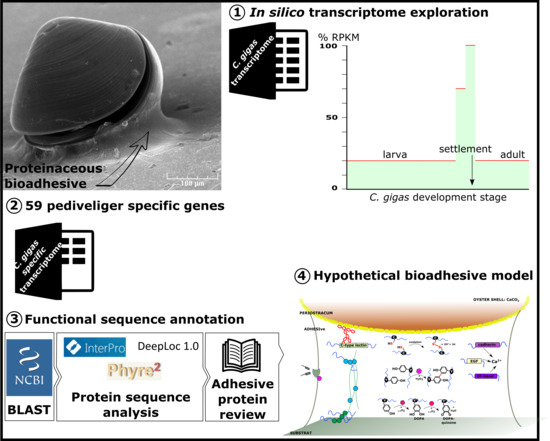
In Silico Analysis of Pacific Oyster (Crassostrea gigas) Transcriptome over Developmental Stages Reveals Candidate Genes for Larval Settlement
Abstract
1. Introduction
2. Results
3. Discussion
3.1. Sequences Involved in Reduction-Oxidation Reactions (Redox)
3.2. Proteases and Enzyme Inhibitors
3.3. Sequences Related to the Extracellular Matrix
3.4. Calcifying Sequences
3.5. Hypothetical Sequences
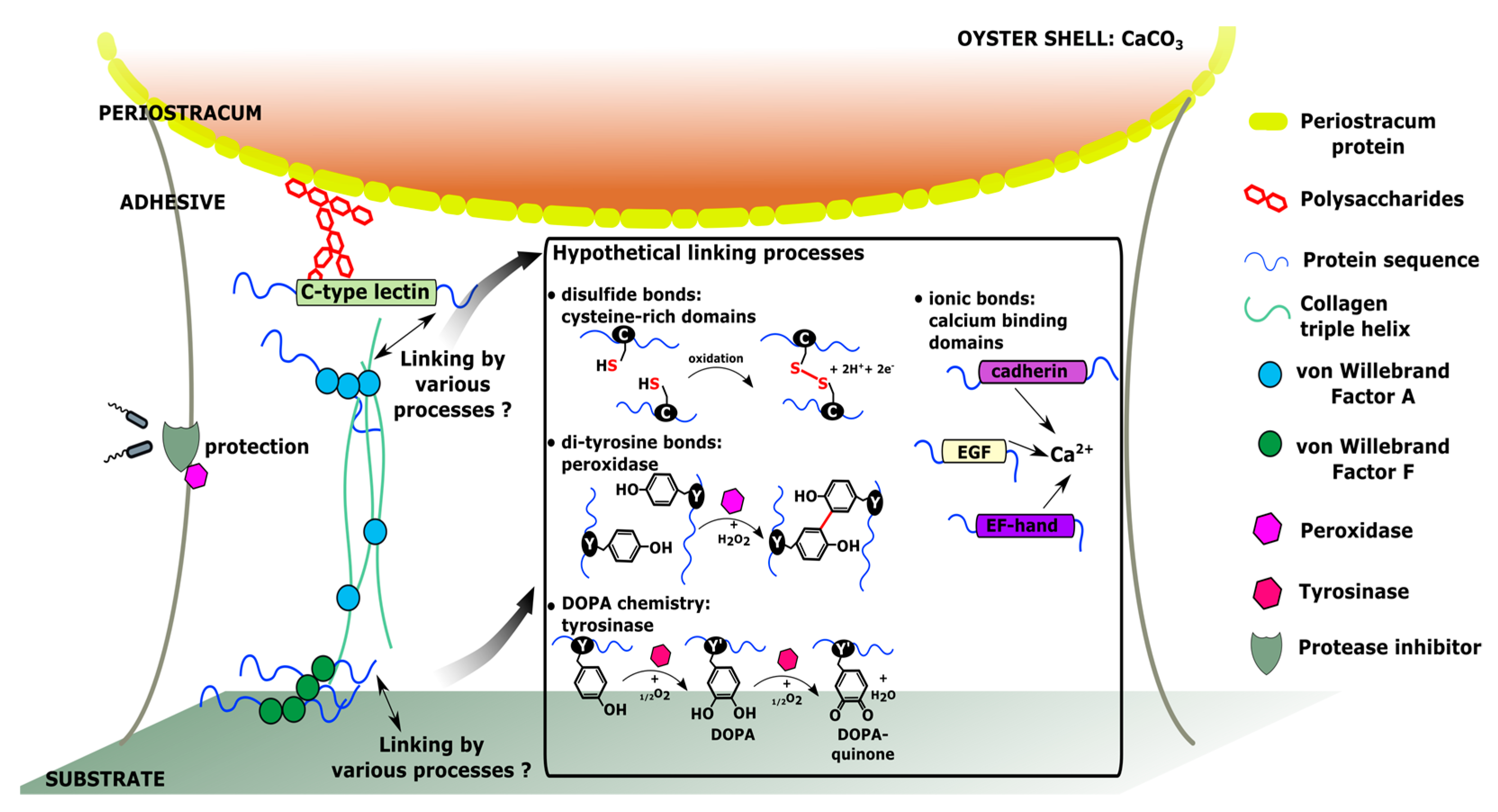
4. Hypothetical Model of Molecular Interactions within C. gigas Adhesive
5. Materials and Methods
- RPKM of stages E to U6, stages S and J, and adult organs less than 20% that of P1 or P2.
- RPKM of stages LU1, LU2 below 70% that of P1 or P2.
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hennebert, E.; Maldonado, B.; Ladurner, P.; Flammang, P.; Santos, R. Experimental strategies for the identification and characterization of adhesive proteins in animals: A review. Interface Focus 2014, 5, 20140064. [Google Scholar] [CrossRef] [PubMed]
- North, M.A.; Del Grosso, C.A.; Wilker, J.J. High strength underwater bonding with polymer mimics of mussel adhesive proteins. ACS Appl. Mater. Interfaces 2017, 9, 7866–7872. [Google Scholar] [CrossRef] [PubMed]
- Foster, L.J.R. Bioadhesives as surgical sealants: A Review. Bioadhesive. Biomim. Nat. Appl. 2015, 203–234. [Google Scholar]
- Foulon, V.; Artigaud, S.; Buscaglia, M.; Bernay, B.; Fabioux, C.; Petton, B.; Elies, P.; Boukerma, K.; Hellio, C.; Guérard, F.; et al. Proteinaceous secretion of bioadhesive produced during crawling and settlement of Crassostrea gigas larvae. Sci. Rep. 2018, 8, 15298. [Google Scholar] [CrossRef] [PubMed]
- Petrone, L.; Ragg, N.L.C.; McQuillan, A.J. In situ infrared spectroscopic investigation of Perna canaliculus mussel larvae primary settlement. Biofouling 2008, 24, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Petrone, L.; Ragg, N.L.C.; Girvan, L.; McQuillan, J.A. Scanning electron microscopy and energy dispersive X-Ray microanalysis of Perna canaliculus mussel larvae adhesive secretion. J. Adhes. 2009, 85, 78–96. [Google Scholar] [CrossRef]
- Cranfield, H.J. The ultrastructure and histochemistry of the larval cement of Ostrea edulis L. J. Mar. Biol. Assoc. UK 1975, 55, 497–503. [Google Scholar] [CrossRef]
- Gruffydd, L.D.; Lane, D.J.W.; Beaumont, A.R. The glands of the larval foot in Pecten maximus L. and possible homologues in other bivalves. J. Mar. Biol. Assoc. UK 1975, 55, 463–476. [Google Scholar] [CrossRef]
- Tibabuzo Perdomo, A.M.; Alberts, E.M.; Taylor, S.D.; Sherman, D.M.; Huang, C.-P.; Wilker, J.J. Changes in cementation of reef building oysters transitioning from larvae to adults. ACS Appl. Mater. Interfaces 2018, 10, 14248–14253. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.; Nott, J. A study of the morphology, fine structure and histochemistry of the foot of the pediveliger of Mytilus edulis L. J. Mar. Biol. Assoc. UK 1975, 55, 477–495. [Google Scholar] [CrossRef]
- Rodrigues, M.; Ostermann, T.; Kremeser, L.; Lindner, H.; Beisel, C.; Berezikov, E.; Hobmayer, B.; Ladurner, P. Profiling of adhesive-related genes in the freshwater cnidarian Hydra magnipapillata by transcriptomics and proteomics. Biofouling 2016, 32, 1115–1129. [Google Scholar] [CrossRef]
- Miao, Y.; Zhang, L.; Sun, Y.; Jiao, W.; Li, Y.; Sun, J.; Wang, Y.; Wang, S.; Bao, Z.; Liu, W. Integration of transcriptomic and proteomic approaches provides a core set of genes for understanding of scallop attachment. Mar. Biotechnol. 2015, 17, 523–532. [Google Scholar] [CrossRef]
- Pan, Q.; Qi, Q.; Bao, L.-F.; Qin, C.-L.; He, J.-Y.; Fan, M.-H.; Liao, Z. Illumina-based De novo sequencing and characterization of Mytilus coruscus foot transcriptome. Chin. J. Biochem. Mol. Biol. 2015, 8, 014. [Google Scholar]
- Buffet, J.-P.; Corre, E.; Duvernois-Berthet, E.; Fournier, J.; Lopez, P.J. Adhesive gland transcriptomics uncovers a diversity of genes involved in glue formation in marine tube-building polychaetes. Acta Biomater. 2018, 72, 316–328. [Google Scholar] [CrossRef]
- Pennati, R.; Rothbächer, U. Bioadhesion in ascidians: A developmental and functional genomics perspective. Interface Focus 2015, 5, 20140061. [Google Scholar] [CrossRef] [PubMed]
- Riviere, G.; Klopp, C.; Ibouniyamine, N.; Huvet, A.; Boudry, P.; Favrel, P. GigaTON: An extensive publicly searchable database providing a new reference transcriptome in the pacific oyster Crassostrea gigas. BMC Bioinform. 2015, 16, 401. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Lengerer, B.; Ostermann, T.; Ladurner, P. Molecular biology approaches in bioadhesion research. Beilstein J. Nanotechnol. 2014, 5, 983. [Google Scholar] [CrossRef]
- Makarev, E.; Schubert, A.D.; Kanherkar, R.R.; London, N.; Teka, M.; Ozerov, I.; Lezhnina, K.; Bedi, A.; Ravi, R.; Mehra, R. In silico analysis of pathways activation landscape in oral squamous cell carcinoma and oral leukoplakia. Cell Death Discov. 2017, 3, 17022. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.; Guerra, D.; Stewart, D.; Breton, S. In silico analyses of mitochondrial ORFans in freshwater mussels (Bivalvia: Unionoida) provide a framework for future studies of their origin and function. BMC Genom. 2016, 17, 597. [Google Scholar] [CrossRef]
- Alkhalili, R.; Canbäck, B. Identification of Putative Novel Class-I Lanthipeptides in Firmicutes: A Combinatorial In Silico Analysis Approach Performed on Genome Sequenced Bacteria and a Close Inspection of Z-Geobacillin Lanthipeptide Biosynthesis Gene Cluster of the Thermophilic Geobacillus sp. Strain ZGt-1. Int. J. Mol. Sci. 2018, 19, 2650. [Google Scholar] [CrossRef]
- Wu, C.; Jiang, Q.; Wei, L.; Cai, Z.; Chen, J.; Yu, W.; He, C.; Wang, J.; Guo, W.; Wang, X. A Rhodopsin-Like gene may be associated with the light-sensitivity of adult Pacific oyster Crassostrea gigas. Front. Physiol. 2018, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Dermit, M.; Dodel, M.; Mardakheh, F.K. Methods for monitoring and measurement of protein translation in time and space. Mol. Biosyst. 2017, 13, 2477–2488. [Google Scholar] [CrossRef] [PubMed]
- Morisaki, T.; Lyon, K.; DeLuca, K.F.; DeLuca, J.G.; English, B.P.; Zhang, Z.; Lavis, L.D.; Grimm, J.B.; Viswanathan, S.; Looger, L.L. Real-time quantification of single RNA translation dynamics in living cells. Science 2016, 352, 1425–1429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Fang, X.; Guo, X.; Li, L.; Luo, R.; Xu, F.; Yang, P.; Zhang, L.; Wang, X.; Qi, H.; et al. The oyster genome reveals stress adaptation and complexity of shell formation. Nature 2012, 490, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Attwood, T.K.; Babbitt, P.C.; Bateman, A.; Bork, P.; Bridge, A.J.; Chang, H.-Y.; Dosztányi, Z.; El-Gebali, S.; Fraser, M. InterPro in 2017—Beyond protein family and domain annotations. Nucleic Acids Res. 2016, 45, D190–D199. [Google Scholar] [CrossRef] [PubMed]
- Almagro Armenteros, J.J.; Sønderby, C.K.; Sønderby, S.K.; Nielsen, H.; Winther, O. DeepLoc: Prediction of protein subcellular localization using deep learning. Bioinformatics 2017, 33, 3387–3395. [Google Scholar] [CrossRef] [PubMed]
- Waite, J.H. Mussel adhesion–essential footwork. J. Exp. Biol. 2017, 220, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.J.; Wang, C.S.; Song, I.T.; Jones, J.P. The role of coacervation and phase transitions in the sandcastle worm adhesive system. Adv. Colloid Interface Sci. 2017, 239, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Martinez Rodriguez, N.R.; Das, S.; Kaufman, Y.; Israelachvili, J.N.; Waite, J.H. Interfacial pH during mussel adhesive plaque formation. Biofouling 2015, 31, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Becker, P.T.; Lambert, A.; Lejeune, A.; Lanterbecq, D.; Flammang, P. Identification, characterization, and expression levels of putative adhesive proteins from the tube-dwelling polychaete Sabellaria alveolata. Biol. Bull. 2012, 223, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.J.; Weaver, J.C.; Morse, D.E.; Waite, J.H. The tube cement of Phragmatopoma californica: A solid foam. J. Exp. Biol. 2004, 207, 4727–4734. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.S.; Stewart, R.J. Multipart copolyelectrolyte adhesive of the sandcastle worm, Phragmatopoma californica (Fewkes): Catechol oxidase catalyzed curing through peptidyl-DOPA. Biomacromolecules 2013, 14, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Cranfield, H.J. A study of the morphology, ultrastructure, and histochemistry of the foot of the pediveliger of Ostrea edulis. Mar. Biol. 1973, 22, 187–202. [Google Scholar] [CrossRef]
- Qin, C.; Pan, Q.; Qi, Q.; Fan, M.; Sun, J.; Li, N.; Liao, Z. In-depth proteomic analysis of the byssus from marine mussel Mytilus coruscus. J. Proteom. 2016, 144, 87–98. [Google Scholar] [CrossRef]
- Mikami, M.; Sonoki, T.; Ito, M.; Funasaka, Y.; Suzuki, T.; Katagata, Y. Glycosylation of tyrosinase is a determinant of melanin production in cultured melanoma cells. Mol. Med. Rep. 2013, 8, 818–822. [Google Scholar] [CrossRef]
- Wang, C.-S.; Pan, H.; Weerasekare, G.M.; Stewart, R.J. Peroxidase-catalysed interfacial adhesion of aquatic caddisworm silk. J. R. Soc. Interface 2015, 12, 20150710. [Google Scholar] [CrossRef]
- Li, S.; Xia, Z.; Chen, Y.; Gao, Y.; Zhan, A. Byssus structure and protein composition in the highly invasive fouling mussel Limnoperna fortunei. Front. Physiol. 2018, 9, 418. [Google Scholar] [CrossRef]
- Liu, C.; Xie, L.; Zhang, R. Ca2+ mediates the self-assembly of the foot proteins of Pinctada fucata from the nanoscale to the microscale. Biomacromolecules 2016, 17, 3347–3355. [Google Scholar] [CrossRef]
- So, C.R.; Scancella, J.M.; Fears, K.P.; Essock-Burns, T.; Haynes, S.E.; Leary, D.H.; Diana, Z.; Wang, C.; North, S.; Oh, C.S. Oxidase activity of the barnacle adhesive interface involves peroxide-dependent catechol oxidase and lysyl oxidase enzymes. ACS Appl. Mater. Interfaces 2017, 9, 11493–11505. [Google Scholar] [CrossRef]
- Zhang, G.; He, L.; Wong, Y.-H.; Xu, Y.; Zhang, Y.; Qian, P. Chemical component and proteomic study of the Amphibalanus (=Balanus) amphitrite shell. PLoS ONE 2015, 10, e0133866. [Google Scholar] [CrossRef]
- Berglin, M.; Delage, L.; Potin, P.; Vilter, H.; Elwing, H. Enzymatic cross-linking of a phenolic polymer extracted from the marine alga Fucus serratus. Biomacromolecules 2004, 5, 2376–2383. [Google Scholar] [CrossRef] [PubMed]
- Vreeland, V.; Waite, J.H.; Epstein, L. Minireview—Polyphenols and oxidases in substratum adhesion by marine algae and mussels. J. Phycol. 1998, 34, 1–8. [Google Scholar] [CrossRef]
- Hopkinson, B.M.; Tansik, A.L.; Fitt, W.K. Internal carbonic anhydrase activity in the tissue of scleractinian corals is sufficient to support proposed roles in photosynthesis and calcification. J. Exp. Biol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, M.; Jia, Z.; Qiu, L.; Wang, L.; Zhang, A.; Song, L. A carbonic anhydrase serves as an important acid–base regulator in pacific oyster Crassostrea gigas exposed to elevated CO2: Implication for physiological responses of mollusk to ocean acidification. Mar. Biotechnol. 2017, 19, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, R. Matrix proteins in the outer shells of molluscks. Mar. Biotechnol. 2006, 8, 572–586. [Google Scholar] [CrossRef] [PubMed]
- McDougall, C.; Degnan, B.M. The evolution of mollusck shells. Wiley Interdiscip. Rev. Dev. Biol. 2018, 7, e313. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.Y.; Iordachescu, M.; Huang, J.; Hennebert, E.; Kim, S.; Rho, S.; Foo, M.; Flammang, P.; Zeng, H.; Hwang, D. Sugary interfaces mitigate contact damage where stiff meets soft. Nat. Commun. 2016, 7, 11923. [Google Scholar] [CrossRef]
- Bayne, B.L. Biology of Oysters; Developments in Aquaculture and Fisheries Science; Elsevier Science: Amsterdam, The Netherlands, 2017; ISBN 978-0-12-803500-9. [Google Scholar]
- Zhang, X.; Dai, X.; Wang, L.; Miao, Y.; Xu, P.; Liang, P.; Dong, B.; Bao, Z.; Wang, S.; Lyu, Q. Characterization of an atypical metalloproteinase inhibitors like protein (Sbp8-1) from scallop byssus. Front. Physiol. 2018, 9, 597. [Google Scholar] [CrossRef]
- Gerdol, M.; Fujii, Y.; Hasan, I.; Koike, T.; Shimojo, S.; Spazzali, F.; Yamamoto, K.; Ozeki, Y.; Pallavicini, A.; Fujita, H. The purplish bifurcate mussel Mytilisepta virgata gene expression atlas reveals a remarkable tissue functional specialization. BMC Genom. 2017, 18, 590. [Google Scholar] [CrossRef]
- Harada, N.; Iijima, S.; Kobayashi, K.; Yoshida, T.; Brown, W.R.; Hibi, T.; Oshima, A.; Morikawa, M. Human IgGFc binding protein (FcγBP) in colonic epithelial cells exhibits mucin-like structure. J. Biol. Chem. 1997, 272, 15232–15241. [Google Scholar] [CrossRef]
- Espinosa, E.P.; Koller, A.; Allam, B. Proteomic characterization of mucosal secretions in the eastern oyster, Crassostrea virginica. J. Proteom. 2016, 132, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Hennebert, E.; Leroy, B.; Wattiez, R.; Ladurner, P. An integrated transcriptomic and proteomic analysis of sea star epidermal secretions identifies proteins involved in defense and adhesion. J. Proteom. 2015, 128, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Takeuchi, Y.; Miki, D.; Odo, S. Mussel adhesive plaque protein gene is a novel member of epidermal growth factor-like gene family. J. Biol. Chem. 1995, 270, 6698–6701. [Google Scholar] [CrossRef] [PubMed]
- Hennebert, E.; Wattiez, R.; Demeuldre, M.; Ladurner, P.; Hwang, D.S.; Waite, J.H.; Flammang, P. Sea star tenacity mediated by a protein that fragments, then aggregates. Proc. Natl. Acad. Sci. USA 2014, 111, 6317–6322. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, S.; Huang, J.; Liu, Y.; Jia, G.; Xie, L.; Zhang, R. Extensible byssus of Pinctada fucata: Ca2+-stabilized nanocavities and a thrombospondin-1 protein. Sci. Rep. 2015, 5, 15018. [Google Scholar] [CrossRef] [PubMed]
- Oberhauser, A.F.; Marszalek, P.E.; Erickson, H.P.; Fernandez, J.M. The molecular elasticity of the extracellular matrix protein tenascin. Nature 1998, 393, 181. [Google Scholar]
- Anlar, B.; Gunel-Ozcan, A. Tenascin-R: Role in the central nervous system. Int. J. Biochem. Cell Biol. 2012, 44, 1385–1389. [Google Scholar] [CrossRef]
- Valcourt, U.; Alcaraz, L.B.; Exposito, J.-Y.; Lethias, C.; Bartholin, L. Tenascin-X: Beyond the architectural function. Cell Adhes. Migr. 2015, 9, 154–165. [Google Scholar] [CrossRef]
- Pasche, D.; Horbelt, N.; Marin, F.; Motreuil, S.; Macías-Sánchez, E.; Falini, G.; Hwang, D.S.; Fratzl, P.; Harrington, M.J. A new twist on sea silk: The peculiar protein ultrastructure of fan shell and pearl oyster byssus. Soft Matter 2018, 14, 5654–5664. [Google Scholar] [CrossRef]
- Suhre, M.H.; Scheibel, T. Structural diversity of a collagen-binding matrix protein from the byssus of blue mussels upon refolding. J. Struct. Biol. 2014, 186, 75–85. [Google Scholar] [CrossRef]
- Waite, J.H. The formation of mussel byssus: Anatomy of a natural manufacturing process. In Structure, Cellular Synthesis and Assembly of Biopolymers; Springer: Berlin, Germany, 1992; pp. 27–54. [Google Scholar]
- Rich, A.; Crick, F. The Structure of Collagen. Nature 1955, 176, 915–916. [Google Scholar] [CrossRef] [PubMed]
- Suhre, M.H.; Gertz, M.; Steegborn, C.; Scheibel, T. Structural and functional features of a collagen-binding matrix protein from the mussel byssus. Nat. Commun. 2014, 5, 3392. [Google Scholar] [CrossRef] [PubMed]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M. Biological Adhesives, 2nd ed.; Springer International Publishing: Berlin, Germany, 2016; Volume VIII, p. 378. ISBN 978-3-319-46082-6. [Google Scholar]
- Huang, J.; Zhang, C.; Ma, Z.; Xie, L.; Zhang, R. A novel extracellular EF-hand protein involved in the shell formation of pearl oyster. Biochim. Biophys. Acta BBA Gen. Subj. 2007, 1770, 1037–1044. [Google Scholar] [CrossRef]
- Li, X.-X.; Yu, W.-C.; Cai, Z.-Q.; He, C.; Wei, N.; Wang, X.-T.; Yue, X.-Q. Molecular cloning and characterization of full-length cDNA of calmodulin gene from Pacific oyster Crassostrea gigas. BioMed Res. Int. 2016, 2016, 5986519. [Google Scholar] [PubMed]
- Takeichi, M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science 1991, 251, 1451–1455. [Google Scholar] [CrossRef] [PubMed]
- Albertin, C.B.; Simakov, O.; Mitros, T.; Wang, Z.Y.; Pungor, J.R.; Edsinger-Gonzales, E.; Brenner, S.; Ragsdale, C.W.; Rokhsar, D.S. The octopus genome and the evolution of cephalopod neural and morphological novelties. Nature 2015, 524, 220. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yu, H.; Kong, L.; Li, Q. Transcriptomic responses to salinity stress in the Pacific oyster Crassostrea gigas. PLoS ONE 2012, 7, e46244. [Google Scholar] [CrossRef] [PubMed]
- Lebesgue, N.; Da Costa, G.; Ribeiro, R.M.; Ribeiro-Silva, C.; Martins, G.G.; Matranga, V.; Scholten, A.; Cordeiro, C.; Heck, A.J.R.; Santos, R. Deciphering the molecular mechanisms underlying sea urchin reversible adhesion: A quantitative proteomics approach. J. Proteom. 2016, 138, 61–71. [Google Scholar] [CrossRef]
- Drickamer, K. C-type lectin-like domains. Curr. Opin. Struct. Biol. 1999, 9, 585–590. [Google Scholar] [CrossRef]
- Blank, S.; Arnoldi, M.; Khoshnavaz, S.; Treccani, L.; Kuntz, M.; Mann, K.; Grathwohl, G.; Fritz, M. The nacre protein perlucin nucleates growth of calcium carbonate crystals. J. Microsc. 2003, 212, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Kiani, C.; Liwen, C.; Wu, Y.J.; Albert, J.Y.; Burton, B.Y. Structure and function of aggrecan. Cell Res. 2002, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, B.T.; Stroud, R.M.; Burden, D.K.; Fears, K.P.; Everett, R.K.; Wahl, K.J. Shell structure and growth in the base plate of the Barnacle Amphibalanus amphitrite. ACS Biomater. Sci. Eng. 2015, 1, 1085–1095. [Google Scholar] [CrossRef]
- Flammang, P.; Demeuldre, M.; Hennebert, E.; Santos, R. Adhesive secretions in echinoderms: A review. In Biological Adhesives; Springer: Berlin, Germany, 2016; pp. 193–222. [Google Scholar]
- Peng, Y.Y.; Glattauer, V.; Skewes, T.D.; McDevitt, A.; Elvin, C.M.; Werkmeister, J.A.; Graham, L.D.; Ramshaw, J.A. Identification of proteins associated with adhesive prints from Holothuria dofleinii Cuvierian tubules. Mar. Biotechnol. 2014, 16, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Peters, W. Occurrence of chitin in Molluscka. Comp. Biochem. Physiol. Part B Comp. Biochem. 1972, 41, 541–550. [Google Scholar] [CrossRef]
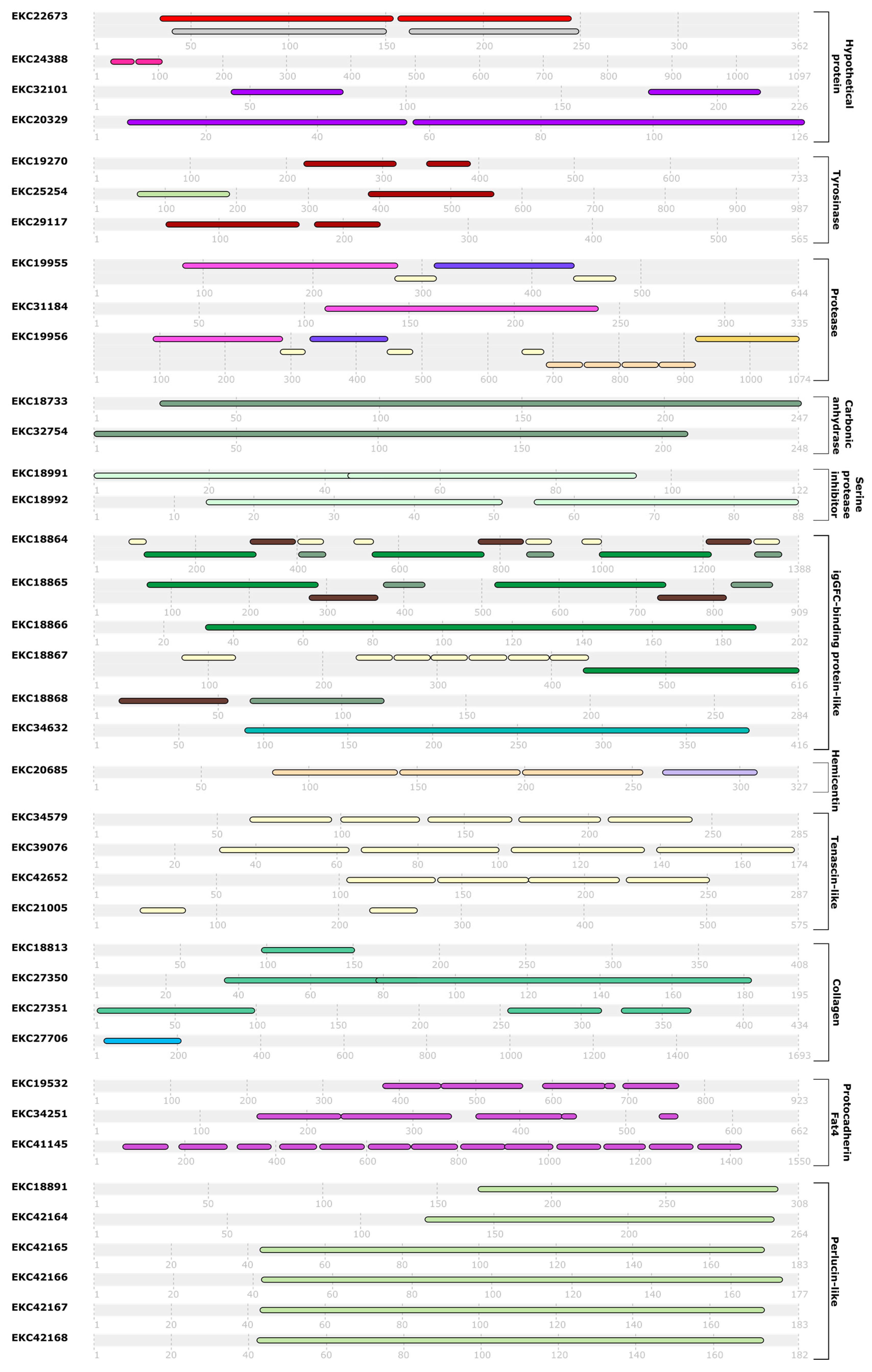
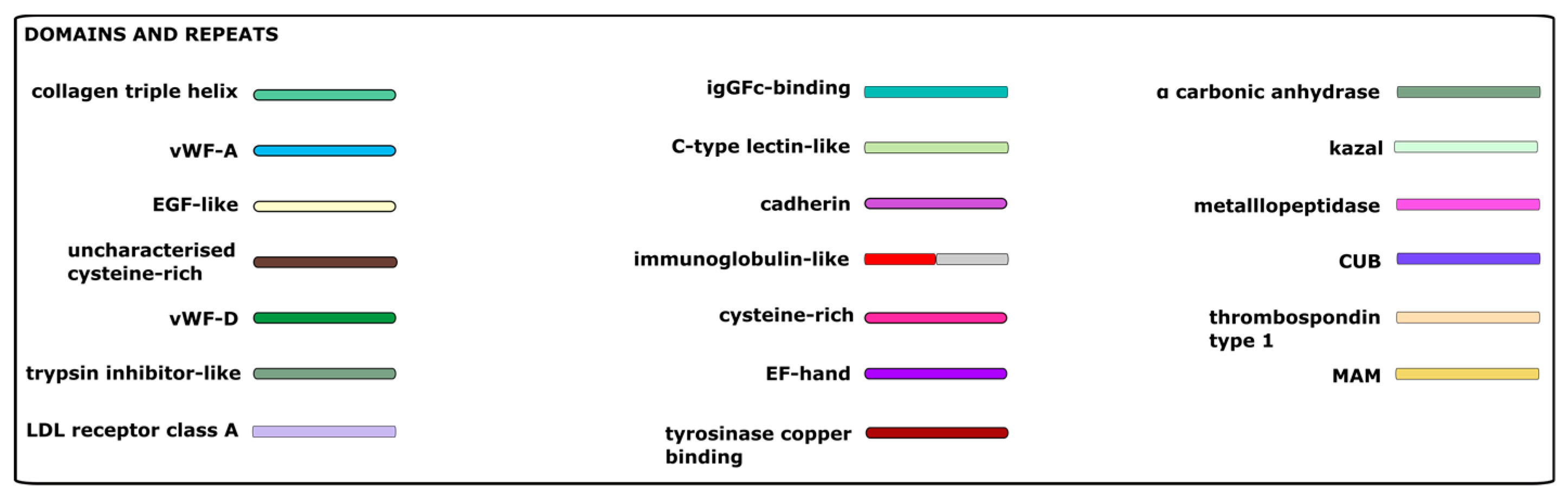
| Group | Ensembl Gene ID | Protein ID | Name | Cell. Loc. |
|---|---|---|---|---|
| Hypothetical protein | CGI_10014580 | EKC18206 | Hypothetical protein CGI_10014580 | Mit 0.58 |
| CGI_10010208 | EKC18972 | Hypothetical protein CGI_10010208 | Ext 1 | |
| CGI_10004853 | EKC21005 | Hypothetical protein CGI_10004853 | Ext 0.42 | |
| CGI_10002578 | EKC22248 | Hypothetical protein CGI_10002578 | Ext 0.89 | |
| CGI_10001746 | EKC22673 | Hypothetical protein CGI_10001746 | Ext 0.90 | |
| CGI_10013335 | EKC23310 | Hypothetical protein CGI_10013335 | Nuc 0.50 | |
| CGI_10013386 | EKC24388 | Hypothetical protein CGI_10013386 | Ext 0.41 | |
| CGI_10005578 | EKC25384 | Hypothetical protein CGI_10005578 | Ext 0.43 | |
| CGI_10013385 | EKC24387 | Hypothetical protein CGI_10013385 | Ext 0.60 | |
| CGI_10003237 | EKC27225 | Hypothetical protein CGI_10003237 | Ext 0.99 | |
| CGI_10025142 | EKC28625 | Hypothetical protein CGI_10025142 | Ext 0.91 | |
| CGI_10009961 | EKC31321 | Hypothetical protein CGI_10009961 | Ext 0.99 | |
| CGI_10025191 | EKC32101 | Hypothetical protein CGI_10025191 | Cyt 0.38 | |
| CGI_10012470 | EKC33059 | Hypothetical protein CGI_10012470 | Ext 0.99 | |
| CGI_10016093 | EKC35263 | Hypothetical protein CGI_10016093 | Ext 0.52 | |
| CGI_10016094 | EKC35264 | Hypothetical protein CGI_10016094 | Ext 0.52 | |
| CGI_10027526 | EKC35968 | Hypothetical protein CGI_10027526 | ER 0.40 | |
| CGI_10026725 | EKC38958 | Hypothetical protein CGI_10026725 | Ext 0.62 | |
| CGI_10022908 | EKC41146 | Hypothetical protein CGI_10022908 | ER 0.25 | |
| CGI_10008429 | EKC41249 | Hypothetical protein CGI_10008429 | Ext 0.90 | |
| CGI_10013282 | EKC42653 | Hypothetical protein CGI_10013282 | Nuc 0.59 | |
| Enzyme | CGI_10009044 | EKC19270 | Putative tyrosinase-like protein tyr 1 | Mem 0.82 |
| CGI_10014286 | EKC25254 | Putative tyrosinase-like protein tyr-3 | Mem 0.99 | |
| CGI_10006802 | EKC29117 | Tyrosinase-like protein 1 | Ext 0.64 | |
| CGI_10016593 | EKC32997 | Peroxidase-like protein | Ext 0.49 | |
| CGI_10010889 | EKC32754 | Carbonic anhydrase 2 | Cyt 0.41 | |
| CGI_10011324 | EKC18733 | Carbonic anhydrase 7 | Ext 0.65 | |
| CGI_10003099 | EKC28981 | Cell surface hyaluronidase-like | Plast 0.39 | |
| CGI_10003100 | EKC28982 | Cell migration-inducing and hyaluronan-binding protein-like | Cyt 0.29 | |
| CGI_10007190 | EKC19955 | Metalloendopeptidase | Ext 0.39 | |
| CGI_10007191 | EKC19956 | Metalloendopeptidase | Ext 0.51 | |
| CGI_10020760 | EKC31184 | Zinc metalloproteinase nas-15 | Ext 0.70 | |
| Protease inhibitor | CGI_10010154 | EKC18991 | Serine protease inhibitor dipetalogastin-like | Ext 0.55 |
| CGI_10010155 | EKC18992 | Serine protease inhibitor dipetalogastin-like | Ext 1 | |
| Structural protein | CGI_10005627 | EKC20685 | Hemicentin-1 | Ext 0.72 |
| CGI_10010553 | EKC18864 | IgGFc-binding protein (zonadhesin-like) | Ext 0.64 | |
| CGI_10010554 | EKC18865 | IgGFc-binding protein (zonadhesin-like) | Ext 0.72 | |
| CGI_10010555 | EKC18866 | IgGFc-binding protein (zonadhesin-like) | Ext 0.93 | |
| CGI_10010556 | EKC18867 | IgGFc-binding protein (zonadhesin-like) | Ext 0.60 | |
| CGI_10010557 | EKC18868 | IgGFc-binding protein (zonadhesin-like) | Ext 0.91 | |
| CGI_10023170 | EKC34632 | IgGFc-binding protein | Ext 0.52 | |
| CGI_10010465 | EKC34579 | Tenascin-X | Ext 0.80 | |
| CGI_10000981 | EKC39076 | Tenascin-R | Ext 0.84 | |
| CGI_10025295 | EKC40994 | Multiple EGF-like domains 10 | Ext 0.42 | |
| CGI_10013281 | EKC42652 | Tenascin-R | Ext 0.99 | |
| CGI_10010827 | EKC18813 | Collagen-like protein 7 | Ext 0.89 | |
| CGI_10010374 | EKC27350 | Collagen-like protein 7 | Mem 0.56 | |
| CGI_10010375 | EKC27351 | Collagen-like protein 7 | Mem 0.72 | |
| CGI_10011175 | EKC27706 | Collagen alpha-5(VI) chain | Ext 0.55 | |
| Calcification-related protein and calcium-binding protein | CGI_10010615 | EKC18891 | Aggrecan core protein | Ext 0.79 |
| CGI_10006917 | EKC42164 | Asialoglycoprotein receptor 2 | Ext 0.99 | |
| CGI_10006919 | EKC42165 | Perlucin-like protein | Ext 0.95 | |
| CGI_10006920 | EKC42166 | C-type mannose receptor 2 | Ext 0.90 | |
| CGI_10006921 | EKC42167 | Perlucin-like protein | Ext 0.99 | |
| CGI_10006922 | EKC42168 | Perlucin-like protein | Ext 0.99 | |
| CGI_10008331 | EKC19532 | Protocadherin Fat 4-like | Lys 0.36 | |
| CGI_10018326 | EKC34251 | Protocadherin Fat 4 | Cyt 0.72 | |
| CGI_10022907 | EKC41145 | Protocadherin Fat 4-like | Cyt 0.44 | |
| CGI_10006247 | EKC20329 | Putative calmodulin | Cyt 0.48 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foulon, V.; Boudry, P.; Artigaud, S.; Guérard, F.; Hellio, C. In Silico Analysis of Pacific Oyster (Crassostrea gigas) Transcriptome over Developmental Stages Reveals Candidate Genes for Larval Settlement. Int. J. Mol. Sci. 2019, 20, 197. https://doi.org/10.3390/ijms20010197
Foulon V, Boudry P, Artigaud S, Guérard F, Hellio C. In Silico Analysis of Pacific Oyster (Crassostrea gigas) Transcriptome over Developmental Stages Reveals Candidate Genes for Larval Settlement. International Journal of Molecular Sciences. 2019; 20(1):197. https://doi.org/10.3390/ijms20010197
Chicago/Turabian StyleFoulon, Valentin, Pierre Boudry, Sébastien Artigaud, Fabienne Guérard, and Claire Hellio. 2019. "In Silico Analysis of Pacific Oyster (Crassostrea gigas) Transcriptome over Developmental Stages Reveals Candidate Genes for Larval Settlement" International Journal of Molecular Sciences 20, no. 1: 197. https://doi.org/10.3390/ijms20010197
APA StyleFoulon, V., Boudry, P., Artigaud, S., Guérard, F., & Hellio, C. (2019). In Silico Analysis of Pacific Oyster (Crassostrea gigas) Transcriptome over Developmental Stages Reveals Candidate Genes for Larval Settlement. International Journal of Molecular Sciences, 20(1), 197. https://doi.org/10.3390/ijms20010197