CDC42 Negatively Regulates Testis-Specific SEPT12 Polymerization
Abstract
1. Introduction
1.1. Septin Family
1.2. Functional Roles of SEPTs in Mammalian Spermatogenesis
1.3. CDC42 and SEPTs
2. Results
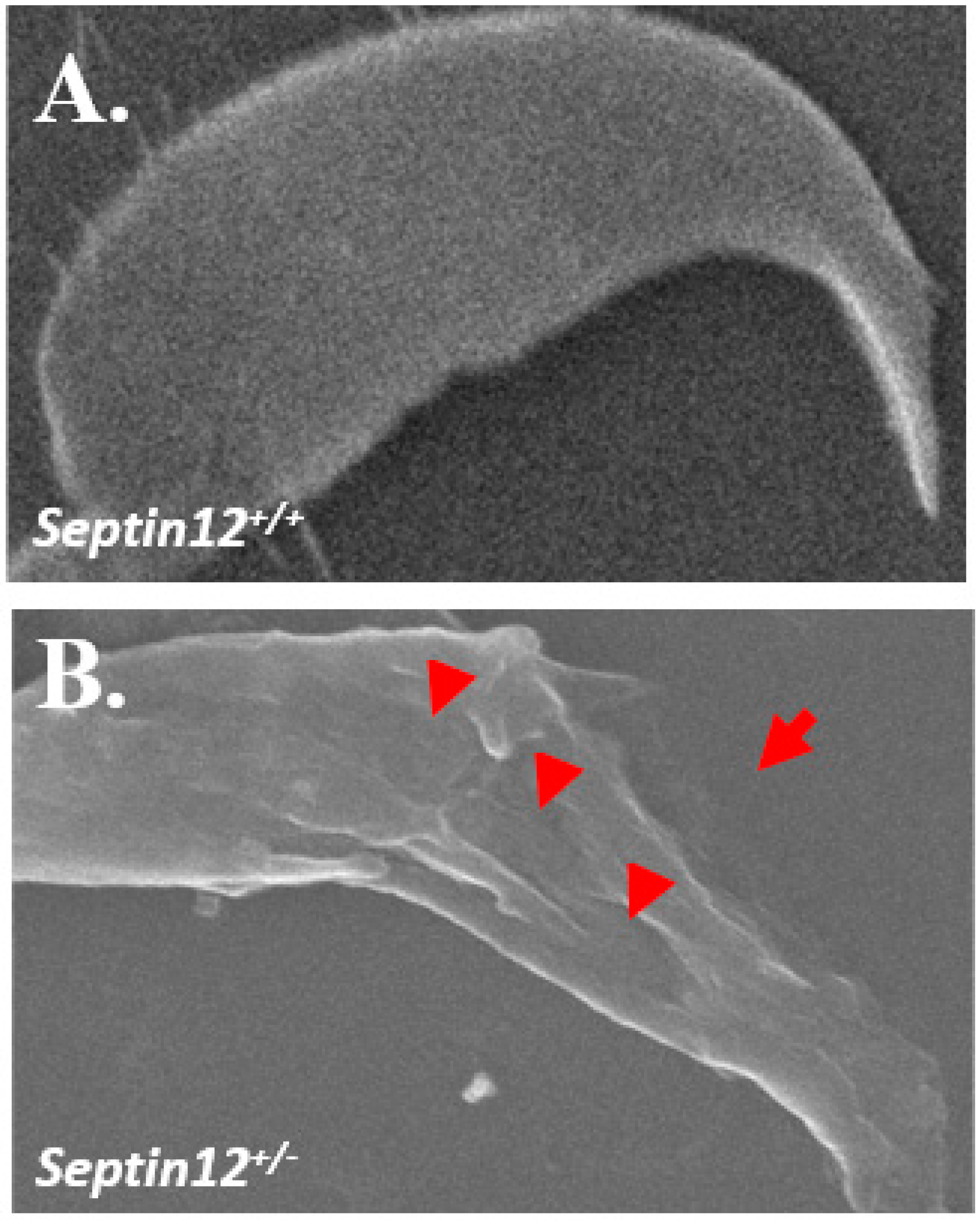
2.1. Using Scanning Electron Microscopy to Evaluate Sperm Heads from Septin12+/− Adult Mice
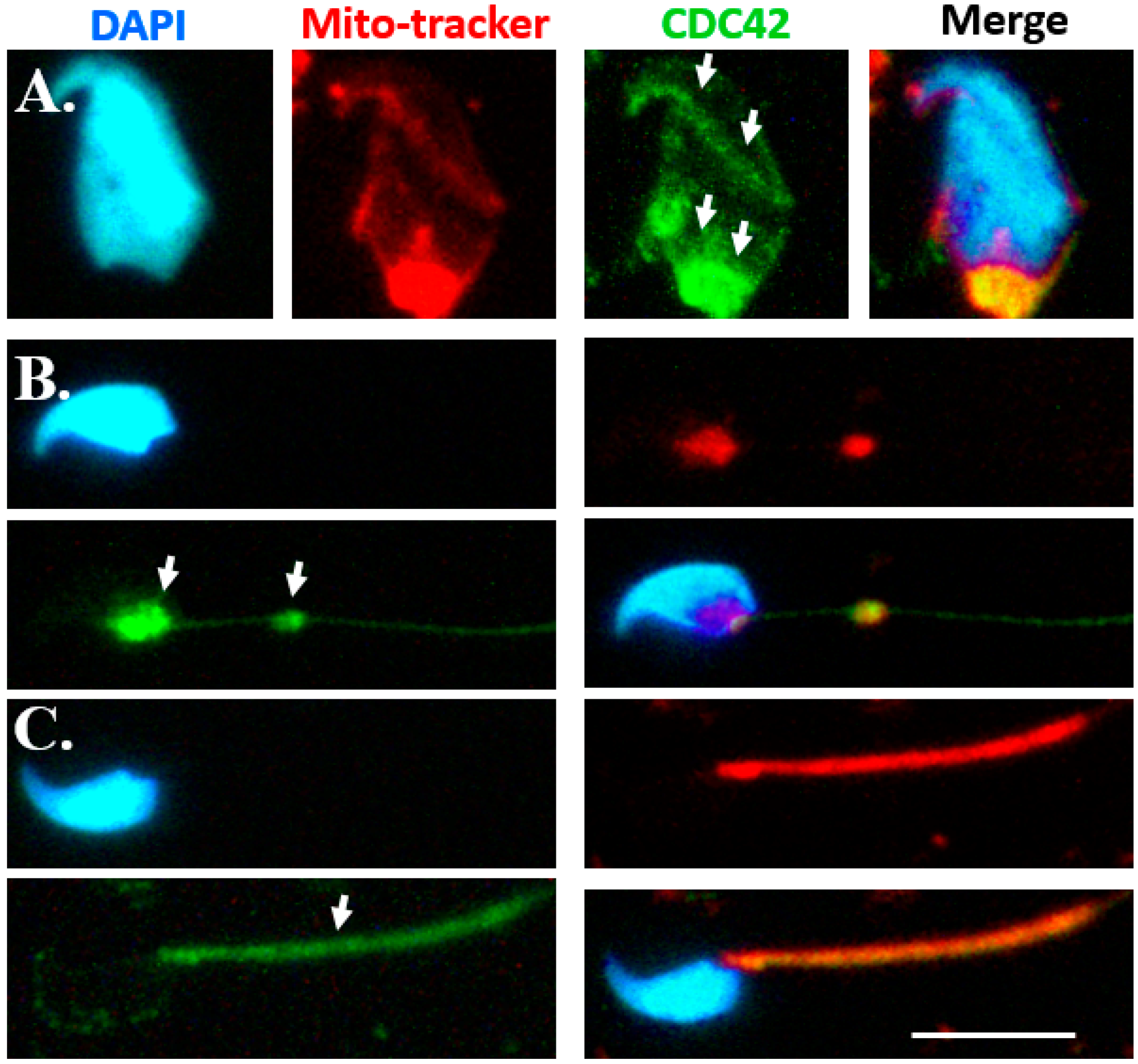
2.2. Dynamic Expression Patterns of CDC42 during Murine Spermiogenesis
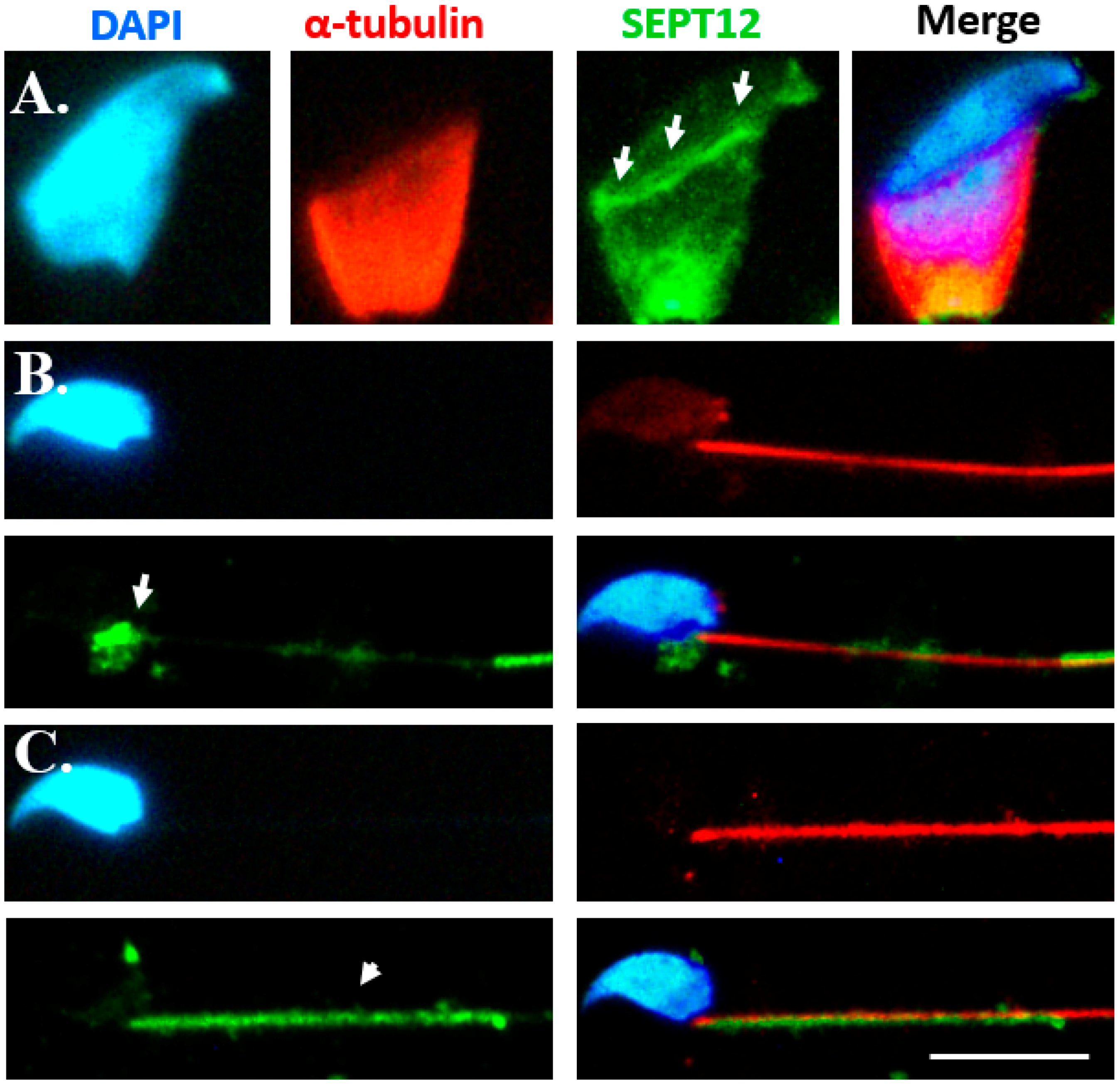
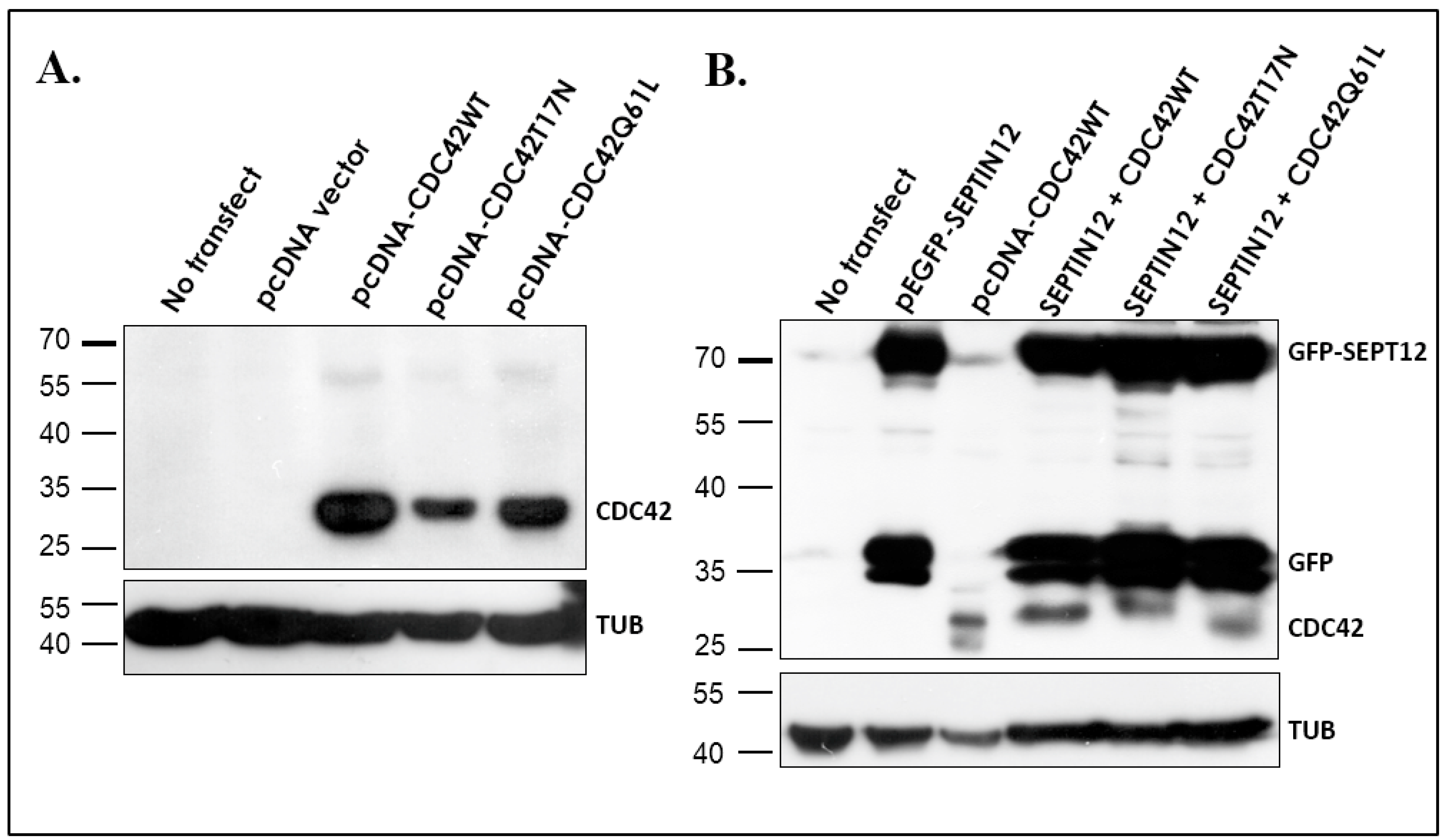
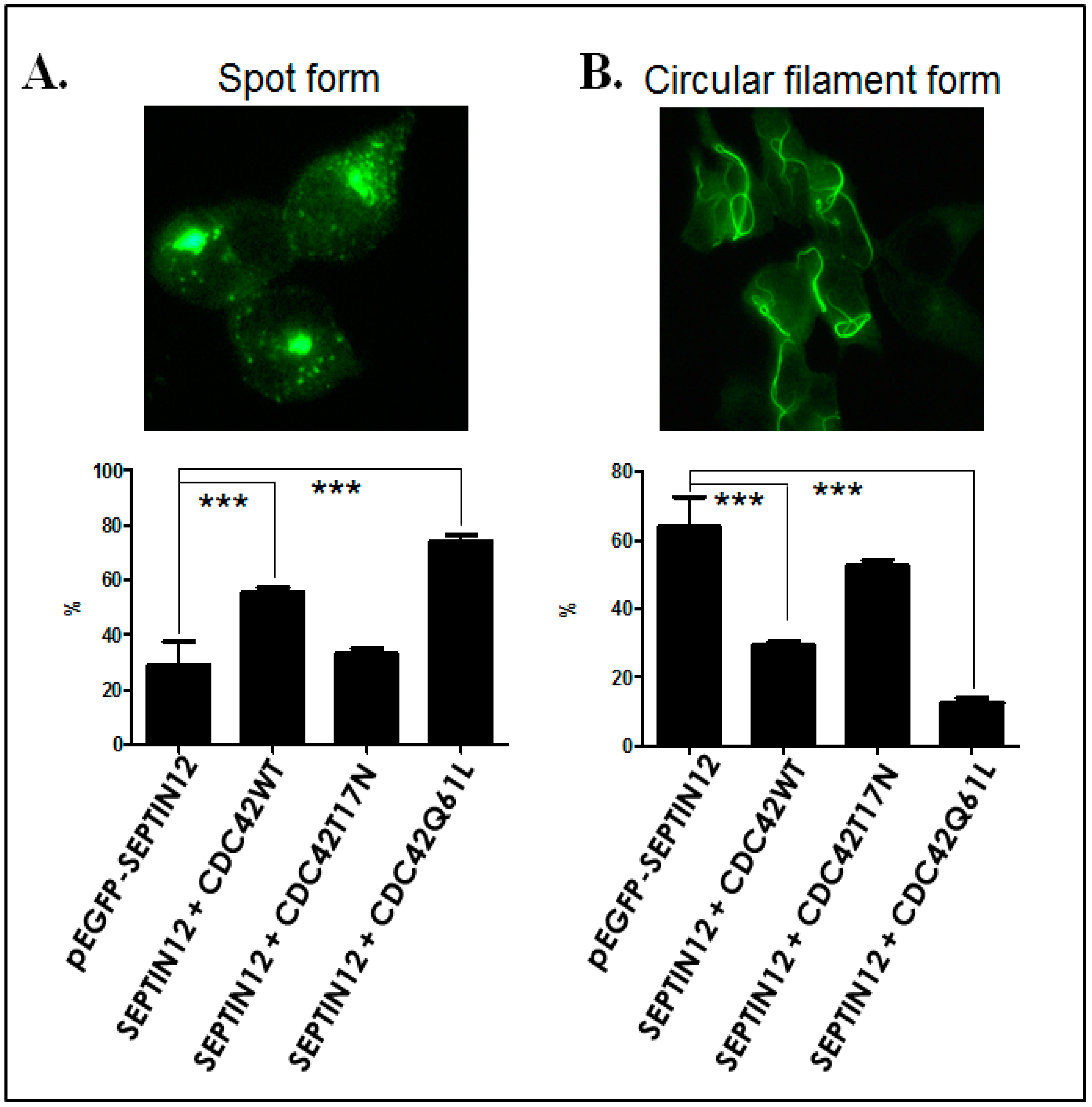
2.3. CDC42 Alters SEPT12 Polymerization
3. Discussion
3.1. Loss of Septin12/SEPTIN12 Damages the Structure of Sperm Heads
3.2. Dynamic Expression of CDC42 and SEPT12 during Mammalian Spermiogenesis
3.3. CDC42 Alters SEPT12 Polymerization
3.4. CDC42/BROG/SEPTs and Terminal Differentiation of Sperm
4. Experimental Section
4.1. Scanning Electron Microscopy
4.2. Testicular Germ Cell Isolation
4.3. Immunofluorescence Staining
4.4. Cloning, Transfection, and Western Blotting
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Mostowy, S.; Cossart, P. Septins: The fourth component of the cytoskeleton. Nat. Rev. Mol. Cell Biol. 2012, 13, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Versele, M.; Thorner, J. Some assembly required: Yeast septins provide the instruction manual. Trends Cell Biol. 2005, 15, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, M. Diversity of septin scaffolds. Curr. Opin. Cell Biol. 2006, 18, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Hall, P.A.; Russell, S.E. The pathobiology of the septin gene family. J. Pathol. 2004, 204, 489–505. [Google Scholar] [CrossRef] [PubMed]
- Hall, P.A.; Jung, K.; Hillan, K.J.; Russell, S.E. Expression profiling the human septin gene family. J. Pathol. 2005, 206, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Sirajuddin, M.; Farkasovsky, M.; Hauer, F.; Kuhlmann, D.; Macara, I.G.; Weyand, M.; Stark, H.; Wittinghofer, A. Structural insight into filament formation by mammalian septins. Nature 2007, 449, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Ihara, M.; Kinoshita, A.; Yamada, S.; Tanaka, H.; Tanigaki, A.; Kitano, A.; Goto, M.; Okubo, K.; Nishiyama, H.; Ogawa, O.; et al. Cortical organization by the septin cytoskeleton is essential for structural and mechanical integrity of mammalian spermatozoa. Dev. Cell 2005, 8, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Kissel, H.; Georgescu, M.M.; Larisch, S.; Manova, K.; Hunnicutt, G.R.; Steller, H. The Sept4 septin locus is required for sperm terminal differentiation in mice. Dev. Cell 2005, 8, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Sugino, Y.; Ichioka, K.; Soda, T.; Ihara, M.; Kinoshita, M.; Ogawa, O.; Nishiyama, H. Septins as diagnostic markers for a subset of human asthenozoospermia. J. Urol. 2008, 180, 2706–2709. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Lin, Y.M.; Teng, Y.N.; Hsieh, T.Y.; Lin, Y.S.; Kuo, P.L. Identification of ten novel genes involved in human spermatogenesis by microarray analysis of testicular tissue. Fertil. Steril. 2006, 86, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Lin, Y.M.; Wang, Y.Y.; Yu, I.S.; Lin, Y.W.; Wang, Y.H.; Wu, C.M.; Pan, H.A.; Chao, S.C.; Yen, P.H.; et al. The expression level of septin12 is critical for spermiogenesis. Am. J. Pathol. 2009, 174, 1857–1868. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Yu, W.; Liu, M.; Shen, S.; Chen, F.; Wan, B.; Yu, L. SEPT12 interacts with SEPT6 and this interaction alters the filament structure of SEPT6 in Hela cells. J. Biochem. Mol. Biol. 2007, 40, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Steels, J.D.; Estey, M.P.; Froese, C.D.; Reynaud, D.; Pace-Asciak, C.; Trimble, W.S. Sept12 is a component of the mammalian sperm tail annulus. Cell Motil. Cytoskelet. 2007, 64, 794–807. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.C.; Lin, Y.H.; Chen, H.I.; Wang, Y.Y.; Chiou, Y.W.; Lin, H.H.; Pan, H.A.; Wu, C.M.; Su, S.M.; Hsu, C.C.; et al. SEPT12 mutations cause male infertility with defective sperm annulus. Hum. Mutat. 2012, 33, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Wang, Y.Y.; Chen, H.I.; Kuo, Y.C.; Chiou, Y.W.; Lin, H.H.; Wu, C.M.; Hsu, C.C.; Chiang, H.S.; Kuo, P.L. SEPTIN12 Genetic Variants Confer Susceptibility to Teratozoospermia. PLoS ONE 2012, 7, e34011. [Google Scholar] [CrossRef] [PubMed]
- Silverman-Gavrila, R.V.; Silverman-Gavrila, L.B. Septins: New microtubule interacting partners. Sci. World J. 2008, 8, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Spiliotis, E.T.; Nelson, W.J. Here come the septins: Novel polymers that coordinate intracellular functions and organization. J. Cell. Sci. 2006, 119, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Gladfelter, A.S.; Bose, I.; Zyla, T.R.; Bardes, E.S.; Lew, D.J. Septin ring assembly involves cycles of GTP loading and hydrolysis by Cdc42p. J. Cell. Biol. 2002, 156, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Cid, V.J.; Adamikova, L.; Sanchez, M.; Molina, M.; Nombela, C. Cell cycle control of septin ring dynamics in the budding yeast. Microbiology 2001, 147, 1437–1450. [Google Scholar] [CrossRef] [PubMed]
- Joberty, G.; Perlungher, R.R.; Sheffield, P.J.; Kinoshita, M.; Noda, M.; Haystead, T.; Macara, I.G. Borg proteins control septin organization and are negatively regulated by Cdc42. Nat. Cell. Biol. 2001, 3, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Sheffield, P.J.; Oliver, C.J.; Kremer, B.E.; Sheng, S.; Shao, Z.; Macara, I.G. Borg/septin interactions and the assembly of mammalian septin heterodimers, trimers, and filaments. J. Biol. Chem. 2003, 278, 3483–3488. [Google Scholar] [CrossRef] [PubMed]
- Chapin, R.E.; Wine, R.N.; Harris, M.W.; Borchers, C.H.; Haseman, J.K. Structure and control of a cell-cell adhesion complex associated with spermiation in rat seminiferous epithelium. J. Androl. 2001, 22, 1030–1052. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Kuo, P.L. The Expression Level of Septin12 Is Critical for Spermiogenesis. J. Physiol. Sci. 2009, 59, 279. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Chou, C.K.; Hung, Y.C.; Yu, I.S.; Pan, H.A.; Lin, S.W.; Kuo, P.L. SEPT12 deficiency causes sperm nucleus damage and developmental arrest of preimplantation embryos. Fertil. Steril. 2011, 95, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Russell, L.D.; Russell, J.A.; MacGregor, G.R.; Meistrich, M.L. Linkage of manchette microtubules to the nuclear envelope and observations of the role of the manchette in nuclear shaping during spermiogenesis in rodents. Am. J. Anat. 1991, 192, 97–120. [Google Scholar] [CrossRef] [PubMed]
- Kierszenbaum, A.L. Spermatid manchette: Plugging proteins to zero into the sperm tail. Mol. Reprod. Dev. 2001, 59, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, A.J.; Calvo, F. Cdc42 regulates CDC42EP3 function in cancer-associated fibroblasts. Small GTPases 2017, 8, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Calvo, F.; Ranftl, R.; Hooper, S.; Farrugia, A.J.; Moeendarbary, E.; Bruckbauer, A.; Batista, F.; Charras, G.; Sahai, E. CDC42EP3/BORG2 and Septin Network Enables Mechano-transduction and the Emergence of Cancer-Associated Fibroblasts. Cell Rep. 2015, 13, 2699–2714. [Google Scholar] [CrossRef] [PubMed]
- Ide, M.; Lewis, D.A. Altered cortical CDC42 signaling pathways in schizophrenia: Implications for dendritic spine deficits. Biol. Psychiatry 2010, 68, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Ageta-Ishihara, N.; Yamazaki, M.; Konno, K.; Nakayama, H.; Abe, M.; Hashimoto, K.; Nishioka, T.; Kaibuchi, K.; Hattori, S.; Miyakawa, T.; et al. A CDC42EP4/septin-based perisynaptic glial scaffold facilitates glutamate clearance. Nat. Commun. 2015, 6, 10090. [Google Scholar] [CrossRef] [PubMed]
- Burbelo, P.D.; Drechsel, D.; Hall, A. A conserved binding motif defines numerous candidate target proteins for both CDC42 and Rac GTPases. J. Biol. Chem. 1995, 270, 29071–29074. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.T.; Lin, T.T.; Chang, J.E. Leaching behavior and ESEM characterization of water-sensitive mudstone in southwestern Taiwan. J. Environ. Sci. Health Part A 2003, 38, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Gopalkrishnan, K.; Anand Kumar, T.C. Scanning electron microscopy in the assessment of sperm morphology. Indian J. Med. Res. 1990, 92, 169–174. [Google Scholar] [PubMed]
- Yeh, Y.C.; Yang, V.C.; Huang, S.C.; Lo, N.W. Stage-dependent expression of extra-embryonic tissue-spermatogenesis-homeobox gene 1 (ESX1) protein, a candidate marker for X chromosome-bearing sperm. Reprod. Fertil. Dev. 2005, 17, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Lin, Y.M.; Kuo, Y.C.; Wang, Y.Y.; Kuo, P.L. Identification and characterization of a novel Rab GTPase-activating protein in spermatids. Int. J. Androl. 2011, 34, e358–e367. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-Y.; Wang, Y.-Y.; Chen, Y.-L.; Chen, M.-F.; Chiang, H.-S.; Kuo, P.-L.; Lin, Y.-H. CDC42 Negatively Regulates Testis-Specific SEPT12 Polymerization. Int. J. Mol. Sci. 2018, 19, 2627. https://doi.org/10.3390/ijms19092627
Huang C-Y, Wang Y-Y, Chen Y-L, Chen M-F, Chiang H-S, Kuo P-L, Lin Y-H. CDC42 Negatively Regulates Testis-Specific SEPT12 Polymerization. International Journal of Molecular Sciences. 2018; 19(9):2627. https://doi.org/10.3390/ijms19092627
Chicago/Turabian StyleHuang, Chia-Yen, Ya-Yun Wang, Ying-Liang Chen, Mei-Feng Chen, Han-Sun Chiang, Pao-Lin Kuo, and Ying-Hung Lin. 2018. "CDC42 Negatively Regulates Testis-Specific SEPT12 Polymerization" International Journal of Molecular Sciences 19, no. 9: 2627. https://doi.org/10.3390/ijms19092627
APA StyleHuang, C.-Y., Wang, Y.-Y., Chen, Y.-L., Chen, M.-F., Chiang, H.-S., Kuo, P.-L., & Lin, Y.-H. (2018). CDC42 Negatively Regulates Testis-Specific SEPT12 Polymerization. International Journal of Molecular Sciences, 19(9), 2627. https://doi.org/10.3390/ijms19092627





