K+ and Rb+ Affinities of the Na,K-ATPase α1 and α2 Isozymes: An Application of ICP-MS for Quantification of Na+ Pump Kinetics in Myofibers
Abstract
1. Introduction
2. Results
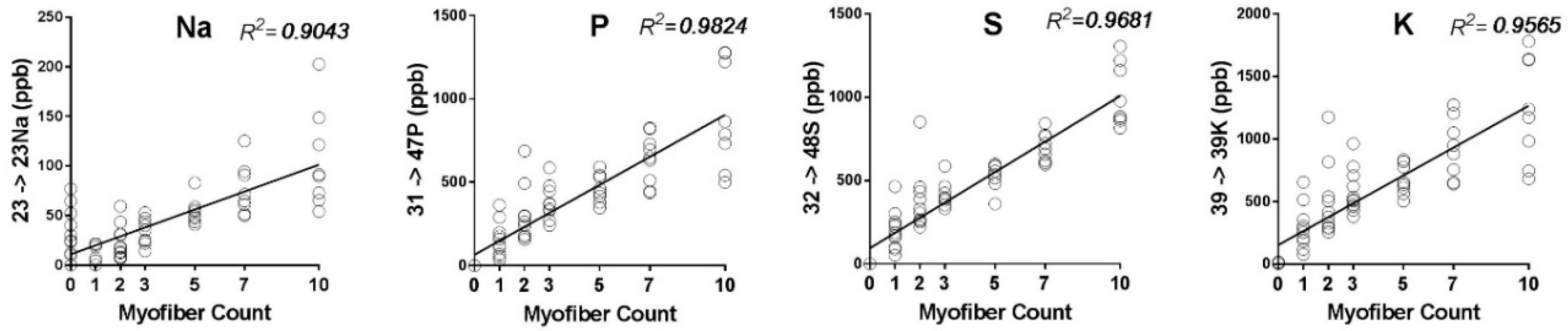
2.1. Detection Limits, Sample Size, and Selection of Elemental Mass Tag
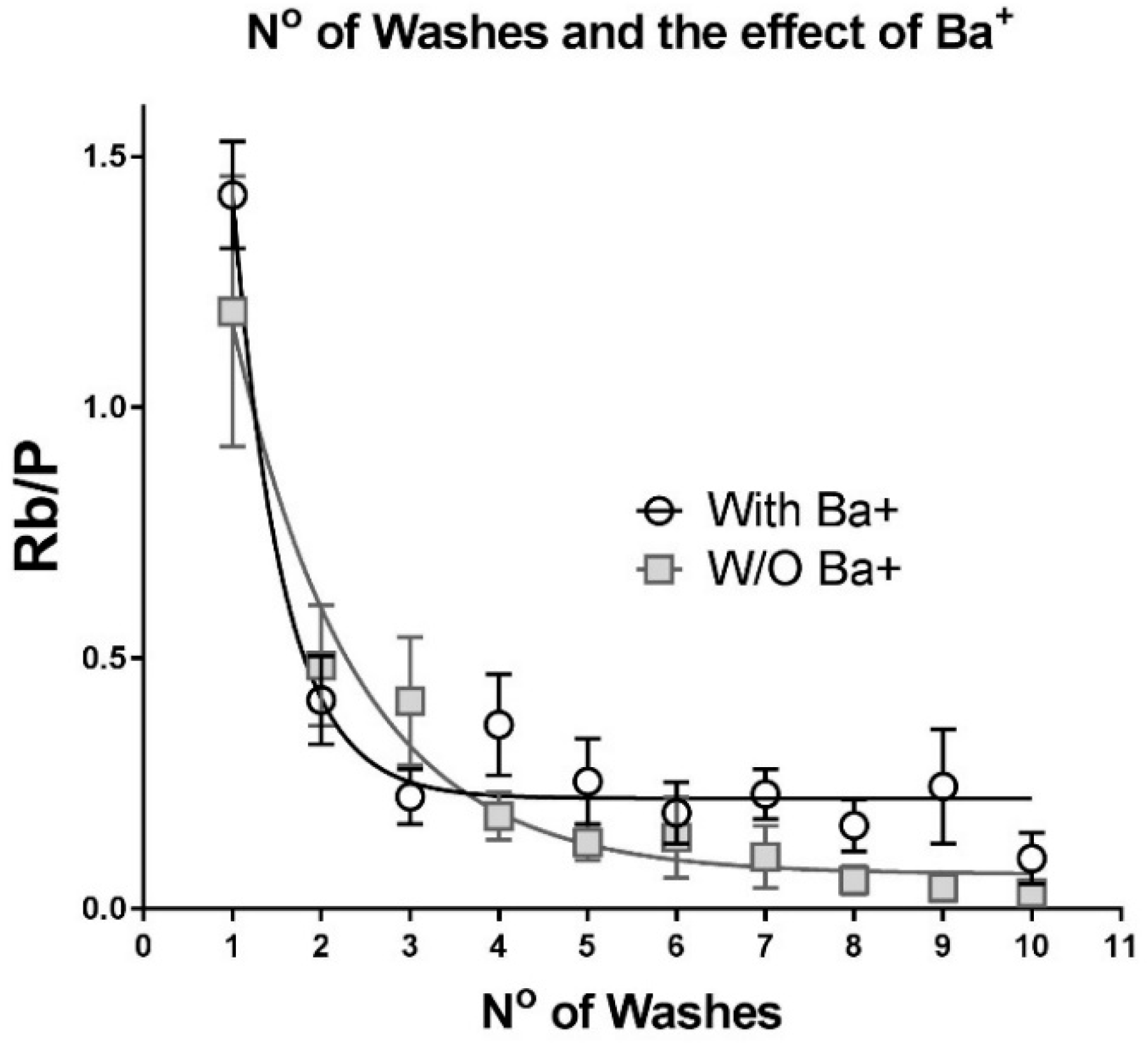
2.2. Optimization of the Wash Procedure
2.3. Na,K-ATPase Mediates the Majority of Active Rb+ Uptake in This Assay
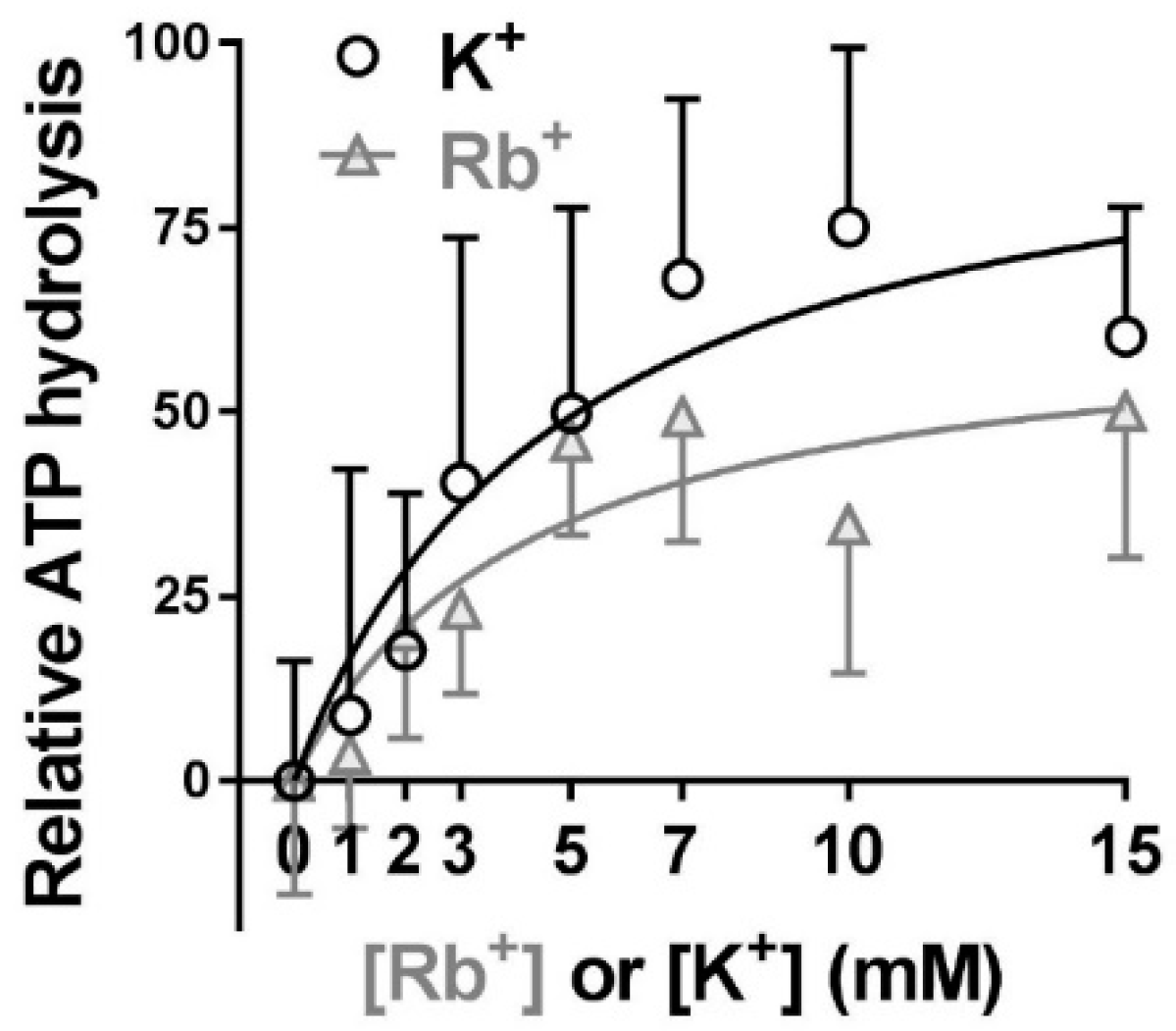
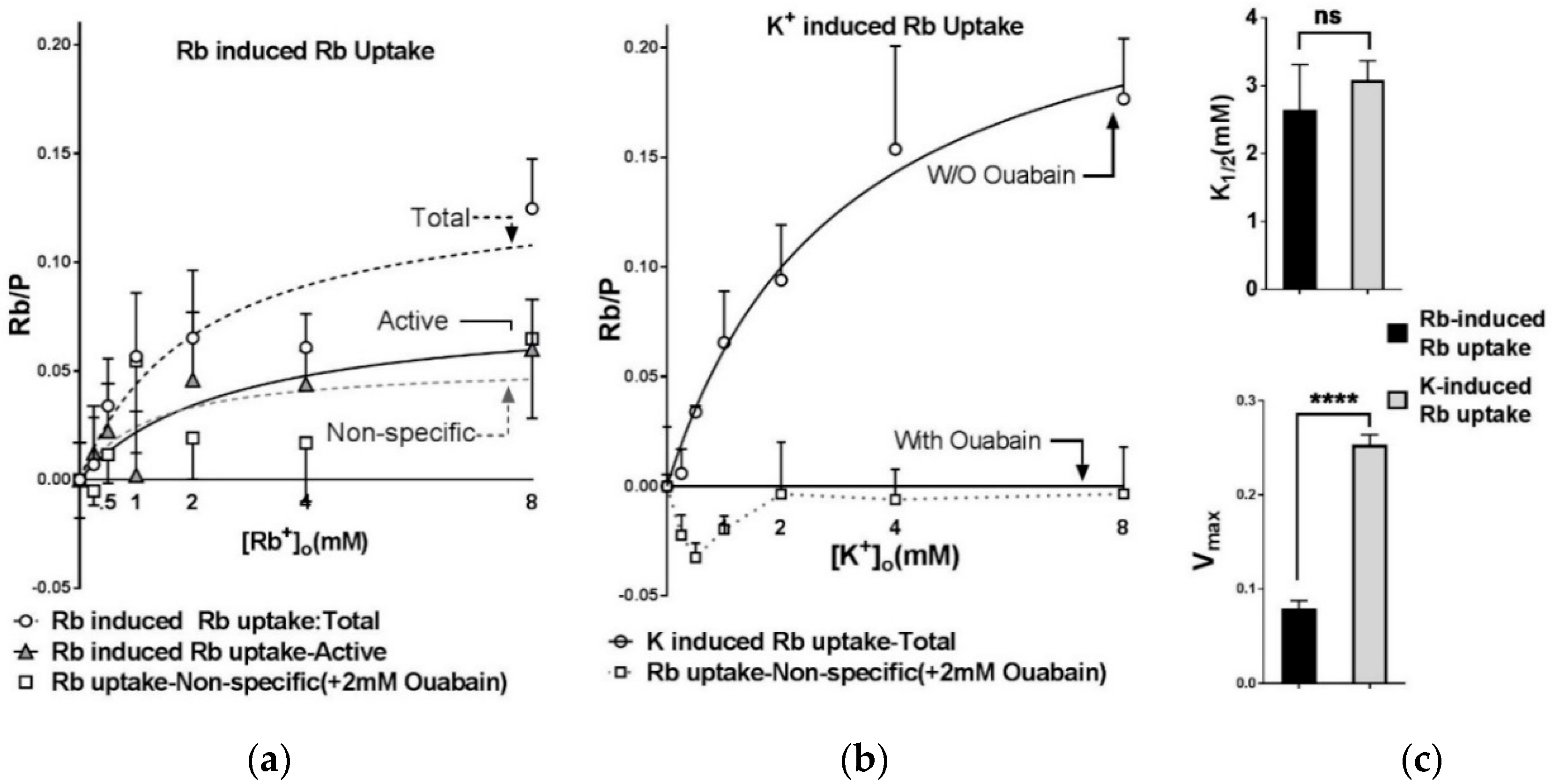
2.4. Extracellular Substrate Affinity of Na,K-ATPase for Rb+ is Identical to K+, but Maximum Turnover Rate is Slower when Rb+ is the Transported Ion
2.5. K Affinity of Na,K-ATPase Measured in Dissociated Fibers
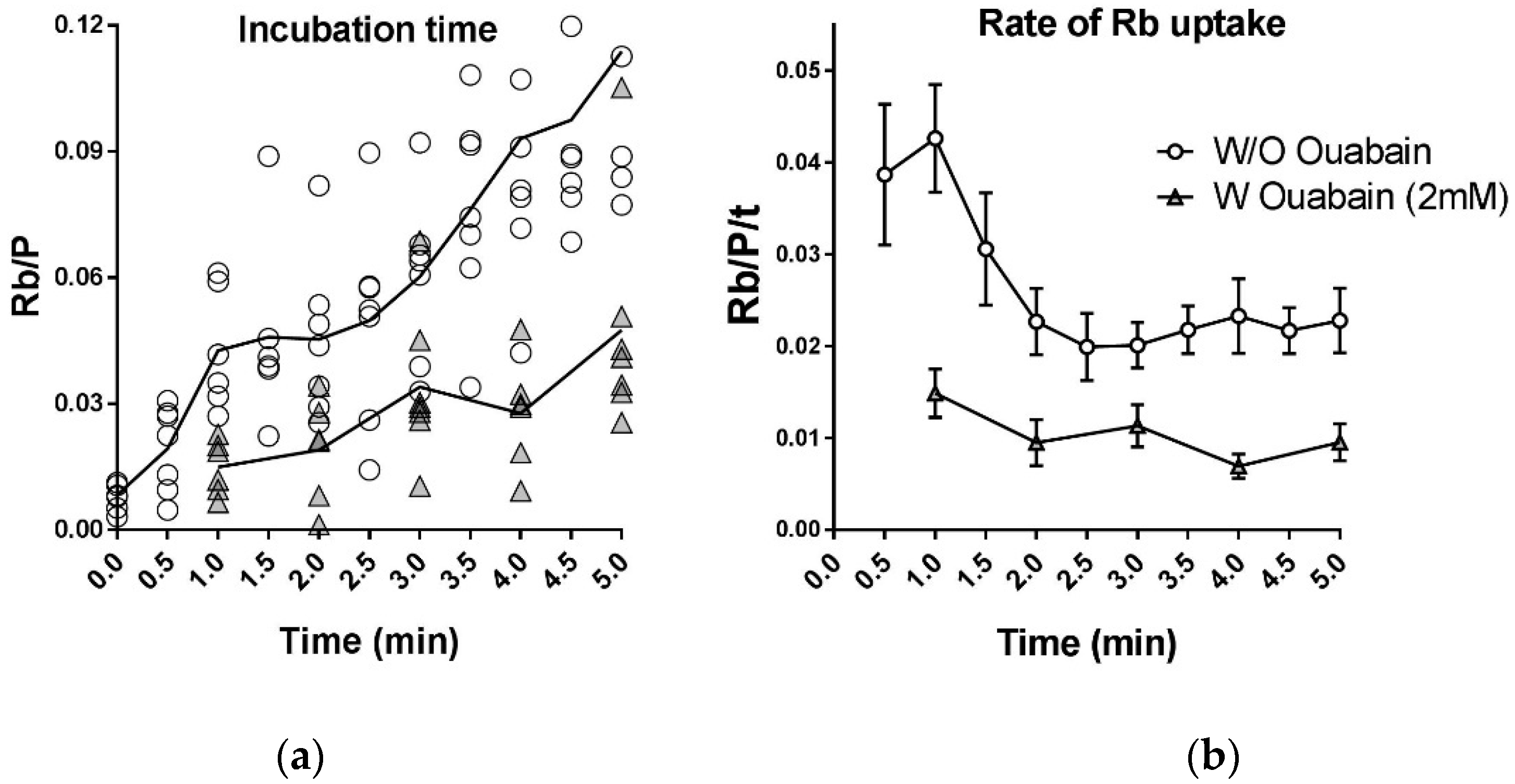
2.6. Instantaneous Rate of Rb+ Uptake by the Na,K-ATPase Is Not Constant
2.7. K Affinity of α1 and α2 Isoforms of Na,K-ATPase in Dissociated Fibers
3. Discussion
- The apparent affinities of Na,K-ATPase α1 and α2 isoforms for K+, measured in intact muscle fibers with unperturbed intracellular environment, are 1.3 and 4 mM, respectively. These affinities are not significantly different from the K+ affinities of α1 and α2 isozymes measured in mouse skeletal muscles using other experimental models [8].
- The extracellular substrate sites of Na,K-ATPase bind Rb+ and K+ with the same apparent affinity. However, pump turnover rate is reduced when Rb+ is the transported ion. Practically, this means that Rb+ can be used at mM concentrations as a congener for K+ for determining extracellular substrate affinity but should be limited to tracer concentrations to accurately measure Vmax of Na,K-ATPase.
- The instantaneous rate of K/Rb transport is not constant but declines to a steady level with a half time of 1–2 min Therefore, Rb+ uptake measured using uptake periods of ≤30 s reports a near-peak uptake rate, and Rb+ uptake measured for uptake periods >5 min reports a near steady-state uptake rate.
- Measurement of Rb+ uptake in intact, dissociated fibers using a multi-well format and quantification of Rb+ content by ICP-MS provides a useful, intermediate throughput assay with good time resolution and statistical power. The use of a WT mice and transgenic mice with altered ouabain affinities allowed us to measure the separate contributions of α1 and α2 isozymes using a single, low concentration of ouabain (10 µM). This strategy provided a better separation of isoform-specific contributions than was possible using WT fibers alone and a 1000-fold range of ouabain concentrations (µM and mM), in which the overlap in ouabain affinities of native α isoforms contributes some overlap to their measured K+ affinities.
3.1. Isoform-Specific Affinities for Extracellular K: Physiological Implications
3.2. Use of Rb+ as a Congener for K+ in Studies of Na,K-ATPase
3.3. Na,K-ATPase Transport Rates Decline in Intact Fibers under Dynamically Changing Substrate Conditions
3.4. Intermediate throughput Assay of Na,K-ATPase Activity in Intact Muscle Cells
3.4.1. Method: ATPase Hydrolysis Assay
- Measurement: Inorganic phosphate released by ATP hydrolysis, detected by fluorimetry.
- Sample: Sarcolemmal-enriched membranes prepared from skeletal muscle muscles.
- Advantages: (a) High reproducibility; (b) substrate concentrations at both extracellular and intracellular facing substrate sites can be fixed; (c) enzyme activity is computed using a single equation with simple stoichiometry.
- Disadvantages: (a) Low yield, purified membranes may not reflect the in vivo distribution of enzyme subunits; (b) physiological sidedness of substrate concentrations is absent.
3.4.2. Method: Na,K-ATPase Specific Current (Ipump)
- Measurement: Outward pump current.
- Sample: Dissociated, intact FDB fibers.
- Advantages: (a) Continuous recording of pump current with millisecond time resolution. (b) Extracellular [K]o can be fixed within seconds. (c) Intracellular [Na+]i can be reasonably fixed by the pipette [Na+]i, due to the high pipette-to-fiber volume ratio. (d) Vm, which influences substrate binding and transport activity, can be fixed.
- Disadvantages: (a) Passive conditions with all other ion transport pathways inhibited are non-physiological; possible interactions between Na,K-ATPase and other transport pathways are lost. (b) Regulation of pump activity by intracellular Ca2+ is prevented by the high concentrations of Ca2+ buffers used to block fiber contraction.
3.4.3. Method: Rb Uptake in whole Muscles Measured by ICP-MS
- Measurement: 85Rb/87Rb uptake.
- Sample: Isolated whole muscle.
- Advantages: (a) Rb uptake is a ‘gold standard’ assay to measure Na,K-ATPase activity in intact tissues and cells with unperturbed intracellular milieu. (b) Close to physiological conditions with intact transport pathways for other ions. Studies of other transport pathways which operate in parallel or in concert with Na,K-ATPase are possible with ICP-MS. (c) Physiological resting Na+ and K+ ion gradients and membrane potential are preserved. (d) Intracellular environment, including the [Na+]i is unperturbed. (e) Uses non-radioactive, naturally abundant 85Rb or 87Rb. (f) Rb is easily detected by ICP-MS due to low interference from other ions. (g) High sensitivity (1 part per billion detection limit).
- Disadvantages: (a) Extracellular diffusion delays through the muscle interstitium limit control of extracellular concentrations and increase Rb+ leak during extended wash times. (b) Compared to dissociated fibers, higher endogenous ionic background and greater non-specific Rb+ uptake by other transport pathways. (c) Limited to a single data point per muscle, increases variance and number of animals. (d) Rb leak during washing procedure.
3.4.4. Method: Rb Uptake by Dissociated Single Fibers Measured with ICP-MS in a Multi-Well Format
- Measurement: 85Rb/87Rb uptake.Sample: Dissociated, intact fibers.
- Advantages: (a) Essentially no diffusion delays, increased time resolution. (b) Unperturbed intracellular milieu, closer to in vivo conditions in which [Na+]i changes dynamically during Na,K-ATPase transport. (c) Medium throughput. (d) Parallel measurements under multiple conditions using fibers from the same animal greatly increases the statistical power. Most useful for dose dependent and time dependent studies.
- Disadvantages: (a) Possible selection bias—only resilient myofibers tolerate the dissociation procedure. (b) Extracellular matrix is compromised by enzymatic dissociation, with unknown consequences on transport pathways. (c) Increases nonspecific membrane leak.
3.4.5. Method: Quantification of Na+ and K+ Content by Flame Photometry
- Measurement: Total Na+ and K+ content.Sample: Isolated whole muscle.
- Advantages: (a) Direct measurement of native cations Na+ and K+ (vs. tracer cations e.g., Rb+ and Li+). (b) High sensitivity and specificity for Na+. (c) Simulations quantification of Na+ and K+ enables determination of net ionic fluxes and changes in intracellular ion content directly. (d) An established method with several available trouble-shooting guides.
- Disadvantages: (a) High background [K]i makes it difficult to detect the relatively small K influx. (b) K+ leaks from cells faster than Na+ during consecutive washes, which may introduce noise in the K+ reading. (c) In milli molar K+ concentrations, the read out of the flame photometric becomes non-linear regarding K+ concentration in solution, which makes it more difficult to use the assay in the millimolar range.
4. Materials and Methods
4.1. Animals
4.2. Single Fiber Dissociation
4.3. Rb Uptake by Dissociated Intact Fibers Measured using a Multi-Well Format
4.4. Quantification of Rb+ Content by ICP-MS
4.5. ATPase Activity
4.6. Data Analysis and Statistics
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| EDL | Extensor digitorum longus |
| FDB | Flexor digitorum brevis |
| ICP-MS | Inductively coupled plasma mass spectrometry |
| ppb | Part per billion |
| RSD | Relative standard deviation |
| SEM | Standard error of mean |
| SD | Standard deviation |
| SK | Skeletal muscle |
References
- Blanco, G. Na,K-ATPase Subunit Heterogeneity as a Mechanism for Tissue-Specific Ion Regulation. Semin. Nephrol. 2005, 25, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.H. Biochemistry of NA,K-ATPase. Annu. Rev. Biochem. 2002, 71, 511–535. [Google Scholar] [CrossRef] [PubMed]
- Geering, K. FXYD proteins: New regulators of Na-K-ATPase. Am. J. Physiol. Renal Physiol. 2006, 290, 241–250. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Shelly, D.A.; Moseley, A.E.; James, P.F.; James, J.H.; Paul, R.J.; Lingrel, J.B. The α1- and α2-isoforms of Na-K-ATPase play different roles in skeletal muscle contractility. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 281, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Radzyukevich, T.L.; Neumann, J.C.; Rindler, T.N.; Oshiro, N.; Goldhamer, D.J.; Lingrel, J.B.; Heiny, J.A. Tissue-specific role of the Na,K-ATPase α2 isozyme in skeletal muscle. J. Biol. Chem. 2013, 288, 1226–1237. [Google Scholar] [CrossRef] [PubMed]
- Clausen, T.; Everts, M.E.; Kjeldsen, K. Quantification of the maximum capacity for active sodium-potassium transport in rat skeletal muscle. J. Physiol. 1987, 388, 163–181. [Google Scholar] [CrossRef] [PubMed]
- Radzyukevich, T.L.; Moseley, A.E.; Shelly, D.A.; Redden, G.A.; Behbehani, M.M.; Lingrel, J.B.; Paul, R.J.; Heiny, J.A. The Na(+)-K(+)-ATPase alpha2-subunit isoform modulates contractility in the perinatal mouse diaphragm. Am. J. Physiol. Cell Physiol. 2004, 287, C1300. [Google Scholar] [CrossRef] [PubMed]
- DiFranco, M.; Hakimjavadi, H.; Lingrel, J.B.; Heiny, J.A. Na,K-ATPase alpha2 activity in mammalian skeletal muscle T-tubules is acutely stimulated by extracellular K+. J. Gen. Physiol. 2015, 146, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Clausen, T. Clearance of extracellular K+ during muscle contraction—Roles of membrane transport and diffusion. J. Gen. Physiol. 2008, 131, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Heyse, S.; Wuddel, I.; Apell, H.J.; Stürmer, W. Partial reactions of the Na,K-ATPase: Determination of rate constants. J. Gen. Physiol. 1994, 104, 197–240. [Google Scholar] [CrossRef] [PubMed]
- Froehlich, J.P.; Fendler, K. The partial reactions of the Na(+)- and Na(+) + K(+)-activated adenosine triphosphatases. Soc. Gen. Physiol. Ser. 1991, 46, 227–247. [Google Scholar] [PubMed]
- Johnson, C.G.; Johnson, C.L.; Schultheis, P.J.; Lingrel, J.B.; Wallick, E.T. Comparison of the Effects of Potassium on Ouabain Binding to Native and Site-Directed Mutants of Na,K-ATPase. Arch. Biochem. Biophys. 1995, 317, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, J.A.; Stiner, C.A.; Radzyukevich, T.L.; Heiny, J.A. Metal ion transport quantified by ICP-MS in intact cells. Sci. Rep. 2016, 6, 20551. [Google Scholar] [CrossRef] [PubMed]
- Stiner, C.; Hakimjavadi, H.; Vignesh, K.S.; Deepe, G., Jr.; Heiny, J.; Figueroa, J.A.L. ICP-MS-MS Analysis of Biological Micro Samples with Heteroatoms as Internal Tag for Mass-Free Quantification of Selected Elements. J. Analyt. At. Spectrom. 2018, in press. [Google Scholar]
- Clausen, T.; Nielsen, O.B. Potassium, Na+,K+-pumps and fatigue in rat muscle. J. Physiol. 2007, 584, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Hasler, U.; Wang, X.; Crambert, G.; Béguin, P.; Jaisser, F.; Horisberger, J.D.; Geering, K. Role of beta-subunit domains in the assembly, stable expression, intracellular routing, and functional properties of Na,K-ATPase. J. Biol. Chem. 1998, 273, 30826–30835. [Google Scholar] [CrossRef] [PubMed]
- Crambert, G.; Hasler, U.; Beggah, A.T.; Yu, C.; Modyanov, N.N.; Horisberger, J.; Lelièvre, L.; Geering, K. Transport and Pharmacological Properties of Nine Different Human Na,K-ATPase Isozymes. J. Biol. Chem. 2000, 275, 1976–1986. [Google Scholar] [CrossRef] [PubMed]
- Kravtsova, V.V.; Petrov, A.M.; Matchkov, V.V.; Bouzinova, E.V.; Vasiliev, A.N.; Benziane, B.; Zefirov, A.L.; Chibalin, A.V.; Heiny, J.A.; Krivoi, I.I. Distinct α2 Na,K-ATPase membrane pools are differently involved in early skeletal muscle remodeling during disuse. J. Gen. Physiol. 2016, 147, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, M.; Juel, C. Na+,K+-ATPase Na+ Affinity in Rat Skeletal Muscle Fiber Types. J. Membr. Biol. 2010, 234, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Munzer, J.S.; Daly, S.E.; Jewell-Motz, E.A.; Lingrel, J.B.; Blostein, R. Tissue- and isoform-specific kinetic behavior of the Na,K-ATPase. J. Biol. Chem. 1994, 269, 16668–16676. [Google Scholar] [PubMed]
- Ashcroft, F.M.; Heiny, J.A.; Vergara, J. T-system optical signals associated with inward rectification in skeletal muscle. Nature 1983, 301, 164–166. [Google Scholar] [CrossRef]
- Nakajima, S.; Gilai, A. Action potentials of isolated single muscle fibers recorded by potential-sensitive dyes. J. Gen. Physiol. 1980, 76, 729–750. [Google Scholar] [CrossRef] [PubMed]
- Despa, S.; Bers, D.M. Na/K pump current and [Na](i) in rabbit ventricular myocytes: Local [Na](i) depletion and Na buffering. Biophys. J. 2003, 84, 4157–4166. [Google Scholar] [CrossRef]
- Lu, F.; Hilgemann, D.W. Na/K pump inactivation, subsarcolemmal Na measurements, and cytoplasmic ion turnover kinetics contradict restricted Na spaces in murine cardiac myocytes. J. Gen. Physiol. 2017, 149, 727–749. [Google Scholar] [CrossRef] [PubMed]
- Lieber, R.L. Muscle Fiber Length and Moment Arm Coordination during Dorsi- and Plantarflexion in the Mouse Hindlimb. Cells Tissues Organs 1997, 159, 84–89. [Google Scholar] [CrossRef]
- Danilov, K.; Sidorenko, S.; Milovanova, K.; Klimanova, E.; Kapilevich, L.V.; Orlov, S.N. Electrical pulse stimulation decreases electrochemical Na+ and K+ gradients in C2C12 myotubes. Biochem. Biophys. Res. Commun. 2017, 493, 875–878. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.K.; Brault, J.J.; Terjung, R.L. Phosphate uptake and PiT-1 protein expression in rat skeletal muscle. Am. J. Physiol. Cell Physiol. 2004, 287, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Dostanic, I.; Schultz, J.E.; Lorenz, J.N.; Lingrel, J.B. The alpha 1 isoform of Na,K-ATPase regulates cardiac contractility and functionally interacts and co-localizes with the Na/Ca exchanger in heart. J. Biol. Chem. 2004, 279, 54053–54061. [Google Scholar] [CrossRef] [PubMed]

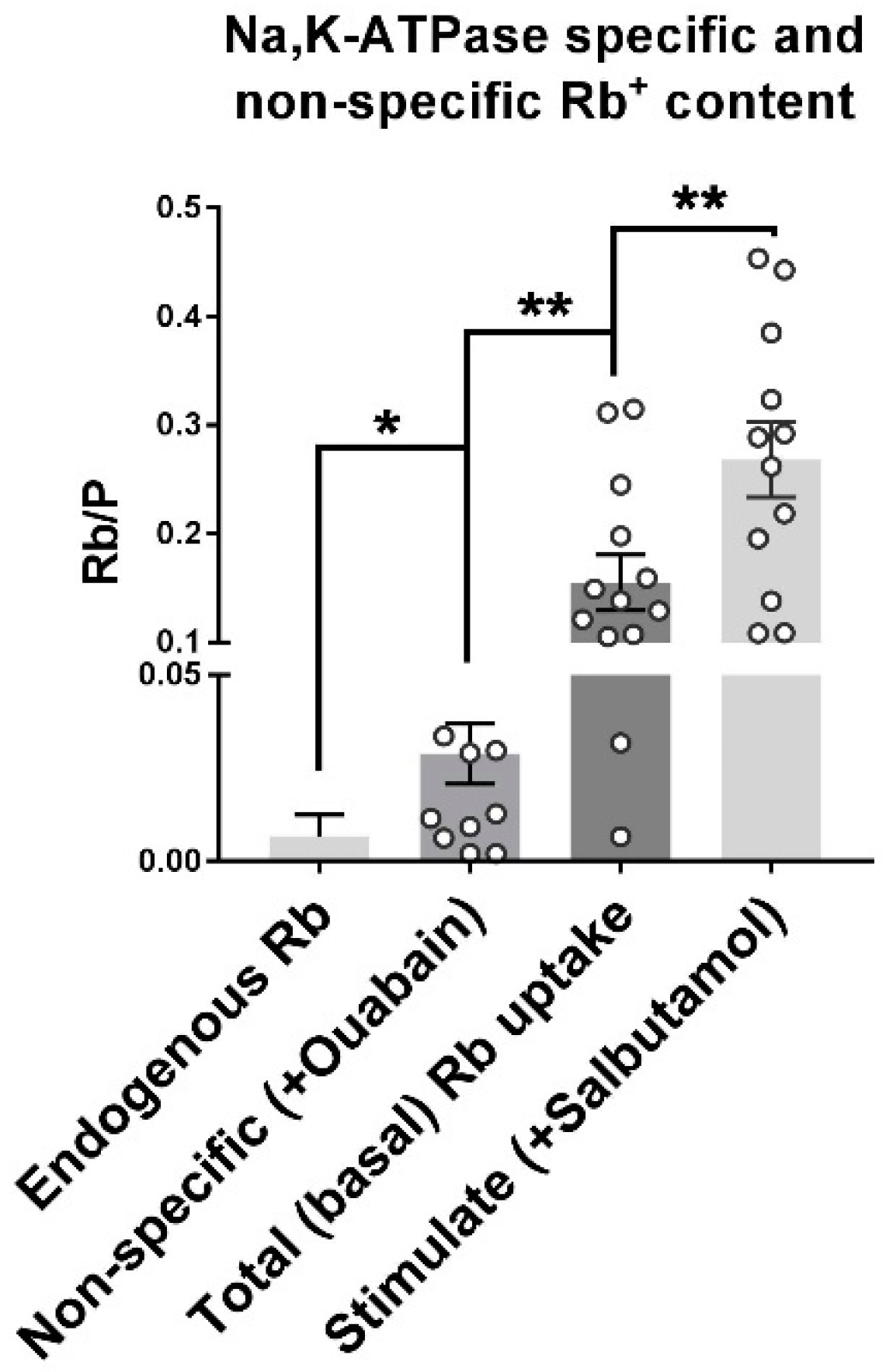

| Mean Rb | SEM | Mean Rb/P | SEM | |
|---|---|---|---|---|
| Endogenous Rb | 1.589 | 0.37 | 0.007 | 0.002 |
| Passive (+ ouabain) | 12.66 | 4.16 | 0.028 | 0.008 |
| Total Rb+ uptake | 35.72 | 6.56 | 0.155 | 0.026 |
| Stimulate (+ Salbutamol) | 65.83 | 9.50 | 0.268 | 0.035 |
| Vmax | K1/2, mM | n | |
|---|---|---|---|
| K | 96.9 ± 47.1 | 4.79 ± 5.71 | 31 |
| Rb | 64.5 ± 26.6 | 4.14 ± 4.41 | 32 |
| p value | 0.002 | 0.621 |
| Mouse Model | Treatment | Active NKA Isoform | K1/2,K | Vmax | No. |
|---|---|---|---|---|---|
| WT | No treatment | α1 + α2 | 3.01 mM ± 1.66 mM | 0.222 ± 0.035 | 46 |
| WT | 10 µM ouabain | α1 | 1.30 mM ± 1.40 mM | 0.088 ± 0.021 | 39 |
| ‘SWAP’ | 10 µM ouabain | α2 | 3.95 mM ± 2.85 mM | 0.181 ± 0.039 | 46 |
| skα2−/− | No treatment | α1 | 1.02 mM ± 0.95 mM | 0.146 ± 0.025 | 46 |
| Source | Measurement | Experimental Model | Vm (mV) | Na+ & K+ Conc. Gradients | α1-K1/2,K, (mM) | α2-K1/2,K (mM) | α1 + α2-K1/2,K (mM) |
|---|---|---|---|---|---|---|---|
| [8] | pump current | voltage-clamped single FDB fibers of WT mice | −90 | Yes | 4.3 ± 0.3 | ||
| [8] | ATPase turnover | sarcolemmal-enriched membranes from hindlimb muscles of WT mice | 0 | No | 1.7 ± 0.6 | 3.6 ± 0.5 | |
| Figure 4 | ATPase turnover | sarcolemmal-enriched membranes from WT mouse EDL | 0 | No | 4.8 ± 5.7 | ||
| Figure 5 | Rb uptake | samples of 2–5 dissociated EDL fibers of WT mice + 10 µM ouabain (α1 active) or ‘SWAP’ mice + 10 µM ouabain (α2 active) | resting | Yes | 1.3 ± 1.4 | 4.0 ± 2.9 | 3.0 ± 1.7 |
| Figure 7 | Rb uptake | samples of 2-5 dissociated EDL fibers from skα2−/− mice (α1 only) | resting | Yes | 1.0 ± 0.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hakimjavadi, H.; Stiner, C.A.; Radzyukevich, T.L.; Lingrel, J.B.; Norman, N.; Landero Figueroa, J.A.; Heiny, J.A. K+ and Rb+ Affinities of the Na,K-ATPase α1 and α2 Isozymes: An Application of ICP-MS for Quantification of Na+ Pump Kinetics in Myofibers. Int. J. Mol. Sci. 2018, 19, 2725. https://doi.org/10.3390/ijms19092725
Hakimjavadi H, Stiner CA, Radzyukevich TL, Lingrel JB, Norman N, Landero Figueroa JA, Heiny JA. K+ and Rb+ Affinities of the Na,K-ATPase α1 and α2 Isozymes: An Application of ICP-MS for Quantification of Na+ Pump Kinetics in Myofibers. International Journal of Molecular Sciences. 2018; 19(9):2725. https://doi.org/10.3390/ijms19092725
Chicago/Turabian StyleHakimjavadi, Hesamedin, Cory A. Stiner, Tatiana L. Radzyukevich, Jerry B. Lingrel, Natalie Norman, Julio A. Landero Figueroa, and Judith A. Heiny. 2018. "K+ and Rb+ Affinities of the Na,K-ATPase α1 and α2 Isozymes: An Application of ICP-MS for Quantification of Na+ Pump Kinetics in Myofibers" International Journal of Molecular Sciences 19, no. 9: 2725. https://doi.org/10.3390/ijms19092725
APA StyleHakimjavadi, H., Stiner, C. A., Radzyukevich, T. L., Lingrel, J. B., Norman, N., Landero Figueroa, J. A., & Heiny, J. A. (2018). K+ and Rb+ Affinities of the Na,K-ATPase α1 and α2 Isozymes: An Application of ICP-MS for Quantification of Na+ Pump Kinetics in Myofibers. International Journal of Molecular Sciences, 19(9), 2725. https://doi.org/10.3390/ijms19092725





