RETRACTED: Uremic Toxins Activates Na/K-ATPase Oxidant Amplification Loop Causing Phenotypic Changes in Adipocytes in In Vitro Models
Abstract
1. Introduction
2. Results
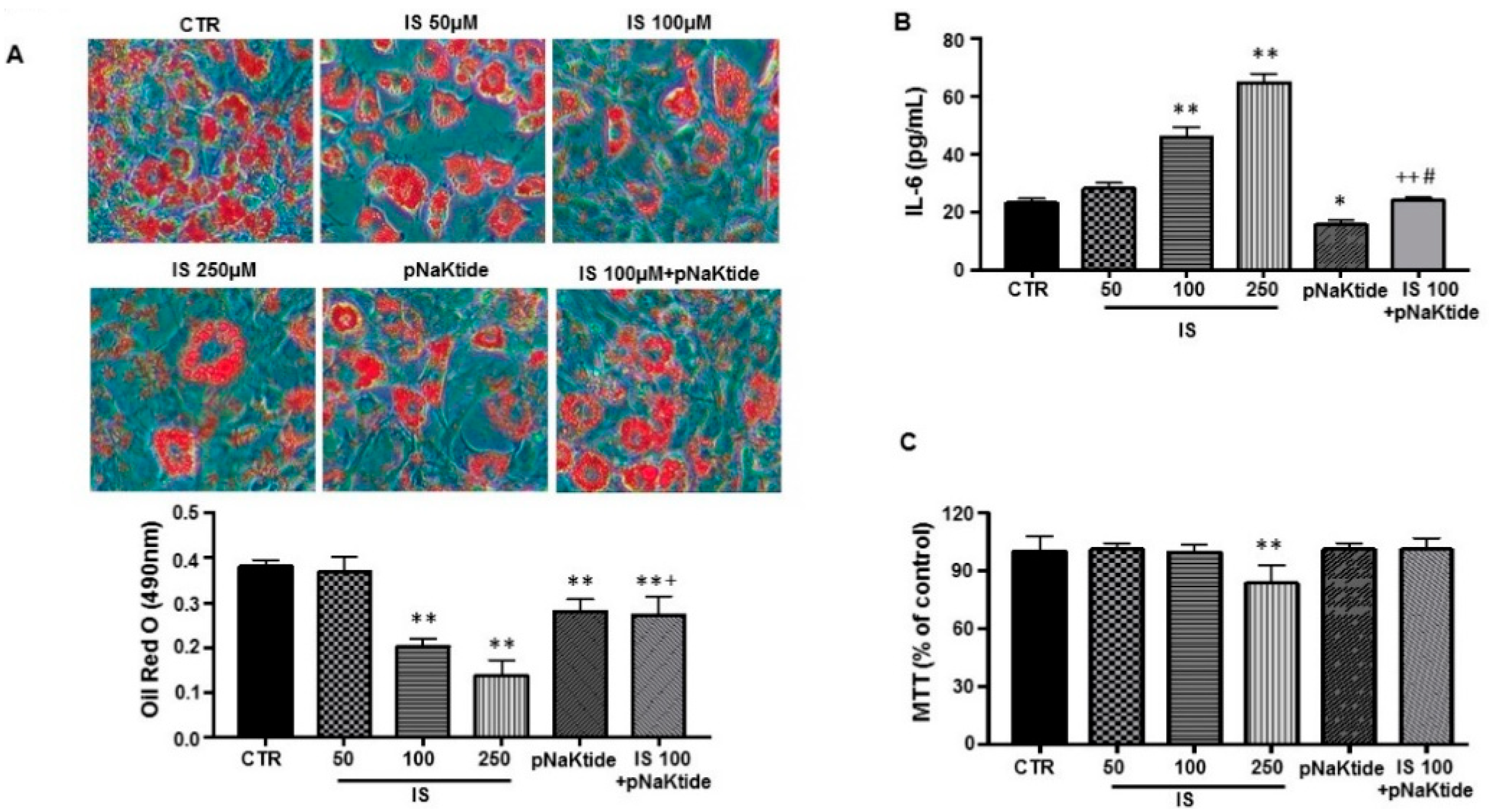
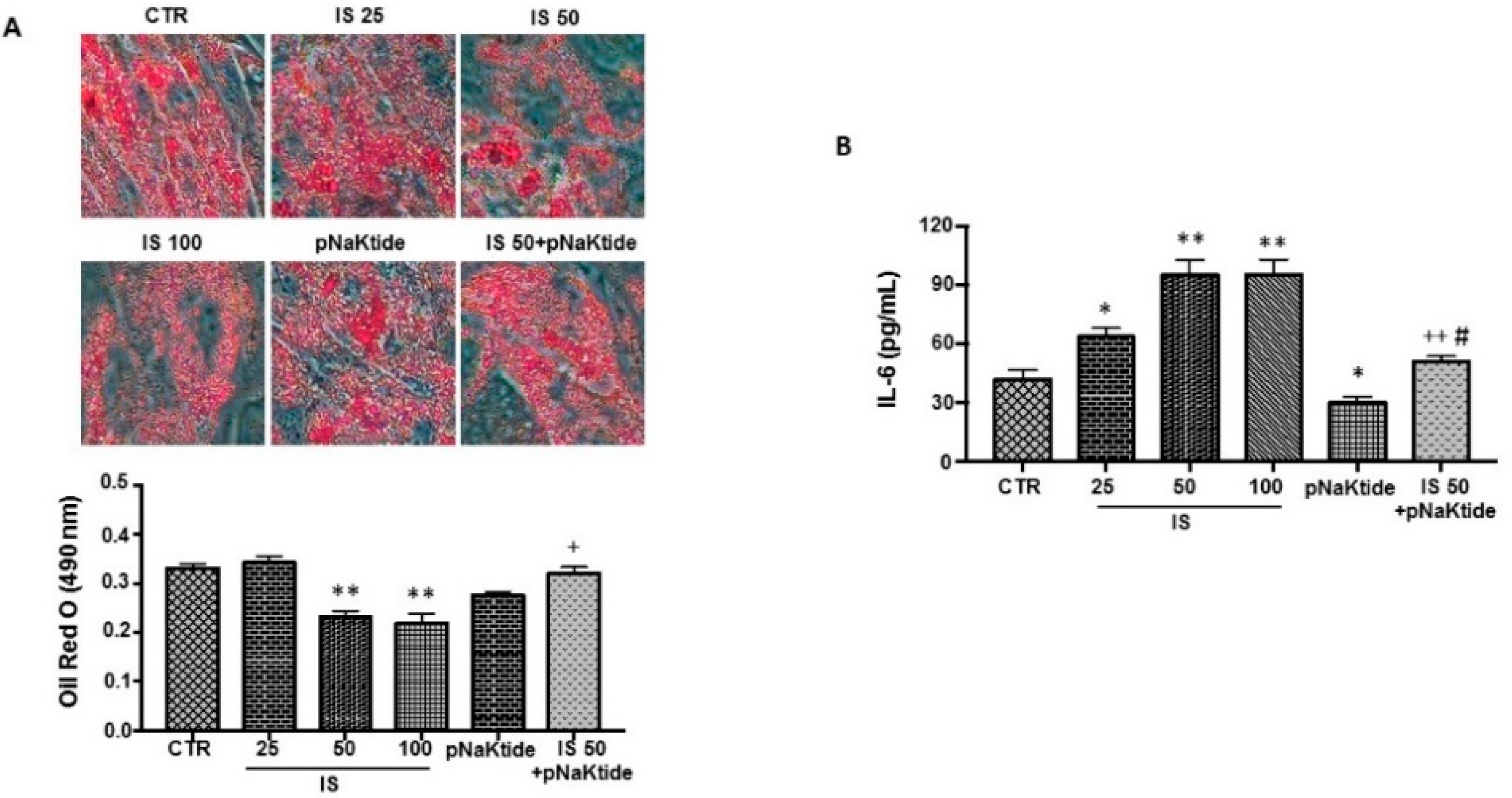
2.1. Effect of IS and pNaKtide on Lipid Accumulation, Inflammatory Cytokines and Cytotoxicity in 3T3-L1 Murine Pre-Adipocytes
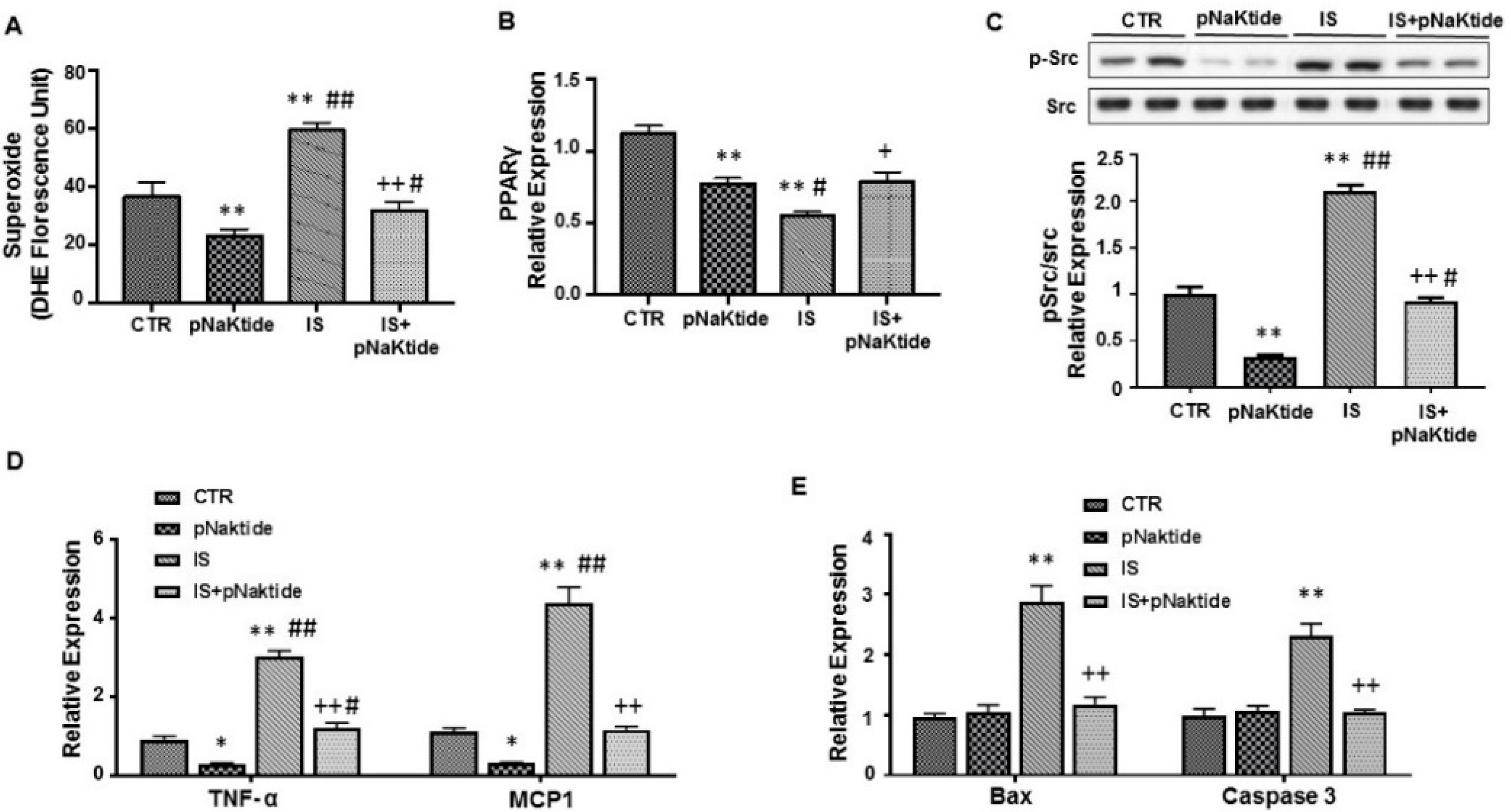
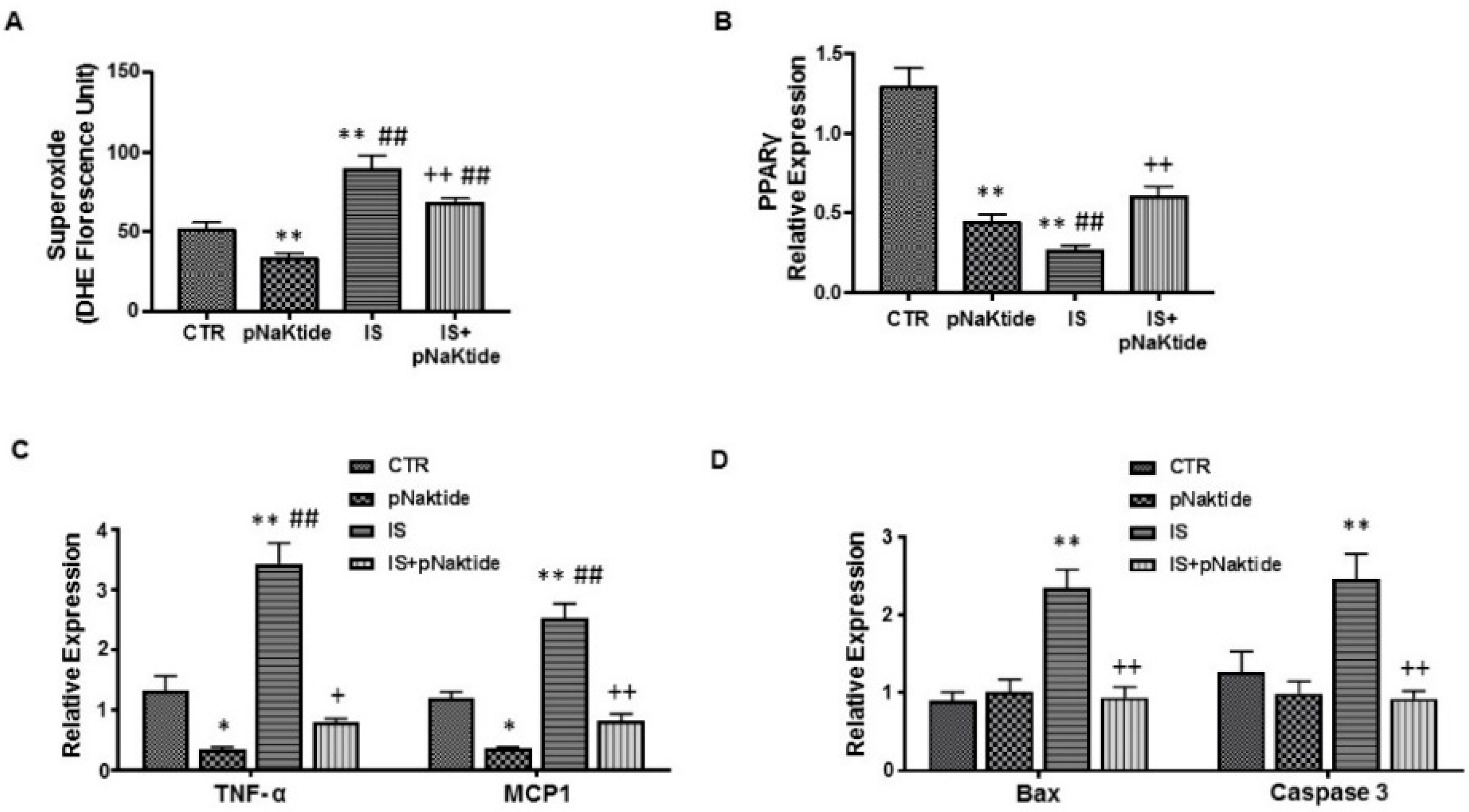
2.2. Effect of IS and pNaKtide on Oxidative Stress, Adipogenic, Apoptotic and Inflammatory Markers in 3T3-L1 Murine Pre-Adipocytes
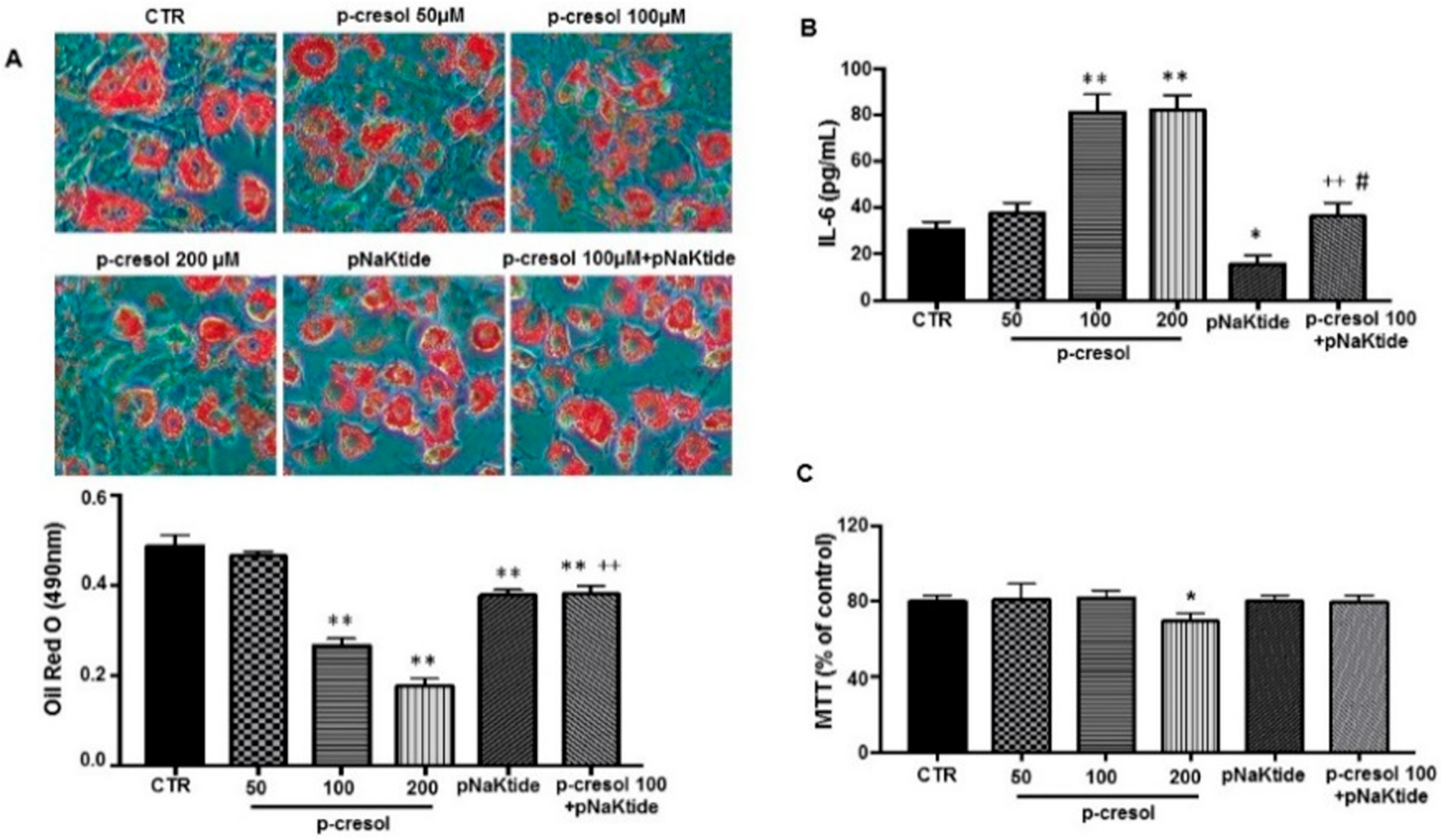
2.3. Effect of p-Cresol and pNaKtide Treatment on Lipid Accumulation, Inflammatory Cytokines and Cytotoxicity in 3T3-L1 Murine Pre-Adipocytes
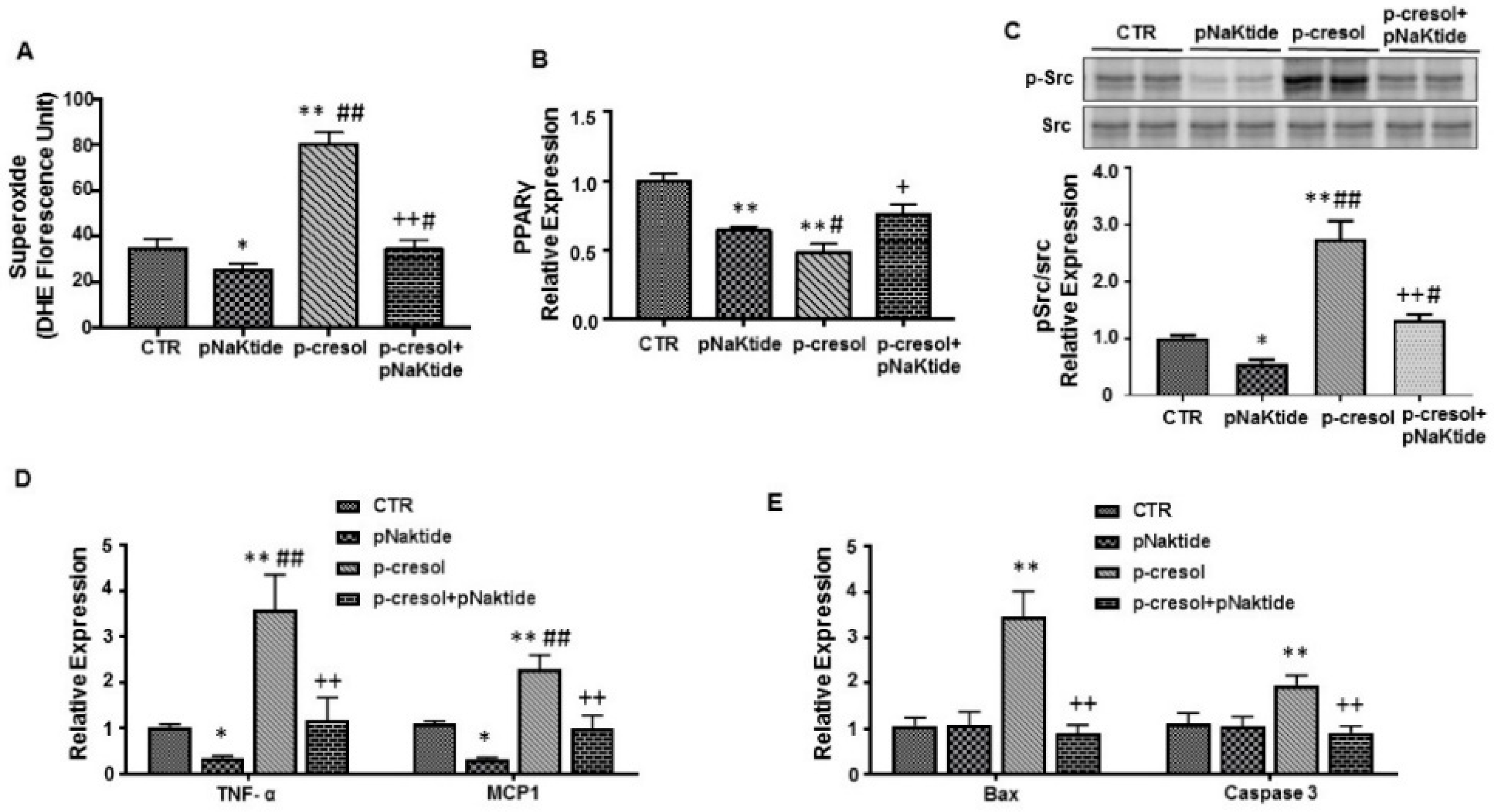
2.4. Effect of p-Cresol and pNaKtide on Oxidative Stress, Adipogenic, Apoptotic and Inflammatory Markers in 3T3-L1 Murine-Adipocytes
2.5. Effect of IS and pNaKtide on Lipid Accumulation, Inflammatory Cytokines and Cytotoxicity in MSC-Derived Adipocytes
2.6. Effect of IS and pNaKtide on Oxidative Stress, Adipogenic, Apoptotic and Inflammatory Markers in MSC-Derived Adipocytes
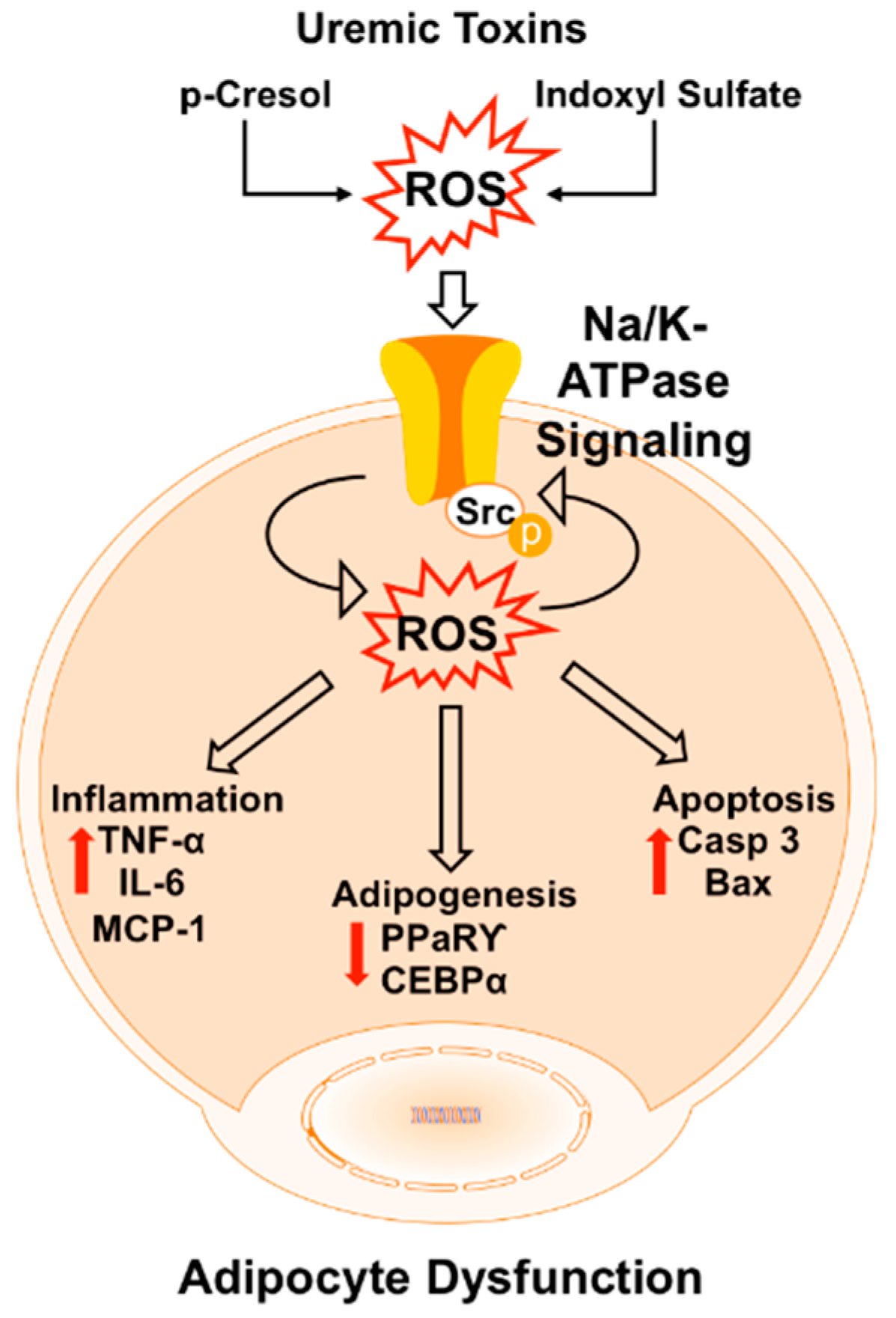
3. Discussion
4. Material and Methods
4.1. Experimental Design for In Vitro Experiment
4.2. Oil Red O Staining for Lipid Accumulation
4.3. Measurement of Superoxide Levels by DHE Staining
4.4. MTT Assays for Cell Proliferation
4.5. Cytokine Measurements
4.6. RNA Extraction and RT-PCR Analysis
4.7. Western Blot Analysis to Demonstrate Phosphorylation of Src
4.8. Statistical Analyses
Author Contributions
Funding
Conflicts of Interest
References
- Fraser, S.D.; Blakeman, T. Chronic kidney disease: Identification and management in primary care. Pragmat. Obs. Res. 2016, 7, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, J.; Drummond, C.A.; Shapiro, J.I. Sodium potassium adenosine triphosphatase (Na/K-ATPase) as a therapeutic target for uremic cardiomyopathy. Expert Opin. Ther. Targets 2017, 21, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Moradi, H.; Sica, D.A.; Kalantar-Zadeh, K. Cardiovascular burden associated with uremic toxins in patients with chronic kidney disease. Am. J. Nephrol. 2013, 38, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Baud, L.; Ardaillou, R. Reactive oxygen species: Production and role in the kidney. Am. J. Physiol. 1986, 251 Pt 2, F765–F776. [Google Scholar] [CrossRef]
- Modaresi, A.; Nafar, M.; Sahraei, Z. Oxidative stress in chronic kidney disease. Iran. J. Kidney Dis. 2015, 9, 165–179. [Google Scholar] [PubMed]
- Kao, M.P.; Ang, D.S.C.; Pall, A.A.; Struthers, A.D. Oxidative stress in renal dysfunction: Mechanisms, clinical sequelae and therapeutic options. J. Hum. Hypertens. 2010, 24, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Parfrey, P.S.; Foley, R.N. The clinical epidemiology of cardiac disease in chronic renal failure. J. Am. Soc. Nephrol. 1999, 10, 1606–1615. [Google Scholar] [PubMed]
- Sarnak, M.J.; Levey, A.S.; Schoolwerth, A.C.; Coresh, J.; Culleton, B.; Hamm, L.L.; Parfrey, P. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension 2003, 42, 1050–1065. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Campbell, K.L.; Johnson, D.W.; Stanton, T.; Vesey, D.A.; Coombes, J.S.; Westone, K.S.; Hawleyac, C.M.; McWhinney, B.C.; Isbel, N.; et al. Protein-bound uremic toxins, inflammation and oxidative stress: A cross-sectional study in stage 3-4 chronic kidney disease. Arch. Med. Res. 2014, 45, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Sedeek, M.; Nasrallah, R.; Touyz, R.M.; Hébert, R.L. NADPH oxidases, reactive oxygen species, and the kidney: Friend and foe. J. Am. Soc. Nephrol. 2013, 24, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Galle, J. Oxidative stress in chronic renal failure. Nephrol. Dial. Transplant. 2001, 16, 2135–2137. [Google Scholar] [CrossRef] [PubMed]
- Oberg, B.P.; McMenamin, E.; Lucas, F.L.E.E.; McMonagle, E.; Morrow, J.; Ikizler, T.A.L.P.; Himmelfarb, J. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004, 65, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Abraham, N.G.; Sodhi, K.; Silvis, A.M.; Vanella, L.; Favero, G.; Rezzani, R.; Lee, C.; Zeldin, D.C.; Schwartzman, M.L. CYP2J2 targeting to endothelial cells attenuates adiposity and vascular dysfunction in mice fed a high-fat diet by reprogramming adipocyte phenotype. Hypertension 2014, 64, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Peterson, S.J.; Sodhi, K.; Vanella, L.; Barbagallo, I.; Rodella, L.F.; Schwartzman, M.L.; Abraham, N.G.; Kappas, A. Heme oxygenase gene targeting to adipocytes attenuates adiposity and vascular dysfunction in mice fed a high-fat diet. Hypertension 2012, 60, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, K.; Maxwell, K.; Yan, Y.; Liu, J.; Chaudhry, M.A.; Getty, M.; Shapiro, J.I. pNaKtide inhibits Na/K-ATPase reactive oxygen species amplification and attenuates adipogenesis. Sci. Adv. 2015, 1, e1500781. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tian, J.; Chaudhry, M.; Maxwell, K.; Yan, Y.; Wang, X.; El-Hamdani, A. Attenuation of Na/K-ATPase Mediated Oxidant Amplification with pNaKtide Ameliorates Experimental Uremic Cardiomyopathy. Sci. Rep. 2016, 6, 34592. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ye, Q.; Liu, C.; Xie, J.X.; Yan, Y.; Lai, F.; Xie, Z. Involvement of Na/K-ATPase in hydrogen peroxide-induced activation of the Src/ERK pathway in LLC-PK1 cells. Free Radic. Biol. Med. 2014, 71, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Shapiro, A.P.; Haller, S.; Katragadda, V.; Liu, L.; Tian, J.; Shapiro, J.I. Involvement of reactive oxygen species in a feed-forward mechanism of Na/K-ATPase-mediated signaling transduction. J. Biol. Chem. 2013, 288, 34249–34258. [Google Scholar] [CrossRef] [PubMed]
- Srikanthan, K.; Shapiro, J.I.; Sodhi, K. The Role of Na/K-ATPase Signaling in Oxidative Stress Related to Obesity and Cardiovascular Disease. Molecules 2016, 21, 1172. [Google Scholar] [CrossRef] [PubMed]
- Lakkis, J.I.; Weir, M.R. Obesity and Kidney Disease. Prog. Cardiovasc. Dis. 2018, 61, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Le Lay, S.; Simard, G.; Martinez, M.C.; Andriantsitohaina, R. Oxidative stress and metabolic pathologies: From an adipocentric point of view. Oxid. Med. Cell Longev. 2014, 2014, 908539. [Google Scholar] [CrossRef] [PubMed]
- Khanh, V.C.; Ohneda, K.; Kato, T.; Yamashita, T.; Sato, F.; Tachi, K.; Ohneda, O. Uremic Toxins Affect the Imbalance of Redox State and Overexpression of Prolyl Hydroxylase 2 in Human Adipose Tissue-Derived Mesenchymal Stem Cells Involved in Wound Healing. Stem Cells Dev. 2017, 26, 948–963. [Google Scholar] [CrossRef] [PubMed]
- Stockler-Pinto, M.B.; Saldanha, J.F.; Yi, D.; Mafra, D.; Fouque, D.; Soulage, C.O. The uremic toxin indoxyl sulfate exacerbates reactive oxygen species production and inflammation in 3T3-L1 adipose cells. Free Radic. Res. 2016, 50, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Yano, S.; Sheikh, A.M.; Nagai, A.; Sugimoto, T. Effects of uremic toxin p-cresol on proliferation, apoptosis, differentiation, and glucose uptake in 3T3-L1 cells. Artif. Organs 2014, 38, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cai, T.; Tian, J.; Xie, J.X.; Zhao, X.; Liu, L.; Xie, Z. NaKtide, a Na/K-ATPase-derived peptide Src inhibitor, antagonizes ouabain-activated signal transduction in cultured cells. J. Biol. Chem. 2009, 284, 21066–21076. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Shapiro, J.I. The physiological and clinical importance of sodium potassium ATPase in cardiovascular diseases. Curr. Opin. Pharmacol. 2016, 27, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Puri, N.; Sodhi, K.; Haarstad, M.; Kim, D.H.; Bohinc, S.; Foglio, E.; Favero, G.; Abraham, N.G. Heme induced oxidative stress attenuates sirtuin1 and enhances adipogenesis in mesenchymal stem cells and mouse pre-adipocytes. J. Cell. Biochem. 2012, 113, 1926–1935. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, K.; Puri, N.; Favero, G.; Stevens, S.; Meadows, C.; Abraham, N.G.; Shapiro, J.I. Fructose Mediated Non-Alcoholic Fatty Liver Is Attenuated by HO-1-SIRT1 Module in Murine Hepatocytes and Mice Fed a High Fructose Diet. PLoS ONE 2015, 10, e0128648. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, K.; Nichols, A.; Mallick, A.; Klug, R.L.; Liu, J.; Wang, X.; Lilly, M.N. The Na/K-ATPase Oxidant Amplification Loop Regulates Aging. Sci. Rep. 2018, 8, 9721. [Google Scholar] [CrossRef] [PubMed]
- Waldman, M.; Bellner, L.; Vanella, L.; Schragenheim, J.; Sodhi, K.; Singh, S.P.; LIn, D.; Lakhkar, A.; Li, J.; Arad, M.; et al. Epoxyeicosatrienoic Acids Regulate Adipocyte Differentiation of Mouse 3T3 Cells, Via PGC-1alpha Activation, Which Is Required for HO-1 Expression and Increased Mitochondrial Function. Stem Cells Dev. 2016, 25, 1084–1094. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Haller, S.; Shapiro, A.; Malhotra, N.; Tian, J.; Xie, Z.; Malhotra, D.; Liu, J. Ouabain-stimulated trafficking regulation of the Na/K-ATPase and NHE3 in renal proximal tubule cells. Mol. Cell. Biochem. 2012, 367, 175–183. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartlett, D.E.; Miller, R.B.; Thiesfeldt, S.; Lakhani, H.V.; Khanal, T.; D. Pratt, R.; Cottrill, C.L.; Klug, R.L.; Adkins, N.S.; Bown, P.C.; et al. RETRACTED: Uremic Toxins Activates Na/K-ATPase Oxidant Amplification Loop Causing Phenotypic Changes in Adipocytes in In Vitro Models. Int. J. Mol. Sci. 2018, 19, 2685. https://doi.org/10.3390/ijms19092685
Bartlett DE, Miller RB, Thiesfeldt S, Lakhani HV, Khanal T, D. Pratt R, Cottrill CL, Klug RL, Adkins NS, Bown PC, et al. RETRACTED: Uremic Toxins Activates Na/K-ATPase Oxidant Amplification Loop Causing Phenotypic Changes in Adipocytes in In Vitro Models. International Journal of Molecular Sciences. 2018; 19(9):2685. https://doi.org/10.3390/ijms19092685
Chicago/Turabian StyleBartlett, David E., Richard B. Miller, Scott Thiesfeldt, Hari Vishal Lakhani, Tilak Khanal, Rebecca D. Pratt, Cameron L. Cottrill, Rebecca L. Klug, Nathaniel Seth Adkins, Paul C. Bown, and et al. 2018. "RETRACTED: Uremic Toxins Activates Na/K-ATPase Oxidant Amplification Loop Causing Phenotypic Changes in Adipocytes in In Vitro Models" International Journal of Molecular Sciences 19, no. 9: 2685. https://doi.org/10.3390/ijms19092685
APA StyleBartlett, D. E., Miller, R. B., Thiesfeldt, S., Lakhani, H. V., Khanal, T., D. Pratt, R., Cottrill, C. L., Klug, R. L., Adkins, N. S., Bown, P. C., Nease, D. B., Shapiro, J. I., & Sodhi, K. (2018). RETRACTED: Uremic Toxins Activates Na/K-ATPase Oxidant Amplification Loop Causing Phenotypic Changes in Adipocytes in In Vitro Models. International Journal of Molecular Sciences, 19(9), 2685. https://doi.org/10.3390/ijms19092685





