Myosin Head Configurations in Resting and Contracting Murine Skeletal Muscle
Abstract
1. Introduction
2. Results
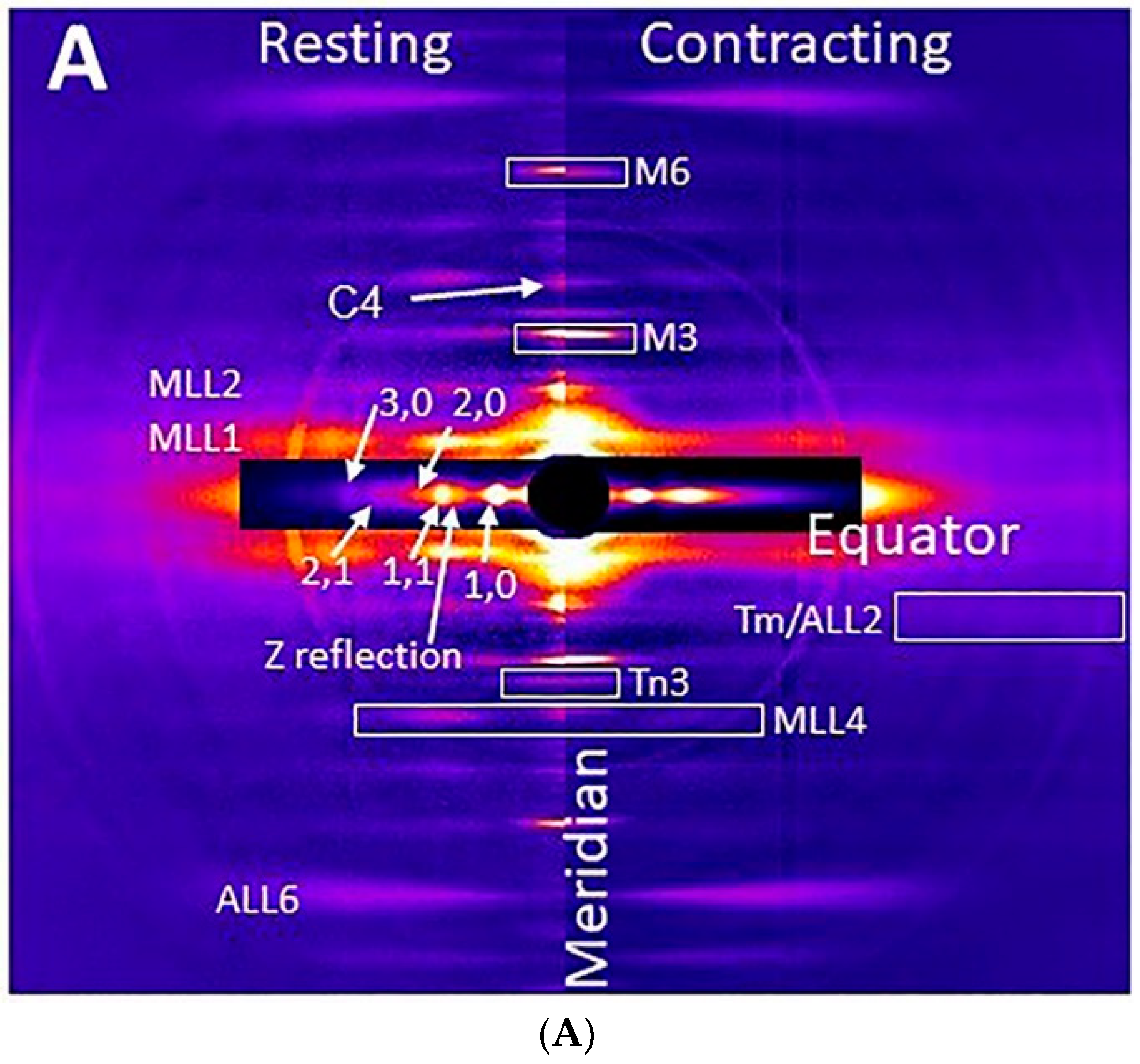
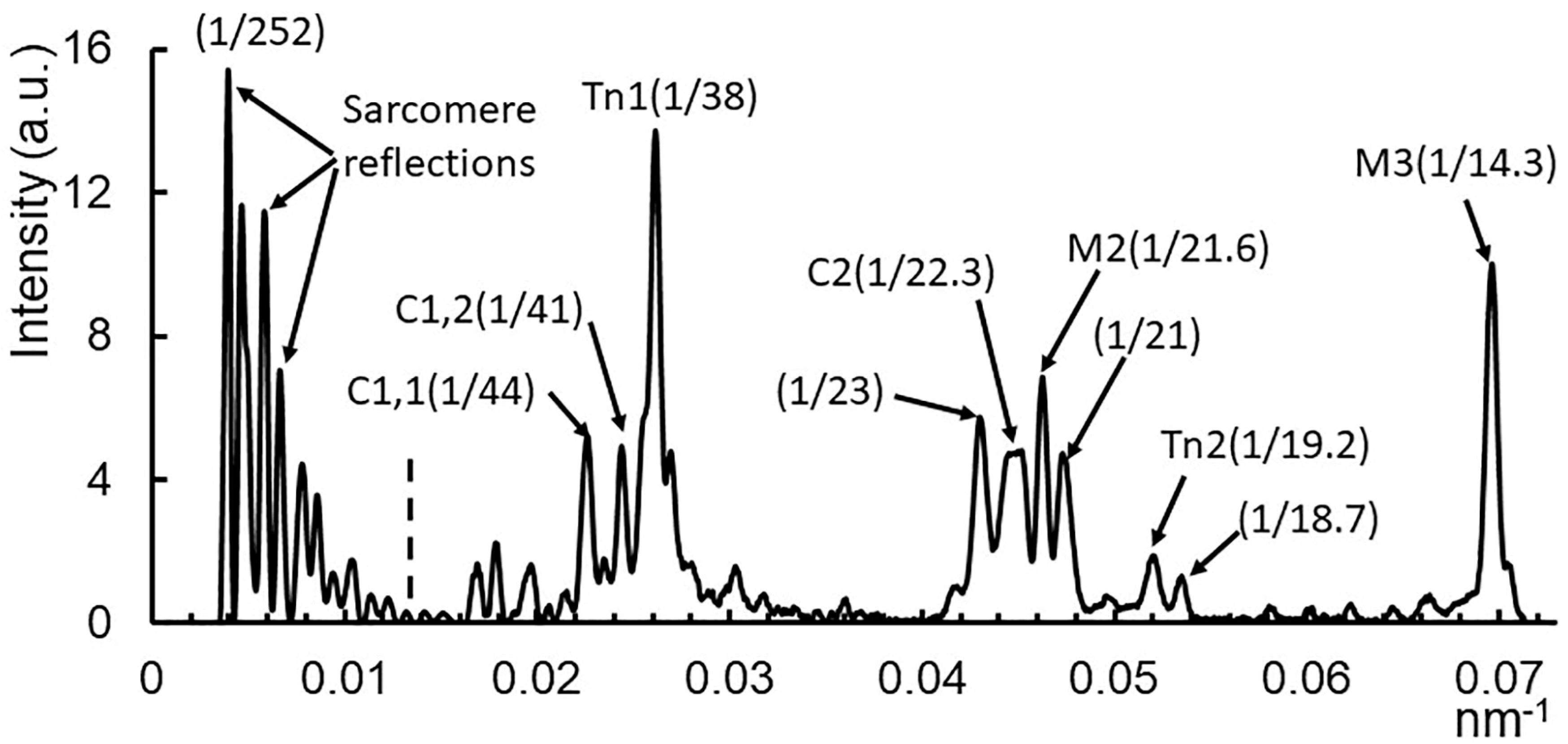
2.1. X-ray Patterns from Mouse EDL Muscle
2.2. Residual Myosin Layer Line Intensity in Contracting Muscle Patterns
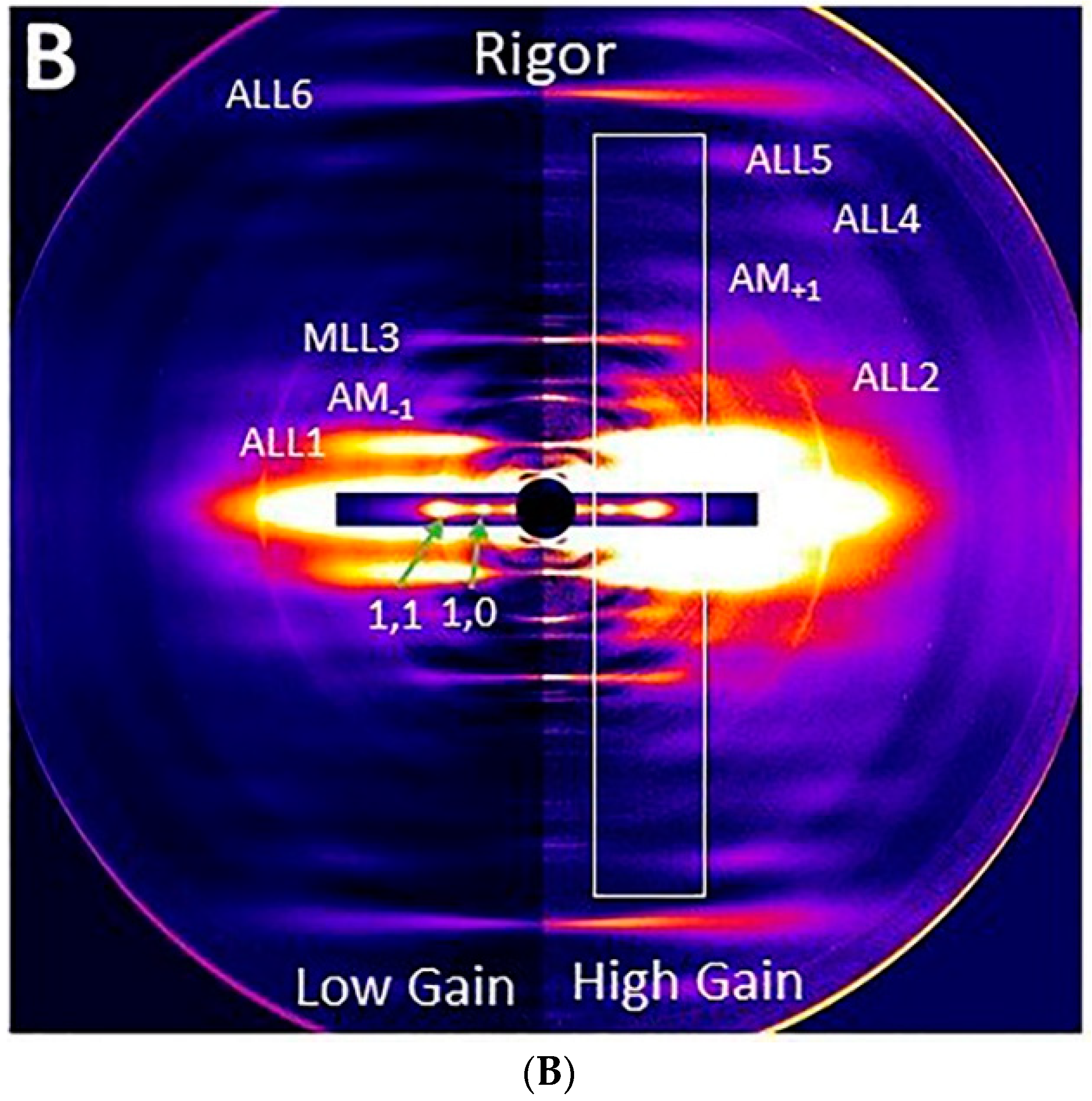
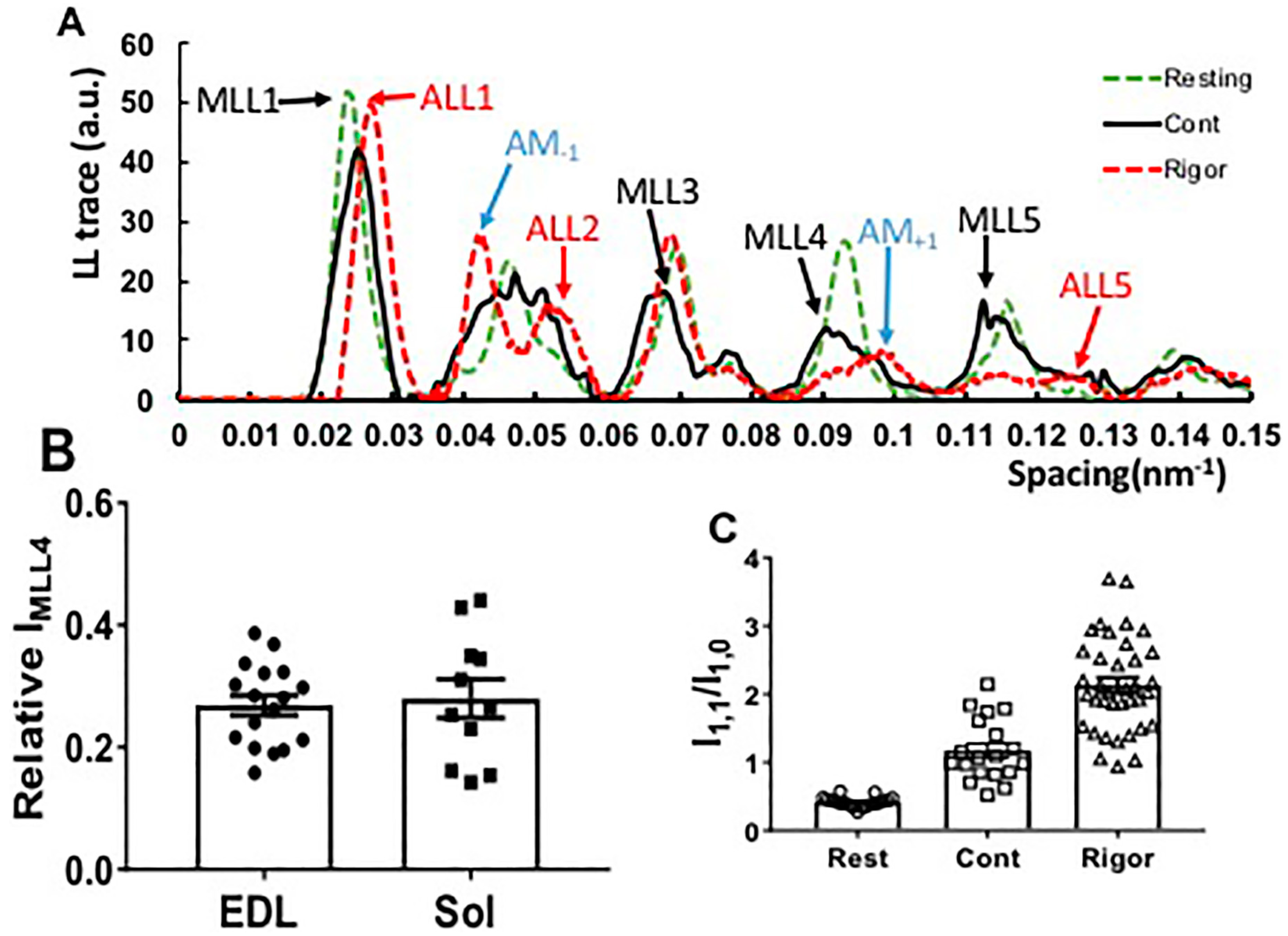
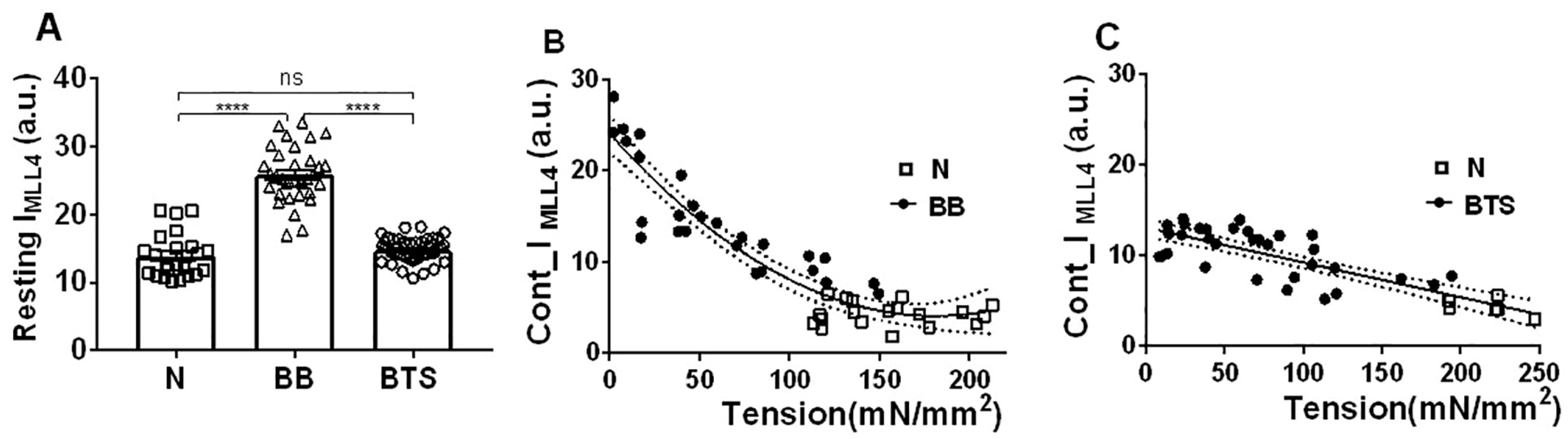
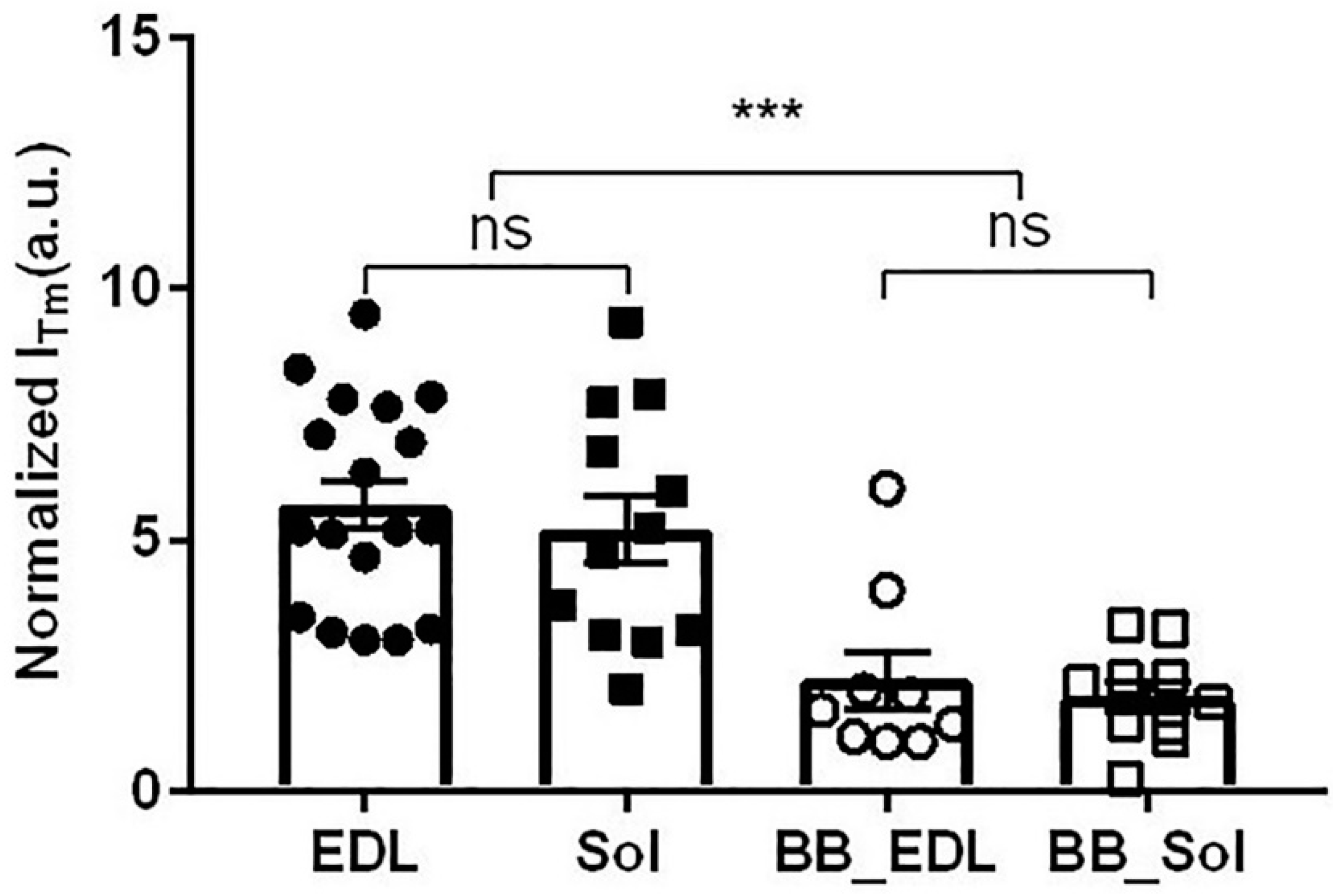
2.3. Effect of Myosin Inhibitors on Myosin Layer Lines
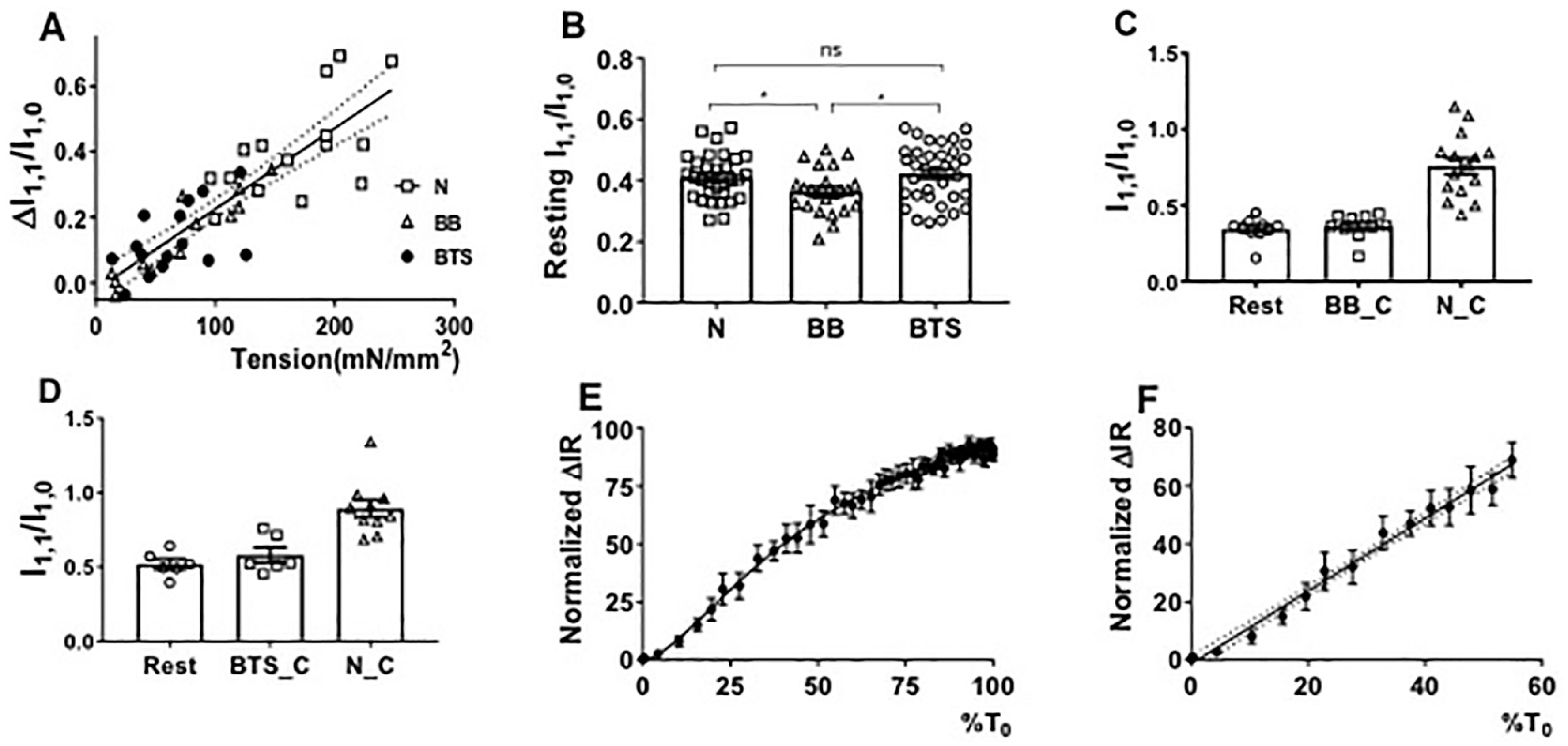
2.4. Equatorial Intensity Ratios
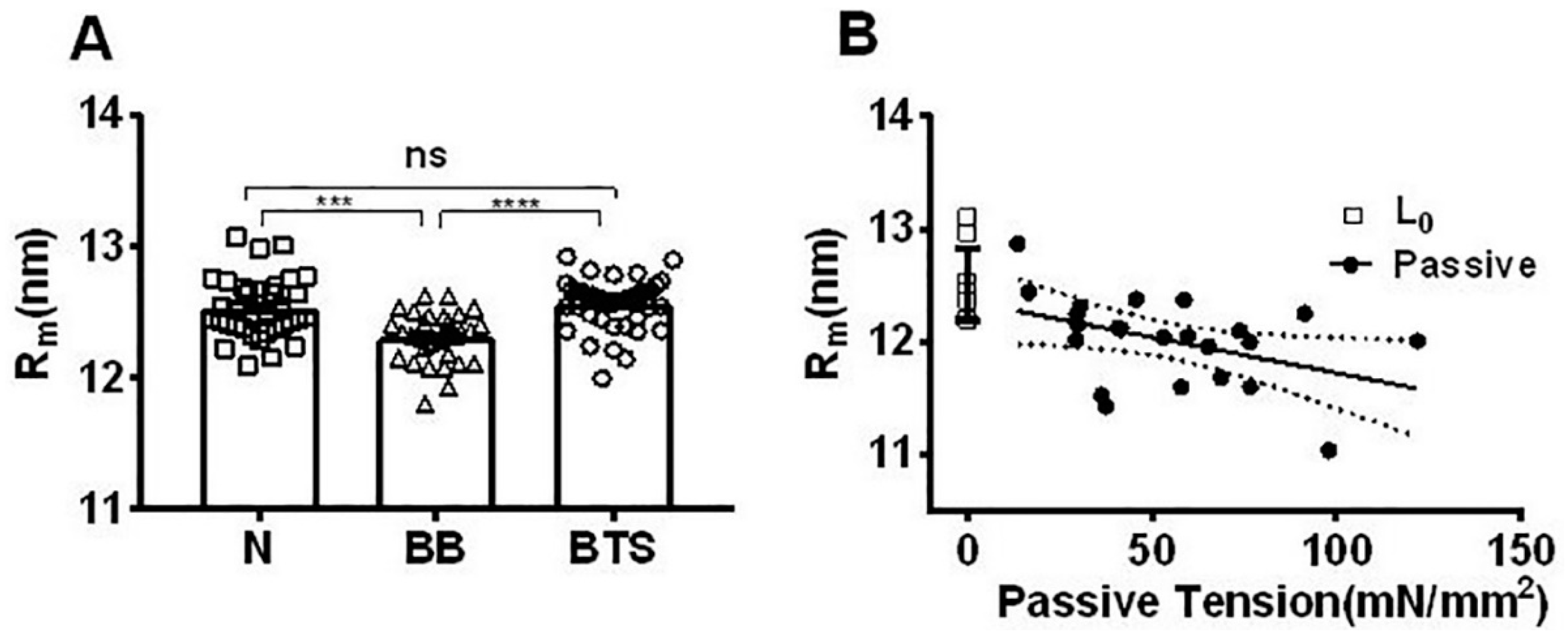
2.5. Effect of Myosin Inhibitors on Radial Spacing of Myosin Heads
2.6. Effect of Passive Tension on Radial Head Position
2.7. Effect of Blebbistatin on Tropomyosin Movement
3. Discussion
3.1. Residual Relaxed Myosin Layer Lines Present during Isometric Contraction
3.2. Estimation of Fraction of Actin-Bound Myosin Heads during Contraction
3.3. Is There More Than One Quasi-Helically Ordered State in Resting Mouse Muscle?
3.4. Interconversion between the Blebbistatin-Stabilized SRX State and Other States
4. Materials and Methods
4.1. Muscle Preparations
4.2. X-ray Diffraction
4.3. Post-Experiment Muscle Treatment
4.4. Data Analysis
4.5. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALL | actin layer line |
| AM | actomyosin |
| BB | blebbistatin |
| BTS | N-benzyl-p-toluene sulfonamide |
| C1 | lowest angle myosin binding protein C reflection |
| C2 | second myosin binding protein C reflection (doublet with C1) |
| C4 | 4th myosin binding protein C reflection (doublet with C3) |
| cKO | conditional knockout |
| DRX | disordered-relaxed state |
| EDL | extensor digitorum longus muscle |
| MLL | myosin layer line |
| M1 | first order myosin meridional X-ray reflection |
| M2 | second order myosin meridional X-ray reflection |
| M3 | third order myosin meridional X-ray reflection |
| M6 | sixth order myosin meridional reflection |
| RRX | regular-relaxed state |
| Tm | tropomyosin |
| Tn1 | first order troponin meridional reflection |
| Tn2 | second order troponin meridional reflection |
| Tn3 | third order troponin meridional reflection |
References
- Brunello, E.; Bianco, P.; Piazzesi, G.; Linari, M.; Reconditi, M.; Panine, P.; Narayanan, T.; Helsby, W.I.; Irving, M.; Lombardi, V. Structural changes in the myosin filament and cross-bridges during active force development in single intact frog muscle fibres: Stiffness and X-ray diffraction measurements. J. Physiol. 2006, 577, 971–984. [Google Scholar] [CrossRef] [PubMed]
- Reconditi, M. Recent improvements in small angle X-ray diffraction for the study of muscle physiology. Rep. Prog. Phys. 2006, 69, 2709–2759. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Wakayama, J.; Inoue, K.; Yagi, N.; Iwamoto, H. Dynamics of thin-filament activation in rabbit skeletal muscle fibers examined by time-resolved X-ray diffraction. Biophys. J. 2009, 96, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Huxley, H.E. Past, present and future experiments on muscle. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000, 355, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Kress, M.; Huxley, H.E.; Faruqi, A.R.; Hendrix, J. Structural changes during activation of frog muscle studied by time-resolved X-ray diffraction. J. Mol. Biol. 1986, 188, 325–342. [Google Scholar] [CrossRef]
- Bekyarova, T.I.; Reedy, M.C.; Baumann, B.A.; Tregear, R.T.; Ward, A.; Krzic, U.; Prince, K.M.; Perz-Edwards, R.J.; Reconditi, M.; Gore, D.; et al. Reverse actin sliding triggers strong myosin binding that moves tropomyosin. Proc. Natl. Acad. Sci. USA 2008, 105, 10372–10377. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Gu, J.; Belknap, B.; White, H.; Yu, L.C. Structural characterization of the binding of myosin ADP pi to actin in permeabilized rabbit psoas muscle. Biophys. J. 2006, 91, 3370–3382. [Google Scholar] [CrossRef] [PubMed]
- Squire, J.M.; Knupp, C. X-ray diffraction studies of muscle and the crossbridge cycle. Adv. Protein Chem. 2005, 71, 195–255. [Google Scholar] [PubMed]
- Perz-Edwards, R.J.; Irving, T.C.; Baumann, B.A.; Gore, D.; Hutchinson, D.C.; Krzic, U.; Porter, R.L.; Ward, A.B.; Reedy, M.K. X-ray diffraction evidence for myosin-troponin connections and tropomyosin movement during stretch activation of insect flight muscle. Proc. Natl. Acad. Sci. USA 2011, 108, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, H.; Inoue, K.; Yagi, N. Fast X-ray recordings reveal dynamic action of contractile and regulatory proteins in stretch-activated insect flight muscle. Biophys. J. 2010, 99, 184–192. [Google Scholar] [CrossRef] [PubMed]
- de Tombe, P.P.; Mateja, R.D.; Tachampa, K.; Ait Mou, Y.; Farman, G.P.; Irving, T.C. Myofilament length dependent activation. J. Mol. Cell. Cardiol. 2010, 48, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Ait-Mou, Y.; Hsu, K.; Farman, G.P.; Kumar, M.; Greaser, M.L.; Irving, T.C.; de Tombe, P.P. Titin strain contributes to the frank-starling law of the heart by structural rearrangements of both thin- and thick-filament proteins. Proc. Natl. Acad. Sci. USA 2016, 113, 2306–2311. [Google Scholar] [CrossRef] [PubMed]
- Reconditi, M.; Caremani, M.; Pinzauti, F.; Powers, J.D.; Narayanan, T.; Stienen, G.J.; Linari, M.; Lombardi, V.; Piazzesi, G. Myosin filament activation in the heart is tuned to the mechanical task. Proc. Natl. Acad. Sci. USA 2017, 114, 3240–3245. [Google Scholar] [CrossRef] [PubMed]
- Ochala, J.; Iwamoto, H.; Larsson, L.; Yagi, N. A myopathy-linked tropomyosin mutation severely alters thin filament conformational changes during activation. Proc. Natl. Acad. Sci. USA 2010, 107, 9807–9812. [Google Scholar] [CrossRef] [PubMed]
- Ochala, J.; Gokhin, D.S.; Iwamoto, H.; Fowler, V.M. Pointed-end capping by tropomodulin modulates actomyosin crossbridge formation in skeletal muscle fibers. FASEB J. 2014, 28, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, J.; Iwamoto, H.; Blanco, G.; Ochala, J. The fraction of strongly bound cross-bridges is increased in mice that carry the myopathy-linked myosin heavy chain mutation myh4(l342q). Dis. Models Mech. 2013, 6, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Huxley, H.E.; Kress, M. Crossbridge behaviour during muscle contraction. J. Muscle Res. Cell Motil. 1985, s6, 153–161. [Google Scholar] [CrossRef]
- Tsaturyan, A.K.; Bershitsky, S.Y.; Koubassova, N.A.; Fernandez, M.; Narayanan, T.; Ferenczi, M.A. The fraction of myosin motors that participate in isometric contraction of rabbit muscle fibers at near-physiological temperature. Biophys. J. 2011, 101, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Linari, M.; Caremani, M.; Piperio, C.; Brandt, P.; Lombardi, V. Stiffness and fraction of myosin motors responsible for active force in permeabilized muscle fibers from rabbit psoas. Biophys. J. 2007, 92, 2476–2490. [Google Scholar] [CrossRef] [PubMed]
- McNamara, J.W.; Li, A.; Dos Remedios, C.G.; Cooke, R. The role of super-relaxed myosin in skeletal and cardiac muscle. Biophys. Rev. 2015, 7, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Naber, N.; Pate, E.; Cooke, R. The myosin inhibitor blebbistatin stabilizes the super-relaxed state in skeletal muscle. Biophys. J. 2014, 107, 1637–1646. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, D.V.; Adhikari, A.S.; Sarkar, S.S.; Ruppel, K.M.; Spudich, J.A. Hypertrophic cardiomyopathy and the myosin mesa: Viewing an old disease in a new light. Biophys. Rev. 2018, 10, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.; Trivedi, D.V.; Sarkar, S.S.; Henze, M.; Ma, W.; Gong, H.; Rogers, C.; Wong, F.; Morck, M.; Ruppel, K.; et al. Mavacamten stabilizes a folded-back sequestered super-relaxed state of β-cardiac myosin. Proc. Natl. Acad. Sci. USA 2018. [Google Scholar] [CrossRef]
- Nogara, L.; Naber, N.; Pate, E.; Canton, M.; Reggiani, C.; Cooke, R. Spectroscopic studies of the super relaxed state of skeletal muscle. PLoS ONE 2016, 11, e0160100. [Google Scholar] [CrossRef] [PubMed]
- Hooijman, P.; Stewart, M.A.; Cooke, R. A new state of cardiac myosin with very slow atp turnover: A potential cardioprotective mechanism in the heart. Biophys. J. 2011, 100, 1969–1976. [Google Scholar] [CrossRef] [PubMed]
- Alamo, L.; Ware, J.S.; Pinto, A.; Gillilan, R.E.; Seidman, J.G.; Seidman, C.E.; Padron, R. Effects of myosin variants on interacting-heads motif explain distinct hypertrophic and dilated cardiomyopathy phenotypes. eLife 2017, 6, e24634. [Google Scholar] [CrossRef] [PubMed]
- Farman, G.P.; Tachampa, K.; Mateja, R.; Cazorla, O.; Lacampagne, A.; de Tombe, P.P. Blebbistatin: Use as inhibitor of muscle contraction. Pflug. Arch. Eur. J. Physiol. 2008, 455, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, M.; Toth, J.; Hetenyi, C.; Malnasi-Csizmadia, A.; Sellers, J.R. Mechanism of blebbistatin inhibition of myosin ii. J. Biol. Chem. 2004, 279, 35557–35563. [Google Scholar] [CrossRef] [PubMed]
- Kampourakis, T.; Zhang, X.; Sun, Y.B.; Irving, M. Omecamtiv mercabil and blebbistatin modulate cardiac contractility by perturbing the regulatory state of the myosin filament. J. Physiol. 2018, 596, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, M.E.; Woodhead, J.L.; Moss, R.L.; Craig, R. Three-dimensional structure of vertebrate cardiac muscle myosin filaments. Proc. Natl. Acad. Sci. USA 2008, 105, 2386–2390. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.Q.; Padron, R.; Craig, R. Blebbistatin stabilizes the helical order of myosin filaments by promoting the switch 2 closed state. Biophys. J. 2008, 95, 3322–3329. [Google Scholar] [CrossRef] [PubMed]
- Haselgrove, J.C.; Huxley, H.E. X-ray evidence for radial cross-bridge movement and for the sliding filament model in actively contracting skeletal muscle. J. Mol. Biol. 1973, 77, 549–568. [Google Scholar] [CrossRef]
- Matsubara, I.; Yagi, N.; Hashizume, H. Use of an X-ray television for diffraction of the frog striated muscle. Nature 1975, 255, 728–729. [Google Scholar] [CrossRef] [PubMed]
- Brenner, B.; Yu, L.C. Equatorial X-ray-diffraction from single skinned rabbit psoas fibers at various degrees of activation. Changes in intensities and lattice spacing. Biophys. J. 1985, 48, 829–834. [Google Scholar] [CrossRef]
- Matsubara, I. X-ray diffraction studies of the heart. Annu. Rev. Biophys. Bioeng. 1980, 9, 81–105. [Google Scholar] [CrossRef] [PubMed]
- Malinchik, S.; Xu, S.; Yu, L.C. Temperature-induced structural changes in the myosin thick filament of skinned rabbit psoas muscle. Biophys. J. 1997, 73, 2304–2312. [Google Scholar] [CrossRef]
- Huxley, H.E. Recent X-ray diffraction studies of muscle contraction and their implications. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004, 359, 1879–1882. [Google Scholar] [PubMed]
- Linari, M.; Piazzesi, G.; Dobbie, I.; Koubassova, N.; Reconditi, M.; Narayanan, T.; Diat, O.; Irving, M.; Lombardi, V. Interference fine structure and sarcomere length dependence of the axial X-ray pattern from active single muscle fibers. Proc. Natl. Acad. Sci. USA 2000, 97, 7226–7231. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, R.; Holmes, K.C. Actin structure and function. Annu. Rev. Biophys. 2011, 40, 169–186. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, K.; Ueno, Y.; Amemiya, Y.; Tanaka, H. Intensity changes of actin-based layer lines from frog skeletal muscles during an isometric contraction. Adv. Exp. Med. Biol. 1988, 226, 353–367. [Google Scholar] [PubMed]
- Li, F.; Buck, D.; De Winter, J.; Kolb, J.; Meng, H.; Birch, C.; Slater, R.; Escobar, Y.N.; Smith, J.E., 3rd; Yang, L.; et al. Nebulin deficiency in adult muscle causes sarcomere defects and muscle-type-dependent changes in trophicity: Novel insights in nemaline myopathy. Hum. Mol. Genet. 2015, 24, 5219–5233. [Google Scholar] [CrossRef] [PubMed]
- Yagi, N.; Iwamoto, H.; Wakayama, J.; Inoue, K. Structural changes of actin-bound myosin heads after a quick length change in frog skeletal muscle. Biophys. J. 2005, 89, 1150–1164. [Google Scholar] [CrossRef] [PubMed]
- Koubassova, N.A.; Bershitsky, S.Y.; Ferenczi, M.A.; Tsaturyan, A.K. Direct modeling of X-ray diffraction pattern from contracting skeletal muscle. Biophys. J. 2008, 95, 2880–2894. [Google Scholar] [CrossRef] [PubMed]
- Kiss, B.; Lee, E.; Ma, W.; Li, F.; Tonino, P.; Mijailovich, S.; Irving, T.C.; Granzier, H. Nebulin stiffens the thin filament and augments cross-bridge interaction in skeletal muscle. Proc. Natl. Acad. Sci. USA 2018, in press. [Google Scholar]
- Huxley, H.E.; Stewart, A.; Sosa, H.; Irving, T. X-ray diffraction measurements of the extensibility of actin and myosin filaments in contracting muscle. Biophys. J. 1994, 67, 2411–2421. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Sugimoto, Y.; Tanaka, H.; Ueno, Y.; Takezawa, Y.; Amemiya, Y. X-ray diffraction evidence for the extensibility of actin and myosin filaments during muscle contraction. Biophys. J. 1994, 67, 2422–2435. [Google Scholar] [CrossRef]
- Xu, S.G.; White, H.D.; Offer, G.W.; Yu, L.C. Stabilization of helical order in the thick filaments by blebbistatin: Further evidence of coexisting multiple conformations of myosin. Biophys. J. 2009, 96, 3673–3681. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, H. Effects of myosin inhibitors on the X-ray diffraction patterns of relaxed and calcium-activated rabbit skeletal muscle fibers. Biophys. Physicobiol. 2018, 15, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.C.; Steven, A.C.; Naylor, G.R.; Gamble, R.C.; Podolsky, R.J. Distribution of mass in relaxed frog skeletal muscle and its redistribution upon activation. Biophys. J. 1985, 47, 311–321. [Google Scholar] [CrossRef]
- Irving, T.; Wu, Y.; Bekyarova, T.; Farman, G.P.; Fukuda, N.; Granzier, H. Thick-filament strain and interfilament spacing in passive muscle: Effect of titin-based passive tension. Biophys. J. 2011, 100, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Martyn, D.; Zaman, J.; Yu, L.C. X-ray diffraction studies of the thick filament in permeabilized myocardium from rabbit. Biophys. J. 2006, 91, 3768–3775. [Google Scholar] [CrossRef] [PubMed]
- Huxley, H.E. The structural basis of muscular contraction. Proc. R. Soc. Lond. B Biol. Sci. 1971, 178, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Parry, D.A.; Squire, J.M. Structural role of tropomyosin in muscle regulation: Analysis of the X-ray diffraction patterns from relaxed and contracting muscles. J. Mol. Biol. 1973, 75, 33–55. [Google Scholar] [CrossRef]
- Poole, K.J.; Lorenz, M.; Evans, G.; Rosenbaum, G.; Pirani, A.; Craig, R.; Tobacman, L.S.; Lehman, W.; Holmes, K.C. A comparison of muscle thin filament models obtained from electron microscopy reconstructions and low-angle X-ray fibre diagrams from non-overlap muscle. J. Struct. Biol. 2006, 155, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Bordas, J.; Diakun, G.P.; Diaz, F.G.; Harries, J.E.; Lewis, R.A.; Lowy, J.; Mant, G.R.; Martin-Fernandez, M.L.; Towns-Andrews, E. Two-dimensional time-resolved X-ray diffraction studies of live isometrically contracting frog sartorius muscle. J. Muscle Res. Cell Motil. 1993, 14, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Kraft, T.; Mattei, T.; Radocaj, A.; Piep, B.; Nocula, C.; Furch, M.; Brenner, B. Structural features of cross-bridges in isometrically contracting skeletal muscle. Biophys. J. 2002, 82, 2536–2547. [Google Scholar] [CrossRef]
- Ferenczi, M.A.; Bershitsky, S.Y.; Koubassova, N.; Siththanandan, V.; Helsby, W.I.; Panine, P.; Roessle, M.; Narayanan, T.; Tsaturyan, A.K. The “roll and lock” mechanism of force generation in muscle. Structure 2005, 13, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Hakim, C.H.; Wasala, N.B.; Duan, D. Evaluation of muscle function of the extensor digitorum longus muscle ex vivo and tibialis anterior muscle in situ in mice. J. Vis. Exp. 2013, 72, 50183. [Google Scholar] [CrossRef] [PubMed]
- Irving, M. Regulation of contraction by the thick filaments in skeletal muscle. Biophys. J. 2017, 113, 2579–2594. [Google Scholar] [CrossRef] [PubMed]
- Irving, T.C.; Millman, B.M. Changes in thick filament structure during compression of the filament lattice in relaxed frog sartorius muscle. J. Muscle Res. Cell Motil. 1989, 10, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.C. Analysis of equatorial X-ray diffraction patterns from skeletal muscle. Biophys. J. 1989, 55, 433–440. [Google Scholar] [CrossRef]
- Malinchik, S.; Yu, L.C. Analysis of equatorial X-ray diffraction patterns from muscle fibers: Factors that affect the intensities. Biophys. J. 1995, 68, 2023–2031. [Google Scholar] [CrossRef]
- Bershitsky, S.Y.; Koubassova, N.A.; Bennett, P.M.; Ferenczi, M.A.; Shestakov, D.A.; Tsaturyan, A.K. Myosin heads contribute to the maintenance of filament order in relaxed rabbit muscle. Biophys. J. 2010, 99, 1827–1834. [Google Scholar] [CrossRef] [PubMed]
- Huxley, H.E.; Faruqi, A.R.; Kress, M.; Bordas, J.; Koch, M.H. Time-resolved X-ray diffraction studies of the myosin layer-line reflections during muscle contraction. J. Mol. Biol. 1982, 158, 637–684. [Google Scholar] [CrossRef]
- Bershitsky, S.Y.; Tsaturyan, A.K.; Bershitskaya, O.N.; Mashanov, G.I.; Brown, P.; Burns, R.; Ferenczi, M.A. Muscle force is generated by myosin heads stereo-specifically attached to actin. Nature 1997, 388, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.; Weisberg, A.; Kulikovskaya, I.; McClellan, G.; Winegrad, S. Multiple structures of thick filaments in resting cardiac muscle and their influence on cross-bridge interactions. Biophys. J. 2001, 81, 1070–1082. [Google Scholar] [CrossRef]
- Squire, J. Muscle regulation: A decade of the steric blocking model. Nature 1981, 291, 614–615. [Google Scholar] [CrossRef] [PubMed]
- Huxley, H. Structural changes in the actin- and myosin-containing filaments during contraction. Cold Spring Harb. Symp.Quant. Biol. 1973, 37, 361–376. [Google Scholar] [CrossRef]
- Haselgrove, J. X-ray evidence for a conformational change in the actin-containing filaments of vertebrate striated muscle. Cold Spring Harb. Symp. Quant. Biol. 1973, 37, 341–352. [Google Scholar] [CrossRef]
- Lehman, W.; Craig, R.; Vibert, P. Ca2+ induced tropomyosin movement in limulus thin filaments revealed by three-dimensional reconstruction. Nature 1994, 368, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Vibert, P.; Craig, R.; Lehman, W. Steric-model for activation of muscle thin filaments. J. Mol. Biol. 1997, 266, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Mckillop, D.F.A.; Geeves, M.A. Regulation of the interaction between actin and myosin subfragment-1: Evidence for 3 states of the thin filament. Biophys. J. 1993, 65, 693–701. [Google Scholar] [CrossRef]
- Allingham, J.S.; Smith, R.; Rayment, I. The structural basis of blebbistatin inhibition and specificity for myosin ii. Nat. Struct. Mol. Biol. 2005, 12, 378–379. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, B.; Yengo, C.M.; Straight, A.F.; Mitchison, T.J.; Sweeney, H.L. Kinetic mechanism of blebbistatin inhibition of nonmuscle myosin iib. Biochemistry 2004, 43, 14832–14839. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Usaj, M.; Rassier, D.E.; Mansson, A. Blebbistatin effects expose hidden secrets in the force-generating cycle of actin and myosin. Biophys. J. 2018, 115, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.; Franks-Skiba, K.; Cooke, R. Myosin regulatory light chain phosphorylation inhibits shortening velocities of skeletal muscle fibers in the presence of the myosin inhibitor blebbistatin. J. Muscle. Res. Cell Motil. 2009, 30, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Colson, B.A.; Patel, J.R.; Chen, P.P.; Bekyarova, T.; Abdalla, M.I.; Tong, C.W.; Fitzsimons, D.P.; Irving, T.C.; Moss, R.L. Myosin binding protein-c phosphorylation is the principal mediator of protein kinase a effects on thick filament structure in myocardium. J. Mol. Cell. Cardiol. 2012, 53, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Millman, B.M. The filament lattice of striated muscle. Physiol. Rev. 1998, 78, 359–391. [Google Scholar] [CrossRef] [PubMed]
- Irving, T.C.; Konhilas, J.; Perry, D.; Fischetti, R.; de Tombe, P.P. Myofilament lattice spacing as a function of sarcomere length in isolated rat myocardium. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H2568–H2573. [Google Scholar] [CrossRef] [PubMed]
- Fusi, L.; Brunello, E.; Yan, Z.; Irving, M. Thick filament mechano-sensing is a calcium-independent regulatory mechanism in skeletal muscle. Nat. Commun. 2016, 7, 13281. [Google Scholar] [CrossRef] [PubMed]
- Spudich, J.A. The myosin mesa and a possible unifying hypothesis for the molecular basis of human hypertrophic cardiomyopathy. Biochem. Soc. Trans. 2015, 43, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Kawana, M.; Sarkar, S.S.; Sutton, S.; Ruppel, K.M.; Spudich, J.A. Biophysical properties of human beta-cardiac myosin with converter mutations that cause hypertrophic cardiomyopathy. Sci. Adv. 2017, 3, e1601959. [Google Scholar] [CrossRef] [PubMed]
- Green, E.M.; Wakimoto, H.; Anderson, R.L.; Evanchik, M.J.; Gorham, J.M.; Harrison, B.C.; Henze, M.; Kawas, R.; Oslob, J.D.; Rodriguez, H.M.; et al. A small-molecule inhibitor of sarcomere contractility suppresses hypertrophic cardiomyopathy in mice. Science 2016, 351, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Kawas, R.F.; Anderson, R.L.; Ingle, S.R.B.; Song, Y.; Sran, A.S.; Rodriguez, H.M. A small-molecule modulator of cardiac myosin acts on multiple stages of the myosin chemomechanical cycle. J. Biol. Chem. 2017, 292, 16571–16577. [Google Scholar] [CrossRef] [PubMed]
- Marian, A.J.; Salek, L.; Lutucuta, S. Molecular genetics and pathogenesis of hypertrophic cardiomyopathy. Minerva Med. 2001, 92, 435–451. [Google Scholar] [PubMed]
- Harris, S.P.; Lyons, R.G.; Bezold, K.L. In the thick of it: Hcm-causing mutations in myosin binding proteins of the thick filament. Circ. Res. 2011, 108, 751–764. [Google Scholar] [CrossRef] [PubMed]
- McNamara, J.W.; Li, A.; Smith, N.J.; Lal, S.; Graham, R.M.; Kooiker, K.B.; van Dijk, S.J.; Remedios, C.G.D.; Harris, S.P.; Cooke, R. Ablation of cardiac myosin binding protein-c disrupts the super-relaxed state of myosin in murine cardiomyocytes. J. Mol. Cell Cardiol. 2016, 94, 65–71. [Google Scholar] [CrossRef] [PubMed]
- McNamara, J.W.; Li, A.; Lal, S.; Bos, J.M.; Harris, S.P.; van der Velden, J.; Ackerman, M.J.; Cooke, R.; Dos Remedios, C.G. Mybpc3 mutations are associated with a reduced super-relaxed state in patients with hypertrophic cardiomyopathy. PLoS ONE 2017, 12, e0180064. [Google Scholar] [CrossRef] [PubMed]
- Kensler, R.W.; Craig, R.; Moss, R.L. Phosphorylation of cardiac myosin binding protein c releases myosin heads from the surface of cardiac thick filaments. Proc. Natl. Acad. Sci. USA 2017, 114, E1355–E1364. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Huxley, H.E. Structural difference between resting and rigor muscle; evidence from intensity changes in the lowangle equatorial X-ray diagram. J. Mol. Biol. 1968, 37, 507–520. [Google Scholar] [CrossRef]
- Fischetti, R.; Stepanov, S.; Rosenbaum, G.; Barrea, R.; Black, E.; Gore, D.; Heurich, R.; Kondrashkina, E.; Kropf, A.J.; Wang, S.; et al. The biocat undulator beamline 18id: A facility for biological non-crystalline diffraction and X-ray absorption spectroscopy at the advanced photon source. J. Synchrotron. Radiat. 2004, 11, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Alexander, R.M.V.A. The dimensions of knee and ankle muscles and the forces they exert. J. Hum. Mov. Stud. 1975, 1, 115–123. [Google Scholar]
- Burkholder, T.J.; Fingado, B.; Baron, S.; Lieber, R.L. Relationship between muscle-fiber types and sizes and muscle architectural properties in the mouse hindlimb. J. Morphol. 1994, 221, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Narici, M.V.; Landoni, L.; Minetti, A.E. Assessment of human knee extensor muscles stress from in vivo physiological cross-sectional area and strength measurements. Eur. J. Appl. Physiol. Occup. Physiol. 1992, 65, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Hammersley, A.P. Fit2d: A multi-purpose data reduction, analysis and visualization program. J. Appl. Crystallogr. 2016, 49, 646–652. [Google Scholar] [CrossRef]
- Jiratrakanvong, J.; Shao, J.; Menendez, M.; Li, X.; Li, J.; Ma, W.; Agam, G.; Irving, T. Musclex: Software suite for diffraction X-ray imaging v1.13.1. 2018. Available online: https://zenodo.org/record/1195579#.W45kKJoRWUk (accessed on 5 September 2018). [CrossRef]
- Vainshtein, B.K. Diffraction of X-rays by Chain Molecules; Elsevier: Amsterdam, The Netherlands, 1966; pp. 216–229. [Google Scholar]
- Burton, K.; Zagotta, W.N.; Baskin, R.J. Sarcomere-length behavior along single frog-muscle fibers at different lengths during isometric tetani. J. Muscle Res. Cell Motil. 1989, 10, 67–84. [Google Scholar] [CrossRef] [PubMed]
| Equatorial Width Parameters | Resting (n = 9) | Contraction (n = 9) | p Value |
|---|---|---|---|
| Axial width (nm−1) | 2.7 ± 0.2 | 2.8 ± 0.2 | ns |
| σd (nm−1) | 1.36 ± 0.09 (×10−3) | 2.21 ± 0.11 (×10−3) | **** |
| σs (nm−1) | 0.54 ± 0.04 (×10−3) | 0.98 ± 0.08 (×10−3) | ** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, W.; Gong, H.; Irving, T. Myosin Head Configurations in Resting and Contracting Murine Skeletal Muscle. Int. J. Mol. Sci. 2018, 19, 2643. https://doi.org/10.3390/ijms19092643
Ma W, Gong H, Irving T. Myosin Head Configurations in Resting and Contracting Murine Skeletal Muscle. International Journal of Molecular Sciences. 2018; 19(9):2643. https://doi.org/10.3390/ijms19092643
Chicago/Turabian StyleMa, Weikang, Henry Gong, and Thomas Irving. 2018. "Myosin Head Configurations in Resting and Contracting Murine Skeletal Muscle" International Journal of Molecular Sciences 19, no. 9: 2643. https://doi.org/10.3390/ijms19092643
APA StyleMa, W., Gong, H., & Irving, T. (2018). Myosin Head Configurations in Resting and Contracting Murine Skeletal Muscle. International Journal of Molecular Sciences, 19(9), 2643. https://doi.org/10.3390/ijms19092643







