Research Advances and Prospects on Mechanism of Sinomenin on Histamine Release and the Binding to Histamine Receptors
Abstract
1. Introduction
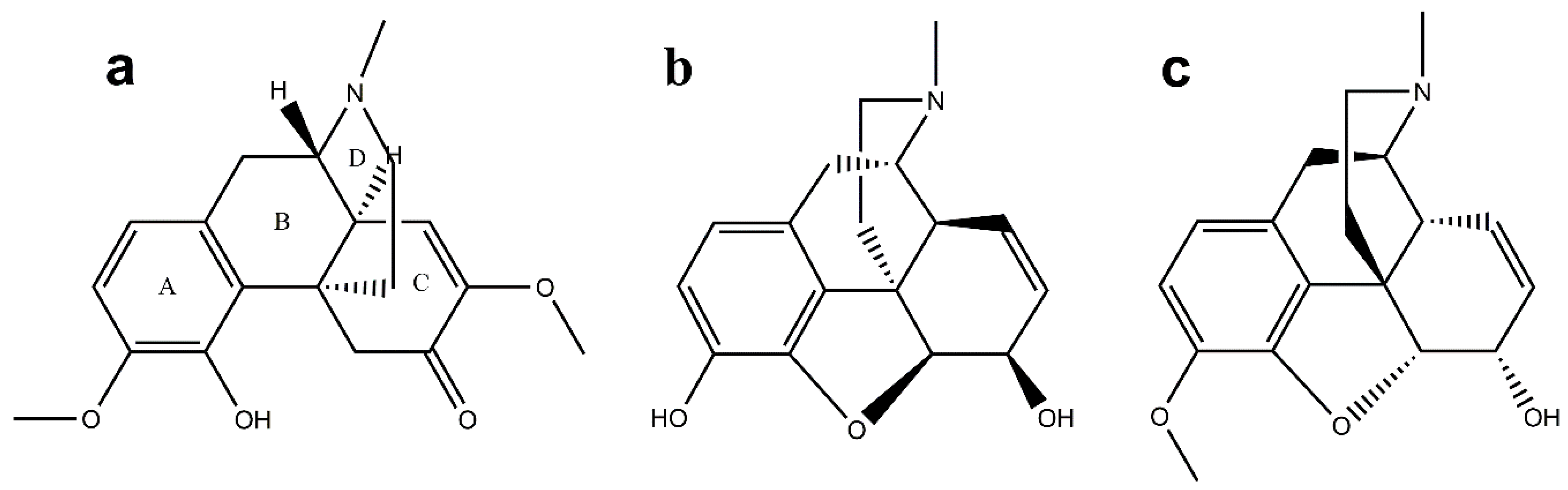
Chemical Structure of SIN
2. Pharmacological Effects of SIN
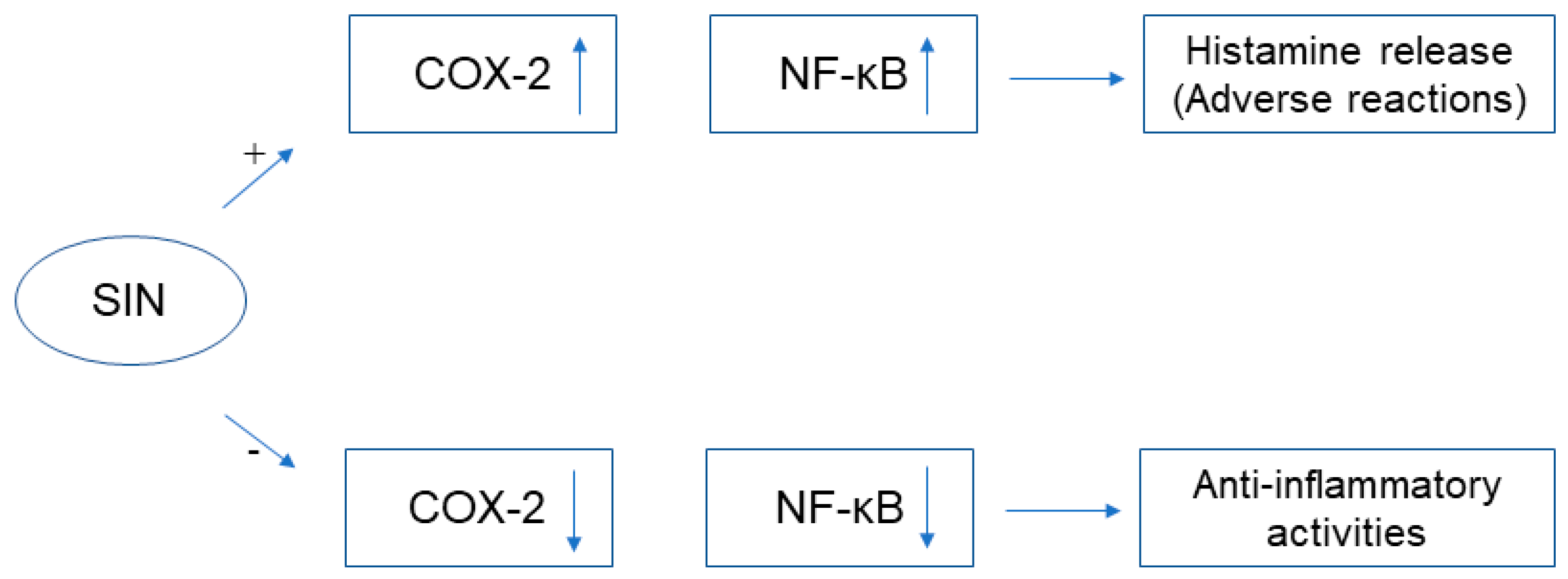
2.1. Immunosuppressive and Anti-Inflammatory Activities
2.2. Analgesic and Sedative Effects
2.3. Anti-Tumor Effects
2.4. Other Pharmacological Effects
3. Clinical Application and Adverse Reactions of SIN
3.1. Clinical Application
3.2. Adverse Reactions
4. Mechanism of Action of SIN and Its Relationship with Histamine/Histamine Receptor
4.1. The Mechanism of Histamine Release in Adverse Reactions of SIN
4.2. The Mechanism of Histamine and Its Receptors in Pharmacological Effects of SIN
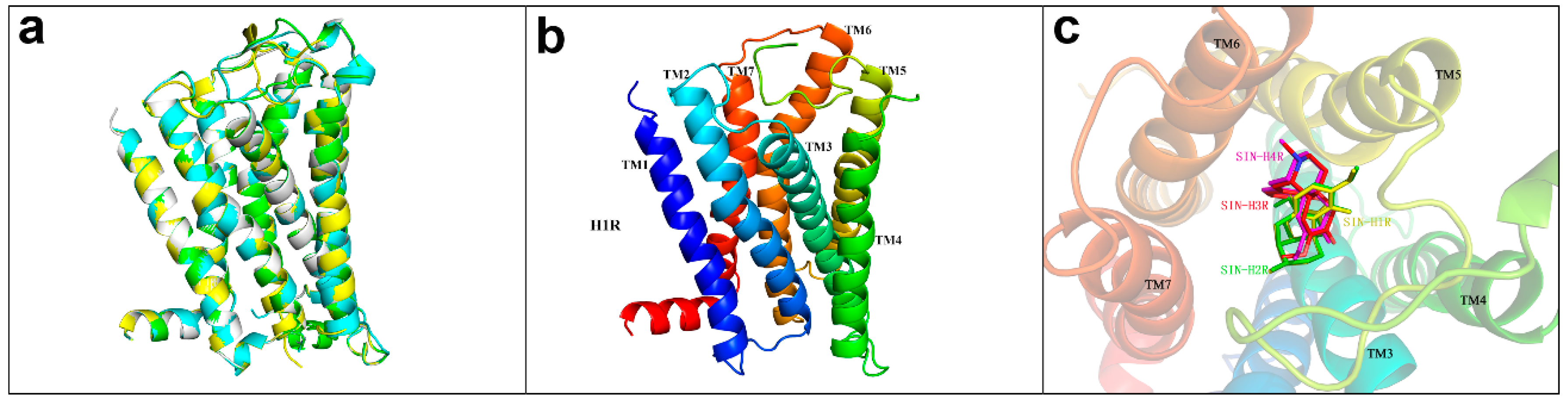
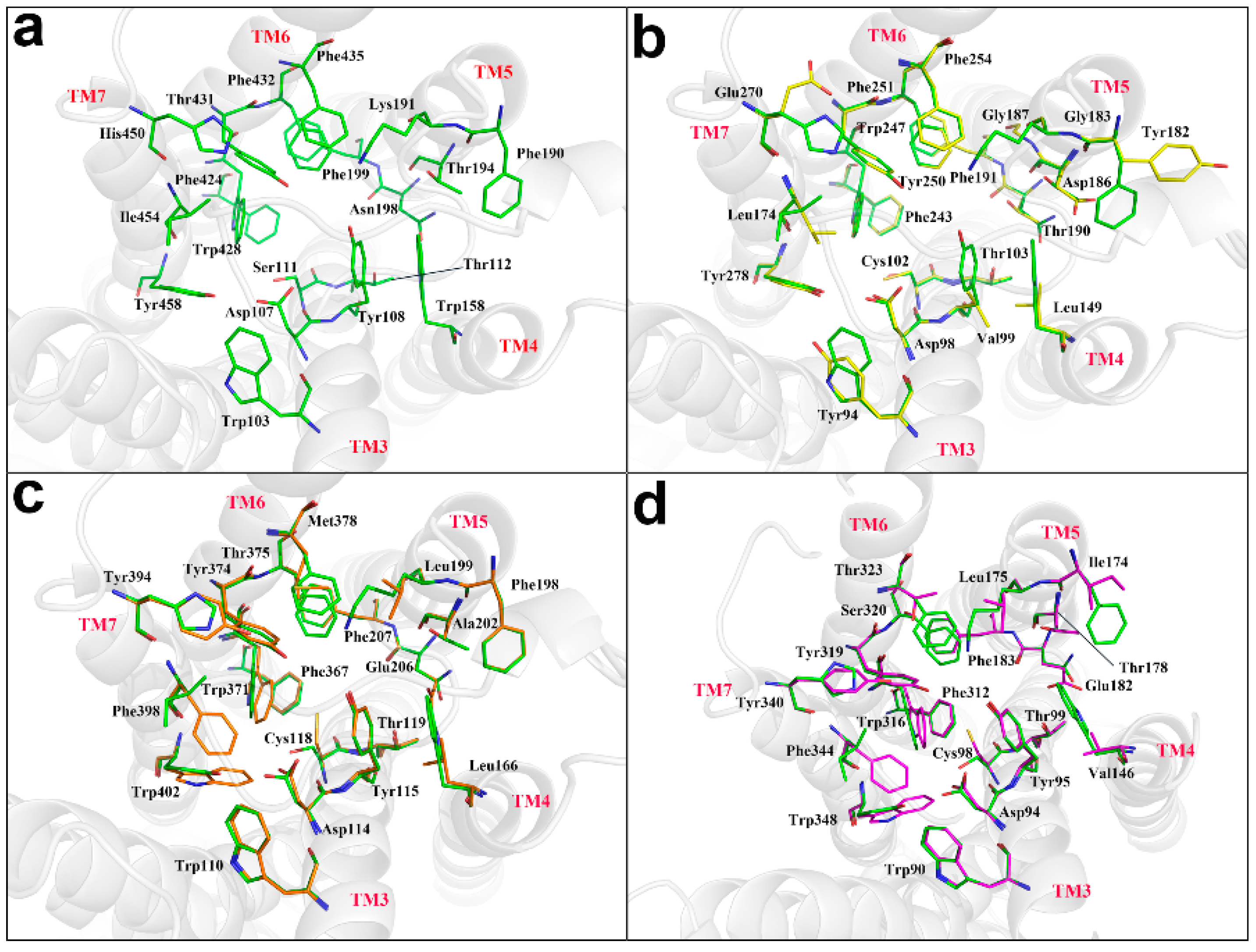
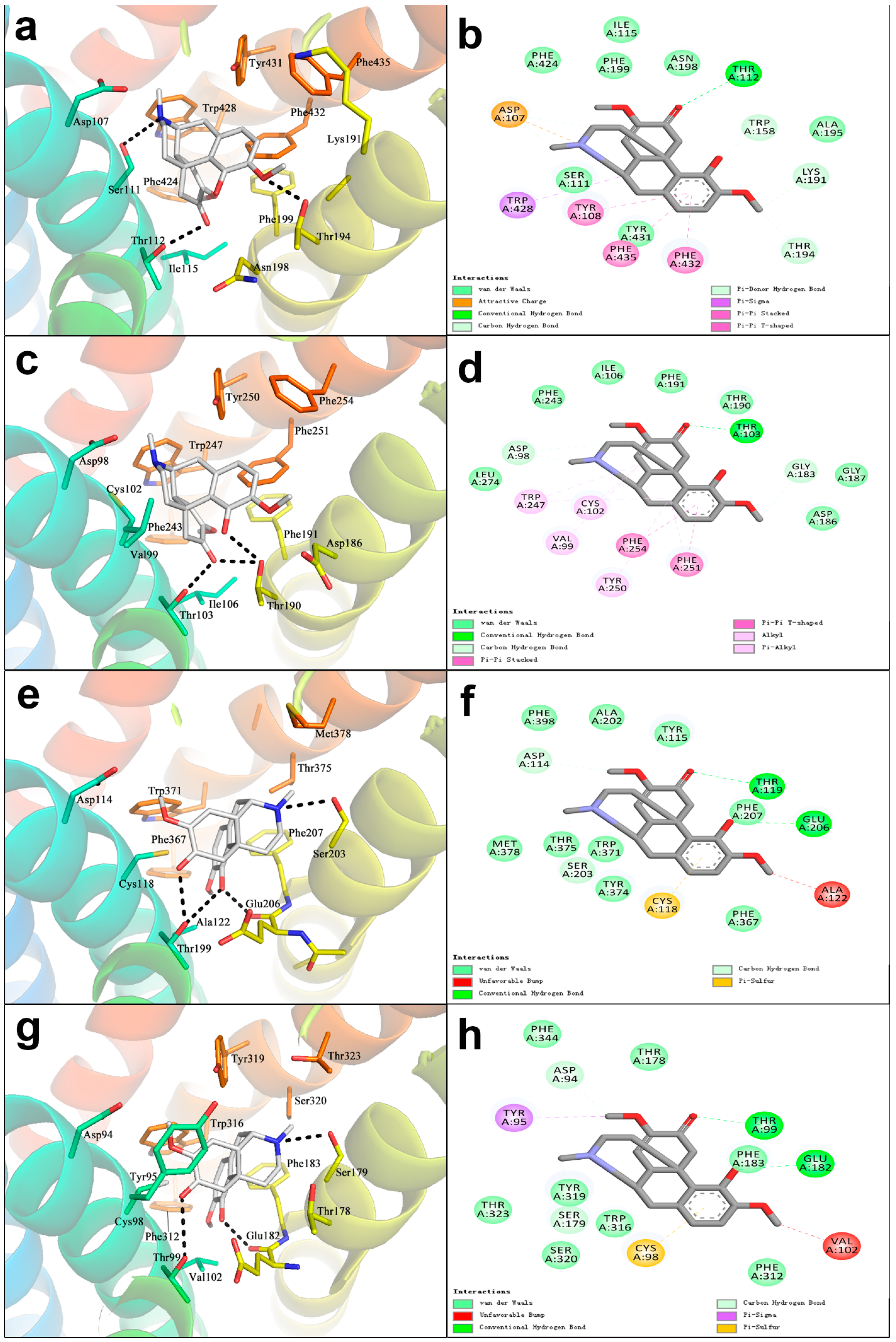
4.3. The Docking of SIN with Histamine Receptors H1R, H2R, H3R, H4R
5. Prospects
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SIN | Sinomenine |
| RA | rheumatic diseases |
| TCM | traditional Chinese Medicine |
| DC | dendritic cells |
| MO | macrophages |
| PGE2 | prostaglandin E2 |
| COX-2 | cyclooxygenase-2 |
| MAPKs | mitogen-activated protein kinases |
| ICAM-1 | intercellular adhesion molecule-1 |
| DSS | dextran sulfate sodium salt |
| GABAA | γ-aminobutyric acid type A |
| CPP | Conditioned Place Preference |
| SHh | Sonic hedgehog |
| LVET | left ventricular ejection time |
| ZQFTN | Zhengqing Fengtongning |
| SHI | Sinomenine Hydrochloride Injection |
| CNKI | China National Knowledge Infrastructure |
| RCT | randomized controlled trial |
| MTX | methotrexate |
| KOA | knee osteoarthritis |
| AS | ankylosing spondylitis |
| GPCR | G-protein coupled receptor |
| ERK | signal-regulated kinase |
| ANXA1 | Annexin A1 |
| cPLA2 | cytosolic phospholipase A2 |
| RBL-2H3 | rat basophilic leukemia 2H3 |
| IP3 | inositol-1,4,5-trisphosphate |
| MRGPRX2 | Mas-related G protein-coupled receptor X2 |
References
- Liu, L.; Resch, K.; Kaever, V. Inhibition of lymphocyte proliferation by the anti-arthritic drug sinomenine. Int. J. Immunopharmacol. 1994, 16, 685–691. [Google Scholar] [CrossRef]
- Yamasaki, H. Pharmacology of sinomenine, an anti-rheumatic alkaloid from Sinomenium acutum. Acta Med. Okayama 1976, 30, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Buchner, E.; Beitze, D.; Schmidt-Weber, C.B.; Kaever, V.; Emmrich, F.; Kinne, R. Amelioration of rat experimental arthritides by treatment with the alkaloid sinomenine. Int. J. Immunopharmacol. 1996, 18, 529–543. [Google Scholar] [CrossRef]
- Qin, F.; Cai, H. Research progress on pharmacological effects of sinomenine. Chin. Med. J. Res. Pract. 2016, 30, 81–86. [Google Scholar] [CrossRef]
- Zhu, S.L.; Chen, D.Z.; Li, Y.; Lin, H.W.; Duan, Y.G. Progress in the study of Sinomenine. J. Jishou Univ. (Nat. Sci. Ed.) 2011, 32, 95–100. [Google Scholar] [CrossRef]
- Chen, W.Y.; Qin, C.H.; Yin, X.G. Progress in anti-tumor effects of sinomenine. China Pharm. 2013, 16, 1902–1904. [Google Scholar] [CrossRef]
- Sun, Y.H.; Zhu, Q.; Li, J.X. Research progress on anti-inflammatory and anti-tumor effects of sinomenine. Chin. Pharmacol. Bull. 2015, 31, 1040–1043. [Google Scholar] [CrossRef]
- Jiang, Y.M.; Wang, D.Q. Research progress on antitumor mechanisms of sinomenine. Drugs Clin. 2016, 31, 1866–1870. [Google Scholar] [CrossRef]
- Li, L.; Zhang, C.L.; Song, B.W. Pharmacological research and clinical application of sinomenine. Tradit. Chin. Drug Res. Clin. Pharmacol. 2006, 17, 310–313. [Google Scholar] [CrossRef]
- Zhao, Z.J. Advances in the study of structural modification of sinomenine. J. Huaihua Univ. 2016, 35, 44–55. [Google Scholar] [CrossRef]
- Liu, Z.H.; Huang, Y.L.; Jiang, F.; Lu, Q. Research progress on synthesis and structure-activity relationship of sinomenine. Guangzhou Chem. Ind. 2017, 45, 48–52. [Google Scholar]
- Ye, X.R.; Yan, K.X.; Wu, K.M.; Feng, X.Z.; Huang, Y.M.; Qiu, P. Synthesis and anti-inflammatory analgesic activities of sinomenine derivatives. Acta. Pharm. Sin. 2004, 39, 180–183. [Google Scholar] [CrossRef]
- Chai, X.Y.; Guan, Z.J.; Yu, S.C.; Zhao, Q.J.; Hu, H.G.; Zou, Y.; Tao, X.; Wu, Q.-Y. Design, synthesis and molecular docking studies of sinomenine derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 5849–5852. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.L.; Liu, H.Q.; Chen, Y.Q.; Li, N.G.; Li, W. Recent advance of sinomenine derivatives. Prog. Mod. Biomed. 2016, 16, 1167–1171. [Google Scholar] [CrossRef]
- Li, X.Z.; Zhao, Q.J.; Dong, J.X.; Jiang, Y.Y.; Ye, G.M. Research progress in sinomenine structural modification. J. Pharm. Pract. 2018, 36, 204–209, 214. [Google Scholar] [CrossRef]
- Tang, J.; Raza, A.; Chen, J.; Xu, H.X. A systematic review on the sinomenine derivatives. Mini-Rev. Med. Chem. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Nerurkar, L.; Siebert, S.; McInnes, I.B.; Cavanagh, J. Rheumatoid arthritis and depression: An inflammatory perspective. Lancet Psychiatry 2018. [Google Scholar] [CrossRef]
- Cai, H.; Yao, R.B. Advances in mechanisms of sinomenine for rheumatoid Arthritis. Guid. J. Tradit. Chin. Med. Pharm. 2015, 21, 94–96. [Google Scholar] [CrossRef]
- Wang, H.Y.; Song, C.H.; Liu, X.P.; Qian, T.L.; Zhu, Y.L.; Hou, X.J. Application of sinomenine in rheumatic diseases. TMR Integr. Med. 2018, 2, 54–61. [Google Scholar] [CrossRef]
- Wang, Q.X.; Li, X.K. Immunosuppressive and anti-inflammatory activities of sinomenine. Int. Immunopharmacol. 2011, 11, 373–376. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.; Yu, K.Q.; Liu, Y.; Chen, X.G. Sinomenine inhibits maturation of monocyte-derived dendritic cells through blocking activation of NF-kappa B. Int. Immunopharmacol. 2007, 7, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.B.; Yin, P.H.; Shi, Z.; Ma, Y.C.; Zhao, C.G.; Zheng, J.; Chen, T. Sinomenine, a COX-2 inhibitor, induces cell cycle arrest and inhibits growth of human colon carcinoma cells in vitro and in vivo. Oncol. Lett. 2016, 11, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.Y.; He, K.W.; Zhu, H.D. Chinese herbal medicinal ingredients affect secretion of NO, IL-10, ICAM-1 and IL-2 by endothelial cells. Immunopharmacol. Immunot. 2015, 37, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.F.; Tian, L.; Zhao, Z.H.; Chen, S.P.; Zhao, Q.Y.; Hong, J.B.; Xie, Y.; Zhou, N.J.; Fu, Y.J. The sinomenine enteric-coated microspheres suppressed the Tlr/nf-κb signaling in Dss-induced experimental colitis. Int. Immunopharmacol. 2017, 50, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Lodge, D.; Headley, P.M.; Duggan, A.W.; Biscoe, T. The effects of morphine, etorphine and sinomenine on the chemical sensitivity and synaptic responses of Renshaw cells and other spinal neurones in the rat. Eur. J. Pharm. 1974, 26, 277–284. [Google Scholar] [CrossRef]
- Lee, J.Y.; Yoon, S.Y.; Won, J.; Kim, H.B.; Kang, Y.; Oh, S.B. Sinomenine produces peripheral analgesic effects via inhibition of voltage-gated sodium currents. Neuroscience 2017, 358, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Sun, Y.H.; Zhu, J.; Fang, T.; Zhang, W.; Li, J.X. Antinociceptive effects of sinomenine in a rat model of neuropathic pain. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.T.; Su, M.; Ling, Y.; Wei, Q.Q.; Pan, F.; Li, J.J.; Li, J.X.; Zhu, Q. Anti-allodynic effects of N-demethylsinomenine, an active metabolite of sinomenine, in a mouse model of postoperative pain. Eur. J. Pharm. 2018, 823, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Mo, Z.X.; Tu, H.H. Efect of sinomenine on morphine dependence in isolated guinea pig ileum. J. First. Mil. Med. Univ. 2003, 23, 329–331. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, J.F.; Yang, L.Q.; Yi, L.; Hu, B. Effects of sinomenine on NO/nNOS system in cerebellum and spinal cord of morphinedependent and withdrawal mice. Acta Physiol. Sin. 2007, 59, 285–292. [Google Scholar] [CrossRef]
- Chen, S.W.; Mi, X.J.; Wang, R.; Wang, W.J.; Kong, W.X.; Zhang, Y.J.; Li, Y.L. Behavioral effects of sinomenine in murine models of anxiety. Life Sci. 2005, 78, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Song, L.Q.; Liu, D.; Zhao, Y.; He, J.J.; Kang, H.F.; Dai, Z.J.; Wang, X.J.; Zhang, S.Q.; Zan, Y. Sinomenine inhibits breast cancer cell invasion and migration by suppressing NF-κB activation mediated by IL-4/miR-324-5p/CUEDC2 axis. Biochem. Biophys. Res. Commun. 2015, 464, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Song, L.Q.; Liu, D.; Zhao, Y.; He, J.J.; Kang, H.F.; Dai, Z.J.; Wang, X.J.; Zhang, S.Q.; Zan, Y.; Xue, X.H. Sinomenine reduces growth and metastasis of breast cancer cells and improves the survival of tumor-bearing mice through suppressing the SHh pathway. Biomed. Pharm. 2018, 98, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.P.; Luan, H.; Liu, Q.P.; Jiang, T.S.; Liang, H.Y.; Dong, X.H.; Shang, H. Activation of PI3K/Akt and ERK signaling pathways antagonized sinomenine-induced lung cancer cell apoptosis. Mol. Med. Rep. 2012, 5, 1256–1260. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Cen, G.D.; Gao, Y.X. Study on P13K/Akt signaling pathway of proliferation and apoptosis induced by sinomenine in Hela Cells. Heilongjiang J. Tradit. Chin. Med. 2010, 5, 43–44. [Google Scholar]
- Hong, Y.; Yang, J.; Shen, X.; Zhu, H.F.; Sun, X.X.; Wen, X.; Bian, J.; Hu, H.Y.; Yuan, L.; Tao, J. Sinomenine hydrochloride enhancement of the inhibitory effects of anti-transferrin receptor antibody-dependent on the COX-2 pathway in human hepatoma cells. Cancer Immunol. Immunother. 2013, 62, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Ma, Y.X.; Liang, L.; Zhang, P.; Feng, J. The pro-apoptosis effect of sinomenine in renal carcinoma via inducing autophagy through inactivating PI3K/AKT/mTOR pathway. Biomed. Pharm. 2018, 97, 1269–1274. [Google Scholar] [CrossRef]
- Zhao, X.X.; Peng, C.; Zhang, H.; Qin, L.P. Sinsomenium acutum: A review of chemistry, pharmacology, pharmacokinetics, and clinical use. Pharm. Biol. 2012, 50, 1053–1061. [Google Scholar] [CrossRef]
- Liu, J.B.; Chen, X.C.; Cao, Q.; Yang, Y.J.; Kang, J. Relationship between plasma concentration of sinomenine hydrochloride and its effects on systolic time intervals. J. Xi’an Med Univ. 1998, 19, 391–394. [Google Scholar]
- Li, L. Effects of sinomenine on proliferation of vascular smooth muscle cells. J. Xi’an Med Univ. 2000, 21, 205–210. [Google Scholar] [CrossRef]
- Zhao, Z.Q.; Guan, R.; Song, S.H.; Zhang, M.J.; Liu, F.; Guo, M.; Guo, W.Y.; Yu, Q.L.; Zhang, L.D.; Wang, Q.X. Sinomenine protects mice against ischemia reperfusion induced renal injury by attenuating inflammatory response and tubular cell apoptosis. Int. J. Clin. Exp. Pathol. 2013, 6, 1702. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.L. Clinical analysis of Zhengqing Fengtongning tablet in treating 80 cases of rheumatoid arthritis. Pharm. Res. 2013, 10, 83. [Google Scholar] [CrossRef]
- Liu, W.W.; Qian, X.; Ji, W.; Lu, Y.; Wei, G.; Wang, Y. Effects and safety of Sinomenine in treatment of rheumatoid arthritis contrast to methotrexate: A systematic review and Meta-analysis. J. Tradit. Chin. Med. 2016, 36, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.S.; Liu, C.X.; Zhang, J.H.; Qin, Y.S.; Zheng, W.K.; Wan, Y.Y. Systematic evaluation and meta-analysis of randomized controlled clinical trials of Zhengqing Fengtongning preparation in the treatment of knee osteoarthritis. Lishizhen Med. Mater. Med. Res. 2018, 29, 1479–1482. [Google Scholar] [CrossRef]
- Liu, M.H.; Long, X.Y. Clinical observation on 67 cases of ankylosing spondylitis treated by Zhengqing Fengtongning. Tradit. Chin. Drug Res. Clin. Pharmacol. 2002, 13, 147–148. [Google Scholar] [CrossRef]
- Liang, S.H. Efficacy and Safety of Traditional Chinese Medicine Therapy for Ankylosing Spondylitis: A Meta-Analysis and Analysis of Drugs. Ph.D. Thesis, Southern Medical University, Guangzhou, China, 2013. [Google Scholar]
- Zhou, J.; Chen, Q.K.; Xu, J.H.; Luo, L.M. Clinical observation of Sinomenium acutum preparation in the treatment of glomerular diseases. Chin. J. Integr. Tradit. West. Nephrol. 2003, 3, 160–162. [Google Scholar] [CrossRef]
- Sun, J.P.; Gao, Y.X.; Dong, H. Clinical observation on treatment of glomerulonephritis with sinomenine preparation: A report of 28 cases. Clin. Focus 2007, 22, 1194–1195. [Google Scholar] [CrossRef]
- Wu, Z.Z.; Long, L.P.; Chen, X.; Chen, G.J. Analysis of 193 cases of adverse drug reactions induced by Zhengqingfengtongning sustained release tablets. Chin. J. Pharm. 2016, 13, 489–491,495. [Google Scholar]
- Chen, J.R.; Zhong, B.; Wang, Y. Agranulocytosis induced by sinomenine hydrochloride. Am. J. Case Rep. 2017, 18, 959–962. [Google Scholar] [CrossRef]
- Mayeda, H. The release of histamine by sinomenine. Jpn. J. Pharm. 1953, 3, 62–72. [Google Scholar] [CrossRef]
- Huang, L.F.; Pi, J.; Wu, J.L.; Zhou, H.; Cai, J.Y.; Li, T.; Liu, L. A rapid and sensitive assay based on particle analysis for cell degranulation detection in basophils and mast cells. Pharmacol. Res. 2016, 111, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Akdis, C.A.; Simons, F.E.R. Histamine receptors are hot in immunopharmacology. Eur. J. Pharm. 2006, 533, 69–76. [Google Scholar] [CrossRef] [PubMed]
- O’mahony, L.; Akdis, M.; Akdis, C.A. Regulation of the immune response and inflammation by histamine and histamine receptors. J. Allergy Clin. Immunol. 2011, 128, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.Q.; Wu, X.; Tan, Y.H. Progress in histamine and histamine receptors. Chin. J. Lung Dis. 2015, 8, 234–237. [Google Scholar] [CrossRef]
- Huang, L.F.; Li, T.; Zhou, H.; Qiu, P.; Wu, J.L.; Liu, L. Sinomenine potentiates degranulation of RBL-2H3 basophils via up-regulation of phospholipase A2 phosphorylation by Annexin A1 cleavage and ERK phosphorylation without influencing on calcium mobilization. Int. Immunopharmacol. 2015, 28, 945–951. [Google Scholar] [CrossRef]
- Wang, N.; Liu, R.; Liu, Y.P.; Zhang, R.R.; He, L.C. Sinomenine potentiates P815 cell degranulation via upregulation of Ca2+ mobilization through the Lyn/PLCγ/IP3R pathway. Int. J. Immunopath. Pharmacol. 2016, 29, 676–683. [Google Scholar] [CrossRef]
- Liu, R.; Che, D.L.; Zhao, T.T.; Pundir, P.; Cao, J.; Lv, Y.N.; Wang, J.; Ma, P.Y.; Fu, J.; Wang, N.N.; et al. MRGPRX2 is essential for sinomenine hydrochloride induced anaphylactoid reactions. Biochem. Pharmacol. 2017, 146, 214–223. [Google Scholar] [CrossRef]
- Huang, L.F.; Dong, Y.; Wu, J.L.; Wang, P.X.; Zhou, H.; Li, T.; Liu, L. Sinomenine-induced histamine release-like anaphylactoid reactions are blocked by tranilast via inhibiting NF-κB signaling. Pharmacol. Res. 2017, 125, 150–160. [Google Scholar] [CrossRef]
- Wang, W.J.; Li, H.; Du, Q.; Wang, P.X.; Liang, R.Y. Liang, Effects of sinomenine on proliferation and degranulation in RBL-2H3 mast cells. Immunol. J. 2009, 25, 261–263. [Google Scholar]
- Zhang, M.F. Pharmacological effects of sinomenine. Shanxi Med J. 1981, 10, 57–60. [Google Scholar]
- Mo, Z.X.; Liang, R.N.; Wang, C.Y. Effects of caulis ainomenii and sinomenine on morphine-induced place preference and cyclic AMP level in mice. Chin. J. Mod. Appl. Pharm. 2004, 21, 87–90. [Google Scholar] [CrossRef]
- Mo, Z.X.; Liang, R.N.; Weng, J.L. Changes in cAMP and cGMP levels in neonatal rat histaminergic neurons of tuberomammillary nucleus following 48-hour morphine exposure and effects of sinomenine intervention. J. First Mil. Meal. Univ. 2005, 25, 1105–1108. [Google Scholar] [CrossRef]
- Mo, Z.X.; He, H.J.; Zhu, Z.H. A study on histamine release from mast cells induced by sinomenine and anti-histamine effect of sinomenine in isolated guinea pig ileumuijie. Pharmacol. Clin. Chin. Mater. Med. 2006, 22, 16–19. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Bienert, S.; Waterhouse, A.; de Beer, T.A.P.; Tauriello, G.; Studer, G.; Bordoli, L.; Schwede, T. The SWISS-MODEL Repository—New features and functionality. Nucleic Acids Res. 2016, 45, D313–D319. [Google Scholar] [CrossRef]
- Liao, F.; Yang, Z.R.; Lu, X.H.; Guo, X.F.; Dong, W.G. Sinomenine sensitizes gastric cancer cells to 5-fluorouracil in vitro and in vivo. Oncol. Lett. 2013, 6, 1604–1610. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Z.R.; Dong, W.G.; Zhang, J.X.; Guo, X.F.; Song, J.; Qiu, S. Cooperative inhibitory effect of sinomenine combined with 5-fluorouracil on esophageal carcinoma. World J. Gastroenterol. 2013, 19, 8292–8300. [Google Scholar] [CrossRef]
- Shi, R.D.; Tian, X.Z. The effect of sinomenine and cisplatin on cell proliferation of human cervical cancer cell line Hela. Chin. Remedies Clin. 2010, 10, 1117–1119. [Google Scholar] [CrossRef]
- Wu, M.; Li, X.G.; Zhang, Y.; Fang, C.Y.; Liu, J.J. The synergistic antiproliferative effect of sinomenine and carboplatin on cervical cancer Hela cells. J. Pract. Obstet. Gynecol. 2009, 25, 473–475. [Google Scholar] [CrossRef]
| Pharmacological Effects of SIN | References |
|---|---|
| Anti-inflammatory activities | [17,18,19,20,21,22,23,24] |
| Analgesic effects | [25,26,27,28] |
| Sedative effects | [29,30,31] |
| Anti-tumor effects | [32,33,34,35,36,37] |
| Antiarrhythmic effects | [38,39] |
| Other pharmacological effects | [40,41] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.-S.; Han, J.-Y.; Iqbal, O.; Liang, A.-H. Research Advances and Prospects on Mechanism of Sinomenin on Histamine Release and the Binding to Histamine Receptors. Int. J. Mol. Sci. 2019, 20, 70. https://doi.org/10.3390/ijms20010070
Zhang Y-S, Han J-Y, Iqbal O, Liang A-H. Research Advances and Prospects on Mechanism of Sinomenin on Histamine Release and the Binding to Histamine Receptors. International Journal of Molecular Sciences. 2019; 20(1):70. https://doi.org/10.3390/ijms20010070
Chicago/Turabian StyleZhang, Yu-Shi, Jia-Yin Han, Omer Iqbal, and Ai-Hua Liang. 2019. "Research Advances and Prospects on Mechanism of Sinomenin on Histamine Release and the Binding to Histamine Receptors" International Journal of Molecular Sciences 20, no. 1: 70. https://doi.org/10.3390/ijms20010070
APA StyleZhang, Y.-S., Han, J.-Y., Iqbal, O., & Liang, A.-H. (2019). Research Advances and Prospects on Mechanism of Sinomenin on Histamine Release and the Binding to Histamine Receptors. International Journal of Molecular Sciences, 20(1), 70. https://doi.org/10.3390/ijms20010070





