Antihistamines for Allergic Rhinitis Treatment from the Viewpoint of Nonsedative Properties
Abstract
1. Introduction
2. Pharmacological Aspects Related to Sedation of Antihistamines
2.1. Histamine and Its Receptors
2.2. Sedative Potentials of Antihistamines and Their Classification Based on Brain H1 Receptor Occupancy
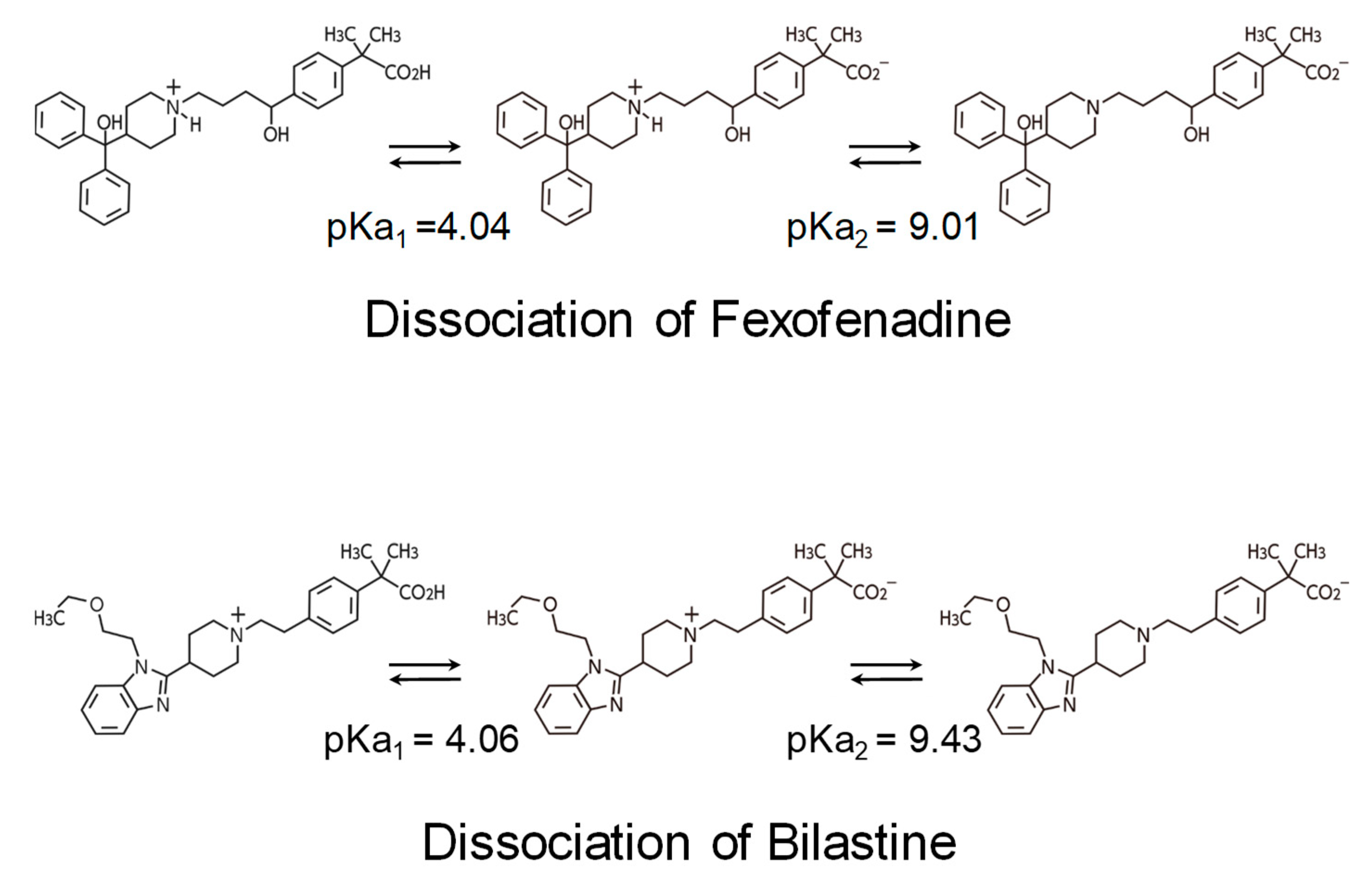
2.3. Non-Brain-Penetrating Antihistamines: Bilastine and Fexofenadine
2.4. Residual Effects by Sedating Antihistamines
3. Clinical Aspects of Non-Sedating Antihistamines
3.1. Clinical Profiles of Representative Second-Generation Antihistamines
3.2. Efficacy for Seasonal Allergic Rhinitis
3.3. Central Nervous System Safety of Bilastine
4. Conclusions
5. Expert Opinion
Article Highlights Box
- In selecting antihistamines for allergic rhinitis, it is particularly important for safety that the selected drug does not have central depressant/sedative properties and anticholinergic effects.
- Differences in sedative effects and anticholinergic effects were observed among the second-generation antihistamines.
- Based on the brain H1 receptor occupancy, which is an index of sedative properties, fexofenadine and bilastine belonging to the non-sedating group can be distinguished as “non-brain-penetrating antihistamines”.
- No major differences in efficacy are observed among recent, representative, non-sedating antihistamines for allergic rhinitis.
- Central nervous system safety of antihistamines needs to be evaluated not only by subjective indices, such as drowsiness, but also by the results of objective performance tests.
- Non-brain-penetrating antihistamines have been confirmed not to show sedative properties even at twice the usual dose and thus are considered to be the first-line antihistamines for allergic rhinitis.
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| H1RO | Brain H1 receptor occupancy |
| GPCR | G-protein-coupled receptors |
| PIR | proportional impairment ratio |
| CONGA | Consensus Group of New Generation of Antihistamines |
| BBB | blood–brain barrier |
| pKa | Acid–Base Dissociation Constant |
| Tmax | time to maximum plasma concentration |
| t½ | elimination half-life |
| ARIA | Allergic Rhinitis and its Impact on Asthma |
| SAR | seasonal allergic rhinitis |
| TSS | total symptom score |
| NSS | nasal symptom score |
| NNSS | non-nasal symptom score |
| PAR | perennial allergic rhinitis |
| FMT | Fine Motoric Test |
| CFF | Critical Flicker-Fusion Frequency Test |
| D2T | “d2” Cancellation Test |
| SRT | Simple Reaction Time |
| SDLP | standard deviations of lateral position |
References
- Bousquet, J.; Khaltaev, N.; Cruz, A.A.; Denburg, J.; Fokkens, W.J.; Togias, A.; Zuberbier, T.; Baena-Cagnani, C.E.; Canonica, G.W.; van Weel, C.; et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008. Allergy 2008, 63 (Suppl. 86), 8–160. [Google Scholar]
- Okubo, K.; Kurono, Y.; Ichimura, K.; Enomoto, T.; Okamoto, Y.; Kawauchi, H.; Suzaki, H.; Fujieda, S.; Masuyama, K. Japanese guidelines for allergic rhinitis 2017. Allergol. Int. 2017, 66, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Kay, A.B. Allergy and allergic diseases. First of two parts. N. Engl. J. Med. 2001, 344, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Clement, P.; Smitz, J.; De Waele, M.; Derde, M.P. Correlations between complaints, inflammatory cells and mediator concentrations in nasalsecretions after nasal allergen challenge and during natural allergen exposure. Int. Arch. Allergy Immunol. 1995, 106, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Mandhane, S.N.; Shah, J.H.; Thennati, R. Allergic rhinitis: An update on disease, present treatments and future prospects. Int. Immunopharmacol. 2011, 11, 1646–1662. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Ghoshal, A.G.; Bin Abdul Muttalif, A.R.; Lin, H.C.; Thanaviratananich, S.; Bagga, S.; Faruqi, R.; Sajjan, S.; Brnabic, A.J.; Dehle, F.C.; et al. Quality of life and economic burden of respiratory disease in Asia-Pacific—Asia-Pacific Burden of Respiratory Diseases Study. Value Health Reg. Issues 2016, 9, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Lim-Jurado, M.; Prepageran, N.; Tantilipikorn, P.; Wang de, Y. Treatment of allergic rhinitis and urticaria: A review of the newest antihistamine drug bilastine. Ther. Clin. Risk Manag. 2016, 12, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Yanai, K.; Yoshikawa, T.; Yanai, A.; Nakamura, T.; Iida, T.; Leurs, R.; Tashiro, M. The clinical pharmacology of non-sedating antihistamines. Pharmacol. Ther. 2017, 178, 148–156. [Google Scholar] [CrossRef]
- Yanai, K.; Zhang, D.; Tashiro, M.; Yoshikawa, T.; Naganuma, F.; Harada, R.; Nakamura, T.; Shibuya, K.; Okamura, N. Positron emission tomography evaluation of sedative properties of antihistamines. Expert Opin. Drug Saf. 2011, 10, 613–622. [Google Scholar] [CrossRef]
- Panula, P.; Chazot, P.L.; Cowart, M.; Gutzmer, R.; Leurs, R.; Liu, W.L.; Stark, H.; Thurmond, R.L.; Haas, H.L. International Union of Basic and Clinical Pharmacology. XCVIII. Histamine receptors. Pharmacol. Rev. 2015, 67, 601–655. [Google Scholar] [CrossRef]
- Leurs, R.; Church, M.K.; Taglialatela, M. H1-antihistamines: Inverse agonism, anti-inflammatory actions and cardiac effects. Clin. Exp. Allergy 2002, 32, 489–498. [Google Scholar] [CrossRef]
- Church, D.S.; Church, M.K. Pharmacology of antihistamines. World Allergy Organ. J. 2011, 4 (Suppl. 3), S22–S27. [Google Scholar] [CrossRef]
- Shimamura, T.; Shiroishi, M.; Weyand, S.; Tsujimoto, H.; Winter, G.; Katritch, V.; Abagyan, R.; Cherezov, V.; Liu, W.; Han, G.W.; et al. Structure of the human histamine H1 receptor complex with doxepin. Nature 2011, 475, 65–70. [Google Scholar] [CrossRef]
- Haas, H.; Panula, P. The role of histamine and the tuberomamillary nucleus in the nervous system. Nat. Rev. Neurosci. 2003, 4, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Haas, H.; Sergeeva, O.A.; Selbach, O. Histamine in the nervous system. Physiol. Rev. 2008, 88, 1183–1241. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Dauvilliers, Y.; Siegel, J.M. Interactions of the histamine and hypocretin systems in CNS disorders. Nat. Rev. Neurol. 2015, 11, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Yanai, K. Studies on functional roles of the histaminergic neuron system by using pharmacological agents, knockout mice and positron emission tomography. Tohoku J. Exp. Med. 2001, 195, 197–217. [Google Scholar] [CrossRef]
- Yanai, K.; Ryu, J.H.; Watanabe, T.; Iwata, R.; Ido, T.; Sawai, Y.; Ito, K.; Itoh, M. Histamine H1 receptor occupancy in human brains after single oral doses of histamine H1 antagonists measured by positron emission tomography. Br. J. Pharmacol. 1995, 116, 1649–1655. [Google Scholar] [CrossRef]
- Okamura, N.; Yanai, K.; Higuchi, M.; Sakai, J.; Iwata, R.; Ido, T.; Sasaki, H.; Watanabe, T.; Itoh, M. Functional neuroimaging of cognition impaired by a classical antihistamine, d-chlorpheniramine. Br. J. Pharmacol. 2000, 129, 115–123. [Google Scholar] [CrossRef]
- Tagawa, M.; Kano, M.; Okamura, N.; Higuchi, M.; Matsuda, M.; Mizuki, Y.; Arai, H.; Iwata, R.; Fujii, T.; Komemushi, S.; et al. Neuroimaging of histamine H1-receptor occupancy in human brain by positron emission tomography (PET): A comparative study of ebastine, a second-generation antihistamine and (+)-chlorpheniramine, a classical antihistamine. Br. J. Clin. Pharmacol. 2001, 52, 501–509. [Google Scholar] [CrossRef]
- Tashiro, M.; Sakurada, Y.; Iwabuchi, K.; Mochizuki, H.; Kato, M.; Aoki, M.; Funaki, Y.; Itoh, M.; Iwata, R.; Wong, D.F.; et al. Central effects of fexofenadine and cetirizine: Measurement of psychomotor performance, subjective sleepiness, and brain histamine H1-receptor occupancy using 11C-doxepin positron emission tomography. J. Clin. Pharmacol. 2004, 44, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, K.; Tashiro, M.; Grobosch, T.; Maurer, M.; Oda, K.; Toyohara, J.; Ishii, K.; Ishiwata, K.; Yanai, K. Brain histamine H1 receptor occupancy measured by PET after oral administration of levocetirizine, a non-sedating antihistamine. Expert Opin. Drug Saf. 2015, 14, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Yanai, K.; Watanabe, T.; Yokoyama, H.; Hatazawa, J.; Iwata, R.; Ishiwata, K.; Meguro, K.; Itoh, M.; Takahashi, T.; Ido, T.; et al. Mapping of histamine H1 receptors in the human brain using [11C]pyrilamine and positron emission tomography. J. Neurochem. 1992, 59, 128–136. [Google Scholar] [CrossRef]
- Yanai, K.; Watanabe, T.; Yokoyama, H.; Meguro, K.; Hatazawa, J.; Itoh, M.; Iwata, R.; Ishiwata, K.; Takahashi, T.; Ido, T. Histamine H1 receptors in human brain visualized in vivo by [11C]doxepin and positron emission tomography. Neurosci. Lett. 1992, 137, 145–148. [Google Scholar] [CrossRef]
- Yanai, K.; Watanabe, T.; Meguro, K.; Yokoyama, H.; Sato, I.; Sasano, H.; Itoh, M.; Iwata, R.; Takahashi, T.; Ido, T. Age-dependent decrease in histamine H1 receptor in human brains revealed by PET. Neuroreport 1992, 3, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, M.; Horikawa, E.; Mochizuki, H.; Sakurada, Y.; Kato, M.; Inokuchi, T.; Ridout, F.; Hindmarch, I.; Yanai, K. Effects of fexofenadine and hydroxyzine on brake reaction time during car-driving with cellular phone use. Hum. Psychopharmacol. 2005, 20, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, Z.; Hindmarch, I. Sedation and antihistamines: A review of inter-drug differences using proportional impairment ratios. Hum. Psychopharmacol. 2000, 15, S3–S30. [Google Scholar] [CrossRef]
- McDonald, K.; Trick, L.; Boyle, J. Sedation and antihistamines: An update. Review of inter-drug differences using proportional impairment ratios. Hum. Psychopharmacol. 2008, 23, 555–570. [Google Scholar] [CrossRef] [PubMed]
- Yanai, K.; Tashiro, M. The physiological and pathophysiological roles of neuronal histamine: An insight from human positron emission tomography studies. Pharmacol. Ther. 2007, 113, 1–15. [Google Scholar] [CrossRef]
- Holgate, S.T.; Canonica, G.W.; Simons, F.E.; Taglialatela, M.; Tharp, M.; Timmerman, H.; Yanai, K. Consensus Group on New-Generation Antihistamines (CONGA): Present status and recommendations. Clin. Exp. Allergy 2003, 33, 1305–1324. [Google Scholar] [CrossRef]
- Farré, M.; Pérez-Mañá, C.; Papaseit, E.; Menoyo, E.; Pérez, M.; Martin, S.; Bullich, S.; Rojas, S.; Herance, J.R.; Trampal, C.; et al. Bilastine vs. hydroxyzine: Occupation of brain histamine H1 -receptors evaluated by positron emission tomography in healthy volunteers. Br. J. Clin. Pharmacol. 2014, 78, 970–980. [Google Scholar] [CrossRef] [PubMed]
- Yanai, K.; Hiraoka, K.; Kárpáti, A.; Naganuma, F.; Okamura, N.; Tashiro, M.; Nakamura, T.; Yoshikawa, T. Histamine H1 receptor occupancy in human brain. In Histamine Receptors: Preclinical and Clinical Aspects. The Receptors; Blandina, P., Passani, B.M., Eds.; Humana Press: Cham, Switzerland, 2016; Volume 28, pp. 311–326. [Google Scholar]
- Obradovic, T.; Dobson, G.G.; Shingaki, T.; Kungu, T.; Hidalgo, I.J. Assessment of the first and second generation antihistamines brain penetration and role of P-glycoprotein. Pharm. Res. 2007, 24, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Lucero, M.L.; Gonzalo, A.; Ganza, A.; Leal, N.; Soengas, I.; Ioja, E.; Gedey, S.; Jahic, M.; Bednarczyk, D. Interactions of bilastine, a new oral H1 antihistamine, with human transporter systems. Drug Chem. Toxicol. 2012, 35 (Suppl. 1), 8–17. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Funahashi, J.; Mori, K.; Hayashi, K.; Yano, H. The noncompetitive antagonism of histamine H1 receptors expressed in Chinese hamster ovary cells by olopatadine hydrochloride: Its potency and molecular mechanism. Pharmacology 2008, 81, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Corcóstegui, R.; Labeaga, L.; Innerárity, A.; Berisa, A.; Orjales, A. Preclinical pharmacology of bilastine, a new selective histamine H1 receptor antagonist: Receptor selectivity and in vitro antihistaminic activity. Drugs R D 2005, 6, 371–384. [Google Scholar] [CrossRef]
- Bosma, R.; van den Bor, J.; Vischer, H.F.; Labeaga, L.; Leurs, R. The long duration of action of the second generation antihistamine bilastine coincides with its long residence time at the histamine H1 receptor. Eur. J. Pharmacol. 2018, 838, 107–111. [Google Scholar] [CrossRef]
- Church, M.K.; Maurer, M.; Simons, F.E.; Bindslev-Jensen, C.; van Cauwenberge, P.; Bousquet, J.; Holgate, S.T.; Zuberbier, T. Risk of first-generation H1-antihistamines: A GA2LEN position paper. Allergy 2010, 65, 459–466. [Google Scholar] [CrossRef]
- Yanai, K.; Rogala, B.; Chugh, K.; Paraskakis, E.; Pampura, A.N.; Boev, R. Safety considerations in the management of allergic diseases: Focus on antihistamines. Curr. Med. Res. Opin. 2012, 28, 623–642. [Google Scholar] [CrossRef]
- Dresser, G.K.; Bailey, D.G.; Leake, B.F.; Schwarz, U.I.; Dawson, P.A.; Freeman, D.J.; Kim, R.B. Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine. Clin. Pharmacol. Ther. 2002, 71, 11–20. [Google Scholar] [CrossRef]
- Kuna, P.; Bachert, C.; Nowacki, Z.; van Cauwenberge, P.; Agache, I.; Fouquert, L.; Roger, A.; Sologuren, A.; Valiente, R. Efficacy and safety of bilastine 20 mg compared with cetirizine 10 mg and placebo for the symptomatic treatment of seasonal allergic rhinitis: A randomized, double-blind, parallel-group study. Clin. Exp. Allergy 2009, 39, 1338–1347. [Google Scholar] [CrossRef]
- Bachert, C.; Kuna, P.; Sanquer, F.; Ivan, P.; Dimitrov, V.; Gorina, M.M.; van de Heyning, P.; Loureiro, A. Comparison of the efficacy and safety of bilastine 20 mg vs desloratadine 5 mg in seasonal allergic rhinitis patients. Allergy 2009, 6, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Horak, F.; Zieglmayer, P.; Zieglmayer, R.; Lemell, P. The effects of bilastine compared with cetirizine, fexofenadine, and placebo on allergen-induced nasal and ocular symptoms in patients exposed to aeroallergen in the Vienna Challenge Chamber. Inflamm. Res. 2010, 59, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, K.; Wakabayashi, K.I.; Togawa, M.; Saito, A.; Okubo, K. Therapeutic effect of bilastine in Japanese cedar pollinosis using an artificial exposure chamber (OHIO Chamber). Allergol. Int. 2017, 66, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Sastre, J.; Mullol, J.; Valero, A.; Valiente, R. Efficacy and safety of bilastine 20 mg compared with cetirizine 10 mg and placebo in the treatment of perennial allergic rhinitis. Curr. Med. Res. Opin. 2012, 28, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Okubo, K.; Gotoh, M.; Asako, M.; Nomura, Y.; Togawa, M.; Saito, A.; Honda, T.; Ohashi, Y. Efficacy and safety of bilastine in Japanese patients with perennial allergic rhinitis: A multicenter, randomized, double-blind, placebo-controlled, parallel-group phase III study. Allergol. Int. 2017, 66, 97–105. [Google Scholar] [CrossRef] [PubMed]
- García-Gea, C.; Martínez-Colomer, J.; Antonijoan, R.M.; Valiente, R.; Barbanoj, M.J. Comparison of peripheral and central effects of single and repeated oral dose administrations of bilastine, a new H1 antihistamine: A dose-range study in healthy volunteers with hydroxyzine and placebo as control treatments. J. Clin. Psychopharmacol. 2008, 28, 675–685. [Google Scholar] [CrossRef]
- Conen, S.; Theunissen, E.L.; Van Oers, A.C.; Valiente, R.; Ramaekers, J.G. Acute and subchronic effects of bilastine (20 and 40 mg) and hydroxyzine (50 mg) on actual driving performance in healthy volunteers. J. Psychopharmacol. 2011, 25, 1517–1523. [Google Scholar] [CrossRef]
- Jáuregui, I.; Ramaekers, J.G.; Yanai, K.; Farré, M.; Redondo, E.; Valiente, R.; Labeaga, L. Bilastine: A new antihistamine with an optimal benefit-to-risk ratio for safety during driving. Expert Opin. Drug Saf. 2016, 15, 89–98. [Google Scholar] [CrossRef]
- García-Gea, C.; Martínez, J.; Ballester, M.R.; Gich, I.; Valiente, R.; Antonijoan, R.M. Psychomotor and subjective effects of bilastine, hydroxyzine, and cetirizine, in combination with alcohol: A randomized, double-blind, crossover, and positive-controlled and placebo-controlled Phase I clinical trials. Hum. Psychopharmacol. 2014, 29, 120–132. [Google Scholar] [CrossRef]
- Brozek, J.L.; Bousquet, J.; Baena-Cagnani, C.E.; Bonini, S.; Canonica, G.W.; Casale, T.B.; van Wijk, R.G.; Ohta, K.; Zuberbier, T.; Schünemann, H.J. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision. J. Allergy Clin. Immunol. 2010, 126, 466–476. [Google Scholar] [CrossRef]
- Senda, M.; Kubo, N.; Adachi, K.; Ikari, Y.; Matsumoto, K.; Shimizu, K.; Tominaga, H. Cerebral histamine H1 receptor binding potential measured with PET under a test dose of olopatadine, an antihistamine, is reduced after repeated administration of olopatadine. J. Nucl. Med. 2009, 50, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Novák, Z.; Yáñez, A.; Kiss, I.; Kuna, P.; Tortajada-Girbés, M.; Valiente, R. Safety and tolerability of bilastine 10 mg administered for 12 weeks in children with allergic diseases. Pediatr. Allergy Immunol. 2016, 27, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Zuberbier, T.; Aberer, W.; Asero, R.; Bindslev-Jensen, C.; Brzoza, Z.; Canonica, G.W.; Church, M.K.; Ensina, L.F.; Giménez-Arnau, A.; Godse, K.; et al. The EAACI/GA2 LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management of urticaria: The 2013 revision and update. Allergy 2014, 69, 868–887. [Google Scholar] [CrossRef] [PubMed]
- Krause, K.; Spohr, A.; Zuberbier, T.; Church, M.K.; Maurer, M. Up-dosing with bilastine results in improved effectiveness in cold contact urticaria. Allergy 2013, 68, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Church, M.K. Safety and efficacy of bilastine: A new H1-antihistamine for the treatment of allergic rhinoconjunctivitis and urticaria. Expert Opin. Drug Saf. 2011, 10, 779–793. [Google Scholar] [CrossRef] [PubMed]
- Antonijoan, R.; Coimbra, J.; García-Gea, C.; Puntes, M.; Gich, I.; Campo, C.; Valiente, R.; Labeaga, L. Comparative efficacy of bilastine, desloratadine and rupatadine in the suppression of wheal and flare response induced by intradermal histamine in healthy volunteers. Curr. Med. Res. Opin. 2017, 33, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Jutel, M.; Watanabe, T.; Klunker, S.; Akdis, M.; Thomet, O.A.; Malolepszy, J.; Zak-Nejmark, T.; Koga, R.; Kobayashi, T.; Blaser, K.; et al. Histamine regulates T-cell and antibody responses by differential expression of H1 and H2 receptors. Nature 2001, 413, 420–425. [Google Scholar] [CrossRef]
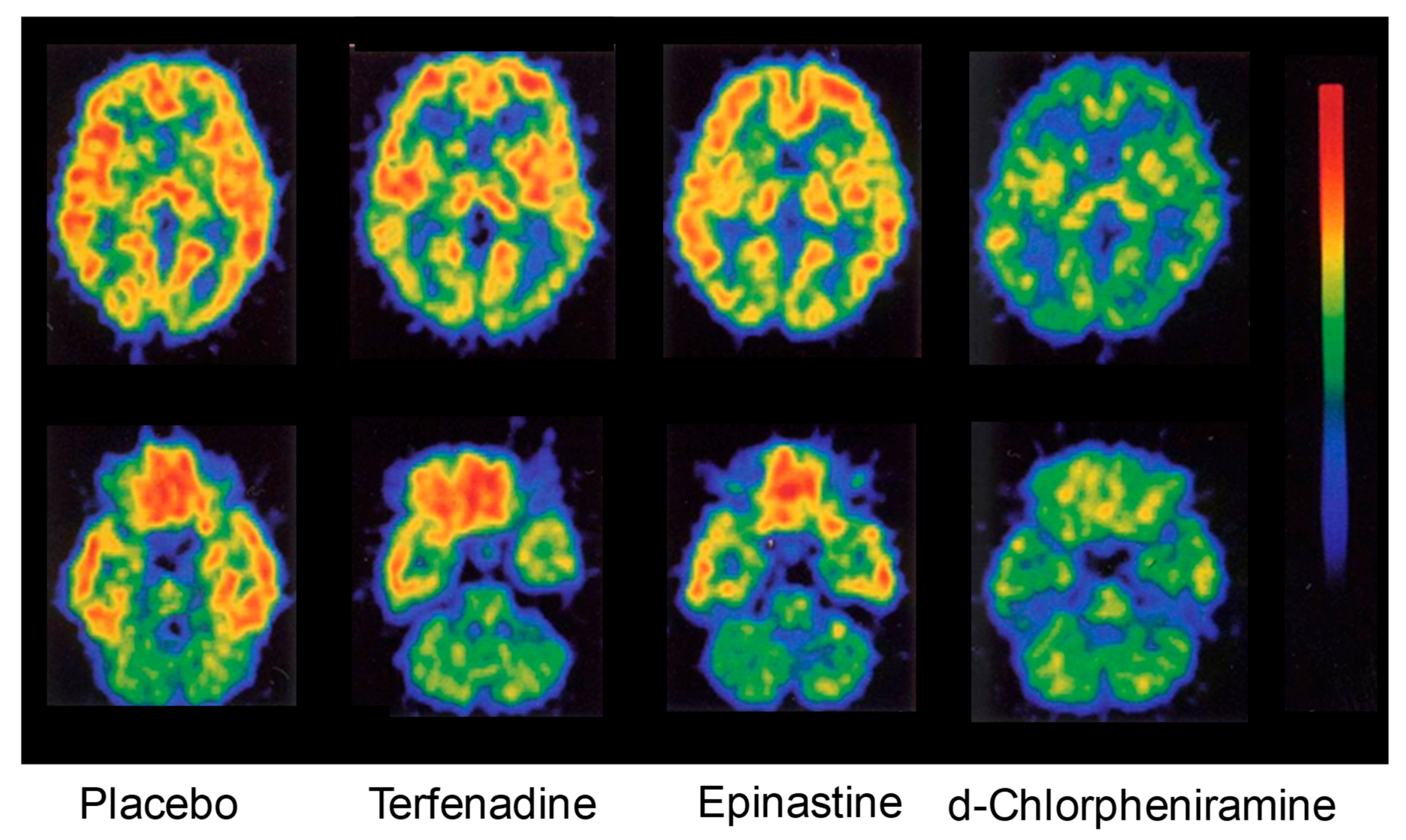
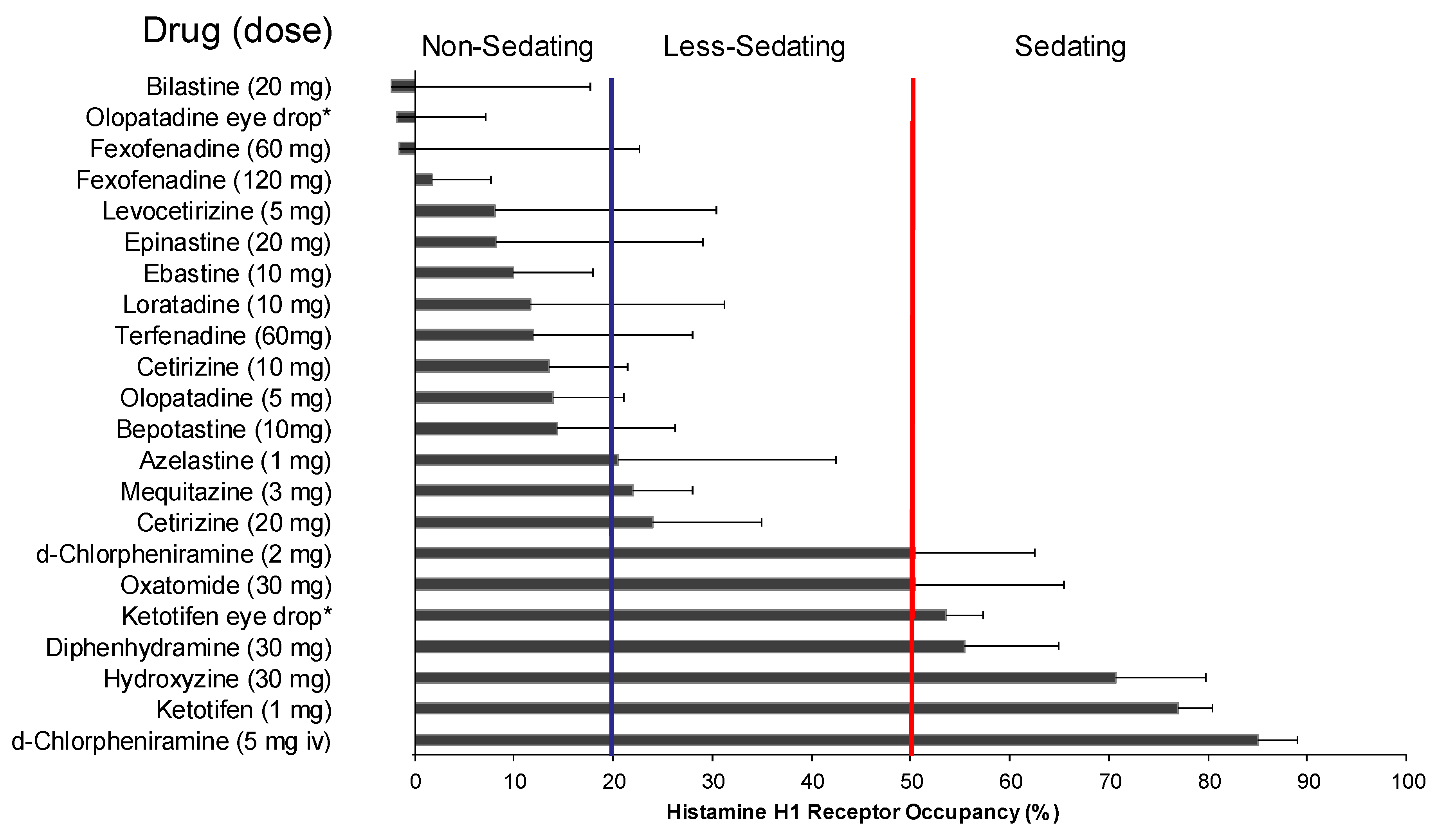
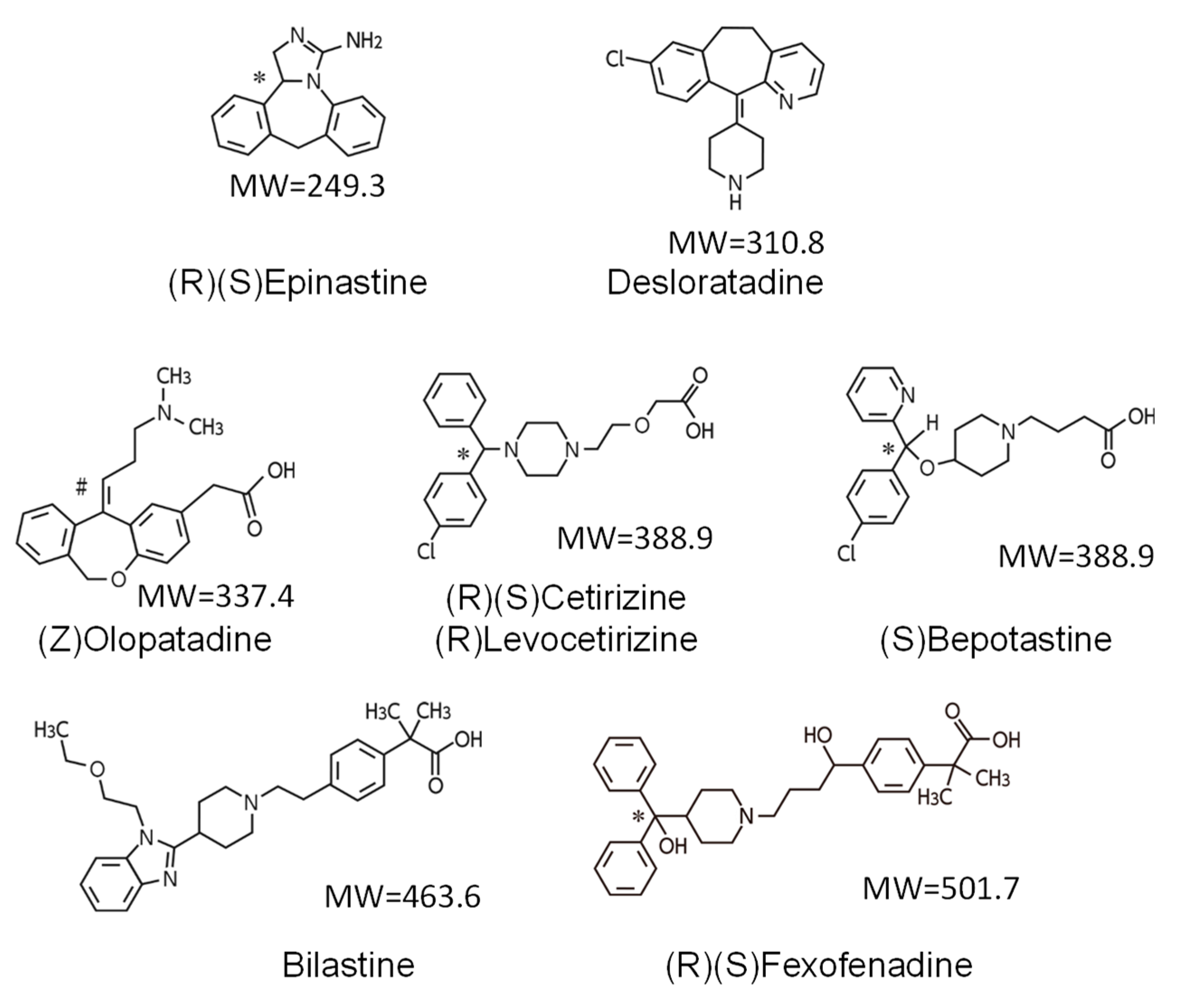
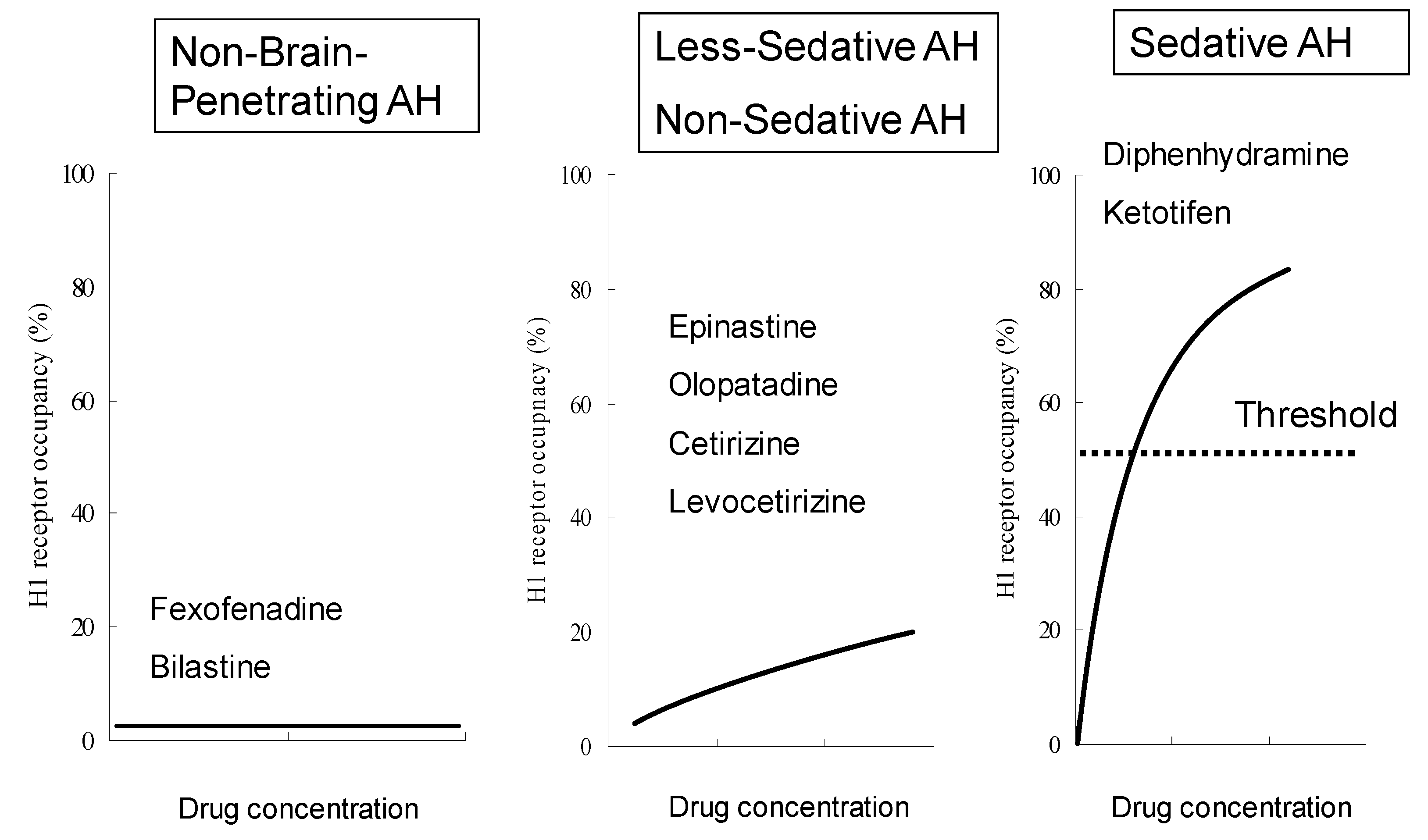
| Characteristic | Bilastine | Fexofenadine | Cetirizine | Levocetirizine | Loratadine | Desloratadine | Ebastine |
|---|---|---|---|---|---|---|---|
| H1 receptor selectivity | +++ | + | + | ++ | + | ++ | ++ |
| Affinity for H2/3 receptors | ± | ± | ± | ± | ± | ± | + |
| Metabolism | Not metabolized | ± | ± | ++ | +++ | +++ | +++ |
| tmax (h) | 1.3 | 1–3 | 1.0 | 0.9 | 1.0–1.5 | 3.0 | 2.6–4.0 (carebastine metabolite) |
| t1/2 (h) | 14.5 | 11–15 | 10.0 | 7.9 | 8.4 | 27.0 | 15–19 (carebastine metabolite) |
| Indicated for allergic rhinoconjunctivitis? | Yes | No | Yes/No (some but not all formulations) | No | No | No | No |
| Indicated for allergic rhinitis? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Indicated for urticaria? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Pediatric indication? | No (ongoing studies) | children > 3 years | children 6–12 years | children > 2 years | children > 2 years | children > 1 year | children > 2 years |
| Dosage adjustment in renal impairment? † | No | No | Yes (in moderate to severe) | Yes (in moderate-to-severe) | Yes | Caution (severe impairment) | Caution |
| Dosage adjustment in hepatic impairment? | No | No | Yes (if concomitant renal dysfunction) | Yes (if concomitant renal dysfunction) | Yes (severe disease) | Not mentioned | Caution (in mild to moderate) |
| Dosage adjustment in elderly? | No | No | No (if renal function OK) | Yes (for concomitant moderate-to-severe renal impairment) | No | Not mentioned | No |
| Interaction with food? | Yes (give on empty stomach) | Not mentioned | No | No | No | No | No |
| Use in pregnancy and lactation? | Caution (very limited data) | No | Caution | Caution | No | No | No |
| Clinically relevant drug interactions? | No | Yes (antacids) | No | Unlikely (no available data) | Potential (with inhibitors of CYP3A4 and CYP2D6) | No | Caution |
| Interaction with alcohol? | No | Not mentioned | Caution | Caution | No | No | No |
| Can patients drive and operate machinery (i.e., lack of sedative potential)? | Yes (caution: drowsiness) | Yes (impairment unlikely) | Yes (check drug response when intending to drive) | Yes (check drug response when intending to drive) | Yes (caution: drowsiness) | Yes (caution: drowsiness) | Yes (caution: somnolence) |
| Contraindications | None | None | Severe renal impairment | Severe renal impairment | None | None | Severe hepatic impairment |
| Number of ARIA recommended antihistamine properties ‡ | 10 | 9.5 | 6 | 6.5 | 6.5 | 6.5 | 6.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawauchi, H.; Yanai, K.; Wang, D.-Y.; Itahashi, K.; Okubo, K. Antihistamines for Allergic Rhinitis Treatment from the Viewpoint of Nonsedative Properties. Int. J. Mol. Sci. 2019, 20, 213. https://doi.org/10.3390/ijms20010213
Kawauchi H, Yanai K, Wang D-Y, Itahashi K, Okubo K. Antihistamines for Allergic Rhinitis Treatment from the Viewpoint of Nonsedative Properties. International Journal of Molecular Sciences. 2019; 20(1):213. https://doi.org/10.3390/ijms20010213
Chicago/Turabian StyleKawauchi, Hideyuki, Kazuhiko Yanai, De-Yun Wang, Koju Itahashi, and Kimihiro Okubo. 2019. "Antihistamines for Allergic Rhinitis Treatment from the Viewpoint of Nonsedative Properties" International Journal of Molecular Sciences 20, no. 1: 213. https://doi.org/10.3390/ijms20010213
APA StyleKawauchi, H., Yanai, K., Wang, D.-Y., Itahashi, K., & Okubo, K. (2019). Antihistamines for Allergic Rhinitis Treatment from the Viewpoint of Nonsedative Properties. International Journal of Molecular Sciences, 20(1), 213. https://doi.org/10.3390/ijms20010213







