Short-Wavelength Sensitive Cone (S-cone) Testing as an Outcome Measure for NR2E3 Clinical Treatment Trials
Abstract
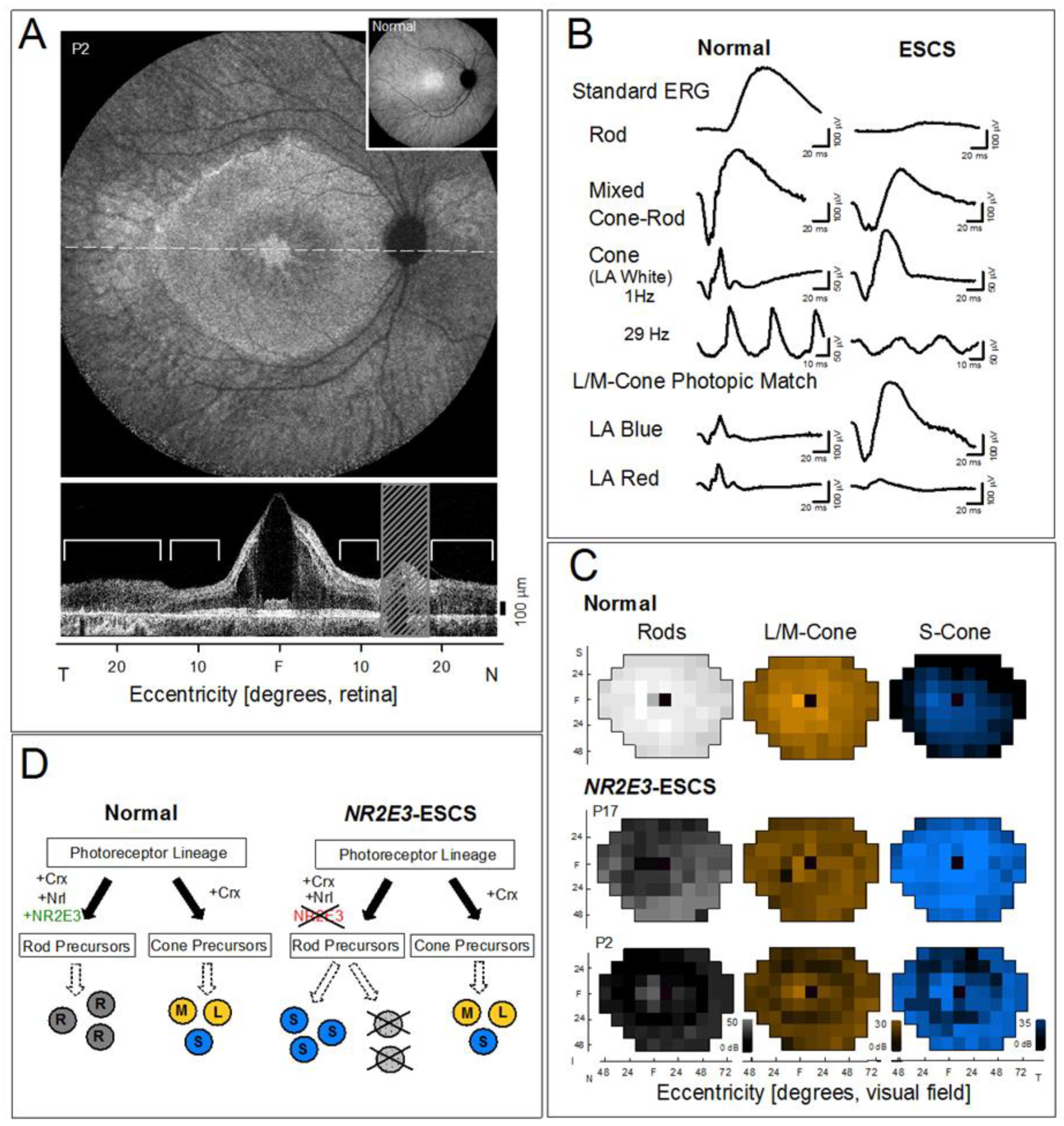
1. Introduction
2. Results and Discussion
2.1. Comparison of Available Computerized Perimeters for S-cone Isolation
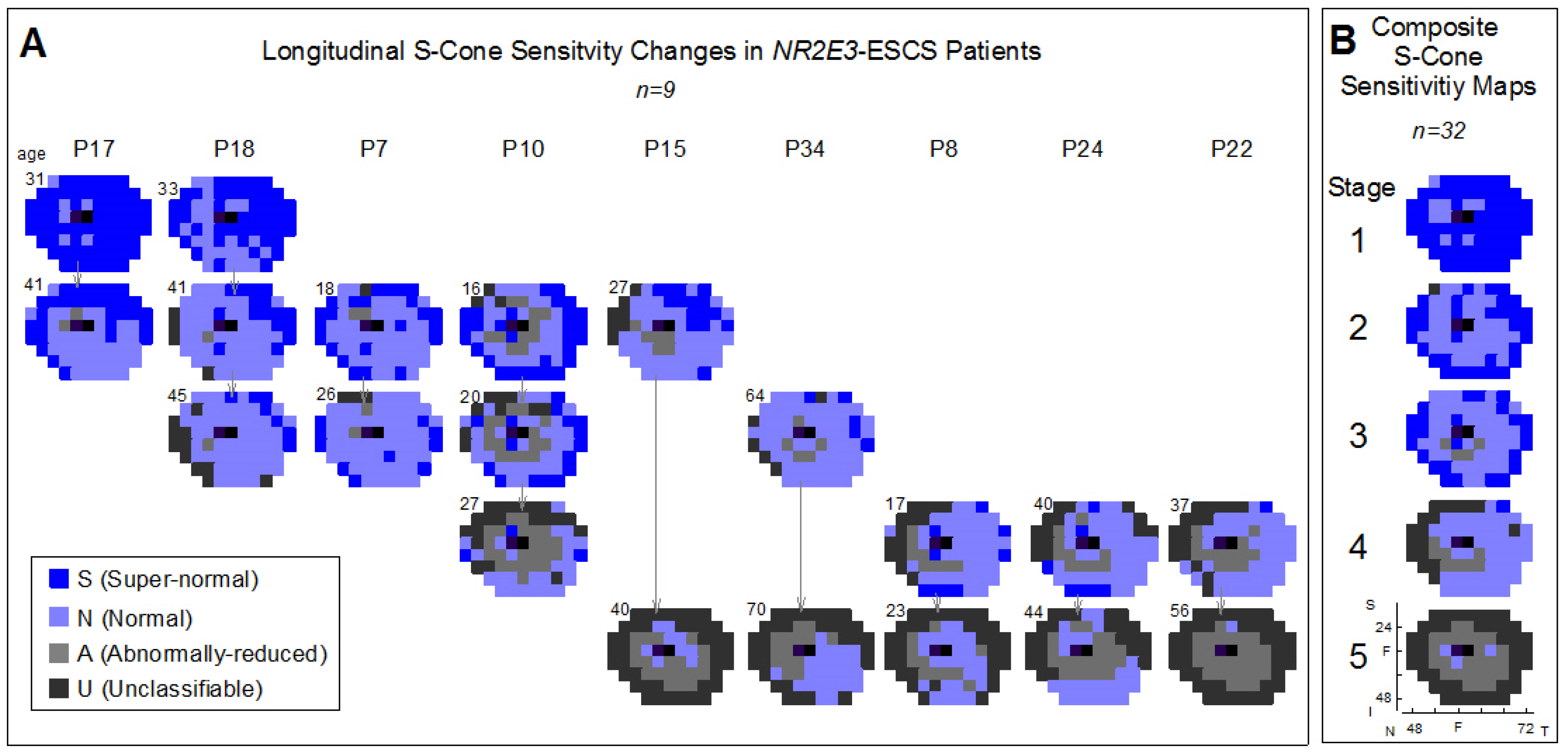
2.2. Using S-cone Perimetry to Describe Disease Progression and Severity of NR2E3-ESCS Patients
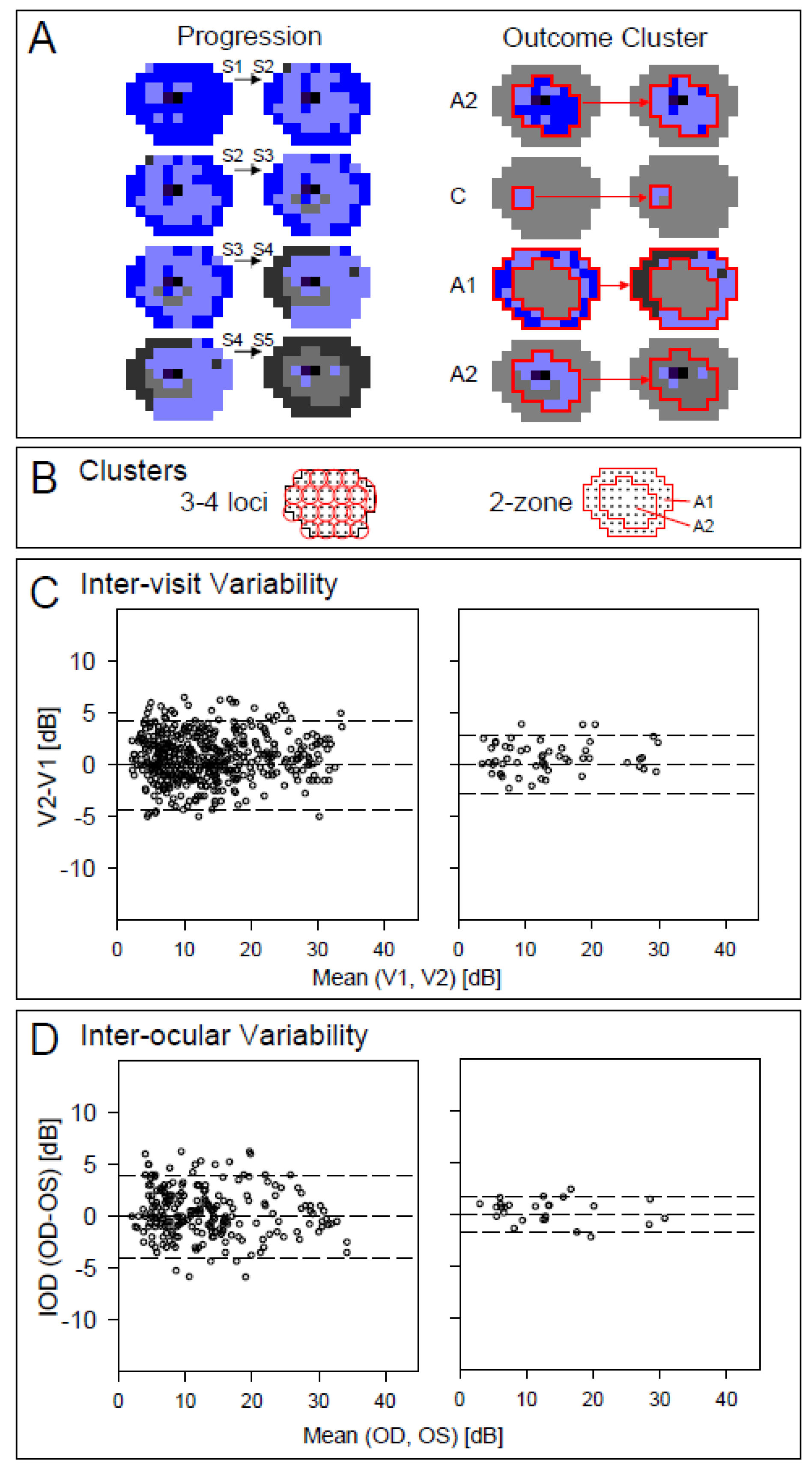
2.3. Regional Variation of S-cone Function Across the Visual Field and Strategies for Monitoring Outcomes
2.4. Results Summary
3. Materials and Methods
3.1. Subjects
3.2. Testing Procedures, Instrumentation, and Analysis
3.3. Mapping Disease Severity of NR2E3-ESCS Patients Using S-cone Perimetry
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jacobson, S.G.; Cideciyan, A.V.; Sumaroka, A.; Roman, A.J.; Charng, J.; Lu, M.; Choi, W.; Sheplock, R.; Swider, M.; Kosyk, M.S.; et al. Outcome measures for clinical trials of Leber congenital amaurosis caused by the intronic mutation in the CEP290 gene. Invest. Ophthalmol. Vis. Sci. 2017, 58, 2609–2622. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, S.G.; Cideciyan, A.V.; Sumaroka, A.; Roman, A.J.; Charng, J.; Lu, M.; Choudhury, S.; Schwartz, S.B.; Heon, E.; Fishman, G.A.; et al. Defining outcomes for clinical trials of Leber congenital amaurosis caused by GUCY2D mutations. Am. J. Ophthalmol. 2017, 177, 44–57. [Google Scholar]
- Calzetti, G.; Levy, R.A.; Cideciyan, A.V.; Garafalo, A.V.; Roman, A.J.; Sumaroka, A.; Charng, J.; Heon, E.; Jacobson, S.G. Efficacy outcome measures for clinical trials of USH2A caused by the common C.2299delG mutation. Am. J. Ophthalmol. 2018, 193, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Csaky, K.G.; Richman, E.A.; Ferris, F.L. Report from the NEI/FDA Ophthalmic clinical trial design and endpoints symposium. Invest. Ophthalmol. Vis. Sci. 2008, 49, 479–489. [Google Scholar] [CrossRef]
- Fishman, G.A.; Jampol, L.M.; Goldberg, M.F. Diagnostic features of the Favre-Goldmann syndrome. Brit. J. Ophthalmol. 1976, 60, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, S.G.; Marmor, M.F.; Kemp, C.M.; Knighton, R.W. SWS (blue) cone hypersensitivity in a newly identified retinal degeneration. Invest. Ophthalmol. Vis. Sci. 1990, 31, 827–838. [Google Scholar]
- Marmor, M.F.; Jacobson, S.G.; Foerster, M.H.; Kellner, U.; Weleber, R.G. Diagnostic clinical findings of a new syndrome with night blindness, maculopathy, and enhanced S cone sensitivity. Am. J. Ophthalmol. 1990, 110, 124–134. [Google Scholar] [CrossRef]
- Jacobson, S.G.; Roman, A.J.; Roman, M.I.; Gass, J.D.; Parker, J.A. Relatively enhanced S cone function in the Goldmann-Favre syndrome. Am. J. Ophthalmol. 1991, 111, 446–453. [Google Scholar]
- Hood, D.C.; Cideciyan, A.V.; Roman, A.J.; Jacobson, S.G. Enhanced S cone syndrome: Evidence for an abnormally large number of S cones. Vision. Res. 1995, 35, 1473–1481. [Google Scholar] [CrossRef]
- Milam, A.H.; Rose, L.; Cideciyan, A.V.; Barakat, M.R.; Tang, W.X.; Gupta, N.; Aleman, T.S.; Wright, A.F.; Stone, E.M.; Sheffield, V.C.; et al. The nuclear receptor NR2E3 plays a role in human retinal photoreceptor differentiation and degeneration. Proc. Natl. Acad. Sci. USA 2002, 99, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Haider, N.B.; Mollema, N.; Gaule, M.; Yang, Y.; Sachs, A.J.; Nuysten, A.M.; Naggert, J.K.; Nishina, P.M. Nr2e3-directed transcriptional regulation of genes involved in photoreceptor development and cell-type specific phototransduction. Exp. Eye Res. 2009, 89, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.F.; Reddick, A.C.; Schwartz, S.B.; Ferguson, J.S.; Aleman, T.S.; Kellner, U.; Jurklies, B.; Schuster, A.; Zrenner, E.; Wissinger, B.; et al. Mutation analysis of NR2E3 and NRL genes in enhanced S Cone syndrome. Hum. Mutat. 2004, 24, 439. [Google Scholar] [CrossRef] [PubMed]
- Garafalo, A.V.; Calzetti, G.; Cideciyan, A.V.; Roman, A.J.; Saxena, S.; Sumaroka, A.; Choi, W.; Wright, A.F.; Jacobson, S.G. Cone vision changes in the enhanced S-cone syndrome caused by NR2E3 gene mutations. Invest. Ophthalmol. Vis. Sci. 2018, 59, 3209–3219. [Google Scholar] [CrossRef] [PubMed]
- Mollema, N.J.; Yuan, Y.; Jelcick, A.S.; Sachs, A.J.; von Alpen, D.; Schorderet, D.; Escher, P.; Haider, N.B. Nuclear receptor Rev-erb Alpha (Nr1d1) functions in concert with Nr2e3 to regulate transcriptional networks in the retina. PLoS ONE 2011, 6, e17494. [Google Scholar] [CrossRef]
- Swaroop, A.; Kim, D.; Forrest, D. Transcriptional regulation of photoreceptor development and homeostasis in the mammalian retina. Nat. Rev. Neurosci. 2010, 11, 563–576. [Google Scholar] [CrossRef]
- Kim, J.W.; Yang, H.J.; Brooks, M.J.; Zelinger, L.; Karakülah, G.; Gotoh, N.; Boleda, A.; Gieser, L.; Giuste, F.; Whitaker, D.T.; et al. NRL-regulated transcriptome dynamics of developing rod photoreceptors. Cell. Rep. 2016, 17, 2460–2473. [Google Scholar] [CrossRef]
- Cruz, N.M.; Yuan, Y.; Leehy, B.D.; Baid, R.; Kompella, U.; DeAngelis, M.M.; Escher, P.; Haider, N.B. Modifier genes as therapeutics: The nuclear hormone receptor Rev Erb alpha (Nr1d1) rescues Nr2e3 associated retinal disease. PLoS ONE 2014, 9, e87942. [Google Scholar] [CrossRef]
- Rabin, J.C.; Kryder, A.C.; Lam, D. Diagnosis of normal and abnormal color vision with cone-specific VEPs. Transl. Vis. Sci. Technol. 2016, 5, 8. [Google Scholar] [CrossRef]
- Huchzermeyer, C.; Kremers, J. Perifoveal L- and M-cone-driven temporal contrast sensitivities at different retinal illuminances. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2016, 33, 1989–1998. [Google Scholar] [CrossRef]
- Stiles, W.S. The directional sensitivity of the retina and the spectral sensitivities of the rods and cones. Proc. Royal Soc. Lond 1939, 127, 64–105. [Google Scholar]
- Sample, P.A.; Weinreb, R.N. Color perimetry for assessment of primary open-angle glaucoma. Invest. Ophthalmol. Vis. Sci. 1990, 31, 1869–1875. [Google Scholar] [PubMed]
- Johnson, C.A. Diagnostic value of short-wavelength automated perimetry. Curr. Opin. Ophthalmol. 1996, 7, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, S.G.; Voigt, W.J.; Parel, J.M.; Apathy, P.P.; Nghiem-Phu, L.; Myers, S.W.; Patella, V.M. Automated light- and dark-adapted perimetry for evaluating retinitis pigmentosa. Ophthalmology. 1986, 93, 1604–1611. [Google Scholar] [CrossRef]
- Sample, P.A.; Johnson, C.A.; Haegerstrom-Portnoy, G.; Adams, A.J. Optimum parameters for short wavelength automated perimetry. J. Glaucoma. 1996, 5, 375–383. [Google Scholar] [CrossRef]
- Demirel, S.; Johnson, C.A. Isolation of short-wavelength sensitive mechanisms in normal and glaucomatous visual field regions. J. Glaucoma. 2000, 9, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Tukey, J.W. Exploratory Data Analysis; Addison-Wesley: Reading, PA, USA, 1977. [Google Scholar]
- McGuigan, D.B.; Heon, E.; Cideciyan, A.V.; Ratnapriya, R.; Lu, M.; Sumaroka, A.; Roman, A.J.; Batmanabane, V.; Garafalo, A.V.; Stone, E.M.; et al. EYS mutations causing autosomal recessive retinitis pigmentosa: Changes of retinal structure and function with disease progression. Genes 2017, 8, 178. [Google Scholar] [CrossRef] [PubMed]
- Wyzecki, G.; Stiles, W.S. Concepts and methods, quantitative data and formulae. In Color Science, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1982. [Google Scholar]
- Parker, R.A.; Weir, C.J.; Rubio, N.; Rabinovich, R.; Pinnock, H.; Hanley, J.; McCloughan, L.; Drost, E.M.; Mantoani, L.C.; MacNee, W.; et al. Application of mixed effects limits of agreement in the presence of multiple sources of variability: Exemplar from the comparison of several devices to measure respiratory rate in COPD patients. PLoS ONE 2016, 11, e0168321. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roman, A.J.; Powers, C.A.; Semenov, E.P.; Sheplock, R.; Aksianiuk, V.; Russell, R.C.; Sumaroka, A.; Garafalo, A.V.; Cideciyan, A.V.; Jacobson, S.G. Short-Wavelength Sensitive Cone (S-cone) Testing as an Outcome Measure for NR2E3 Clinical Treatment Trials. Int. J. Mol. Sci. 2019, 20, 2497. https://doi.org/10.3390/ijms20102497
Roman AJ, Powers CA, Semenov EP, Sheplock R, Aksianiuk V, Russell RC, Sumaroka A, Garafalo AV, Cideciyan AV, Jacobson SG. Short-Wavelength Sensitive Cone (S-cone) Testing as an Outcome Measure for NR2E3 Clinical Treatment Trials. International Journal of Molecular Sciences. 2019; 20(10):2497. https://doi.org/10.3390/ijms20102497
Chicago/Turabian StyleRoman, Alejandro J., Christian A. Powers, Evelyn P. Semenov, Rebecca Sheplock, Valeryia Aksianiuk, Robert C. Russell, Alexander Sumaroka, Alexandra V. Garafalo, Artur V. Cideciyan, and Samuel G. Jacobson. 2019. "Short-Wavelength Sensitive Cone (S-cone) Testing as an Outcome Measure for NR2E3 Clinical Treatment Trials" International Journal of Molecular Sciences 20, no. 10: 2497. https://doi.org/10.3390/ijms20102497
APA StyleRoman, A. J., Powers, C. A., Semenov, E. P., Sheplock, R., Aksianiuk, V., Russell, R. C., Sumaroka, A., Garafalo, A. V., Cideciyan, A. V., & Jacobson, S. G. (2019). Short-Wavelength Sensitive Cone (S-cone) Testing as an Outcome Measure for NR2E3 Clinical Treatment Trials. International Journal of Molecular Sciences, 20(10), 2497. https://doi.org/10.3390/ijms20102497




