Selenite Reduction and the Biogenesis of Selenium Nanoparticles by Alcaligenes faecalis Se03 Isolated from the Gut of Monochamus alternatus (Coleoptera: Cerambycidae)
Abstract
1. Introduction
2. Results and Discussion
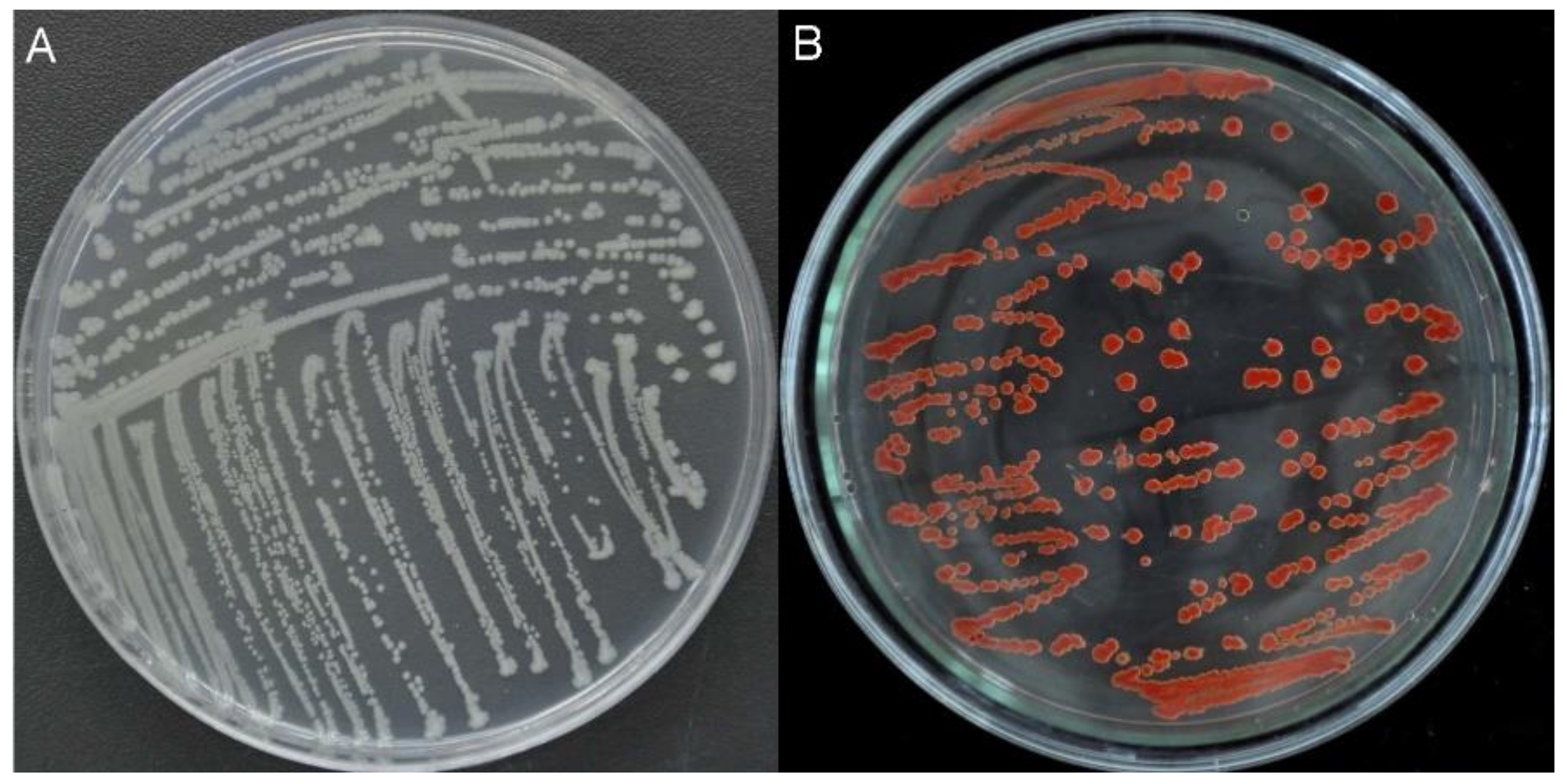
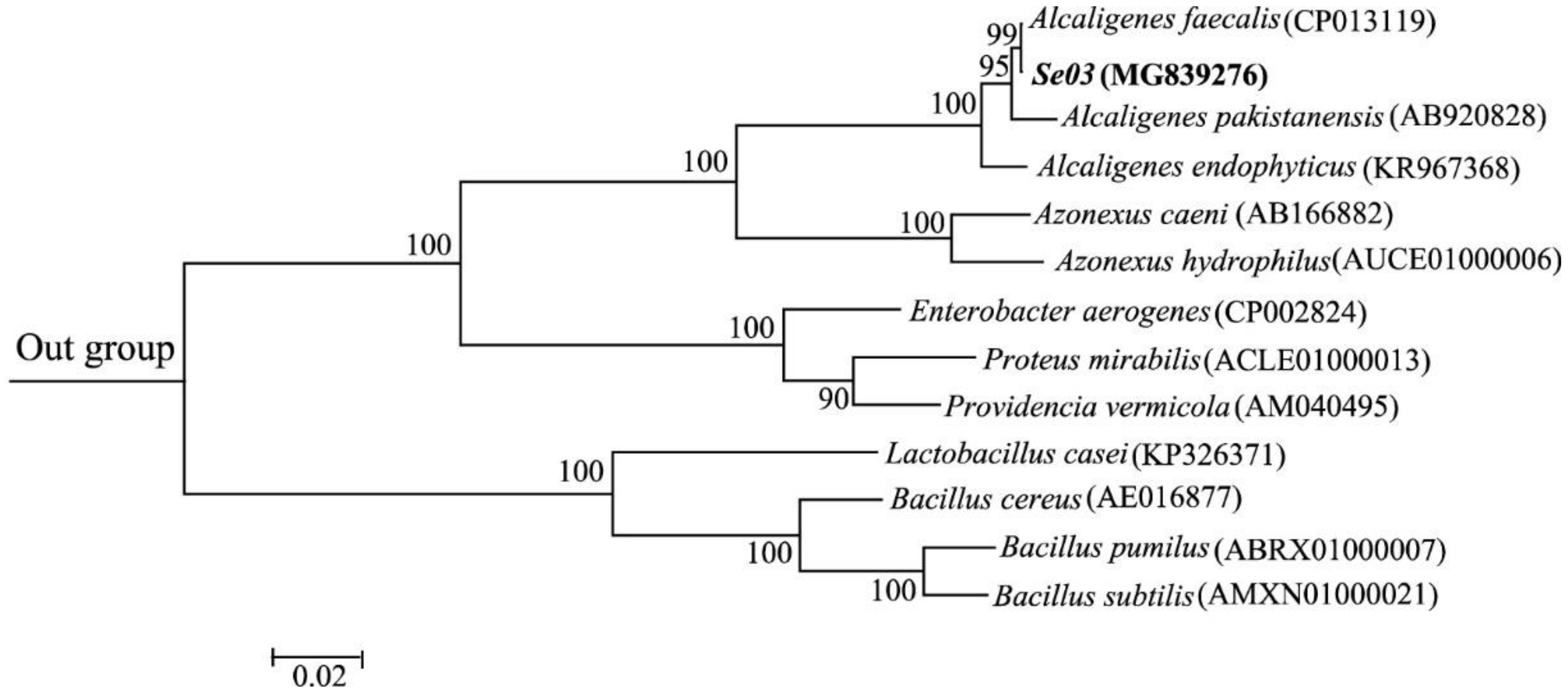
2.1. Isolation, Characterization, and Identification of Bacterial Strains
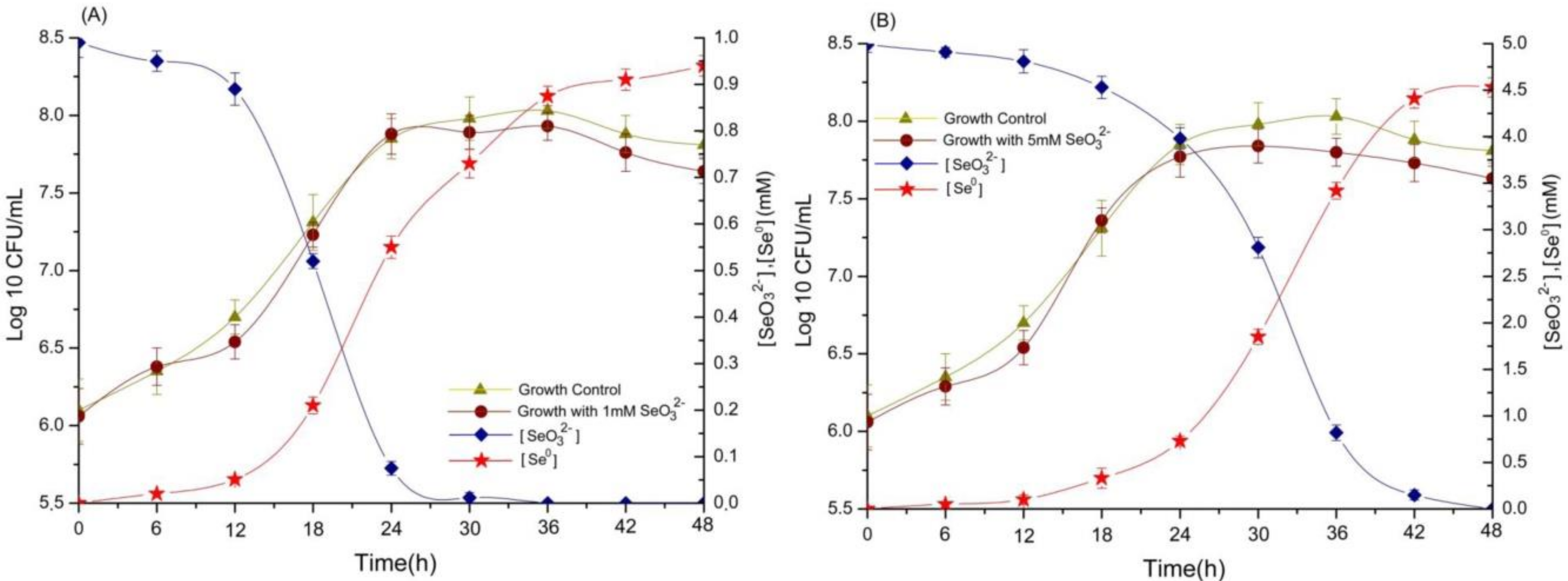
2.2. Selenite Reduction and the Formation of Elemental Selenium
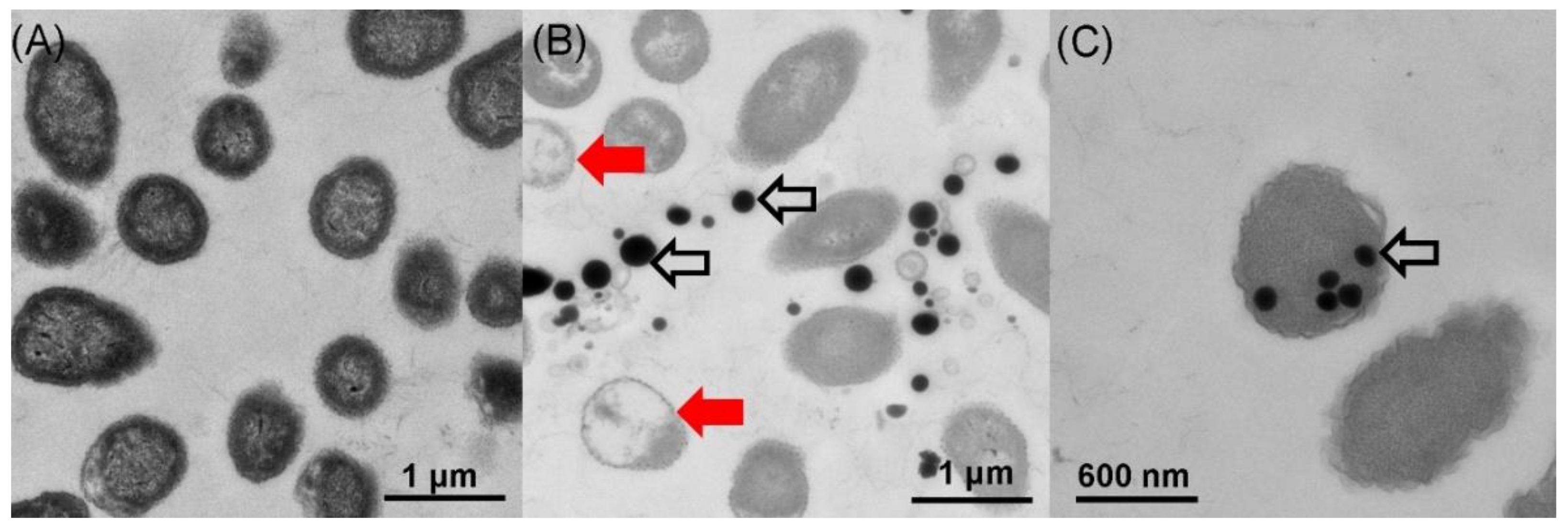
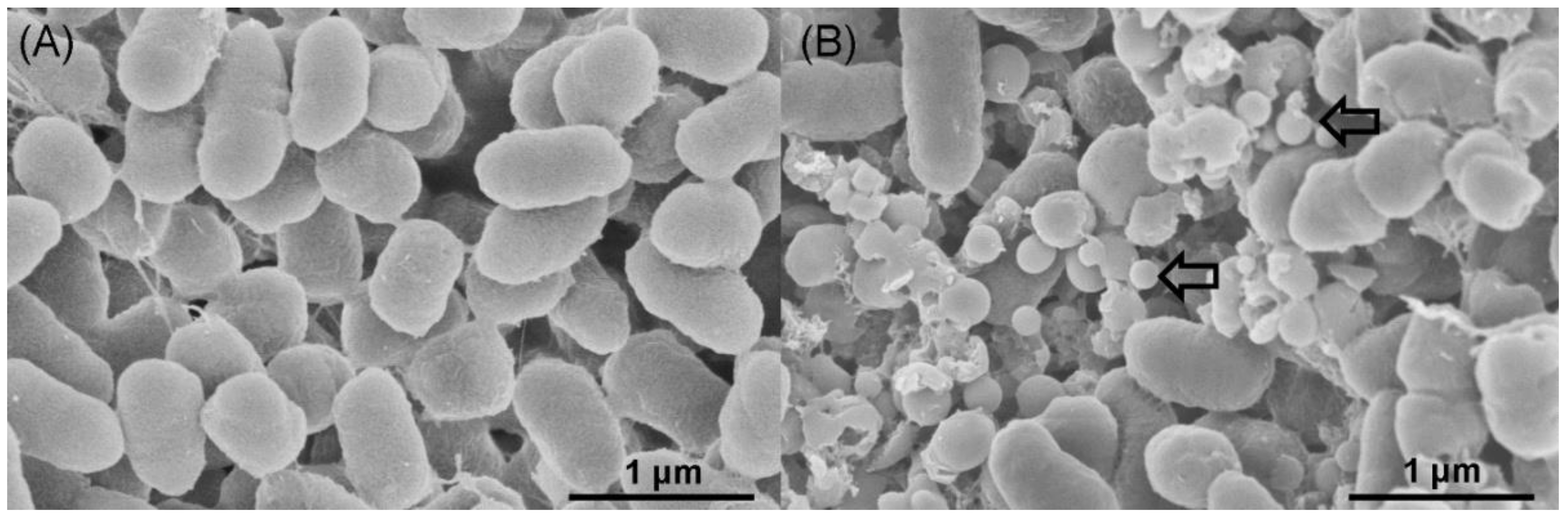
2.3. Location of SeNPs within Cell Cultures of A. faecalis Se03
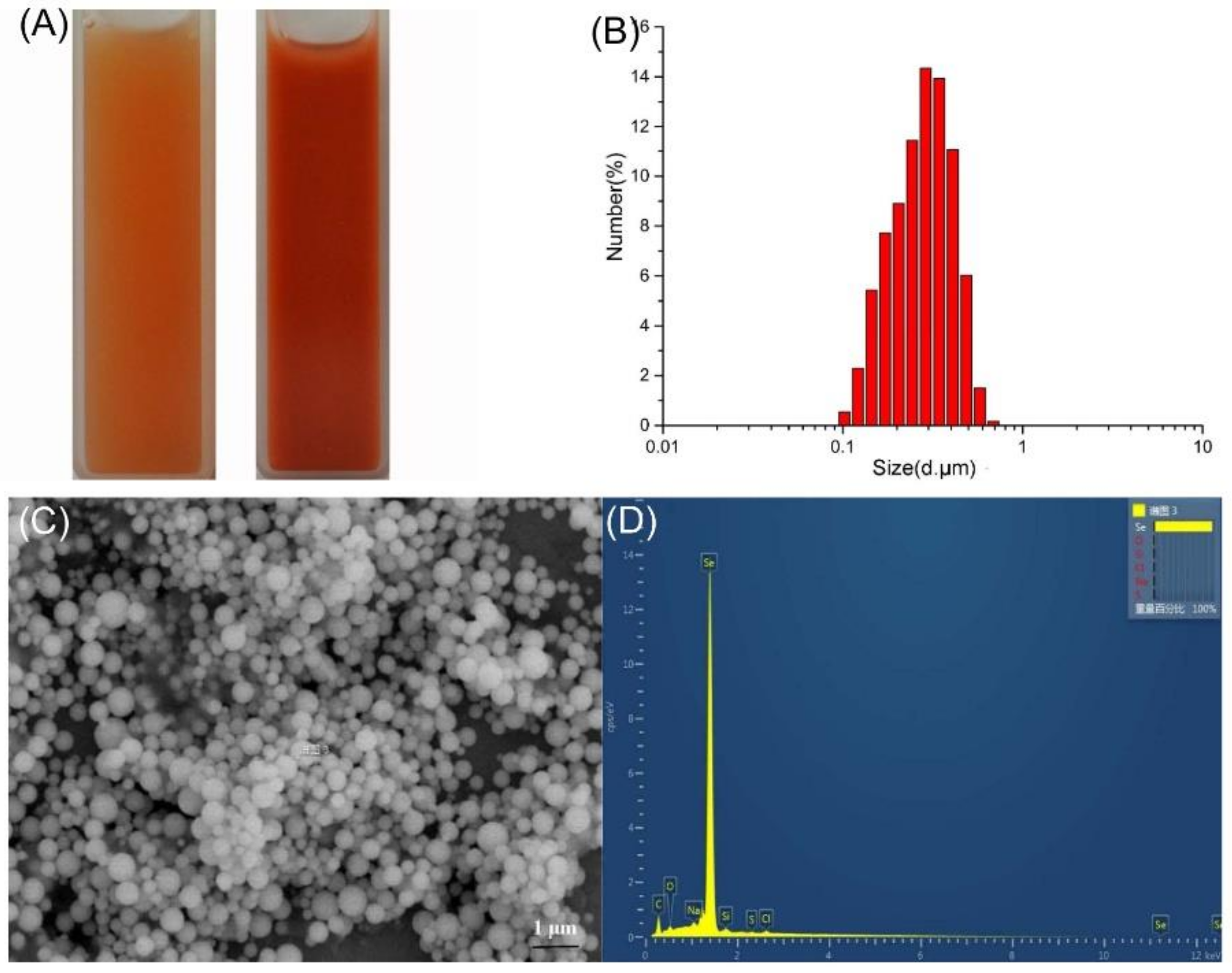
2.4. Characterization of SeNPs Produced by A. faecalis Se03
2.4.1. DLS Analyses and SEM-EDX
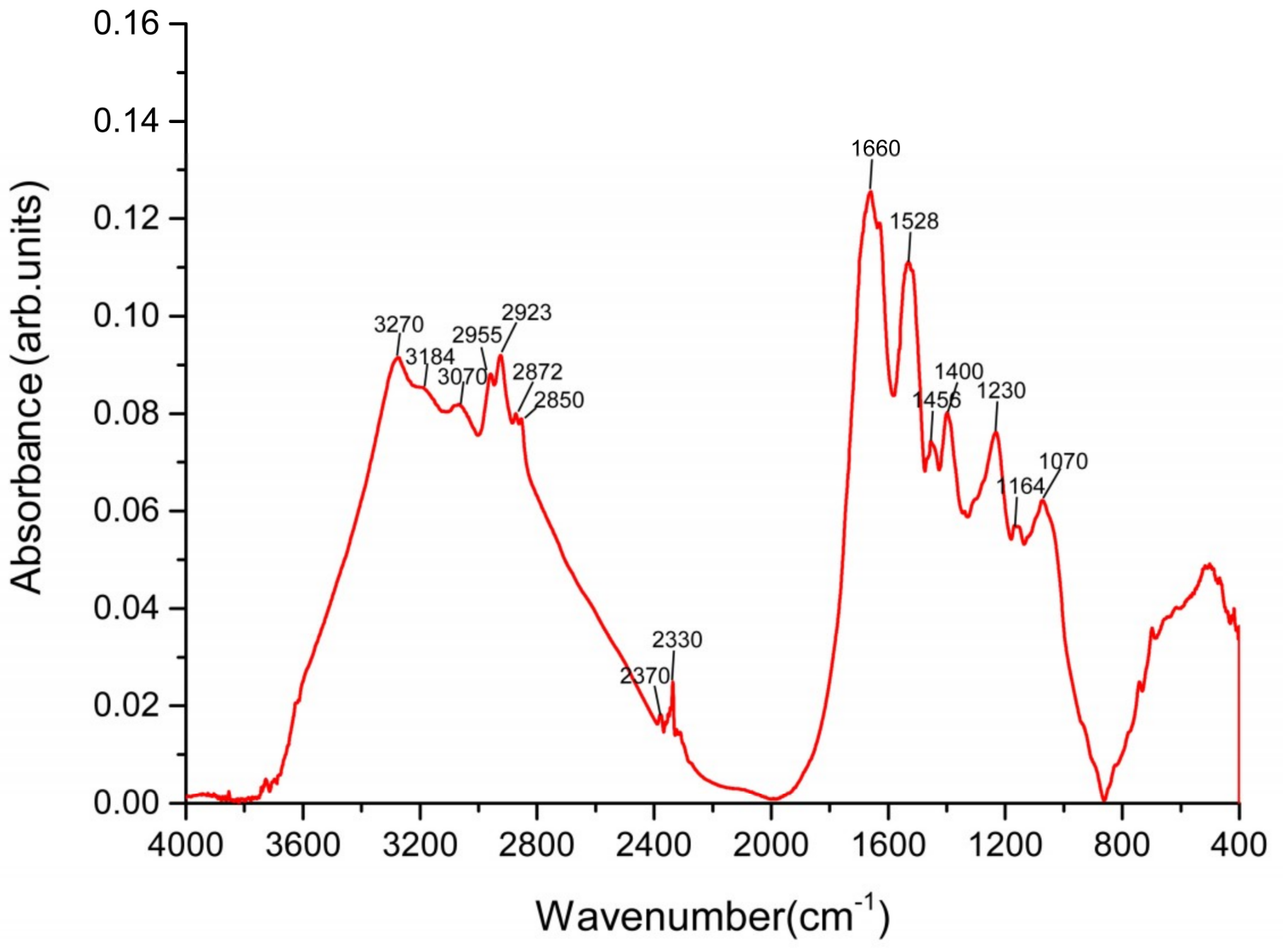
2.4.2. FTIR Analysis
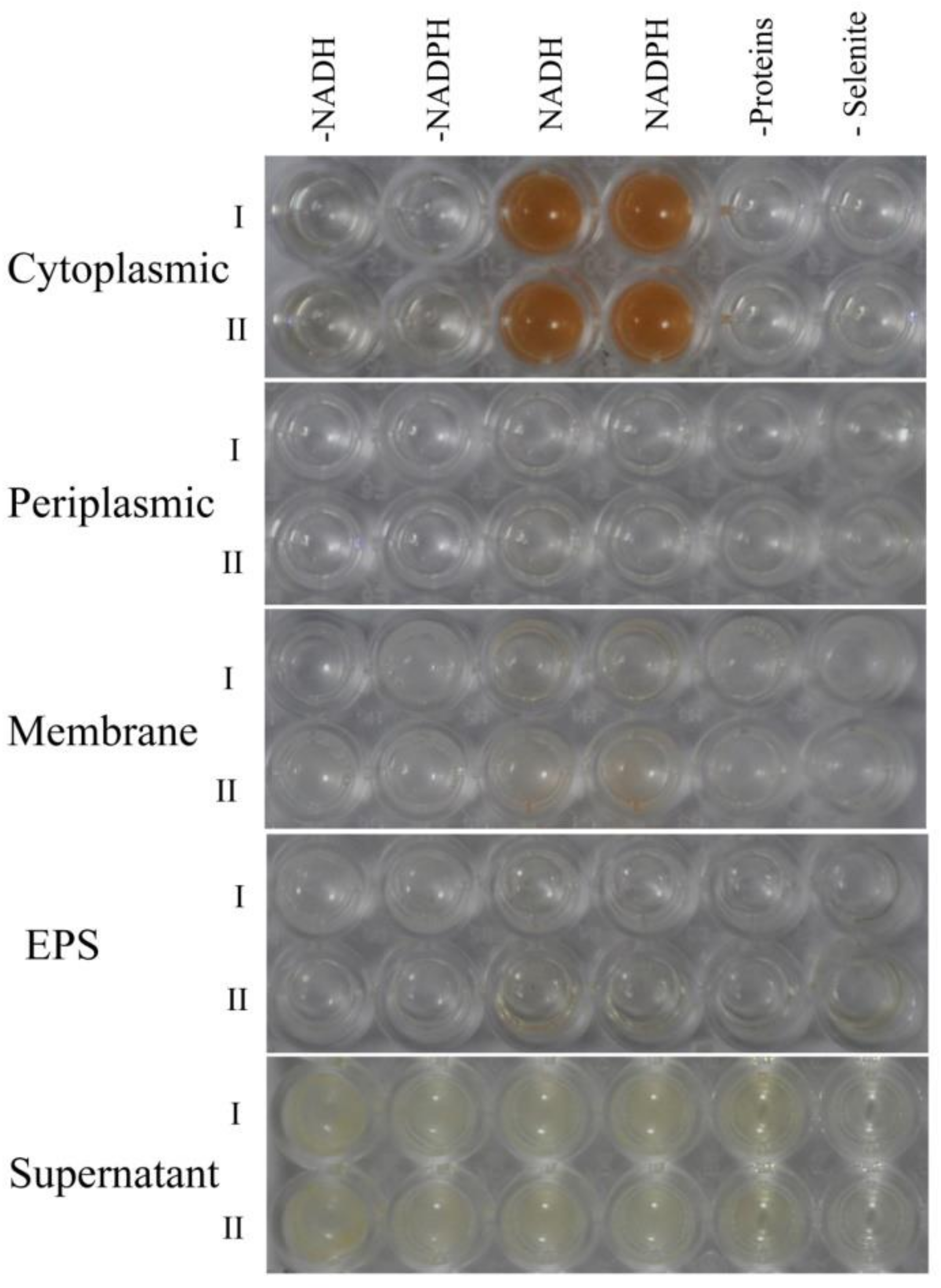
2.5. Biocatalytic Selenite Reduction Activity Assays
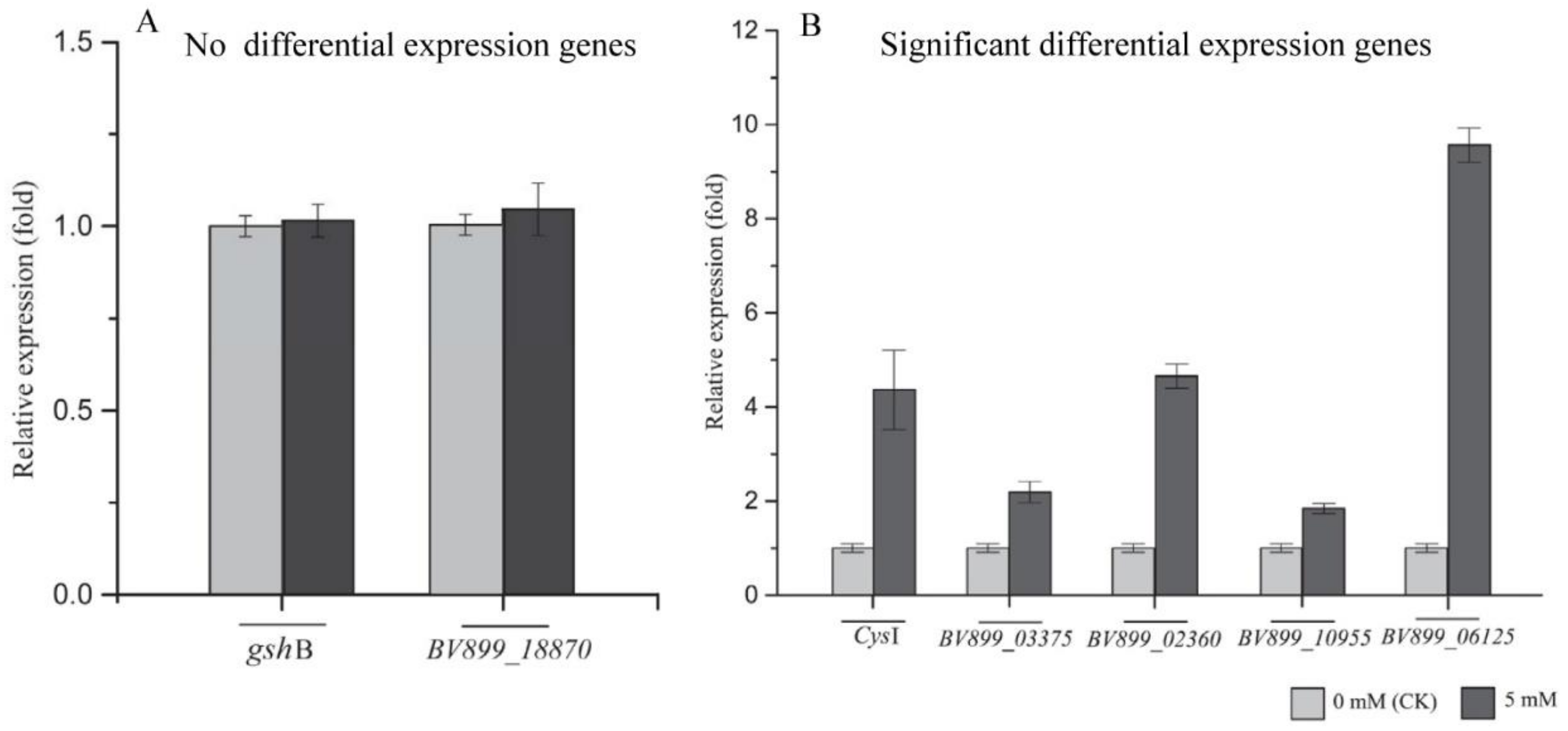
2.6. Real-time PCR Analysis
3. Materials and Methods
3.1. Chemicals and Culture Medium
3.2. Bacterial Isolation and Identification
3.3. Selenite Sensitivity Tests
3.4. Evaluation of the Reduction Efficiency of Se03
3.4.1. Evaluation of Bacterial Growth Dynamics after Exposure to SeO32−
3.4.2. Assessment of SeO32− Bio-Reduction Efficiency
3.4.3. Se0 Content Analysis
3.5. Location of SeNPs within the Bacterial Cells
3.5.1. TEM Analysis
3.5.2. SEM Analysis
3.6. Analysis of SeNPs
3.6.1. Preparation of Biogenic SeNPs
3.6.2. Dynamic Light Scattering (DLS) Analysis
3.6.3. Scanning Electron Microscopy and Electron Dispersive Spectrometry (SEM-EDS) Analysis
3.6.4. Fourier Transform Infrared (FTIR) Spectroscopy Analysis
3.7. Biocatalytic Selenite Reduction Assays
3.7.1. Protein Extraction
3.7.2. EPS Extraction
3.7.3. Supernatant Preparation
3.7.4. Selenite Reducing Reduction Activity Assays
3.8. Real-Time Quantitative PCR (qPCR)
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SeNPs | Se nanoparticles |
| Se0 | Elemental Selenium |
| TEM | Transmission electron microscopy |
| SEM | Scanning electron microscopy |
| EDX | Energy dispersive X-ray analysis |
| MIC | Minimum inhibitory concentration |
| NADH | Nicotinamide adenine dinucleotide |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| qPCR | Real-time quantitative PCR |
References
- Hunter, W.J. Pseudomonas seleniipraecipitans proteins potentially involved in selenite reduction. Curr. Microbiol. 2014, 69, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Kessi, J.; Ramuz, M.; Wehrli, E.; Spycher, M.; Bachofen, R. Reduction of selenite and detoxification of elemental selenium by the phototrophic bacterium Rhodospirillum rubrum. Appl. Environ. Microbiol. 1999, 65, 4734–4740. [Google Scholar] [PubMed]
- Bajaj, M.; Schmidt, S.; Winter, J. Formation of se (0) nanoparticles by Duganella sp. And Agrobacterium sp. Isolated from se-laden soil of north-east punjab, india. Microb. Cell Fact. 2012, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.K.; Park, Y.; Yu, J.; Lee, T. Microbial selenite reduction with organic carbon and electrode as sole electron donor by a bacterium isolated from domestic wastewater. Bioresource. Technol. 2016, 212, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Whanger, P.D. Selenocompounds in plants and animals and their biological significance. J. Am. Coll. Nutr. 2002, 21, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Sors, T.G.; Ellis, D.R.; Salt, D.E. Selenium uptake, translocation, assimilation and metabolic fate in plants. Photosynth. Res. 2005, 86, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Nancharaiah, Y.V.; Lens, P.N.L. Selenium biomineralization for biotechnological applications. Trends Biotechnol. 2015, 33, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Hashimoto, H.; Nakayama, M. Removal of selenium(vi) from aqueous solution with polyamine-type weakly basic ion exchange resin. Sep. Sci. Technol. 2007, 42, 3155–3167. [Google Scholar] [CrossRef]
- Chung, J.; Nerenberg, R.; Rittmann, B.E. Bioreduction of selenate using a hydrogen-based membrane biofilm reactor. Environ. Sci. Technol. 2006, 40, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Khoei, N.S.; Lampis, S.; Zonaro, E.; Yrjälä, K.; Bernardi, P.; Vallini, G. Insights into selenite reduction and biogenesis of elemental selenium nanoparticles by two environmental isolates of Burkholderia fungorum. New Biotechnol. 2017, 34, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yee, N.; Ma, J.; Dalia, A.; Boonfueng, T.; Kobayashi, D. Se(VI) reduction and the precipitation of se(0) by the facultative bacterium Enterobacter cloacae SLD1a-1 are regulated by FNR. Appl. Environ. Microbiol. 2007, 73, 1914–1920. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Llamosas, H.; Castro, L.; Blázquez, M.L.; Díaz, E.; Carmona, M. Speeding up bioproduction of selenium nanoparticles by using Vibrio natriegens as microbial factory. Sci. Rep. 2017, 7, 16046. [Google Scholar] [CrossRef] [PubMed]
- Avendaño, R.; Chaves, N.; Fuentes, P.; Sánchez, E.; Jiménez, J.I.; Chavarría, M. Production of selenium nanoparticles in Pseudomonas putida kt2440. Sci. Rep. 2016, 6, 37155. [Google Scholar] [CrossRef] [PubMed]
- Dhanjal, S.; Cameotra, S.S. Aerobic biogenesis of selenium nanospheres by Bacillus cereus isolated from coalmine soil. Microb. Cell Fact. 2010, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Lampis, S.; Zonaro, E.; Bertolini, C.; Bernardi, P.; Butler, C.S.; Vallini, G. Delayed formation of zero-valent selenium nanoparticles by Bacillus mycoides SelTE01 as a consequence of selenite reduction under aerobic conditions. Microb. Cell Fact. 2014, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Li, D.B.; Cheng, Y.Y.; Wu, C.; Li, W.W.; Li, N.; Yang, Z.C.; Tong, Z.H.; Yu, H.Q. Selenite reduction by Shewanella oneidensis MR-1 is mediated by fumarate reductase in periplasm. Sci. Rep. 2014, 4, 3735. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Błażejak, S.; Gientka, I.; Bzducha-Wróbel, A. Accumulation and metabolism of selenium by yeast cells. Appl. Microbiol. Biotechnol. 2015, 99, 5373–5382. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-lamana, J.; Abadálvaro, I.; Bierla, K.; Laborda, F.; Szpunar, J.; Lobinski, R. Detection and characterization of biogenic selenium nanoparticles in selenium-rich yeast by single particle ICPMS. J. Anal. At. Spectrom. 2018, 33, 452–460. [Google Scholar] [CrossRef]
- Fernández-Llamosas, H.; Castro, L.; Blázquez, M.L.; Díaz, E.; Carmona, M. Biosynthesis of selenium nanoparticles by Azoarcus sp. CIB. Microb. Cell Fact. 2016, 15, 109. [Google Scholar] [CrossRef] [PubMed]
- Wadhwani, S.A.; Shedbalkar, U.U.; Singh, R.; Chopade, B.A. Biogenic selenium nanoparticles: Current status and future prospects. Appl. Microbiol. Biotechnol. 2016, 100, 2555–2566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, Z.; Liu, H.; Zhang, L.; Gao, P.; Li, D. Biosynthesis and structural characteristics of selenium nanoparticles by Pseudomonas alcaliphila. Colloids Surf. B Biointerfaces 2011, 88, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Che, L.; Dong, Y.; Wu, M.; Zhao, Y.; Liu, L.; Zhou, H. Characterization of selenite reduction by Lysinibacillus sp. ZYM-1 and photocatalytic performance of biogenic selenium nanospheres. ACS Sustain. Chem. Eng. 2017, 5, 2535–2543. [Google Scholar] [CrossRef]
- Ramamurthy, C.; Sampath, K.S.; Arunkumar, P.; Kumar, M.S.; Sujatha, V.; Premkumar, K.; Thirunavukkarasu, C. Green synthesis and characterization of selenium nanoparticles and its augmented cytotoxicity with doxorubicin on cancer cells. Bioprocess Biosyst. Eng. 2013, 36, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.; Basu, A.; Sen, T.; Biswas, J.; Bhattacharya, S. Nano-se as a novel candidate in the management of oxidative stress related disorders and cancer. Nucleus 2017, 60, 137–145. [Google Scholar] [CrossRef]
- Srivastava, P.; Braganca, J.M.; Kowshik, M. In vivo synthesis of selenium nanoparticles by Halococcus salifodinae bk18 and their anti-proliferative properties against hela cell line. Biotechnol. Prog. 2014, 30, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.-S.; Hirai, M.; Shoda, M. Characteristics of ammonium removal by heterotrophic nitrification-aerobic denitrification by Alcaligenes faecalis no. 4. J. Biosci. Bioeng. 2005, 100, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Singha, L.P.; Kotoky, R.; Pandey, P. Draft genome sequence of Alcaligenes faecalis BDB4, a polyaromatic hydrocarbon-degrading bacterium isolated from crude oil-contaminated soil. Genome Announc. 2017, 5, e01346-17. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Rong, Y.; Rui, W.; Dan, W.; Wang, G.; Zheng, S. Reduction of selenite to Se(0) nanoparticles by filamentous bacterium Streptomyces sp. ES2-5 isolated from a selenium mining soil. Microb. Cell Fact. 2016, 15, 157. [Google Scholar] [CrossRef] [PubMed]
- Lampis, S.; Zonaro, E.; Bertolini, C.; Cecconi, D.; Monti, F.; Micaroni, M.; Turner, R.J.; Butler, C.S.; Vallini, G. Selenite biotransformation and detoxification by Stenotrophomonas maltophilia SelTE02: Novel clues on the route to bacterial biogenesis of selenium nanoparticles. J. Hazard. Mater. 2017, 324, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.W.; Zhang, H.Y.; Marshall, S.; Jackson, T.A. The scarab gut: A potential bioreactor for bio-fuel production. Insect Sci. 2010, 17, 175–183. [Google Scholar] [CrossRef]
- Undugoda, L.J.S.; Kannangara, S.; Sirisena, D.M. Genetic basis of naphthalene and phenanthrene degradation by phyllosphere bacterial strains Alcaligenes faecalis and Alcaligenes sp. 11SO. J. Bioremediat. Biodegrad. 2016, 7, 2. [Google Scholar]
- Silver, S.; Phung, L.T. Genes and enzymes involved in bacterial oxidation and reduction of inorganic arsenic. Appl. Environ. Microbiol. 2005, 71, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Shakoori, F.R.; Tabassum, S.; Rehman, A.; Shakoori, A.R. Isolation and characterization of Cr6+ reducing bacteria and their potential use in bioremediation of chromium containing wastewater. Pak. J. Zool. 2010, 42, 651–658. [Google Scholar]
- Staicu, L.C.; Ackerson, C.J.; Cornelis, P.; Ye, L.; Berendsen, R.L.; Hunter, W.J.; Noblitt, S.D.; Henry, C.S.; Cappa, J.J.; Montenieri, R.L. Pseudomonas moraviensis subsp. Stanleyae, a bacterial endophyte of hyperaccumulator stanleya pinnata, is capable of efficient selenite reduction to elemental selenium under aerobic conditions. J. Appl. Microbiol. 2015, 119, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Van Fleet-Stalder, V.; Chasteen, T.G.; Pickering, I.J.; George, G.N.; Prince, R.C. Fate of selenate and selenite metabolized by Rhodobacter sphaeroides. Appl. Environ. Microbiol. 2000, 66, 4849–4853. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Błażejak, S.; Bzducha-Wróbel, A.; Kurcz, A. Effects of selenium on morphological changes in Candida utilis atcc 9950 yeast cells. Biol. Trace Element Res. 2016, 169, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Switzer, B.J.; Burns, B.A.; Buzzelli, J.; Stolz, J.F.; Oremland, R.S. Bacillus arsenicoselenatis, sp. nov. and Bacillus selenitireducens, sp. nov.: two haloalkaliphiles, from Mono Lake, California that respire oxyanions, of selenium and arsenic. Arch. Microbiol. 1998, 171, 19–30. [Google Scholar] [CrossRef]
- Debieux, C.M.; Dridge, E.J.; Mueller, C.M.; Splatt, P.; Paszkiewicz, K.; Knight, I.; Florance, H.; Love, J.; Titball, R.W.; Lewis, R.J.; et al. A bacterial process for selenium nanosphere assembly. Proc. Natl. Acad. Sci. USA 2011, 108, 13480–13485. [Google Scholar] [CrossRef] [PubMed]
- Hunter, W.J. A rhizobium selenitireducens protein showing selenite reductase activity. Curr. Microbiol. 2014, 68, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Wu, S.; Li, N.; Wang, D.; Zheng, S.; Wang, G. Novel bacterial selenite reductase csrf responsible for se(iv) and cr(vi) reduction that produces nanoparticles in Alishewanella sp. WH16-1. J. Hazard. Mater. 2018, 342, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Kulp, A.; Kuehn, M.J. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 2010, 64, 163–184. [Google Scholar] [CrossRef] [PubMed]
- Pearce, C.I.; Coker, V.S.; Charnock, J.M.; Pattrick, R.A.; Mosselmans, J.F.; Law, N.; Beveridge, T.J.; Lloyd, J.R. Microbial manufacture of chalcogenide-based nanoparticles via the reduction of selenite using Veillonella atypica: An in situ EXAFS study. Nanotechnology 2008, 19, 155603. [Google Scholar] [CrossRef] [PubMed]
- Nancharaiah, Y.V.; Lens, P.N.L. Ecology and biotechnology of selenium-respiring bacteria. Microbiol. Mol. Biol. Rev. 2015, 79, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Staicu, L.C.; Barton, L.L. Bacterial metabolism of selenium-for survival or profit. In Bioremediation of Selenium Contaminated Wastewater; Van Hullebusch, E., Ed.; Spinger: Cham, Switzerland; Heidelberg, Germany, 2017; pp. 1–31. [Google Scholar]
- Tomei, F.A.; Barton, L.L.; Lemanski, C.L.; Zocco, T.G.; Fink, N.H.; Sillerud, L.O. Transformation of selenate and selenite to elemental selenium by Desulfovibrio desulfuricans. J. Industr. Microbiol. 1995, 14, 329–336. [Google Scholar] [CrossRef]
- Song, D.; Li, X.; Cheng, Y.; Xiao, X.; Lu, Z.; Wang, Y.; Wang, F. Aerobic biogenesis of selenium nanoparticles by Enterobacter cloacae Z0206 as a consequence of fumarate reductase mediated selenite reduction. Sci. Rep. 2017, 7, 3239. [Google Scholar] [CrossRef] [PubMed]
- Tugarova, A.V.; Kamnev, A.A. Proteins in microbial synthesis of selenium nanoparticles. Talanta 2017, 174, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, V.; Hillestrøm, P.R.; Patil, K.; Larsen, E.H.; Olsson, L. The interplay between sulphur and selenium metabolism influences the intracellular redox balance in Saccharomyces cerevisiae. FEMS Yeast Res. 2012, 12, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Tetteh, A.Y.; Sun, K.H.; Hung, C.Y.; Kittur, F.S.; Ibeanu, G.C.; Williams, D.; Xie, J. Transcriptional response of selenopolypeptide genes and selenocysteine biosynthesis machinery genes in Escherichia coli during selenite reduction. Int. J. Microbiol. 2014, 394835. [Google Scholar]
- Sarret, G.; Avoscan, L.; Carrière, M.; Collins, R.; Geoffroy, N.; Carrot, F.; Covès, J.; Gouget, B. Chemical forms of selenium in the metal-resistant bacterium Ralstonia metallidurans CH34 exposed to selenite and selenate. Appl. Environ. Microbiol. 2005, 71, 2331–2337. [Google Scholar] [CrossRef] [PubMed]
- Harrison, G.; Curle, C.; Laishley, E.J. Purification and characterization of an inducible dissimilatory type sulfite reductase from Clostridium pasteurianum. Arch. Microbiol. 1984, 138, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Björnstedt, M.; Holmgren, A. Selenite is a substrate for calf thymus thioredoxin reductase and thioredoxin and elicits a large non-stoichiometric oxidation of NADPH in the presence of oxygen. Eur. J. Biochem. 2010, 207, 435–439. [Google Scholar] [CrossRef]
- Bébien, M.; Lagniel, G.; Garin, J.; Touati, D.; Verméglio, A.; Labarre, J. Involvement of superoxide dismutases in the response of Escherichia coli to selenium oxides. J. Bacteriol. 2002, 184, 1556–1564. [Google Scholar] [CrossRef] [PubMed]
- Lenz, M.; Kolvenbach, B.; Gygax, B.; Moes, S.; Corvini, P.F. Shedding light on selenium biomineralization: Proteins associated with bionanominerals. Appl. Environ. Microbiol. 2011, 77, 4676–4680. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Sheng, P.; Zhang, H. Isolation and identification of cellulolytic bacteria from the gut of Holotrichia parallela larvae (coleoptera: Scarabaeidae). Int. J. Mol. Sci. 2012, 13, 2563–2577. [Google Scholar] [CrossRef] [PubMed]
- Holt, J.G.; Krieg, N.R.; Snealth, P.H.A.; Staley, J.T.; Williams, S.T. Bergey’s Manual of Determinative Bacteriology, 9th ed.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2000; pp. 175–289. [Google Scholar]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing ezbiocloud: A taxonomically united database of 16s rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. Mega7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, F.; Ahmad, R.; Ashraf, M.Y.; Waraich, E.A.; Khan, S.Z. Effect of selenium foliar spray on physiological and biochemical processes and chemical constituents of wheat under drought stress. Ecotoxicol. Environ. Saf. 2015, 113, 191–200. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Result | Characteristic | Result |
|---|---|---|---|
| Gram-staining | − | Assimilation of | |
| Nitrite reduction | + | Glycine | − |
| Motility | + | Lactose | + |
| Oxidase | + | Maltose | + |
| Catalase | + | d-fructose | + |
| Indole test | − | d-Raffinose | + |
| Nitrate reduction | + | Cellobiose | + |
| Urease | + | Rhamnose | + |
| Hydrolysis of | Sucrose | + | |
| Starch | − | d-xylose | − |
| Casein | − | Arginine dihydrolase | + |
| Gelatin | − | Growth in 5% NaCl | − |
| Growth at 5 °C | + | Growth in 10% NaCl | − |
| Growth at 42 °C | + |
| Target Gene | Primer Sequence | Product Size (bp) |
|---|---|---|
| Glutathione synthetase (A0A1Y1PZ31) | Forward: 5′-CCCAAAGTCGGGTTCGT-3′ Reverse: 5′-CAAGTGCGTGGAATAGGAGTA-3′ | 181 |
| Sulfite reductase (A0A1Y1PQF9) | Forward: 5′-TCAAGAGTGGGCTGACAAGA-3′ Reverse: 5′-CACATCATTCAAGGGAGGC-3′ | 186 |
| Sulfate transporter subunit (A0A1Y1PY15) | Forward: 5′-CAAAGAGCAAACGGGTGA-3′ Reverse: 5′-ACAATCGTGGAGGTGTAAGG-3′ | 209 |
| Flavoprotein sulfite reductase (A0A1Y1PQH1) | Forward: 5′-TTCCTGCCGCCCAATCTA-3′ Reverse: 5′-TGCCTTCCAGCGACAACTC-3′ | 161 |
| Thioredoxin reductase (A0A1Y1PXH0) | Forward: 5′-CAGGCTGCGGCGATTCAA-3′ Reverse: 5′-TCTGGGCGTCCTCGGTCAA-3′ | 249 |
| Peroxiredoxin (A0A1Y1PUT6) | Forward: 5′-CATTGAAACCGCCACAGA-3′ Reverse: 5′-CGCCCATTACGAAAGGAT-3′ | 223 |
| Superoxide dismutase (A0A1Y1PZA2) | Forward: 5′-CCCGATGCCGATAGCACCCT-3′ Reverse: 5′-ACTGAGCCAAGCCCAGCCAC-3′ | 134 |
| 16S r RNA | Forward: 5′-AGAGTTTGATCCTGGCTCAG-3′ Reverse: 5′-CTGCTGCCTCCCGTAGGAGT-3′ | 330 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Shu, X.; Zhou, Q.; Fan, T.; Wang, T.; Chen, X.; Li, M.; Ma, Y.; Ni, J.; Hou, J.; et al. Selenite Reduction and the Biogenesis of Selenium Nanoparticles by Alcaligenes faecalis Se03 Isolated from the Gut of Monochamus alternatus (Coleoptera: Cerambycidae). Int. J. Mol. Sci. 2018, 19, 2799. https://doi.org/10.3390/ijms19092799
Wang Y, Shu X, Zhou Q, Fan T, Wang T, Chen X, Li M, Ma Y, Ni J, Hou J, et al. Selenite Reduction and the Biogenesis of Selenium Nanoparticles by Alcaligenes faecalis Se03 Isolated from the Gut of Monochamus alternatus (Coleoptera: Cerambycidae). International Journal of Molecular Sciences. 2018; 19(9):2799. https://doi.org/10.3390/ijms19092799
Chicago/Turabian StyleWang, Yuting, Xian Shu, Qing Zhou, Tao Fan, Taichu Wang, Xue Chen, Minghao Li, Yuhan Ma, Jun Ni, Jinyan Hou, and et al. 2018. "Selenite Reduction and the Biogenesis of Selenium Nanoparticles by Alcaligenes faecalis Se03 Isolated from the Gut of Monochamus alternatus (Coleoptera: Cerambycidae)" International Journal of Molecular Sciences 19, no. 9: 2799. https://doi.org/10.3390/ijms19092799
APA StyleWang, Y., Shu, X., Zhou, Q., Fan, T., Wang, T., Chen, X., Li, M., Ma, Y., Ni, J., Hou, J., Zhao, W., Li, R., Huang, S., & Wu, L. (2018). Selenite Reduction and the Biogenesis of Selenium Nanoparticles by Alcaligenes faecalis Se03 Isolated from the Gut of Monochamus alternatus (Coleoptera: Cerambycidae). International Journal of Molecular Sciences, 19(9), 2799. https://doi.org/10.3390/ijms19092799






