.; He, P.; Bush, J.; Cheng, S.H.; Sheen, J. Differential innate immune signalling via Ca(2+) sensor protein kinases. Nature 2010, 464, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.H.; Willmann, M.R.; Chen, H.C.; Sheen, J. Calcium signaling through protein kinases. The Arabidopsis calcium-dependent protein kinase gene family. Plant Physiol. 2002, 129, 469–485. [Google Scholar] [CrossRef] [PubMed]
- Liese, A.; Romeis, T. Biochemical regulation of in vivo function of plant calcium-dependent protein kinases (CDPK). Biochim. Biophys. Acta 2013, 1833, 1582–1589. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, M.; Lu, L.; He, M.; Qu, W.; Xu, Q.; Qi, X.; Chen, X. Genome-wide analysis and expression of the calcium-dependent protein kinase gene family in cucumber. Mol. Genet. Genom. MGG 2015, 290, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.P.; Xu, Y.P.; Munyampundu, J.P.; Liu, T.Y.; Cai, X.Z. Calcium-dependent protein kinase (CDPK) and CDPK-related kinase (CRK) gene families in tomato: Genome-wide identification and functional analyses in disease resistance. Mol. Genet. Genom. MGG 2016, 291, 661–676. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, C.; Yang, X.; Chen, H.; Yang, Y.; Mo, Y.; Li, H.; Zhang, Y.; Ma, J.; Yang, J.; et al. Genome-wide identification and expression analysis of calciumdependent protein kinase and its related kinase gene families in melon (Cucumis melo L.). PLoS ONE 2017, 12, e0176352. [Google Scholar]
- Ray, S.; Agarwal, P.; Arora, R.; Kapoor, S.; Tyagi, A.K. Expression analysis of calcium-dependent protein kinase gene family during reproductive development and abiotic stress conditions in rice (Oryza sativa L. ssp. indica). Mol. Genet. Genom. MGG 2007, 278, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Hrabak, E.M.; Chan, C.W.; Gribskov, M.; Harper, J.F.; Choi, J.H.; Halford, N.; Kudla, J.; Luan, S.; Nimmo, H.G.; Sussman, M.R.; et al. The Arabidopsis CDPK-SnRK superfamily of protein kinases. Plant Physiol. 2003, 132, 666–680. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Tanaka, N.; Yang, G.; Hayashi, N.; Komatsu, S. Genome-wide identification of the rice calcium-dependent protein kinase and its closely related kinase gene families: Comprehensive analysis of the CDPKs gene family in rice. Plant Cell Physiol. 2005, 46, 356–366. [Google Scholar] [CrossRef]
- Cai, H.; Cheng, J.; Yan, Y.; Xiao, Z.; Li, J.; Mou, S.; Qiu, A.; Lai, Y.; Guan, D.; He, S. Genome-wide identification and expression analysis of calcium-dependent protein kinase and its closely related kinase genes in Capsicum annuum. Front. Plant Sci. 2015, 6, 737. [Google Scholar] [CrossRef]
- Zhou, L.; Lan, W.; Jiang, Y.; Fang, W.; Luan, S. A calcium-dependent protein kinase interacts with and activates a calcium channel to regulate pollen tube growth. Mol. Plant 2014, 7, 369–376. [Google Scholar] [CrossRef]
- Zhu, S.Y.; Yu, X.C.; Wang, X.J.; Zhao, R.; Li, Y.; Fan, R.C.; Shang, Y.; Du, S.Y.; Wang, X.F.; Wu, F.Q.; et al. Two calcium-dependent protein kinases, CPK4 and CPK11, regulate abscisic acid signal transduction in Arabidopsis. Plant Cell 2007, 19, 3019–3036. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.Y.; Wu, W.H. AtCPK23 functions in Arabidopsis responses to drought and salt stresses. Plant Mol. Biol. 2007, 65, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Han, Y.T.; Zhao, F.L.; Hu, Y.; Gao, Y.R.; Ma, Y.F.; Zheng, Y.; Wang, Y.J.; Wen, Y.Q. Genome-wide Identification and Expression Analysis of the CDPK Gene Family in Grape, Vitis spp. BMC Plant Biol. 2015, 15, 164. [Google Scholar] [CrossRef]
- Chen, F.; Fasoli, M.; Tornielli, G.B.; Dal Santo, S.; Pezzotti, M.; Zhang, L.; Cai, B.; Cheng, Z.M. The evolutionary history and diverse physiological roles of the grapevine calcium-dependent protein kinase gene family. PLoS ONE 2013, 8, e80818. [Google Scholar] [CrossRef] [PubMed]
- Myers, C.; Romanowsky, S.M.; Barron, Y.D.; Garg, S.; Azuse, C.L.; Curran, A.; Davis, R.M.; Hatton, J.; Harmon, A.C.; Harper, J.F. Calcium-dependent protein kinases regulate polarized tip growth in pollen tubes. Plant J. Cell Mol. Biol. 2009, 59, 528–539. [Google Scholar] [CrossRef]
- Huang, S.; Li, R.; Zhang, Z.; Li, L.; Gu, X.; Fan, W.; Lucas, W.J.; Wang, X.; Xie, B.; Ni, P.; et al. The genome of the cucumber, Cucumis sativus L. Nat. Genet. 2009, 41, 1275–1281. [Google Scholar] [CrossRef]
- Garcia-Mas, J.; Benjak, A.; Sanseverino, W.; Bourgeois, M.; Mir, G.; Gonzalez, V.M.; Henaff, E.; Camara, F.; Cozzuto, L.; Lowy, E.; et al. The genome of melon (Cucumis melo L.). Proc. Natl. Acad. Sci. USA 2012, 109, 11872–11877. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, J.; Sun, H.; Salse, J.; Lucas, W.J.; Zhang, H.; Zheng, Y.; Mao, L.; Ren, Y.; Wang, Z.; et al. The draft genome of watermelon (Citrullus lanatus) and resequencing of 20 diverse accessions. Nat. Genet. 2013, 45, 51–58. [Google Scholar] [CrossRef]
- Montero-Pau, J.; Blanca, J.; Bombarely, A.; Ziarsolo, P.; Esteras, C.; Marti-Gomez, C.; Ferriol, M.; Gomez, P.; Jamilena, M.; Mueller, L.; et al. De novo assembly of the zucchini genome reveals a whole-genome duplication associated with the origin of the Cucurbita genus. Plant Biotechnol. J. 2018, 16, 1161–1171. [Google Scholar] [CrossRef]
- Sun, H.; Wu, S.; Zhang, G.; Jiao, C.; Guo, S.; Ren, Y.; Zhang, J.; Zhang, H.; Gong, G.; Jia, Z.; et al. Karyotype Stability and Unbiased Fractionation in the Paleo-Allotetraploid Cucurbita Genomes. Mol. Plant 2017, 10, 1293–1306. [Google Scholar] [CrossRef]
- Wu, S.; Shamimuzzaman, M.; Sun, H.; Salse, J.; Sui, X.; Wilder, A.; Wu, Z.; Levi, A.; Xu, Y.; Ling, K.S.; Fei, Z. The bottle gourd genome provides insights into Cucurbitaceae evolution and facilitates mapping of a Papaya ring-spot virus resistance locus. Plant J. Cell Mol. Biol. 2017, 92, 963–975. [Google Scholar] [CrossRef]
- Valmonte, G.R.; Arthur, K.; Higgins, C.M.; MacDiarmid, R.M. Calcium-dependent protein kinases in plants: evolution, expression and function. Plant Cell Physiol. 2014, 55, 551–569. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, S.; Bai, Y.; Sun, H.; Jiao, C.; Guo, S.; Zhao, K.; Blanca, J.; Zhang, Z.; Huang, S.; et al. Cucurbit Genomics Database (CuGenDB): A central portal for comparative and functional genomics of cucurbit crops. Nucleic Acids Res. 2019, 47, D1128–D1136. [Google Scholar] [CrossRef]
- Liu, W.; Li, W.; He, Q.; Daud, M.K.; Chen, J.; Zhu, S. Genome-wide survey and expression analysis of calcium-dependent protein kinase in Gossypium raimondii. PLoS ONE 2014, 9, e98189. [Google Scholar] [CrossRef]
- Vision, T.J.; Brown, D.G.; Tanksley, S.D. The origins of genomic duplications in Arabidopsis. Science 2000, 290, 2114–2117. [Google Scholar] [CrossRef]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef]
- Coca, M.; San Segundo, B. AtCPK1 calcium-dependent protein kinase mediates pathogen resistance in Arabidopsis. Plant J. Cell Mol. Biol. 2010, 63, 526–540. [Google Scholar] [CrossRef]
- Zou, J.J.; Wei, F.J.; Wang, C.; Wu, J.J.; Ratnasekera, D.; Liu, W.X.; Wu, W.H. Arabidopsis calcium-dependent protein kinase CPK10 functions in abscisic acid- and Ca2+-mediated stomatal regulation in response to drought stress. Plant Physiol. 2010, 154, 1232–1243. [Google Scholar] [CrossRef]
- Li, Z.; Takahashi, Y.; Scavo, A.; Brandt, B.; Nguyen, D.; Rieu, P.; Schroeder, J.I. Abscisic acid-induced degradation of Arabidopsis guanine nucleotide exchange factor requires calcium-dependent protein kinases. Proc. Natl. Acad. Sci. USA 2018, 115, E4522–E4531. [Google Scholar] [CrossRef]
- Zuo, R.; Hu, R.; Chai, G.; Xu, M.; Qi, G.; Kong, Y.; Zhou, G. Genome-wide identification, classification, and expression analysis of CDPK and its closely related gene families in poplar (Populus trichocarpa). Mol. Biol. Rep. 2013, 40, 2645–2662. [Google Scholar] [CrossRef]
- Kong, X.; Lv, W.; Jiang, S.; Zhang, D.; Cai, G.; Pan, J.; Li, D. Genome-wide identification and expression analysis of calcium-dependent protein kinase in maize. BMC Genom. 2013, 14, 433. [Google Scholar] [CrossRef]
- Xu, G.; Guo, C.; Shan, H.; Kong, H. Divergence of duplicate genes in exon-intron structure. Proc. Natl. Acad. Sci. USA 2012, 109, 1187–1192. [Google Scholar] [CrossRef]
- Roelfsema, M.R.G.; Hedrich, R. In the light of stomatal opening: New insights into ‘the Watergate’. New Phytol. 2005, 167, 665–691. [Google Scholar] [CrossRef]
- Xu, J.; Tian, Y.-S.; Peng, R.-H.; Xiong, A.-S.; Zhu, B.; Jin, X.-F.; Gao, F.; Fu, X.-Y.; Hou, X.-L.; Yao, Q.-H. AtCPK6, a functionally redundant and positive regulator involved in salt/drought stress tolerance in Arabidopsis. Planta 2010, 231, 1251–1260. [Google Scholar] [CrossRef]
- Yang, Y.; Mo, Y.; Yang, X.; Zhang, H.; Wang, Y.; Li, H.; Wei, C.; Zhang, X. Transcriptome Profiling of Watermelon Root in Response to Short-Term Osmotic Stress. PLoS ONE 2016, 11, e0166314. [Google Scholar] [CrossRef] [PubMed]
- Gutermuth, T.; Lassig, R.; Portes, M.-T.; Maierhofer, T.; Romeis, T.; Borst, J.-W.; Hedrich, R.; Feijó, J.A.; Konrad, K.R. Pollen tube growth regulation by free anions depends on the interaction between the anion channel SLAH3 and calcium-dependent protein kinases CPK2 and CPK20. Plant Cell 2013, 25, 4525–4543. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Mo, Y.; Yang, R.; Liu, L.; Gu, X.; Yang, X.; Wang, Y.; Zhang, X.; Li, H. Growth, photosynthesis and adaptive responses of wild and domesticated watermelon genotypes to drought stress and subsequent re-watering. Plant Growth Regul. 2016, 79, 229–241. [Google Scholar] [CrossRef]
- Song, Q.; Li, D.; Dai, Y.; Liu, S.; Huang, L.; Hong, Y.; Zhang, H.; Song, F. Characterization, expression patterns and functional analysis of the MAPK and MAPKK genes in watermelon (Citrullus lanatus). BMC Plant Biol. 2015, 15, 298. [Google Scholar] [CrossRef]
- Jing-Hua, Y.; Yuan, G.; Yan-Man, L.; Xiao-Hua, Q.; Zhang, M.-F. Salicylic acid-induced enhancement of cold tolerance through activation of antioxidative capacity in watermelon. Sci. Hortic. 2008, 118, 200–205. [Google Scholar] [CrossRef]
- Yang, X.; Li, H.; Yang, Y.; Wang, Y.; Mo, Y.; Zhang, R.; Zhang, Y.; Ma, J.; Wei, C.; Zhang, X. Identification and expression analyses of WRKY genes reveal their involvement in growth and abiotic stress response in watermelon (Citrullus lanatus). PLoS ONE 2018, 13, e0191308. [Google Scholar] [CrossRef]
- Kong, Q.; Yuan, J.; Gao, L.; Zhao, S.; Jiang, W.; Huang, Y.; Bie, Z. Identification of suitable reference genes for gene expression normalization in qRT-PCR analysis in watermelon. PLoS ONE 2014, 9, e90612. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).