Monocytes and Macrophages as Viral Targets and Reservoirs
Abstract
1. Introduction
2. Monocytes and Tissue Macrophages
3. Viruses Infect Monocytes and Macrophages
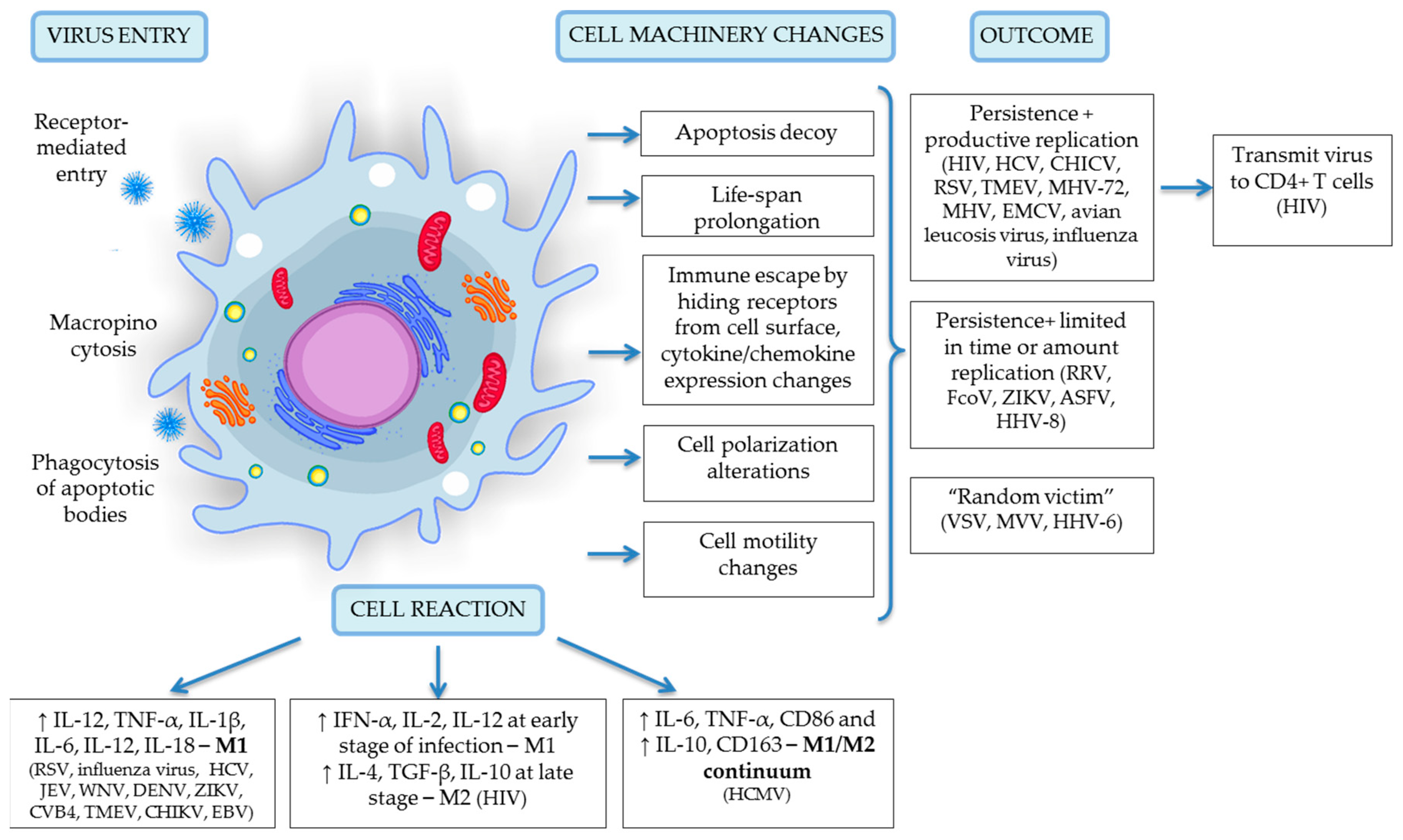
4. Viral Entry into Monocytes and Macrophages
5. Productive Viral Infection
6. Latent Viral Infection and Inflammation
7. Macrophages as a Target for Therapeutic Intervention
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Mo | Monocyte |
| Mφ | Macrophage |
| MDM | Monocyte-derived macrophages |
| HC | Hofbauer cells |
| HIV | Human immunodeficiency virus |
| HCMV | Human cytomegalovirus |
| RSV | Respiratory syncytial virus |
| VSV | Vesicular stomatitis virus |
| dsDNA | Double stranded DNA |
| ssRNA | Single stranded RNA |
| CSFV | Classical swine fever virus |
| EMCV | Encephalomyocarditis virus |
| MHV | Mouse hepatitis virus |
| ZIKV | Zika virus |
| MVV | Maedi-visna virus |
| EBV | Epstein-Barr virus |
| KSHV | Kaposi’s sarcoma-associated herpesvirus |
| HHV-6 | Human herpesvirus 6 |
| VZV | Simian varicella-zoster virus |
| JEV | Japanese encephalitis virus |
| AIDS | Acquired immune deficiency syndrome |
| CHIKV | Chikungunya alphavirus |
| TMEV | Theiler’s murine encephalomyelitis virus |
References
- Liu, Y.; Tang, X.P.; McArthur, J.C.; Scott, J.; Gartner, S. Analysis of human immunodežciency virus type 1 evidence for monocyte trafžcking into brain. J. Neurovirol. 2000, 6, S70–S81. [Google Scholar] [PubMed]
- Fischer-Smith, T.; Rappaport, J. Evolving paradigms in the pathogenesis of hiv-1-associated dementia. Expert Rev. Mol. Med. 2005, 7, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Boeuf, P.; Drummer, H.E.; Richards, J.S.; Scoullar, M.J.; Beeson, J.G. The global threat of zika virus to pregnancy: Epidemiology, clinical perspectives, mechanisms, and impact. BMC Med. 2016, 14, 112. [Google Scholar] [CrossRef] [PubMed]
- Tauber, A.I. Metchnikoff and the phagocytosis theory. Nat. Rev. Mol. Cell Boil. 2003, 4, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Okabe, Y.; Medzhitov, R. Tissue biology perspective on macrophages. Nat. Immunol. 2016, 17, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Plüddemann, A.; Martinez Estrada, F. Macrophage heterogeneity in tissues: Phenotypic diversity and functions. Immunol. Rev. 2014, 262, 36–55. [Google Scholar] [CrossRef] [PubMed]
- Epelman, S.; Lavine, K.J.; Randolph, G.J. Origin and functions of tissue macrophages. Immunity 2014, 41, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Sintiprungrat, K.; Singhto, N.; Sinchaikul, S.; Chen, S.-T.; Thongboonkerd, V. Alterations in cellular proteome and secretome upon differentiation from monocyte to macrophage by treatment with phorbol myristate acetate: Insights into biological processes. J. Proteom. 2010, 73, 602–618. [Google Scholar] [CrossRef] [PubMed]
- Kzhyshkowska, J.; Gudima, A.; Moganti, K.; Gratchev, A.; Orekhov, A. Perspectives for monocyte/macrophage-based diagnostics of chronic inflammation. Transfus. Med. Hemother. 2016, 43, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of human macrophage polarization in inflammation during infectious diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef] [PubMed]
- Herbein, G.; Varin, A. The macrophage in hiv-1 infection: From activation to deactivation? Retrovirology 2010, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M. The Molecular Mechanisms of Chronic Inflammation Development; Frontiers E-books: Lausanne, Switzerland, 2012; ISBN 2889190919. [Google Scholar]
- Wardley, R.; Wilkinson, P. The growth of virulent African swine fever virus in pig monocytes and macrophages. J. Gen. Virol. 1978, 38, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Gazzolo, L.; Moscovici, C.; Moscovici, M. Persistence of avian oncoviruses in chicken macrophages. Infect. Immun. 1979, 23, 294–297. [Google Scholar] [PubMed]
- Nagra, R.M.; Wong, P.K.; Wiley, C.A. Expression of major histocompatibility complex antigens and serum neutralizing antibody in murine retroviral encephalitis. J. Neuropathol. Exp. Neurol. 1993, 52, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Clatch, R.J.; Miller, S.D.; Metzner, R.; Dal Canto, M.C.; Lipton, H.L. Monocytes/macrophages isolated from the mouse central nervous system contain infectious theiler’s murine encephalomyelitis virus (TMEV). Virology 1990, 176, 244–254. [Google Scholar] [CrossRef]
- Stevenson, E.V.; Collins-McMillen, D.; Kim, J.H.; Cieply, S.J.; Bentz, G.L.; Yurochko, A.D. Hcmv reprogramming of infected monocyte survival and differentiation: A goldilocks phenomenon. Viruses 2014, 6, 782–807. [Google Scholar] [CrossRef] [PubMed]
- Abbas, W.; Tariq, M.; Iqbal, M.; Kumar, A.; Herbein, G. Eradication of HIV-1 from the macrophage reservoir: An uncertain goal? Viruses 2015, 7, 1578–1598. [Google Scholar] [CrossRef] [PubMed]
- Hoarau, J.-J.; Bandjee, M.-C.J.; Trotot, P.K.; Das, T.; Li-Pat-Yuen, G.; Dassa, B.; Denizot, M.; Guichard, E.; Ribera, A.; Henni, T. Persistent chronic inflammation and infection by chikungunya arthritogenic alphavirus in spite of a robust host immune response. J. Immunol. 2010, 184, 5914–5927. [Google Scholar] [CrossRef] [PubMed]
- Jaffar-Bandjee, M.C.; Das, T.; Hoarau, J.J.; Trotot, P.K.; Denizot, M.; Ribera, A.; Roques, P.; Gasque, P. Chikungunya virus takes centre stage in virally induced arthritis: Possible cellular and molecular mechanisms to pathogenesis. Microbes Infect. 2009, 11, 1206–1218. [Google Scholar] [CrossRef] [PubMed]
- Ruelas, D.S.; Greene, W.C. An integrated overview of hiv-1 latency. Cell 2013, 155, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Kundu, K.; Dutta, K.; Nazmi, A.; Basu, A. Japanese encephalitis virus infection modulates the expression of suppressors of cytokine signaling (SOCS) in macrophages: Implications for the hosts’ innate immune response. Cell. Immunol. 2013, 285, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Costers, S.; Delputte, P.L.; Nauwynck, H.J. Porcine reproductive and respiratory syndrome virus-infected alveolar macrophages contain no detectable levels of viral proteins in their plasma membrane and are protected against antibody-dependent, complement-mediated cell lysis. J. Gen. Virol. 2006, 87, 2341–2351. [Google Scholar] [CrossRef] [PubMed]
- Psalla, D.; Psychas, V.; Spyrou, V.; Billinis, C.; Papaioannou, N.; Vlemmas, I. Pathogenesis of experimental encephalomyocarditis: A histopathological, immunohistochemical and virological study in mice. J. Comp. Pathol. 2006, 135, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Robert, J.; Abramowitz, L.; Gantress, J.; Morales, H.D. Xenopus laevis: A possible vector of ranavirus infection? J. Wildl. Dis. 2007, 43, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Panuska, J.R.; Cirino, N.M.; Midulla, F.; Despot, J.; McFadden, E., Jr.; Huang, Y. Productive infection of isolated human alveolar macrophages by respiratory syncytial virus. J. Clin. Investig. 1990, 86, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Toledo, E.; Torres-González, L.; Gómez, B. Respiratory syncytial virus persistence in murine macrophages impairs ifn-β response but not synthesis. Viruses 2015, 7, 5361–5374. [Google Scholar] [CrossRef] [PubMed]
- Nakamura-Lopez, Y.; Villegas-Sepúlveda, N.; Gómez, B. Rsv p-protein impairs extrinsic apoptosis pathway in a macrophage-like cell line persistently infected with respiratory syncytial virus. Virus Res. 2015, 204, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Gladkov, S.; Zinserling, V.; Shtro, A.; Belyaevskaya, S.; Zarubaev, V. postmortem diagnosis of influenza during its epidemic and interepidemic periods. Arkhiv Patol. 2014, 77, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Pleskov, V.; Zarubaev, V.; Pleskova, I. reticuloendothelial system and persistence of influenza virus in the body. Vopr. Virusol. 1995, 41, 53–58. [Google Scholar]
- Perrone, L.A.; Plowden, J.K.; García-Sastre, A.; Katz, J.M.; Tumpey, T.M. H5n1 and 1918 pandemic influenza virus infection results in early and excessive infiltration of macrophages and neutrophils in the lungs of mice. PLoS Pathog. 2008, 4, e1000115. [Google Scholar] [CrossRef] [PubMed]
- Simon, I.D.; van Rooijen, N.; Rose, J.K. Vesicular stomatitis virus genomic rna persists in vivo in the absence of viral replication. J. Virol. 2010, 84, 3280–3286. [Google Scholar] [CrossRef] [PubMed]
- Kipar, A.; Meli, M.L.; Baptiste, K.E.; Bowker, L.J.; Lutz, H. Sites of feline coronavirus persistence in healthy cats. J. Gen. Virol. 2010, 91, 1698–1707. [Google Scholar] [CrossRef] [PubMed]
- Cipková-Jarčušková, J.; Chalupkova, A.; Hrabovska, Z.; Wagnerova, M.; Mistrikova, J. Biological and pathogenetic characterization of different isolates of murine gammaherpesvirus 68 (mhv-68) in the context of study of human oncogenic gammaherpesviruses. Acta Virol. 2013, 57, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Glass, W.G.; Chen, B.P.; Liu, M.T.; Lane, T.E. Mouse hepatitis virus infection of the central nervous system: Chemokine-mediated regulation of host defense and disease. Viral Immunol. 2002, 15, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Wang, C.; Chien, M. Virus antigen expression and alterations in peripheral blood mononuclear cell subpopulations after classical swine fever virus infection. Vet. Microbiol. 1999, 67, 17–29. [Google Scholar] [CrossRef]
- Radkowski, M.; Gallegos-Orozco, J.F.; Jablonska, J.; Colby, T.V.; Walewska-Zielecka, B.; Kubicka, J.; Wilkinson, J.; Adair, D.; Rakela, J.; Laskus, T. Persistence of hepatitis c virus in patients successfully treated for chronic hepatitis c. Hepatology 2005, 41, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.; Radkowski, M.; Eschbacher, J.M.; Laskus, T. Activation of brain macrophages/microglia cells in hepatitis c infection. Gut 2010, 59, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Myint, K.S.A.; Kipar, A.; Jarman, R.G.; Gibbons, R.V.; Perng, G.C.; Flanagan, B.; Mongkolsirichaikul, D.; Van Gessel, Y.; Solomon, T. Neuropathogenesis of japanese encephalitis in a primate model. PLoS Negl. Trop. Dis. 2014, 8, e2980. [Google Scholar] [CrossRef] [PubMed]
- Ashhurst, T.M.; van Vreden, C.; Munoz-Erazo, L.; Niewold, P.; Watabe, K.; Terry, R.L.; Deffrasnes, C.; Getts, D.R.; King, N.J.C. Antiviral macrophage responses in flavivirus encephalitis. Indian J. Med. Res. 2013, 138, 632–647. [Google Scholar] [PubMed]
- Quicke, K.M.; Bowen, J.R.; Johnson, E.L.; McDonald, C.E.; Ma, H.; O’Neal, J.T.; Rajakumar, A.; Wrammert, J.; Rimawi, B.H.; Pulendran, B.; et al. Zika virus infects human placental macrophages. Cell Host Microbe 2016, 20, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Alidjinou, E.K.; Sané, F.; Trauet, J.; Copin, M.-C.; Hober, D. Coxsackievirus b4 can infect human peripheral blood-derived macrophages. Viruses 2015, 7, 6067–6079. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yue, Y.; Xiong, S. Nk-derived ifn-γ/il-4 triggers the sexually disparate polarization of macrophages in cvb3-induced myocarditis. J. Mol. Cell. Cardiol. 2014, 76, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Ghazarian, L.; Diana, J.; Beaudoin, L.; Larsson, P.G.; Puri, R.K.; Van Rooijen, N.; Flodström-Tullberg, M.; Lehuen, A. Protection against type 1 diabetes upon coxsackievirus b4 infection and inkt-cell stimulation role of suppressive macrophages. Diabetes 2013, 62, 3785–3796. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, Z.R.; Corbett, J.A. Macrophage expression of inflammatory genes in response to emcv infection. Biomolecules 2015, 5, 1938–1954. [Google Scholar] [CrossRef] [PubMed]
- Himeda, T.; Okuwa, T.; Muraki, Y.; Ohara, Y. Cytokine/chemokine profile in j774 macrophage cells persistently infected with da strain of theiler’s murine encephalomyelitis virus (tmev). J. Neurovirol. 2010, 16, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Bröer, S.; Käufer, C.; Haist, V.; Li, L.; Gerhauser, I.; Anjum, M.; Bankstahl, M.; Baumgärtner, W.; Löscher, W. Brain inflammation, neurodegeneration and seizure development following picornavirus infection markedly differ among virus and mouse strains and substrains. Exp. Neurol. 2016, 279, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Watson, N.B.; Schneider, K.M.; Massa, P.T. Shp-1–dependent macrophage differentiation exacerbates virus-induced myositis. J. Immunol. 2015, 194, 2796–2809. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Jaffar-Bandjee, M.-C.; Giry, C.; de Kerillis, L.C.; Merits, A.; Gasque, P.; Hoarau, J.-J. Mouse macrophage innate immune response to chikungunya virus infection. Virol. J. 2012, 9, 313. [Google Scholar] [CrossRef] [PubMed]
- Labadie, K.; Larcher, T.; Joubert, C.; Mannioui, A.; Delache, B.; Brochard, P.; Guigand, L.; Dubreil, L.; Lebon, P.; Verrier, B. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J. Clin. Investig. 2010, 120, 894–906. [Google Scholar] [CrossRef] [PubMed]
- Suhrbier, A.; La Linn, M. Clinical and pathologic aspects of arthritis due to ross river virus and other alphaviruses. Curr. Opin. Rheumatol. 2004, 16, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Way, S.J.; Lidbury, B.A.; Banyer, J.L. Persistent ross river virus infection of murine macrophages: An in vitro model for the study of viral relapse and immune modulation during long-term infection. Virology 2002, 301, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Assunção-Miranda, I.; Cruz-Oliveira, C.; Da Poian, A.T. Molecular mechanisms involved in the pathogenesis of alphavirus-induced arthritis. BioMed Res. Int. 2013, 2013, 973516. [Google Scholar] [CrossRef] [PubMed]
- Cheevers, W.; Cordery-Cotter, R.; McGuire, T.; DeMartini, J. Neutralizing antibody responses and evolution of antigenic variants in monozygotic twin lambs infected with phenotypically distinct ovine lentiviruses. Virology 1999, 258, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Herbein, G.; Gras, G.; Khan, K.A.; Abbas, W. Review macrophage signaling in hiv-1 infection. Retrovirology 2010, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Le Douce, V.; Herbein, G.; Rohr, O.; Schwartz, C. Molecular mechanisms of hiv-1 persistence in the monocyte-macrophage lineage. Retrovirology 2010, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Thormar, H. Maedi-visna virus and its relationship to human immunodeficiency virus. AIDS Rev. 2005, 7, 233–245. [Google Scholar] [PubMed]
- Oura, C.; Powell, P.; Parkhouse, R. Detection of african swine fever virus in infected pig tissues by immunocytochemistry and in situ hybridisation. J. Virol. Methods 1998, 72, 205–217. [Google Scholar] [CrossRef]
- Donofrio, G.; van Santen, V.L. A bovine macrophage cell line supports bovine herpesvirus-4 persistent infection. J. Gen. Virol. 2001, 82, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Noriega, V.M.; Haye, K.K.; Kraus, T.A.; Kowalsky, S.R.; Ge, Y.; Moran, T.M.; Tortorella, D. Human cytomegalovirus modulates monocyte-mediated innate immune responses during short-term experimental latency in vitro. J. Virol. 2014, 88, 9391–9405. [Google Scholar] [CrossRef] [PubMed]
- Münz, C. Epstein Barr Virus Volume 2. One Herpes Virus: Many Diseases; Springer: Berlin, Germany, 2015. [Google Scholar]
- Shimakage, M. Significant role of macrophages in human cancers associated with epstein-barr virus (review). Oncol. Rep. 2014, 32, 1763–1771. [Google Scholar] [CrossRef] [PubMed]
- Lusso, P. Hhv-6 and the immune system: Mechanisms of immunomodulation and viral escape. J. Clin. Virol. 2006, 37, S4–S10. [Google Scholar] [CrossRef]
- Kondo, K.; Kondo, T.; Okuno, T.; Takahashi, M.; Yamanishi, K. Latent human herpesvirus 6 infection of human monocytes/macrophages. J. Gen. Virol. 1991, 72, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Santoro, F.; Di Lullo, G.; Dagna, L.; Verani, A.; Lusso, P. Selective suppression of il-12 production by human herpesvirus 6. Blood 2003, 102, 2877–2884. [Google Scholar] [CrossRef] [PubMed]
- Rappocciolo, G.; Jenkins, F.J.; Hensler, H.R.; Piazza, P.; Jais, M.; Borowski, L.; Watkins, S.C.; Rinaldo, C.R. Dc-sign is a receptor for human herpesvirus 8 on dendritic cells and macrophages. J. Immunol. 2006, 176, 1741–1749. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Freitas, E.; Sullivan, R.; Mohan, S.; Bacelieri, R.; Branch, D.; Romano, M.; Kearney, P.; Oates, J.; Plaisance, K. Upregulation of xct by kshv-encoded micrornas facilitates kshv dissemination and persistence in an environment of oxidative stress. PLoS Pathog. 2010, 6, e1000742. [Google Scholar] [CrossRef] [PubMed]
- Henning, J.D.; Bunker, C.H.; Patrick, A.L.; Jenkins, F.J. Human herpesvirus 8 establishes a latent infection in prostates of tobago men resulting in increased macrophage infiltration. Prostate 2016, 76, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Mistrikova, J.; Remenova, A.; Lesso, J.; Stancekova, M. Replication and persistence of murine herpesvirus 72 in lymphatic system and peripheral blood mononuclear cells of balb/c mice. Acta Virol. 1994, 38, 151–156. [Google Scholar] [PubMed]
- Gowrishankar, K.; Steain, M.; Cunningham, A.L.; Rodriguez, M.; Blumbergs, P.; Slobedman, B.; Abendroth, A. Characterization of the host immune response in human ganglia after herpes zoster. J. Virol. 2010, 84, 8861–8870. [Google Scholar] [CrossRef] [PubMed]
- Traina-Dorge, V.; Doyle-Meyers, L.A.; Sanford, R.; Manfredo, J.; Blackmon, A.; Wellish, M.; James, S.; Alvarez, X.; Midkiff, C.; Palmer, B.E.; et al. Simian varicella virus is present in macrophages, dendritic cells, and t cells in lymph nodes of rhesus macaques after experimental reactivation. J. Virol. 2015, 89, 9817–9824. [Google Scholar] [CrossRef] [PubMed]
- Grove, J.; Marsh, M. The cell biology of receptor-mediated virus entry. J. Cell Biol. 2011, 195, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- Kerr, M.C.; Teasdale, R.D. Defining macropinocytosis. Traffic 2009, 10, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Tayyari, F.; Marchant, D.; Moraes, T.J.; Duan, W.; Mastrangelo, P.; Hegele, R.G. Identification of nucleolin as a cellular receptor for human respiratory syncytial virus. Nat. Med. 2011, 17, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Pawelek, K.A.; Dor, D., Jr.; Salmeron, C.; Handel, A. Within-host models of high and low pathogenic influenza virus infections: The role of macrophages. PLoS ONE 2016, 11, e0150568. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.M.; Cavanagh, D. The molecular biology of coronaviruses. Adv. Virus Res. 1997, 48, 1–100. [Google Scholar] [PubMed]
- Brahic, M.; Bureau, J.-F.; Michiels, T. The genetics of the persistent infection and demyelinating disease caused by theiler’s virus. Annu. Rev. Microbiol. 2005, 59, 279–298. [Google Scholar] [CrossRef] [PubMed]
- Lichty, B.D.; Power, A.T.; Stojdl, D.F.; Bell, J.C. Vesicular stomatitis virus: Re-inventing the bullet. Trends Mol. Med. 2004, 10, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Otake, Y.; Soundararajan, S.; Sengupta, T.K.; Kio, E.A.; Smith, J.C.; Pineda-Roman, M.; Stuart, R.K.; Spicer, E.K.; Fernandes, D.J. Overexpression of nucleolin in chronic lymphocytic leukemia cells induces stabilization of bcl2 mrna. Blood 2007, 109, 3069–3075. [Google Scholar] [CrossRef] [PubMed]
- Hallak, L.K.; Collins, P.L.; Knudson, W.; Peeples, M.E. Iduronic acid-containing glycosaminoglycans on target cells are required for efficient respiratory syncytial virus infection. Virology 2000, 271, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Ariza, M.-E.; Glaser, R.; Kaumaya, P.T.; Jones, C.; Williams, M.V. The ebv-encoded dutpase activates nf-κb through the tlr2 and myd88-dependent signaling pathway. J. Immunol. 2009, 182, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Thorp, S.C. Cell surface heparan sulfate and its roles in assisting viral infections. Med. Res. Rev. 2002, 22, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Hober, D.; Sane, F.; Jaidane, H.; Riedweg, K.; Goffard, A.; Desailloud, R. Immunology in the clinic review series; focus on type 1 diabetes and viruses: Role of antibodies enhancing the infection with coxsackievirus-b in the pathogenesis of type 1 diabetes. Clin. Exp. Immunol. 2012, 168, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Maréchal, V.; Prevost, M.-C.; Petit, C.; Perret, E.; Heard, J.-M.; Schwartz, O. Human immunodeficiency virus type 1 entry into macrophages mediated by macropinocytosis. J. Virol. 2001, 75, 11166–11177. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, J.R.; Rogers, R.; Molina, R.M.; Dangond, F.; McLane, M.F.; Essex, M.; Brain, J.D. Noninfectious entry of hiv-1 into peripheral and brain macrophages mediated by the mannose receptor. Proc. Natl. Acad. Sci. USA 2007, 104, 5097–5102. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Kondo, T.; Shimada, K.; Amo, K.; Miyagawa, H.; Yamanishi, K. Strong interaction between human herpesvirus 6 and peripheral blood monocytes/macrophages during acute infection. J. Med Virol. 2002, 67, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Spear, P.G.; Longnecker, R. Herpesvirus entry: An update. J. Virol. 2003, 77, 10179–10185. [Google Scholar] [CrossRef] [PubMed]
- Zerboni, L.; Sen, N.; Oliver, S.L.; Arvin, A.M. Molecular mechanisms of varicella zoster virus pathogenesis. Nat. Rev. Microbiol. 2014, 12, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Maginnis, M.S. Virus-receptor interactions: The key to cellular invasion. J. Mol. Boil. 2018, 430, 2590–2611. [Google Scholar] [CrossRef] [PubMed]
- Van Lent, P.L.; Nabbe, K.; Blom, A.B.; Holthuysen, A.E.; Sloetjes, A.; Van De Putte, L.B.; Verbeek, S.; Van Den Berg, W.B. Role of activatory fcγri and fcγriii and inhibitory fcγrii in inflammation and cartilage destruction during experimental antigen-induced arthritis. Am. J. Pathol. 2001, 159, 2309–2320. [Google Scholar] [CrossRef]
- Coyne, C.B.; Shen, L.; Turner, J.R.; Bergelson, J.M. Coxsackievirus entry across epithelial tight junctions requires occludin and the small gtpases rab34 and rab5. Cell Host Microbe 2007, 2, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.G.; Hildreth, J.E. Involvement of macrophage mannose receptor in the binding and transmission of hiv by macrophages. Eur. J. Immunol. 2003, 33, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, D.Y.; Huong, S.-M.; Huang, E.-S. Integrin αvβ3 is a coreceptor for human cytomegalovirus. Nat. Med. 2005, 11, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Beutler, B. Inferences, questions and possibilities in toll-like receptor signalling. Nature 2004, 430, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, I.; Pan, J.; Takizawa, T.; Nakanishi, Y. Virus clearance through apoptosis-dependent phagocytosis of influenza a virus-infected cells by macrophages. J. Virol. 2000, 74, 3399–3403. [Google Scholar] [CrossRef] [PubMed]
- Dutta, K.; Mishra, M.K.; Nazmi, A.; Kumawat, K.L.; Basu, A. Minocycline differentially modulates macrophage mediated peripheral immune response following japanese encephalitis virus infection. Immunobiology 2010, 215, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.; Nogalski, M.T.; Bentz, G.L.; Smith, M.S.; Parmater, A.; Yurochko, A.D. Pi3k-dependent upregulation of mcl-1 by human cytomegalovirus is mediated by epidermal growth factor receptor and inhibits apoptosis in short-lived monocytes. J. Immunol. 2010, 184, 3213–3222. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, P.N.F.; Croci, D.O.; Riva, D.A.; Bibini, M.; Luzzi, R.; Saracco, M.; Mersich, S.E.; Rabinovich, G.A.; Peralta, L.M. Apoptosis resistance in hiv-1 persistently-infected cells is independent of active viral replication and involves modulation of the apoptotic mitochondrial pathway. Retrovirology 2008, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Avalos, C.R.; Abreu, C.M.; Queen, S.E.; Li, M.; Price, S.; Shirk, E.N.; Engle, E.L.; Forsyth, E.; Bullock, B.T.; Mac Gabhann, F. Brain macrophages in simian immunodeficiency virus-infected, antiretroviral-suppressed macaques: A functional latent reservoir. MBio 2017, 8, e01186-17. [Google Scholar] [CrossRef] [PubMed]
- Couturier, J.; Lewis, D.E. Hiv persistence in adipose tissue reservoirs. Curr. HIV/AIDS Rep. 2018, 15, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, S.; Graf, E.H.; Dahl, V.; Strain, M.C.; Yukl, S.A.; Lysenko, E.S.; Bosch, R.J.; Lai, J.; Chioma, S.; Emad, F. Comparative analysis of measures of viral reservoirs in hiv-1 eradication studies. PLoS Pathog. 2013, 9, e1003174. [Google Scholar] [CrossRef] [PubMed]
- Aquaro, S.; Bagnarelli, P.; Guenci, T.; De Luca, A.; Clementi, M.; Balestra, E.; Caliò, R.; Perno, C.F. Long-term survival and virus production in human primary macrophages infected by human immunodeficiency virus. J. Med. Virol. 2002, 68, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Gama, L.; Abreu, C.; Shirk, E.N.; Queen, S.E.; Beck, S.E.; Pate, K.A.M.; Bullock, B.T.; Zink, M.C.; Mankowski, J.L.; Clements, J.E. SIV latency in macrophages in the CNS. In Current Topics in Microbiology and Immunology; Springer: Berlin, Germany, 2018. [Google Scholar]
- Geleziunas, R.; Xu, W.; Takeda, K.; Ichijo, H.; Greene, W.C. Hiv-1 nef inhibits ask1-dependent death signalling providing a potential mechanism for protecting the infected host cell. Nature 2001, 410, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Andrade, V.M.; Stevenson, M. Host and viral factors influencing interplay between the macrophage and hiv-1. J. Neuroimmune Pharmacol. 2018, 1–11. [Google Scholar] [CrossRef]
- Ballon, G.; Ometto, L.; Righetti, E.; Cattelan, A.M.; Masiero, S.; Zanchetta, M.; Chieco-Bianchi, L.; De Rossi, A. Human immunodeficiency virus type 1 modulates telomerase activity in peripheral blood lymphocytes. J. Infect. Dis. 2001, 183, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.; Bhuyan, F.; Hashimoto, M.; Nasser, H.; Maekawa, T.; Suzu, S. M-csf inhibits anti–hiv-1 activity of il-32, but they enhance m2-like phenotypes of macrophages. J. Immunol. 2014, 192, 5083–5089. [Google Scholar] [CrossRef] [PubMed]
- Torre, D.; Gennero, L.; Baccino, F.; Speranza, F.; Biondi, G.; Pugliese, A. Impaired macrophage phagocytosis of apoptotic neutrophils in patients with human immunodeficiency virus type 1 infection. Clin. Diagn. Lab. Immunol. 2002, 9, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Fadok, V.A.; Bratton, D.L.; Konowal, A.; Freed, P.W.; Westcott, J.Y.; Henson, P.M. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving tgf-beta, pge2, and paf. J. Clin. Investig. 1998, 101, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Söderberg-Nauclér, C. Hcmv microinfections in inflammatory diseases and cancer. J. Clin. Virol. 2008, 41, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Nogalski, M.T.; Collins-McMillen, D.; Yurochko, A.D. Overview of human cytomegalovirus pathogenesis. Methods Mol. Biol. 2014, 1119, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Söderberg-Nauclér, C. Does cytomegalovirus play a causative role in the development of various inflammatory diseases and cancer? J. Intern. Med. 2006, 259, 219–246. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.S.; Bentz, G.L.; Smith, P.M.; Bivins, E.R.; Yurochko, A.D. Hcmv activates pi (3) k in monocytes and promotes monocyte motility and transendothelial migration in a pi (3) k-dependent manner. J. Leukoc. Boil. 2004, 76, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Dubuisson, J.; Cosset, F.-L. Virology and cell biology of the hepatitis c virus life cycle—An update. J. Hepatol. 2014, 61, S3–S13. [Google Scholar] [CrossRef] [PubMed]
- Revie, D.; Salahuddin, S.Z. Role of macrophages and monocytes in hepatitis c virus infections. World J. Gastroenterol. 2014, 20, 2777–2784. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.; Gartner, S.; Kawano, T.; Benoit, N.; Cheng-Mayer, C. Hla-a2 down-regulation on primary human macrophages infected with an m-tropic egfp-tagged hiv-1 reporter virus. J. Leukoc. Boil. 2005, 78, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Toledo, C.M.; Wietgrefe, S.W.; Duan, L.; Schacker, T.W.; Reilly, C.S.; Haase, A.T. The immunosuppressive role of il-32 in lymphatic tissue during hiv-1 infection. J. Immunol. 2011, 186, 6576–6584. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.; Cheng, Y.; Rolfe, A.; Hammack, C.; Vera, D.; Kyle, K.; Wang, J.; Meissner, T.B.; Ren, Y.; Cowan, C. An hpsc-derived tissue-resident macrophage model reveals differential responses of macrophages to zikv and denv infection. Stem Cell Rep. 2018, 11, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Venzke, S.; Keppler, O.T. Role of macrophages in hiv infection and persistence. Expert Rev. Clin. Immunol. 2006, 2, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.-K.; Gu, Y.; Diaz, A.; Marlett, J.; Takahashi, Y.; Li, M.; Suzuki, K.; Xu, R.; Hishida, T.; Chang, C.-J.; et al. Use of the crispr/cas9 system as an intracellular defense against hiv-1 infection in human cells. Nat. Commun. 2015, 6, 6413. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, R.; Chen, Y.; Fischer, T.; Tedaldi, E.; Napoli, A.; Zhang, Y.; Karn, J.; Hu, W.; Khalili, K. Elimination of hiv-1 genomes from human t-lymphoid cells by crispr/cas9 gene editing. Sci. Rep. 2016, 6, 22555. [Google Scholar] [CrossRef] [PubMed]
- Lund Laursen, T.; Brøckner Siggard, C.; Kazankov, K.; Damgaard Sandahl, T.; Møller, H.J.; Ong, A.; Douglas, M.W.; George, J.; Tarp, B.; Hagelskjaer Kristensen, L.; et al. Rapid and persistent decline in soluble cd163 with successful direct-acting antiviral therapy and associations with chronic hepatitis c histology. Scand. J. Gastroenterol. 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.E.; DeBiasi, R.; Goade, D.E.; Haaland, K.Y.; Harrington, J.A.; Harnar, J.B.; Pergam, S.A.; King, M.K.; DeMasters, B.; Tyler, K.L. West nile virus neuroinvasive disease. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2006, 60, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Seitz, S.; Clarke, P.; Tyler, K.L. Pharmacologic depletion of microglia increases viral load in the brain and enhances mortality in murine models of flavivirus-induced encephalitis. J. Virol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Rulli, N.E.; Guglielmotti, A.; Mangano, G.; Rolph, M.S.; Apicella, C.; Zaid, A.; Suhrbier, A.; Mahalingam, S. Amelioration of alphavirus-induced arthritis and myositis in a mouse model by treatment with bindarit, an inhibitor of monocyte chemotactic proteins. Arthr. Rheum. Off. J. Am. Coll. Rheumatol. 2009, 60, 2513–2523. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J. Epidemic polyarthritis and ross river virus disease. Clin. Rheum. Dis. 1986, 12, 369–388. [Google Scholar] [PubMed]
- Kang, Y.; Cai, Y. Gut microbiota and hypertension: From pathogenesis to new therapeutic strategies. Clin. Res. Hepatol. Gastroenterol. 2018, 42, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Sterling, R.; Betrapally, N.; Nixon, D.; Fuchs, M.; Daita, K.; Heuman, D.; Sikaroodi, M.; Hylemon, P.; White, M.; et al. Hcv eradication does not impact gut dysbiosis or systemic inflammation in cirrhotic patients. Aliment. Pharmacol. Ther. 2016, 44, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.M.; Adel, A.; El-Gendy, A.O.; Essam, T.M.; Aziz, R.K. Gut microbiome alterations in patients with stage 4 hepatitis c. Gut Pathog. 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Nowak, P.; Troseid, M.; Avershina, E.; Barqasho, B.; Neogi, U.; Holm, K.; Hov, J.R.; Noyan, K.; Vesterbacka, J.; Svärd, J.; et al. Gut microbiota diversity predicts immune status in hiv-1 infection. Aids 2015, 29, 2409–2418. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Nakayama, J.; Moriya, K.; Kawaratani, H.; Momoda, R.; Ito, K.; Iio, E.; Nojiri, S.; Fujiwara, K.; Yoneda, M.; et al. Gut dysbiosis associated with hepatitis c virus infection. Clin. Infect. Dis. 2018, 67, 869–877. [Google Scholar] [CrossRef] [PubMed]
| Virus | Family | Genome | Host | Disease | Model | Type of Cells | Virus Persistence and Survival in Mφ | Reaction of Mφ | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Respiratory syncytial virus (RSV) | Paramyxoviridae | (−) ssRNA | Human | Bronchiolitis and pneumonia, severe acute lower-respiratory tract disease in children | -In vitro model of persistently RSV-infected Mφ-like cell line P388D1 (MφP) -RAW264.7 Mφ -Human tissue Mφ | -Murine Mφ-cell line -Human alveolar Mφ | -RSV persist in in vitro model for over 87 passages. -Alveolar Mφ support a productive RSV infection in vitro at least for 25 days. -Lack of response of infected Mφ to the IFN-beta. | -Infected Mφ produces high level of proinflammatory cytokines (class II HLA-DR, IL-1ß and TNFα—immunofluorescent staining). -Reduced cytotoxic effect in MφP cells (activation of caspase-9 along with impairment of caspase-8 activity). | [26,27,28] |
| 2 | Influenza virus | Orthomyxoviridae | (−) ssRNA | Human | Influenza | -Autopsies -In vitro model, BALB/c mice -Human primary Mφ | -Mouse lung Mφ -Human primary Mφ | -Low virulence persistence of influenza virus in the alveolar Mφ. -Productive replication of H5N1 virus in alveolar Mφ. | -Infected Mφ produces high level IL-1ß, IL-6, TNFα (flow cytometry). -Mφ demonstrated no cytopathic changes (visual examination of monolayers). | [29,30,31] |
| 3 | Vesicular stomatitis virus (VSV) | Rhabdoviridae | (−) ssRNA | Insects, cattle, horses, pigs (zoonotic virus) | Flu-like illness in infected humans | BALB/c mice | Tissue Mφ (lymph nodes, lungs, spleens, liver, muscle) | -Mφ are not the major reservoirs of VSV gRNA at late times (>60 days). -No replication in Mφ. | NS | [32] |
| 4 | Porcine reproductive and respiratory syndrome virus (PRRSV) | Arteriviridae | (+) ssRNA | Pig | Respiratory syndrome | Pig | Porcine alveolar Mφ | Productive replication of virus in alveolar Mφ in in vivo model. | In vitro infected Mφ are protected against complement-mediated cell lysis. | [23] |
| 5 | Feline coronavirus (FCoV) | Coronaviridae | (+) ssRNA | Cats | Infectious peritonitis | Specific-pathogen-free cats | Tissue Mφ | Virus persists in tissue Mφ (mostly in colon) up to 80 days after inoculation. | NS | [33] |
| 6 | Mouse hepatitis virus (MHV) | Coronaviridae | (+) ssRNA | Mouse | Model of multiple sclerosis | Mouse | Mouse peritoneal Mφ | -Mφ disseminate virus into CNS. -Mouse peritoneal Mφ are permissive for virus. Virus persists in the cells up to 8 months. | Infected Mφ express CCR1, CCR2 and CCR5 that lead to recruitment of Mφ into the CNS. | [34,35] |
| 7 | Classical swine fever virus (CSFV) | Flaviviridae | (+) ssRNA | Swine | Lethal fever | Pig tissue monocytes and Mφ | Tissue monocytes and Mφ | Productive replication of virus in alveolar Mφ and monocytes. | NS | [36] |
| 8 | Hepatitis C virus (HCV) | Flaviviridae | (+) ssRNA | Human | Hepatitis C | -Patients -Cell culture (THP-1)/tissue Mφ | -PBMC, Mφ culture (THP-1), microglial Mφ (CD68+ and CD45+) | -HCV persists in liver Mφ and lymphocytes for up to 9 years. -Productive replication of virus in a relatively non-specific manner in Mφ. | Infected Mφ/microglial cells express higher level of IL-1α, TNFα, IL-1β, IL-12, IL-18 (PCR analysis). | [37,38] |
| 9 | Japanese encephalitis virus (JEV), West Nile virus (WNV), Dengue virus (DENV) | Flaviviridae | (+) ssRNA | Human | Neurotropic, CNS | -Raw264.7 cells -BALB/c mice -Macaques | -Murine monocyte-derived Mφ (MDM) -Perivascular Mφ | -Productive JEV and WNV replication in murine and human Mφ. -Intracellular persistence of virus in Mφ. -Flaviviruses evade and/or subvert the Mφ response to favour survival and replication. | -Infected Mφ produce TNF-α, IL-6, IFN-α and CCL2, inducible nitric oxide synthase (iNOS) and nitrotyrosine (NT) in response to JEV in vitro (immunofluorescent staining, IFN bioassay, Cytometric Bead Array). -Both human and rodent microglia produce CCL2, CXCL9 and CXCL10 upon JEV exposure (flow cytometry). | [22,39,40] |
| 10 | Zika virus (ZIKV) | Flaviviridae | (+) ssRNA | Human | Foetal brain abnormalities and microcephaly, Guillain–Barré syndrome | Primary human placental Mφ | Human placental Mφ (Hofbauer cells, HC) | -Hofbauer cells are permissive to productive ZIKV infection. | Infected HCs produce high level of IFNα, IL-6, chemokines MCP-1 and IP-10 (flow cytometry). | [41] |
| 11 | Coxsackieviruses (CVB4, CVB3) | Picornaviridae | (+) ssRNA | Human | -Type 1 diabetes; -Myocarditis | -Human MDM; -Mouse | -MDM -Pancreas Mφ -Myocardial Mφ (activation of JAK1-STAT1 and JAK3-STAT6 pathways) | -CVB4 replicates and persist in MDM and tissue Mφ. | -CVB4-infected Mφ produce high levels of pro-inflammatory cytokines (IL-6 and TNFα—ELISA) in both M-CSF MDM and GM-CSF MDM cultures. -Virus infected pancreas Mφ showed M1 polarization (Ly-6C+/CD115+—flow cytometry). -Mφ polarization depends on gender (M1 phenotype detected in males and M2a phenotype in females). | [42,43,44] |
| 12 | Encephalomyocarditis virus (EMCV) | Picornaviridae | (+) ssRNA | Rodents, pigs | -Lethal acute myocarditis, fatal illness in primates and captive wild animals | -Mouse tissue Mφ -RAW264.7, naive mouse Mφ | -Tissue Mφ (brain, heart, pancreas, kidney, Peyer’s patches, spleen, lung and thymus) | -Virus persists in the thymus Mφ up to 62 days post infection. -Productive viral replication in Mφ. | EMCV activates pro-inflammatory signalling in Mφ within minutes during virus infection and type I IFNs response afterwards. | [24,45] |
| 13 | Theiler’s murine encephalomyelitis virus (TMEV) | Picornaviridae | (+) ssRNA | Mouse | Skeletal muscle infection and inflammation, encephalomyelitis and multiple sclerosis, epilepsy | -J774.1 Mφ -RAW264.7 Mφ cell line -C57BL/6 (B6) mouse -Primary peritoneal Mφ | Mφ cell lines, tissue Mφ | -TMEV persists in Mφ during the chronic demyelinating phase. -Productive replication of TMEV in Mφ. | -Infected Mφ in wild type animals showed M1 polarization (CD45+CD11b+Ly6c+) while muscle-infiltrating Mφ displayed an immature phenotype in SHP-1-deficient mice (flow cytometry). -Infected peritoneal Mφ produce high level of IFNα and TNFα (ELISA). -TMEV replication cause restricted induced apoptosis of Mφ. | [16,46,47,48] |
| 14 | Chikungunya alphavirus (CHIKV) | Togaviridae | (+) ssRNA | Human | Articular disease/ myalgia | -Cell line -Patients -Immunocompetent cynomolgus macaques | -RAW264.7 Mφ -Perivascular synovial Mφ | -Productive replication of CHIKV in RAW264.7 Mφ but in in vivo—low viral replication and release of non-infectious viral particles. -Virus persists in the cells after 18 months of chronic disease. -Mφ are the main cellular reservoirs during the late stages of CHIKV infection in vivo. | No induced apoptosis in infected RAW264.7 cells. | [19,49,50] |
| 15 | Sindbis virus (SINV), Mayaro virus (MAYV), O’nyong-nyong virus (ONNV) and Barmah Forest virus (BFV), Ross River virus (RRV), CHIKV | Togaviridae | (+) ssRNA | Mosquitoes, marsupials, humans | Articular disease/myalgia | -Patients -RAW 264.7 Mφ | -RAW264.7 Mφ -Perivascular synovial Mφ | -Productive viral gene expression in synovial Mφ. -RRV persist in RAW 264.7 Mφ up to 170 days in vitro. | -Infected Mφ displayed M1 polarization (CD68+) in vivo. -CHIKV infection cause induced apoptosis in vivo leading to viral dissemination into apoptotic blebs. -CHIKV-infected RAW264.7 Mφ showed high production of TNF-α, IL-6 and GM-CSF (QPCR). -RRV-infected Mφ in vitro displayed restricted cytopathic effects. | [20,51,52,53] |
| 16 | Avian oncoviruses | Retroviridae | ssRNA-RT | Many species | Cancer | Chicken | Tissue Mφ, MDM | -Avian leukosis viruses persist in Mφ of peripheral blood up to about 3 years. -Avian sarcoma viruses were never found in Mφ. | NS | [14] |
| 17 | Murine leukaemia viruses (MuLVs) | Retroviridae | ssRNA-RT | Mouse | A model for non-inflammatory degeneration of the central nervous system | BALB/c and C3H mice | Tissue Mφ | Virus infects Mφ/microglia and persists during later stages (8 weeks after infection). | NS | [15] |
| 18 | Ovine lentivirus OvLV | Retroviridae | ssRNA-RT | Sheep | Encephalitis and chronic pneumonitis | Lamb | Tissue Mφ | OvLV variants persist in alveolar Mφ. | NS | [54] |
| 19 | Human immunodeficiency virus (HIV), Simian immunodeficiency virus (SIV) | Retroviridae | ssRNA-RT | Human | Immune deficiency syndrome (AID), cancer | Human (U937, THP-1) and mouse cell lines, human and monkey, macaque tissue Mφ | MDM, monocytes, tissue Mφ | -CD14+CD16+ monocytes are permissive to productive infection. -Mφ serves as a major reservoir for HIV. -Infected Mφ escape immune response. -Infected Mφ showed impaired phagocytic activity. -Mφ dissiminate HIV to CD4+ T cells and central nervous system (“Trojan horse hypothesis”). | -Infected Mφ showed M1 polarization during early stages of infection (with high production of IFN-γ, IL-2, IL-12—ELISA). There is a shift of M1 to M2 at later stages of infection (with high production of IL-4, TGF-β and IL-10—ELISA). -HIV-1 infection enhances the survival of Mφ by upregulating antiapoptotic genes through different pathways (activation of NF-kB and PI3K signalling, delay of TNF-induced apoptosis; modulation of mitochondrial pathways; increase telomerase activity). | [18,55,56] |
| 20 | Maedi-visna (MVV) | Retroviridae | ssRNA-RT | Sheep | Fatal lymphoproliferative disease | Sheep | Bone marrow monocytes, PBMC | Limited virus replication in bone marrow monocytes. | NS | [57] |
| 21 | African swine fever virus (ASFV) | Asfarviridae | dsDNA | Pig, warthogs, bushpigs, soft ticks | Lethal haemorrhagic fever | -Pig -Porcine alveolar Mφ | -Cell culture derived from bone marrow, PBMC -Alveolar and bone marrow cells | -Virus persists in tissue Mφ. -Moderate virus replication continued for at least 3 months in alveolar and bone marrow Mφs. | -Virus caused cytotoxic effect within 2–3 days in monocytes but not in Mφ (visual examination of monolayers). -Virus leads to full morphological differentiation of Mφ (visual examination of cell morphology). | [13,58] |
| 22 | Bovine herpesvirus-4 (BHV-4) | Herpesviridae | dsDNA | Cattle, rabbits | Endometritis, vulvovaginitis and mastitis | Bovine Mφ cell line (BOMAC). | Cell culture | -Virus cause cell death of the majority of BOMAC cells and persists in surviving cells. | NS | [59] |
| 23 | Cytomegalovirus (CMV) | Herpesviridae | dsDNA | Human | Chronical inflammation, cardiovascular diseases, some types of cancers | -Murine cytomegalovirus model (MCMV), MDM/Allo-MDM | -Human monocytes -MDM | -Productive replication of CMV in human Mφ up to 16 weeks but not monocytes. -Monocytes disseminate virus in organism. | -HCMV induces specific phenotype within M1/M2 continuum (skewed towards M1). Simultaneous expression of M1-associated molecules (IL-6, TNF-α, CD86) and M2-associated molecules (IL-10 and CD163) by infected Mφ. Data analysed using PCR, flow cytometry (M1 cells were CD68+, M2—CD163+), microarray analysis for more than 2000 genes. -Decoy of induced apoptosis of infected monocytes due to prolonged expression of the anti-apoptotic molecule, Mcl-1. -Infected cells escape the cellular antiviral pro-apoptotic response due to specific cytokine/chemokine expression (the “Goldilocks” phenomenon). -Infected cells utilize EGFR receptor and integrins. | Review [17,60] |
| 24 | Epstein-Barr virus infection (EBV) | Herpesviridae | dsDNA | Human | Inflammation, some types of cancers | Human cancer tissues, human smears, rhesus macaques, Mφ culture (RAW 264.7 cells), Balb/c and IL-10KO mice. | MDM, tissue Mφ, submucosal monocytes, tumour-associated Mφ (TAMs) | -EBV replicates in Mφ. -Monocytes disseminate virus in organism. | -Infected Mφ produce high level of IL-8, MCP-1 due to TLR9 and TLR-2 activation (ELISA). -Monocytes produce high level of IFNα in response to EBV (ELISA). -IL-10-dependent M2 polarization of infected TAMs (ELISA). | A book [61], review [62] |
| 25 | Human herpesvirus 6 (HHV-6) | Herpesviridae | dsDNA | Human | Multiple sclerosis | Human | -PBMC | -Latent persistence of HHV-6 in Mφs for more than 1 month. | Selective downregulation of IL-12 in infected Mφ (ELISA), which is not dependent upon productive viral infection. | [63,64,65] |
| 26 | Kaposi’s sarcoma-associated herpesvirus KSHV (HHV-8) | Herpesviridae | dsDNA | Human | Cancer | -Tumour microenvironment, cell culture. -MDM -Prostate cancer samples | Tissue Mφ, RAW 264.7 cells | -HHV-8 led to production of viral proteins in intralesional Mφ, with little production of viral DNA. -Virus persists in a latent form in Mφ/monocytes. -Lytic gene expression in Mφ in prostate stroma. | KSHV miRNAs protect Mφ from cell death through the upregulation of xCT. | [66,67,68] |
| 27 | Murine herpesvirus 72 (MHV-72) | Herpesviridae | dsDNA | Mouse | Acute infection of lung epithelial cells | Balb/c mice | Lung mononuclear cells | Virus persists in alveolar and peritoneal lung mononuclear cells and Mφ of peripheral blood up to 8 months. | NS | [69] |
| Varicella-zoster virus (VZV), simian varicella virus (SVV) | Herpesviridae | dsDNA | Human, nonhuman primates | Varicella-zoster, “multiple sclerosis-like” pathology | -Human ganglia. -Rhesus macaques. | Alveolar Mφ | -SVV IE63 proteins are present in Mφ in lymph nodes after SVV reactivation in monkeys. -SVV infects alveolar Mφ and transmit virus to T cells. | SVV-infected Mφs were CD163+ (immunofluorescence analysis) after virus reactivation but not during latency. | [70,71] | |
| 29 | Frog virus 3 (FV3) | Iridoviridae | dsDNA | Amphibian species | Acute systemic FV3 infection | Xenopus laevis | Peritoneal Mφ | FV3 persist in peritoneal Mφ in vitro. | No cytopathic effect on infected Mφ. | [25] |
| Virus | Entry Type | Receptor(s) Used for Viral Entry/Attachment | Virus Fate | Reference | |
|---|---|---|---|---|---|
| 1 | Respiratory syncytial virus | Macropinosome formation | Nucleolin, heparan sulphate proteoglycans | Replication | [75,81] |
| 2 | Influenza virus | Endocytosis, Phagocytosis | Sialic acid sugars | Replication | [76] |
| 3 | Vesicular stomatitis virus | endocytosis | Phosphatidylserine | No replication | [32,79] |
| 4 | Mouse hepatitis virus | Phagocytosis | * Sialic acid sugars and glycolipids N- * acetilneuraminic acid receptor | No replication | [34,77] |
| 5 | Japanese encephalitis virus, West Nile virus, Dengue virus | Phagocytosis | * DC-SIGN or * DC-SIGNR TLR-2, TLR-3 and TLR-7, RIG-I | Replication | [39,40] |
| 6 | Coxsackieviruses | Macropinocytosis | * CAR and IgG Fc fraction receptors (FcγRII and FcγRIII), occludin | Replication | [42,73,84] |
| 7 | Theiler’s murine encephalomyelitis virus | Endocytosis | Sialic acid sugars | Replication | [46,78] |
| 8 | Human immunodeficiency virus, Simian immunodeficiency virus | Endocytosis, macropinocytosis | Human mannose receptor C-type 1 * CD4 and a * coreceptors CXCR4 or CCR5, heparan sulphate proteoglycans | Replication | [11,85,86] |
| 9 | Cytomegalovirus | Endocytosis | Heparin sulphate proteoglycans following by the binding to the β1 and β3 integrins, EGFR, TLR2, TLR3 and TLR9 (murine CMV) | Replication | [17,82] |
| 10 | Epstein-Barr virus infection | Endocytosis | * CR2 or CD21, TLR2 and TLR3 | Replication | [82] |
| 11 | Human herpesvirus 6 | Endocytosis | * CD46 | Non-productive infection | [63,87] |
| 12 | Kaposi’s sarcoma-associated herpesvirus | * Macropinosome membrane fusion | xCT, DC-SIGN, * surface heparan sulphate, * integrin α3β1 (CD49c/29)? | Replication | [66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88] |
| 13 | Varicella-zoster virus, Simian varicella virus | Fusion with the plasma membrane or endocytosis | * Mannose-6-phosphate receptor, myelin-associated glycoprotein | not clear | [71,89] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikitina, E.; Larionova, I.; Choinzonov, E.; Kzhyshkowska, J. Monocytes and Macrophages as Viral Targets and Reservoirs. Int. J. Mol. Sci. 2018, 19, 2821. https://doi.org/10.3390/ijms19092821
Nikitina E, Larionova I, Choinzonov E, Kzhyshkowska J. Monocytes and Macrophages as Viral Targets and Reservoirs. International Journal of Molecular Sciences. 2018; 19(9):2821. https://doi.org/10.3390/ijms19092821
Chicago/Turabian StyleNikitina, Ekaterina, Irina Larionova, Evgeniy Choinzonov, and Julia Kzhyshkowska. 2018. "Monocytes and Macrophages as Viral Targets and Reservoirs" International Journal of Molecular Sciences 19, no. 9: 2821. https://doi.org/10.3390/ijms19092821
APA StyleNikitina, E., Larionova, I., Choinzonov, E., & Kzhyshkowska, J. (2018). Monocytes and Macrophages as Viral Targets and Reservoirs. International Journal of Molecular Sciences, 19(9), 2821. https://doi.org/10.3390/ijms19092821