Magnetoferritin: Process, Prospects, and Their Biomedical Applications
Abstract
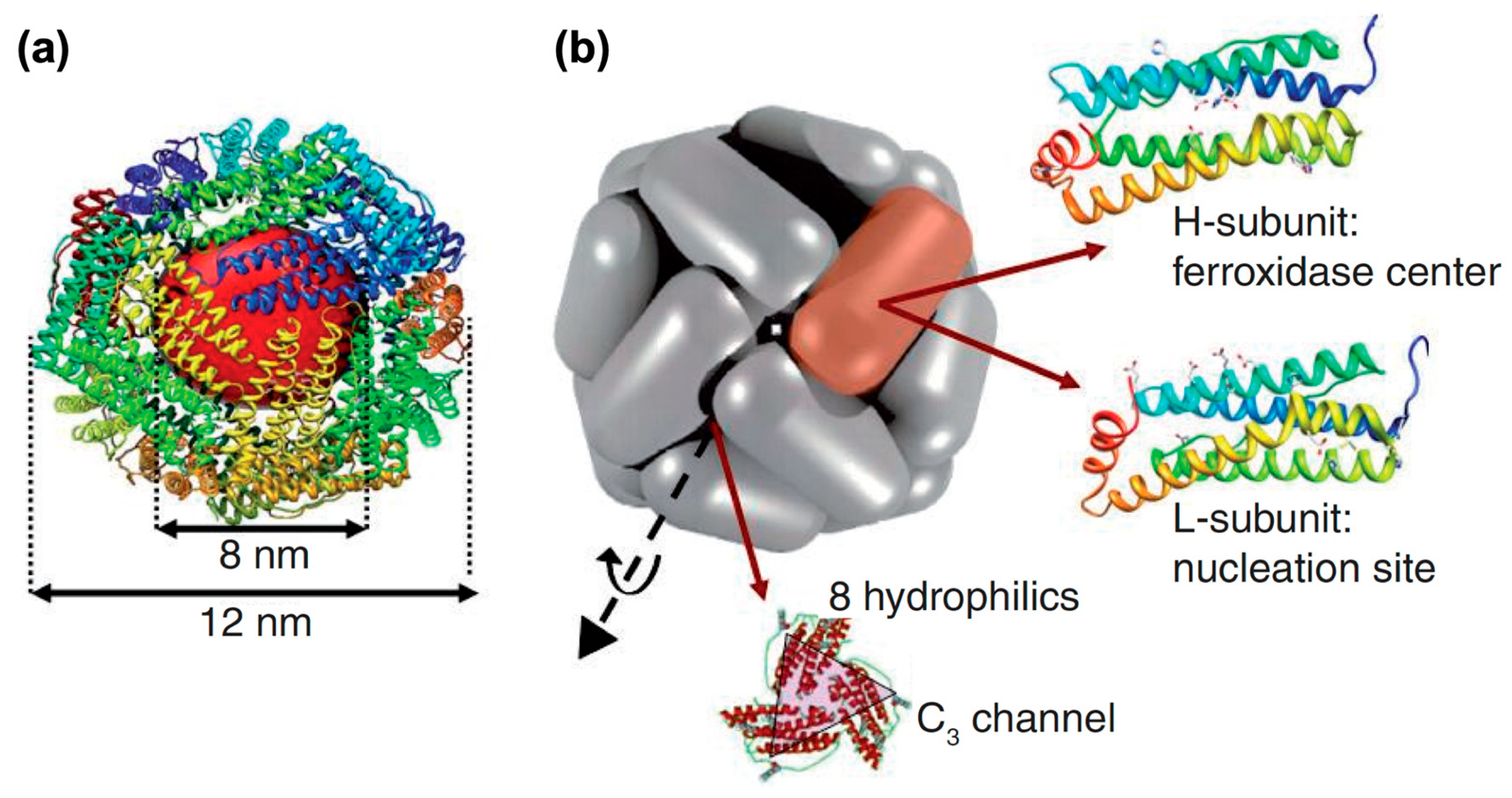
:1. Introduction of Ferritin and Magnetoferritin
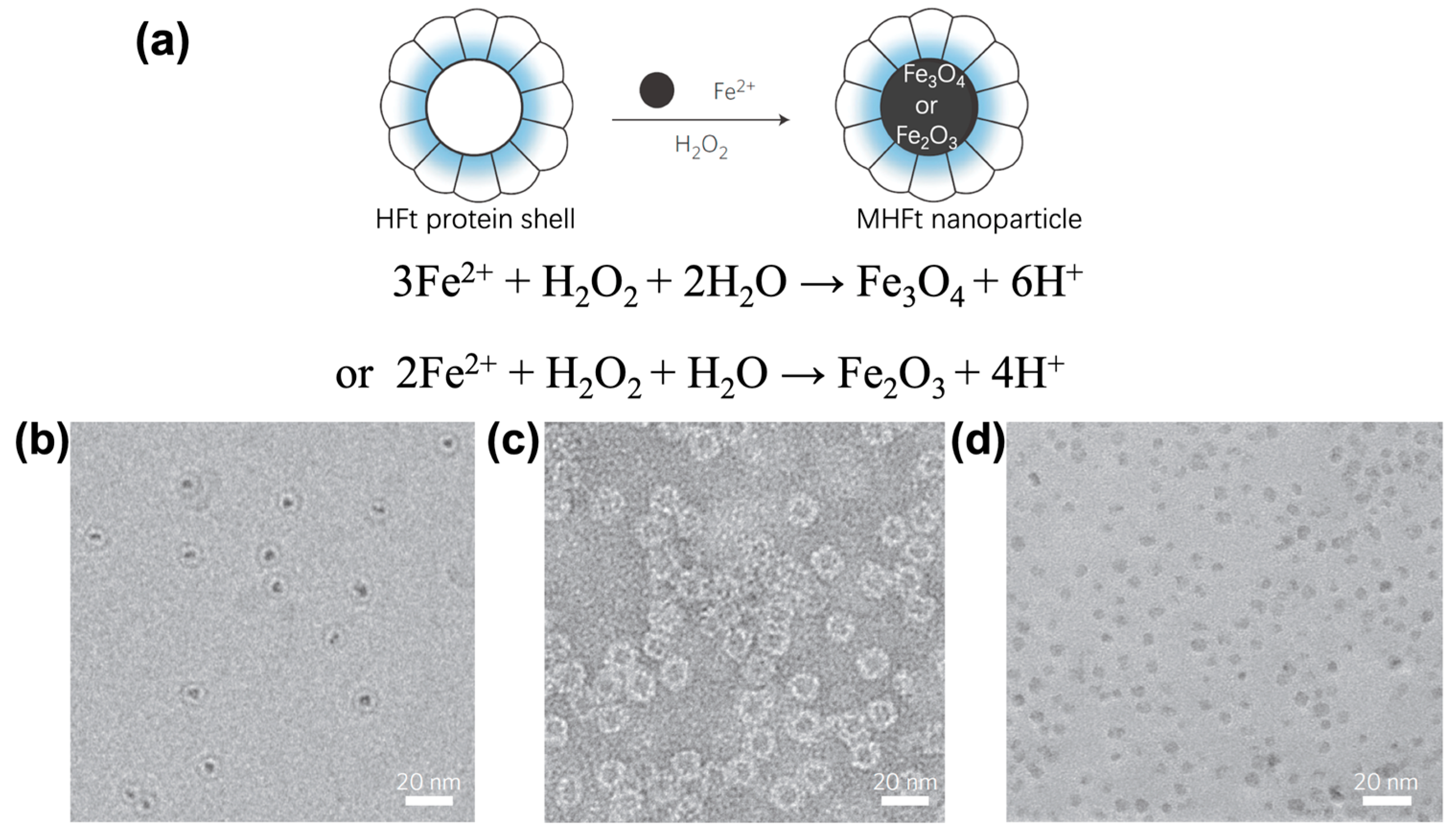
2. Synthesis of Magnetoferritin
3. Magnetic Properties of Magnetoferritin
4. Biomedical Applications
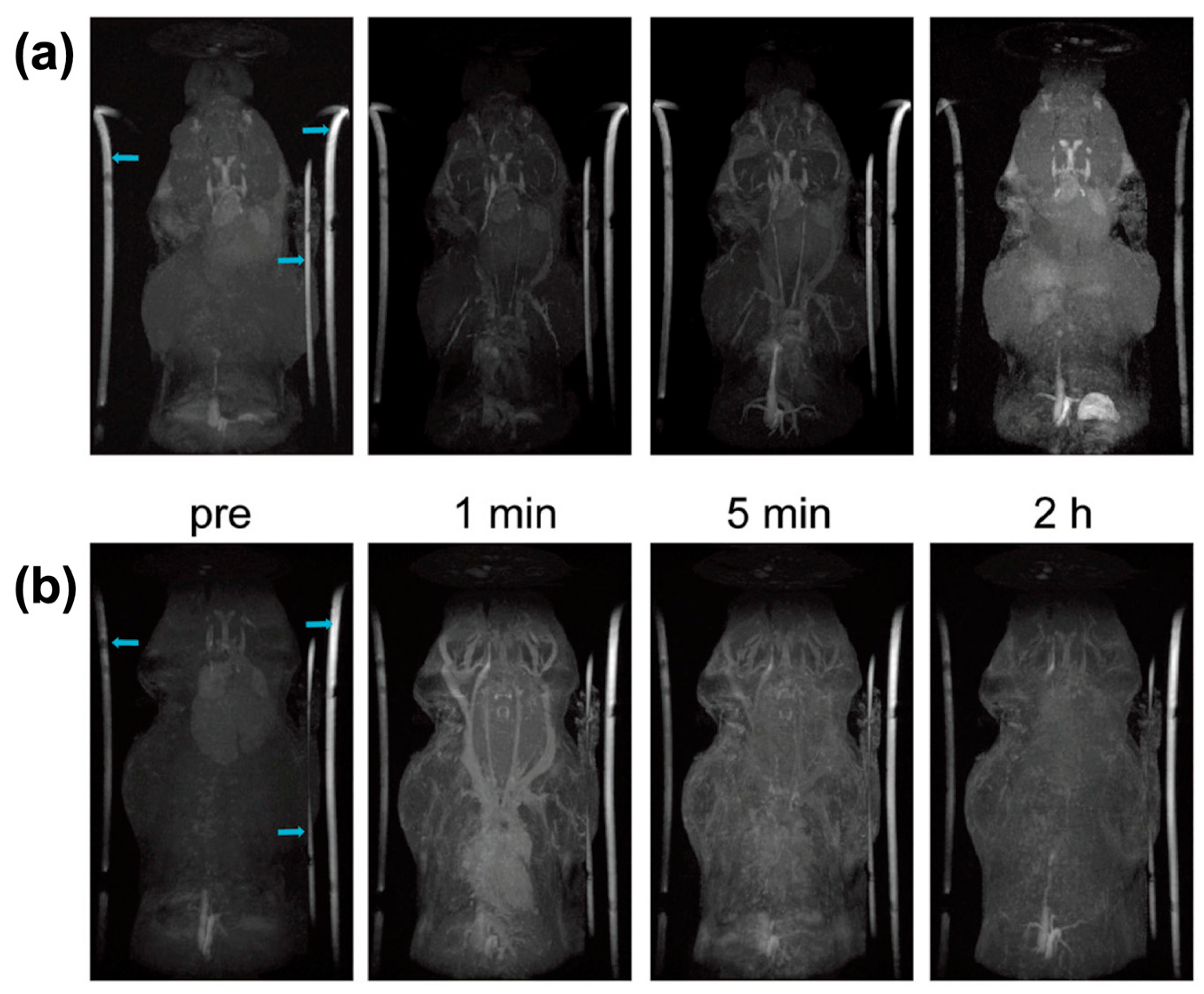
4.1. Magnetic Resonance Imaging
4.2. Multimodal Imaging
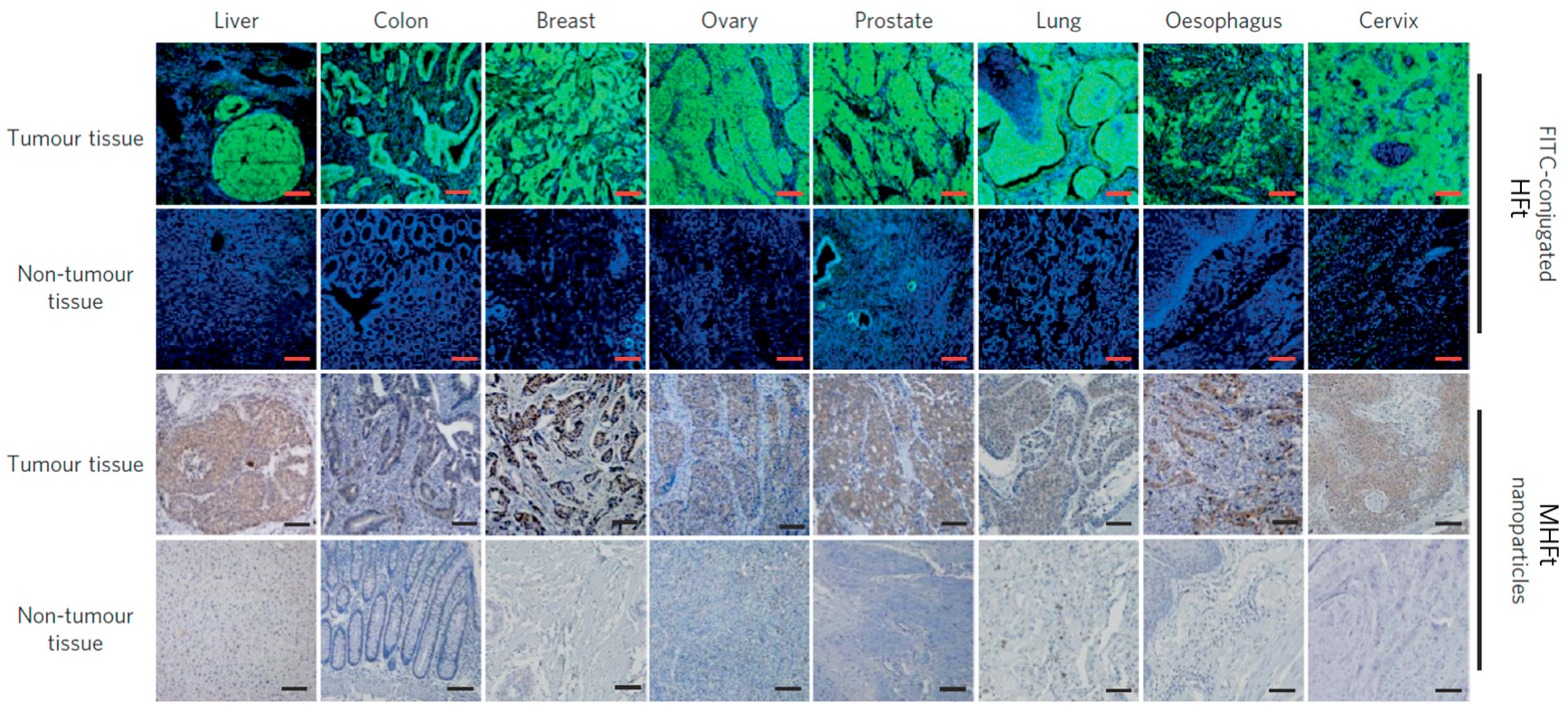
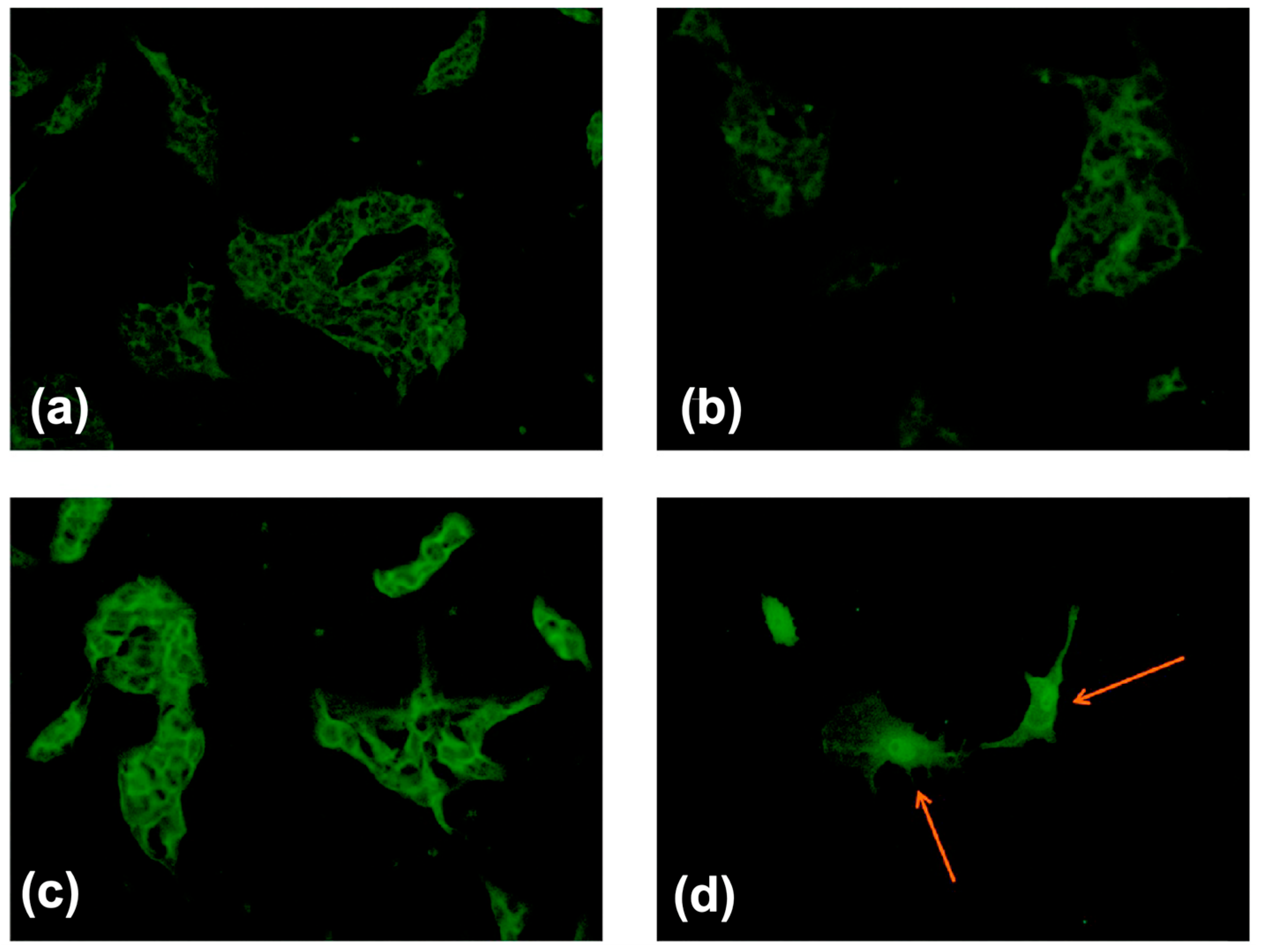
4.3. Tumor Diagnosis In Vitro
4.4. Therapy of Cancer
4.5. Assembly of Ferritin for Biomedical Applications
4.6. Other Bioapplications of Magnetoferritin
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MFt | Magnetoferritin |
| HFt | H chain ferritin |
| apoFt | Apoferritin |
| MRI | Magnetic resonance imaging |
| MHFt | Magnetic HFt |
| TfR1 | Transferrin receptor 1 |
| PfFt | Hyperthermophilic archaeon P. furiosus |
| AfFt | Archaeoglobus fulgidus ferritin |
| EELS | Electron energy-loss spectroscopy |
| SPECT | Single-Photon Emission Computed Tomography |
| SQUID | Superconducting quantum interference device |
| THG | Harmonic generation microscopy |
| OA | Optoacoustic |
| MNP | Magnetic nanoparticle |
| NIRF | Near-infrared fluorescence |
| α-MSH | α-melanocyte-stimulating hormone |
| BBB | Blood–brain barrier |
| ECs | Endothelial cells |
| Dox | Doxorubicin |
| ROS | Reactive Oxygen Species |
References
- Fan, K.; Gao, L.; Yan, X. Human ferritin for tumor detection and therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 287–298. [Google Scholar] [CrossRef]
- Jutz, G.; van Rijn, P.; Santos Miranda, B.; Böker, A. Ferritin: A Versatile Building Block for Bionanotechnology. Chem. Rev. 2015, 115, 1653–1701. [Google Scholar] [CrossRef]
- Kourosh, H.E.; Peter-Leon, H.; Hagen, W.R. Unity in the biochemistry of the iron-storage proteins ferritin and bacterioferritin. Chem. Rev. 2015, 115, 295–326. [Google Scholar]
- Grossman, M.J.; Hinton, S.M.; Minak-Bernero, V.; Slaughter, C.; Stiefel, E.I. Unification of the ferritin family of proteins. Proc. Natl. Acad. Sci. USA 1992, 89, 2419–2423. [Google Scholar] [CrossRef]
- Harrison, P.M.; Arosio, P. The ferritins: Molecular properties, iron storage function and cellular regulation. Biochim. Biophys. Acta Bioenerg. 1996, 1275, 161–203. [Google Scholar] [CrossRef]
- Towe, K.M. Structural distinction between ferritin and iron-dextran (imferon). An electron diffraction comparison. J. Biol. Chem. 1981, 256, 9377–9378. [Google Scholar]
- Domínguez-Vera, J.M.; Fernández, B.; Gálvez, N. Native and synthetic ferritins for nanobiomedical applications: Recent advances and new perspectives. Future Med. Chem. 2010, 2, 609–618. [Google Scholar] [CrossRef]
- Gossuin, Y.; Muller, R.N.; Gillis, P. Relaxation induced by ferritin: A better understanding for an improved MRI iron quantification. NMR Biomed. 2004, 17, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Boyd, D.; Vecoli, C.; Belcher, D.M.; Jain, S.K.; Drysdale, J.W. Structural and functional relationships of human ferritin H and L chains deduced from cDNA clones. J. Biol. Chem. 1985, 260, 11755–11761. [Google Scholar]
- Cozzi, A.; Corsi, B.; Levi, S.; Santambrogio, P.; Albertini, A.; Arosio, P. Overexpression of wild type and mutated human ferritin H-chain in HeLa cells: In vivo role of ferritin ferroxidase activity. J. Biol. Chem. 2000, 275, 25122–25129. [Google Scholar] [CrossRef]
- Levi, S.; Yewdall, S.J.; Harrison, P.M.; Santambrogio, P.; Cozzi, A.; Rovida, E.; Albertini, A.; Arosio, P. Evidence of H- and L-chains have co-operative roles in the iron-uptake mechanism of human ferritin. Biochem. J. 1992, 288, 591–596. [Google Scholar] [CrossRef] [Green Version]
- Alkhateeb, A.A.; Connor, J.R. Nuclear ferritin: A new role for ferritin in cell biology. Biochim. Biophy. Acta, Gen. Subj. 2010, 1800, 793–797. [Google Scholar] [CrossRef]
- Di Sanzo, M.; Chirillo, R.; Aversa, I.; Biamonte, F.; Santamaria, G.; Giovannone, E.D.; Faniello, M.C.; Cuda, G.; Costanzo, F. shRNA targeting of ferritin heavy chain activates H19/miR-675 axis in K562 cells. Gene 2018, 657, 92–99. [Google Scholar] [CrossRef]
- Runsheng, L.; Cherry, L.; Marjelo, M.; Jingwu, Z.; Guo-Huang, F. Chemokine CXCL12 induces binding of ferritin heavy chain to the chemokine receptor CXCR4, alters CXCR4 signaling, and induces phosphorylation and nuclear translocation of ferritin heavy chain. J. Biol. Chem. 2006, 281, 37616–37627. [Google Scholar]
- Flavia, B.; Fabiana, Z.; Andrea, B.; Maddalena, D.S.; Claudia, S.; Domenica, S.; Ilenia, A.; Mariafranca, P.; Maria Concetta, F.; Stefania, B. H-ferritin-regulated microRNAs modulate gene expression in K562 cells. PloS ONE 2015, 10, e0122105. [Google Scholar]
- Zolea, F.; Battaglia, A.M.; Chiarella, E.; Malanga, D.; Marco, C.; Bond, H.M.; Morrone, G.; Costanzo, F.; Biamonte, F. Ferritin Heavy Subunit Silencing Blocks the Erythroid Commitment of K562 Cells via miR-150 up-Regulation and GATA-1 Repression. Int. J. Mol. Sci. 2017, 18, 2167. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.J.; Kwok, Z.H.; Chew, X.H.; Zhang, B.; Liu, C.; Soong, T.W.; Yang, H.; Tay, Y. A FTH1 gene:pseudogene: MicroRNA network regulates tumorigenesis in prostate cancer. Nucleic Acids Res. 2018, 46, 1998–2011. [Google Scholar] [CrossRef]
- Aversa, I.; Chirillo, R.; Chiarella, E.; Zolea, F.; Di Sanzo, M.; Biamonte, F.; Palmieri, C.; Costanzo, F. Chemoresistance in H-Ferritin Silenced Cells: The Role of NF-kappaB. Int. J. Mol. Sci. 2018, 19, 2969. [Google Scholar] [CrossRef] [PubMed]
- Van de Walle, A.; Plan Sangnier, A.; Abou-Hassan, A.; Curcio, A.; Hemadi, M.; Menguy, N.; Lalatonne, Y.; Luciani, N.; Wilhelm, C. Biosynthesis of magnetic nanoparticles from nano-degradation products revealed in human stem cells. Proc. Natl. Acad. Sci. USA 2019, 116, 4044–4053. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.Y.; Uprichard, J. Ferritin and iron studies in anaemia and chronic disease. Ann. Clin. Biochem. 2017, 54, 43–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumida, Y.; Nakajima, A.; Itoh, Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J. Gastroenterol. 2014, 20, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Prados, M.C.; Alvarez-Sala, R.; Garcia Rio, F.J.; Villamor, J. Prospective investigation of tumor markers and risk assessment in early cancer screening. Cancer 2015, 73, 1946–1953. [Google Scholar]
- Chekhun, S.V.; Lukyanova, N.Y.; Shvets, Y.V.; Burlaka, A.P.; Buchinska, L.G. Significance of ferritin expression in formation of malignant phenotype of human breast cancer cells. Exp. Oncol. 2014, 36, 179–183. [Google Scholar] [PubMed]
- Haghgoo, S.M.; Sharafi, H.; Alavian, S.M. Serum cytokines, adipokines and ferritin for non-invasive assessment of liver fibrosis in chronic liver disease: A systematic review. Clin. Chem. Lab. Med. 2019, 57, 577–610. [Google Scholar] [CrossRef]
- Ćujić, D.; Stefanoska, I.; Golubović, S. Serum Ferritin in Healthy Women and Breast Cancer Patients. J. Med. Biochem. 2011, 30, 33–37. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Song, A.; Eo, W. Serum Ferritin as a Prognostic Biomarker for Survival in Relapsed or Refractory Metastatic Colorectal Cancer. J. Cancer 2016, 7, 957–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theil, E.C. Ferritin: Structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu. Rev. Biochem. 2003, 56, 289–315. [Google Scholar] [CrossRef]
- Meldrum, F.C.; Heywood, B.R.; Mann, S. Magnetoferritin: In vitro synthesis of a novel magnetic protein. Science 1992, 257, 522–523. [Google Scholar] [CrossRef]
- Jolley, C.C.; Uchida, M.; Reichhardt, C.; Harrington, R.; Kang, S.; Klem, M.T.; Parise, J.B.; Douglas, T. Size and crystallinity in protein-templated inorganic nanoparticles. Chem. Mater. 2010, 22, 4612–4618. [Google Scholar] [CrossRef]
- Yamashita, I.; Kirimura, H.; Okuda, M.; Nishio, K.; Sano, K.I.; Shiba, K.; Hayashi, T.; Hara, M.; Mishima, Y. Selective Nanoscale Positioning of Ferritin and Nanoparticles by Means of Target-Specific Peptides. Small 2006, 2, 1148–1152. [Google Scholar] [CrossRef] [PubMed]
- Mark, A.; Debbie, W.; Mark, Y.; Trevor, D. Constrained synthesis of cobalt oxide nanomaterials in the 12-subunit protein cage from Listeria innocua. Inorg. Chem. 2003, 42, 6300–6305. [Google Scholar]
- Sang, H.M.; San, B.H.; Kulkarni, A.; Kim, T.; Kim, K.K.; Contribution, E. Synthesis and electric characterization of protein-shelled CdSe quantum dots. J. Mater. Chem. C 2013, 1, 2412–2415. [Google Scholar]
- Song, L.; Lee, A.R.; Ji, H.K.; Sang, J.P. Effect of Temperature on Synthesis of ZnSe Quantum Dots in Apoferritin. Sci. Adv. Mater. 2015, 7, 2743–2746. [Google Scholar]
- Cornell, T.A.; Orner, B.P. Medium Throughput Cage State Stability Screen of Conditions for the Generation of Gold Nanoparticles Encapsulated Within A Mini-Ferritin. Bioorg. Med. Chem. 2018, 26, 5253–5258. [Google Scholar] [CrossRef]
- Dobson, J. Nanoscale biogenic iron oxides and neurodegenerative disease. FEBS Lett. 2001, 496, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Dario, F.; Paolo, A. Biology of ferritin in mammals: An update on iron storage, oxidative damage and neurodegeneration. Arch. Toxicol. 2014, 88, 1787–1802. [Google Scholar]
- Kostiainen, M.A.; Pierpaolo, C.; Manuela, F.; Panu, H.; Oksana, K.; Nolte, R.J.M.; Cornelissen, J.J.L.M.; Desautels, R.D.; Johan, V.L. Hierarchical self-assembly and optical disassembly for controlled switching of magnetoferritin nanoparticle magnetism. Acs Nano 2011, 5, 6394–6402. [Google Scholar] [CrossRef]
- Correia Carreira, S.; Armstrong, J.P.; Seddon, A.M.; Perriman, A.W.; Hartley-Davies, R.; Schwarzacher, W. Ultra-fast stem cell labelling using cationised magnetoferritin. Nanoscale 2016, 8, 7474–7483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charlton, J.R.; Pearl, V.M.; Denotti, A.R.; Lee, J.B.; Swaminathan, S.; Scindia, Y.M.; Charlton, N.P.; Baldelomar, E.J.; Beeman, S.C.; Bennett, K.M. Biocompatibility of ferritin-based nanoparticles as targeted MRI contrast agents. Nanomedicine 2016, 12, 1735–1745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masaki, U.; Flenniken, M.L.; Mark, A.; Willits, D.A.; Crowley, B.E.; Susan, B.; Willis, A.F.; Larissa, J.; Mark, J.; Young, M.J. Targeting of cancer cells with ferrimagnetic ferritin cage nanoparticles. J. Am. Chem. Soc. 2006, 128, 16626–16633. [Google Scholar]
- Fan, K.; Cao, C.; Pan, Y.; Lu, D.; Yang, D.; Feng, J.; Song, L.; Liang, M.; Yan, X. Magnetoferritin nanoparticles for targeting and visualizing tumour tissues. Nat. Nanotechnol. 2012, 7, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Lambert, L.A.; Mitchell, S.L. Molecular Evolution of the Transferrin Receptor/Glutamate Carboxypeptidase II Family. J. Mo. Evol. 2007, 64, 113–128. [Google Scholar] [CrossRef]
- Li, L.; Fang, C.J.; Ryan, J.C.; Niemi, E.C.; Lebrón, J.A.; Bj?Rkman, P.J.; Hisashi, A.; Torti, F.M.; Torti, S.V.; Nakamura, M.C. Binding and uptake of H-ferritin are mediated by human transferrin receptor-1. Proc. Natl. Acad. Sci. USA 2010, 107, 3505–3510. [Google Scholar] [CrossRef] [Green Version]
- Lang, J.; Zhao, X.; Wang, X.; Zhao, Y.; Li, Y.; Zhao, R.; Cheng, K.; Li, Y.; Han, X.; Zheng, X.; et al. Targeted Co-delivery of the Iron Chelator Deferoxamine and a HIF1α Inhibitor Impairs Pancreatic Tumor Growth. ACS Nano 2019, 13, 2176–2189. [Google Scholar] [CrossRef] [PubMed]
- Melníková, L.; Mitróová, Z.; Timko, M.; Kováč, J.; Avdeev, M.V.; Petrenko, V.I.; Garamus, V.M.; Almásy, L.; Kopčanský, P. Structural characterization of magnetoferritin. Mendeleev Commun. 2014, 24, 80–81. [Google Scholar] [CrossRef] [Green Version]
- Elvira, F.; Claudia, I.; Matteo, Z.; Maria, F.; Elisabetta, F.; Miriam, C.; Valbona, S.; Lorenzo, D.C.M.; Carla, G.; Anna Maria, F. A smart platform for hyperthermia application in cancer treatment: Cobalt-doped ferrite nanoparticles mineralized in human ferritin cages. Acs Nano 2014, 8, 4705–4719. [Google Scholar]
- Cai, Y.; Wang, Y.; Xu, H.; Cao, C.; Zhu, R.; Tang, X.; Zhang, T.; Pan, Y. Positive magnetic resonance angiography using ultrafine ferritin-based iron oxide nanoparticles. Nanoscale 2019, 11, 2644–2654. [Google Scholar] [CrossRef] [PubMed]
- Bulte, J.W.; Douglas, T.; Mann, S.; Frankel, R.B.; Moskowitz, B.M.; Brooks, R.A.; Baumgarner, C.D.; Vymazal, J.; Strub, M.P.; Frank, J.A. Magnetoferritin: Characterization of a novel superparamagnetic MR contrast agent. J. Magn. Reson. Imaging Jmri 2010, 4, 497–505. [Google Scholar] [CrossRef]
- Wong, K.K.W.; Douglas, T.; Gider, S.; Awschalom, D.D.; Mann, S. Biomimetic Synthesis and Characterization of Magnetic Proteins (Magnetoferritin). Chem. Mater. 1998, 10, 279–285. [Google Scholar] [CrossRef]
- Fan, K.; Jia, X.; Zhou, M.; Wang, K.; Conde, J.; He, J.; Tian, J.; Yan, X. Ferritin Nanocarrier Traverses the Blood Brain Barrier and Kills Glioma. ACS Nano 2018, 12, 4105–4115. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Fan, K.; Zhou, M.; Duan, D.; Zheng, J.; Yang, D.; Feng, J.; Yan, X. H-ferritin-nanocaged doxorubicin nanoparticles specifically target and kill tumors with a single-dose injection. Proc. Natl. Acad. Sci. USA 2014, 111, 14900–14905. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.J.; Allen, M.A.; Ramsay, B.; Klem, M.T.; Young, M.; Douglas, T. Expanding the Temperature Range of Biomimetic Synthesis Using a Ferritin from the Hyperthermophile Pyrococcus furiosus. Chem. Mater. 2008, 20, 1541–1547. [Google Scholar] [CrossRef]
- Sana, B.; Johnson, E.; Le Magueres, P.; Criswell, A.; Cascio, D.; Lim, S. The role of nonconserved residues of Archaeoglobus fulgidus ferritin on its unique structure and biophysical properties. J. Biol. Chem. 2013, 288, 32663–32672. [Google Scholar] [CrossRef] [PubMed]
- Sana, B.; Johnson, E.; Sheah, K.; Poh, C.L.; Lim, S. Iron-based ferritin nanocore as a contrast agent. Biointerphases 2010, 5, FA48–FA52. [Google Scholar] [CrossRef] [PubMed]
- Klem, M.T.; Resnick, D.A.; Keith, G.; Mark, Y.; Idzerda, Y.U.; Trevor, D. Synthetic control over magnetic moment and exchange bias in all-oxide materials encapsulated within a spherical protein cage. J. Am. Chem. Soc. 2007, 129, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Cao, C.; Tang, X.; Cai, Y.; Yang, C.; Pan, Y. Enhanced peroxidase activity and tumour tissue visualization by cobalt-doped magnetoferritin nanoparticles. Nanotechnology 2017, 28, 045704. [Google Scholar] [CrossRef]
- Hiemstra, T. Surface structure controlling nanoparticle behavior: Magnetism of ferrihydrite, magnetite, and maghemite. Environ. Sci. Nano 2018, 5, 752–764. [Google Scholar] [CrossRef]
- Bo, Z.; Harb, J.N.; Davis, R.C.; Jae-Woo, K.; Sang-Hyon, C.; Sang, C.; Tim, M.; Watt, G.D. Kinetic and thermodynamic characterization of the cobalt and manganese oxyhydroxide cores formed in horse spleen ferritin. Inorg. Chem. 2005, 44, 3738–3745. [Google Scholar]
- Resnick, D.; Gilmore, K.; Idzerda, Y.U.; Klem, M.; Smith, E.; Douglas, T. Modeling of the magnetic behavior of γ-Fe2O3 nanoparticles mineralized in ferritin. J. Appl. Phys. 2004, 95, 7127–7129. [Google Scholar] [CrossRef]
- Geninatti Crich, S.; Cadenazzi, M.; Lanzardo, S.; Conti, L.; Ruiu, R.; Alberti, D.; Cavallo, F.; Cutrin, J.C.; Aime, S. Targeting ferritin receptors for the selective delivery of imaging and therapeutic agents to breast cancer cells. Nanoscale 2015, 7, 6527–6533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhushan, B.; Kumar, S.U.; Matai, I.; Sachdev, A.; Dubey, P.; Gopinath, P. Ferritin Nanocages: A Novel Platform for Biomedical Applications. J. Biomed. Nanotechnol. 2014, 10, 2950–2976. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Tian, L.; Liu, Q.; Liu, W.; Chen, G.; Pan, Y. Magnetic characterization of noninteracting, randomly oriented, nanometer-scale ferrimagnetic particles. J. Geophys. Res. 2010, 115, B07103. [Google Scholar] [CrossRef]
- Jordan, V.C.; Caplan, M.R.; Bennett, K.M. Simplified synthesis and relaxometry of magnetoferritin for magnetic resonance imaging. Magn. Reson. Med. 2010, 64, 1260–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales, M.P.; Veintemillas-Verdaguer, S.; Montero, M.I.; Serna, C.J.; Roig, A.; Casas, L.; Martínez, B.; Sandiumenge, F. Surface and Internal Spin Canting in γ-Fe2O3 Nanoparticles. Chem. Mater. 1999, 11, 3058–3064. [Google Scholar] [CrossRef]
- Li, H.; Klem, M.T.; Sebby, K.B.; Singel, D.J.; Young, M.; Douglas, T.; Idzerda, Y.U. Determination of anisotropy constants of protein encapsulated iron oxide nanoparticles by electron magnetic resonance. J. Magn. Magn. Mater. 2009, 321, 175–180. [Google Scholar] [CrossRef]
- Martínez-Pérez, M.J.; de Miguel, R.; Carbonera, C.; Martínez-Júlvez, M.; Lostao, A.; Piquer, C.; Gómez-Moreno, C.; Bartolomé, J.; Luis, F. Size-dependent properties of magnetoferritin. Nanotechnology 2010, 21, 465707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moro, F.; de Miguel, R.; Jenkins, M.; Gómez-Moreno, C.; Sells, D.; Tuna, F.; McInnes, E.J.L.; Lostao, A.; Luis, F.; van Slageren, J. Magnetic anisotropy of polycrystalline magnetoferritin investigated by SQUID and electron magnetic resonance. J.Magn. Magn. Mater. 2014, 361, 188–196. [Google Scholar] [CrossRef] [Green Version]
- Walls, M.G.; Cao, C.; Yu-Zhang, K.; Li, J.; Che, R.; Pan, Y. Identification of ferrous-ferric Fe3O4 nanoparticles in recombinant human ferritin cages. Microsc. Microanal. 2013, 19, 835–841. [Google Scholar] [CrossRef]
- Comes Franchini, M.; Baldi, G.; Bonacchi, D.; Gentili, D.; Giudetti, G.; Lascialfari, A.; Corti, M.; Marmorato, P.; Ponti, J.; Micotti, E.; et al. Bovine serum albumin-based magnetic nanocarrier for MRI diagnosis and hyperthermic therapy: A potential theranostic approach against cancer. Small 2010, 6, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Fantechi, E.; Campo, G.; Carta, D.; Corrias, A.; de Julián Fernández, C.; Gatteschi, D.; Innocenti, C.; Pineider, F.; Rugi, F.; Sangregorio, C. Exploring the Effect of Co Doping in Fine Maghemite Nanoparticles. J. Phys. Chem. C 2012, 116, 8261–8270. [Google Scholar] [CrossRef]
- Clavijo Jordan, M.V.; Beeman, S.C.; Baldelomar, E.J.; Bennett, K.M. Disruptive chemical doping in a ferritin-based iron oxide nanoparticle to decrease r2 and enhance detection with T1-weighted MRI. Contrast Media Mol. Imaging 2014, 9, 323–332. [Google Scholar] [CrossRef]
- Szybowicz, M.; Koralewski, M.; Karoń, J.; Melnikova, L. Micro-Raman Spectroscopy of Natural and Synthetic Ferritins and Their Mimetics. Acta Phys. Pol. A 2015, 127, 534–536. [Google Scholar] [CrossRef]
- Koralewski, M.; Balejcikova, L.; Mitroova, Z.; Pochylski, M.; Baranowski, M.; Kopcansky, P. Morphology and Magnetic Structure of the Ferritin Core during Iron Loading and Release by Magnetooptical and NMR Methods. ACS Appl. Mater. Interfaces 2018, 10, 7777–7787. [Google Scholar] [CrossRef] [PubMed]
- Koralewski, M.; Kłos, J.W.; Baranowski, M.; Mitróová, Z.; Kopčanský, P.; Melníková, L.; Okuda, M.; Schwarzacher, W. The Faraday effect of natural and artificial ferritins. Nanotechnology 2012, 23, 255704. [Google Scholar] [CrossRef]
- Koralewski, M.; Pochylski, M.; Mitróová, Z.; Timko, M.; Kopčanský, P.; Melníková, L. Magnetic birefringence of natural and synthetic ferritin. J. Magn. Magn. Mater. 2011, 323, 2413–2417. [Google Scholar] [CrossRef]
- Pan, Y.; Cao, C.; He, X.; Yang, C.; Tian, L.; Zhu, R.; Cai, Y. Enhanced magnetic resonance imaging and staining of cancer cells using ferrimagnetic H-ferritin nanoparticles with increasing core size. Int. J. Nanomed. 2015, 10, 2619–2634. [Google Scholar] [CrossRef] [Green Version]
- Tahka, S.; Laiho, A.; Kostiainen, M.A. Diblock-copolymer-mediated self-assembly of protein-stabilized iron oxide nanoparticle clusters for magnetic resonance imaging. Chemistry 2014, 20, 2718–2722. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, L.; Falvo, E.; Fornara, M.; Di Micco, P.; Benada, O.; Krizan, J.; Svoboda, J.; Hulikova-Capkova, K.; Morea, V.; Boffi, A.; et al. Selective targeting of melanoma by PEG-masked protein-based multifunctional nanoparticles. Int. J. Nanomed. 2012, 7, 1489–1509. [Google Scholar] [Green Version]
- Massner, C.; Sigmund, F.; Pettinger, S.; Seeger, M.; Hartmann, C.; Ivleva, N.P.; Niessner, R.; Fuchs, H.; de Angelis, M.H.; Stelzl, A.; et al. Genetically Controlled Lysosomal Entrapment of Superparamagnetic Ferritin for Multimodal and Multiscale Imaging and Actuation with Low Tissue Attenuation. Adv. Funct. Mater. 2018, 28, 1706793. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, M.; Li, X.; Fan, K.; Xiao, J.; Li, Y.; Shi, H.; Wang, F.; Choi, H.S.; Cheng, D.; et al. Bioengineered Magnetoferritin Nanoprobes for Single-Dose Nuclear-Magnetic Resonance Tumor Imaging. ACS Nano 2016, 10, 4184–4191. [Google Scholar] [CrossRef]
- Cao, C.; Wang, X.; Cai, Y.; Sun, L.; Tian, L.; Wu, H.; He, X.; Lei, H.; Liu, W.; Chen, G.; et al. Targeted in vivo imaging of microscopic tumors with ferritin-based nanoprobes across biological barriers. Adv. Mater. 2014, 26, 2566–2571. [Google Scholar] [CrossRef]
- Aslan, T.N.; Aşık, E.; Volkan, M. Preparation and labeling of surface-modified magnetoferritin protein cages with a rhenium(i) carbonyl complex for magnetically targeted radiotherapy. RSC Adv. 2016, 6, 8860–8869. [Google Scholar] [CrossRef]
- Ducasse, R.; Wang, W.A.; Navarro, M.G.; Debons, N.; Colin, A.; Gautier, J.; Guigner, J.M.; Guyot, F.; Gueroui, Z. Programmed Self-Assembly of a Biochemical and Magnetic Scaffold to Trigger and Manipulate Microtubule Structures. Sci. Rep. 2017, 7, 11344. [Google Scholar] [CrossRef]
- Chen, L.; Zang, F.; Wu, H.; Li, J.; Xie, J.; Ma, M.; Gu, N.; Zhang, Y. Using PEGylated magnetic nanoparticles to describe the EPR effect in tumor for predicting therapeutic efficacy of micelle drugs. Nanoscale 2018, 10, 1788–1797. [Google Scholar] [CrossRef]
- Singh, A.; Sahoo, S.K. Magnetic nanoparticles: A novel platform for cancer theranostics. Drug Discov. Today 2014, 19, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Mattix, B.; Olsen, T.R.; Gu, Y.; Casco, M.; Herbst, A.; Simionescu, D.T.; Visconti, R.P.; Kornev, K.G.; Alexis, F. Biological magnetic cellular spheroids as building blocks for tissue engineering. Acta Biomater. 2014, 10, 623–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masaki, U.; Masahiro, T.; Cunningham, C.H.; Yoriyasu, S.; Willits, D.A.; Willis, A.F.; Yang, P.C.; Tsao, P.S.; Mcconnell, M.V.; Young, M.J. A human ferritin iron oxide nano-composite magnetic resonance contrast agent. Magn Reson Med. Magn. Reson. Med. 2010, 60, 1073–1081. [Google Scholar]
- Melnikova, L.; Petrenko, V.I.; Avdeev, M.V.; Ivankov, O.I.; Bulavin, L.A.; Garamus, V.M.; Almásy, L.; Mitroova, Z.; Kopcansky, P. SANS contrast variation study of magnetoferritin structure at various iron loading. J. Magn. Magn. Mater. 2015, 377, 77–80. [Google Scholar] [CrossRef] [Green Version]
- Nandwana, V.; Ryoo, S.R.; Kanthala, S.; Kumar, A.; Sharma, A.; Castro, F.C.; Li, Y.; Hoffman, B.; Lim, S.; Dravid, V.P. Engineered ferritin nanocages as natural contrast agents in magnetic resonance imaging. RSC Adv. 2017, 7, 34892–34900. [Google Scholar] [CrossRef] [Green Version]
- Jensen, J.H.; Tang, H.; Tosti, C.L.; Swaminathan, S.V.; Nunez, A.; Hultman, K.; Szulc, K.U.; Wu, E.X.; Kim, D.; Sheth, S.; et al. Separate MRI quantification of dispersed (ferritin-like) and aggregated (hemosiderin-like) storage iron. Magn. Reson. Med. 2010, 63, 1201–1209. [Google Scholar] [CrossRef]
- Wu, L.; Mendoza-Garcia, A.; Li, Q.; Sun, S. Organic Phase Syntheses of Magnetic Nanoparticles and Their Applications. Chem. Rev. 2016, 116, 10473–10512. [Google Scholar] [CrossRef]
- Lee, N.; Yoo, D.; Ling, D.; Cho, M.H.; Hyeon, T.; Cheon, J. Iron Oxide Based Nanoparticles for Multimodal Imaging and Magnetoresponsive Therapy. Chem. Rev. 2015, 115, 10637–10689. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, Y.J.; Zhang, G.B.; Ling, D.; Wang, M.Q.; Zhou, Y.; Wu, Y.D.; Wu, T.; Hackett, M.J.; Hyo Kim, B.; et al. Iron oxide nanoclusters for T1 magnetic resonance imaging of non-human primates. Nat. Biomed. Eng. 2017, 1, 637–643. [Google Scholar] [CrossRef]
- Bartelle, B.B.; Berrios-Otero, C.A.; Rodriguez, J.J.; Friedland, A.E.; Aristizabal, O.; Turnbull, D.H. Novel genetic approach for in vivo vascular imaging in mice. Circ. Res. 2012, 110, 938–947. [Google Scholar] [CrossRef]
- Lizeng, G.; Jie, Z.; Leng, N.; Jinbin, Z.; Yu, Z.; Ning, G.; Taihong, W.; Jing, F.; Dongling, Y.; Sarah, P. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar]
- Sathya, A.; Guardia, P.; Brescia, R.; Silvestri, N.; Pugliese, G.; Nitti, S.; Manna, L.; Pellegrino, T. CoxFe3–xO4 Nanocubes for Theranostic Applications: Effect of Cobalt Content and Particle Size. Chem. Mater. 2016, 28, 1769–1780. [Google Scholar] [CrossRef]
- Verma, P.; Baldrian, P.; Nerud, F. Decolorization of structurally different synthetic dyes using cobalt(II)/ascorbic acid/hydrogen peroxide system. Chemosphere 2003, 50, 975–979. [Google Scholar] [CrossRef]
- Fan, K.; Xi, J.; Lei, F.; Wang, P.; Zhu, C.; Yan, T.; Xu, X.; Liang, M.; Bing, J.; Yan, X. In vivo guiding nitrogen-doped carbon nanozyme for tumor catalytic therapy. Nat. Commun. 2018, 9, 1140. [Google Scholar] [CrossRef] [PubMed]
- Valimaki, S.; Mikkila, J.; Liljestrom, V.; Rosilo, H.; Ora, A.; Kostiainen, M.A. Hierarchically ordered supramolecular protein-polymer composites with thermoresponsive properties. Int. J. Mol. Sci. 2015, 16, 10201–10213. [Google Scholar] [CrossRef] [PubMed]
- Stanley, S.A.; Sauer, J.; Kane, R.S.; Dordick, J.S.; Friedman, J.M. Remote regulation of glucose homeostasis in mice using genetically encoded nanoparticles. Nat. Med. 2014, 21, 92–98. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, M.A.; Smith, C.J.; Ottolini, M.; Barker, B.S.; Purohit, A.M.; Grippo, R.M.; Gaykema, R.P.; Spano, A.J.; Beenhakker, M.P.; Kucenas, S.; et al. Genetically targeted magnetic control of the nervous system. Nat. Neurosci. 2016, 19, 756–761. [Google Scholar] [CrossRef] [Green Version]
- Meister, M. Physical limits to magnetogenetics. eLife 2016, 5. Available online: https://cdn.elifesciences.org/articles/17210/elife-17210-v2.pdf (accessed on 16 August 2016). [CrossRef]
- Anikeeva, P.; Jasanoff, A. Problems on the back of an envelope. Elife 2016, 5. [Google Scholar] [CrossRef]
- Qin, S.; Yin, H.; Yang, C.; Dou, Y.; Liu, Z.; Zhang, P.; Yu, H.; Huang, Y.; Feng, J.; Hao, J.; et al. A magnetic protein biocompass. Nat. Mater. 2016, 15, 217–226. [Google Scholar] [CrossRef]
- Cao, Y.; Yan, P. Role of atomic spin-mechanical coupling in the problem of a magnetic biocompass. Phys. Rev. E 2018, 97, 042409. [Google Scholar] [CrossRef] [Green Version]
| Ferritin Shell | Extra Modification | Properties of Iron Core | Applications | References |
|---|---|---|---|---|
| apoFt | Superparamagnetic | MRI (T2) | [48] | |
| W doped | Paramagnetic | MRI (T1) | [71] | |
| HFt | Superparamagnetic | MRI (T2) | [63] | |
| Peroxidase | Tumor diagnosis | [41,76] | ||
| Superparamagnetic | Native tumor targeting | [41] | ||
| Polymer mediated | Enhanced relaxivity | MRI (T2) | [77] | |
| RGD linked | Superparamagnetic | Tumor targeting, MRI (T2) | [40] | |
| α-MSH linked | Superparamagnetic | Selective targeting | [78] | |
| Ultrafine Fe2O3 | Superparamagnetic | MRI (T1) | [47] | |
| Co doped | Superparamagnetic, Enhanced relaxivity | Magnetize cells, MRI (T2) | [38] | |
| Co doped | Peroxidase | Cancer diagnosis | [56] | |
| Semigenetic | Superparamagnetic | THG, OA and MRI (T2) | [79] | |
| 125I conjugated | Superparamagnetic | SPECT imaging, MRI (T2) | [80] | |
| Co doped | Enhanced magnetic Anisotropy | Hyperthermia | [46] | |
| Cy5.5 labeled | High relaxivity | NIRF imaging, MRI (T2) | [81] | |
| Re conjugated | Superparamagnetic | Radiotherapy, MRI (T2) | [82] | |
| Self-organized | Magnetic scaffold | Control bioprocess | [83] | |
| AfFt-AA | Enhanced relaxivity | MRI (T2) | [54] | |
| PfFt | Assembly | Superparamagnetic | Control drug delivery | [37] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, L.; Deng, D.; Sun, J. Magnetoferritin: Process, Prospects, and Their Biomedical Applications. Int. J. Mol. Sci. 2019, 20, 2426. https://doi.org/10.3390/ijms20102426
Xue L, Deng D, Sun J. Magnetoferritin: Process, Prospects, and Their Biomedical Applications. International Journal of Molecular Sciences. 2019; 20(10):2426. https://doi.org/10.3390/ijms20102426
Chicago/Turabian StyleXue, Le, Dawei Deng, and Jianfei Sun. 2019. "Magnetoferritin: Process, Prospects, and Their Biomedical Applications" International Journal of Molecular Sciences 20, no. 10: 2426. https://doi.org/10.3390/ijms20102426
APA StyleXue, L., Deng, D., & Sun, J. (2019). Magnetoferritin: Process, Prospects, and Their Biomedical Applications. International Journal of Molecular Sciences, 20(10), 2426. https://doi.org/10.3390/ijms20102426