The Use of Vascular Endothelial Growth Factor Inhibitors and Complementary Treatment Options in Polypoidal Choroidal Vasculopathy: A Subtype of Neovascular Age-Related Macular Degeneration
Abstract
1. Introduction
2. Epidemiology of PCV
3. Risk Factors
4. Clinical Features of PCV
5. Overview of Anti-VEGF Treatment of nAMD
6. Anti-VEGF Monotherapy in PCV
7. Application of the Photosensitizer Verteporfin in Photodynamic Therapy
8. Landmark Trials and PDT Combination Therapy
9. Current Treatment Recommendations for PCV
Author Contributions
Conflicts of Interest
References
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-related macular degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Klein, R.; Klein, B.E.; Linton, K.L. Prevalence of age-related maculopathy. The beaver dam eye study. Ophthalmology 1992, 99, 933–943. [Google Scholar] [CrossRef]
- Coleman, H.R.; Chan, C.C.; Ferris, F.L., 3rd; Chew, E.Y. Age-related macular degeneration. Lancet 2008, 372, 1835–1845. [Google Scholar] [CrossRef]
- Bressler, N.M. Age-related macular degeneration is the leading cause of blindness. JAMA 2004, 291, 1900–1901. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.F.; Maguire, M.G.; Fine, S.L.; Ying, G.S.; Jaffe, G.J.; Grunwald, J.E.; Toth, C.; Redford, M.; Ferris, F.L., 3rd. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: Two-year results. Ophthalmology 2012, 119, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Yannuzzi, L.A.; Sorenson, J.; Spaide, R.F.; Lipson, B. Idiopathic polypoidal choroidal vasculopathy (ipcv). Retina 1990, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, R.M.; Stanga, P.E.; Vingerling, J.R.; Reck, A.C.; Bird, A.C. Polypoidal choroidal vasculopathy in exudative and haemorrhagic pigment epithelial detachments. Br. J. Ophthalmol. 2000, 84, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Cackett, P.; Htoon, H.; Wong, D.; Yeo, I. Haemorrhagic pigment epithelial detachment as a predictive feature of polypoidal choroidal vasculopathy in a chinese population. Eye 2010, 24, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.Z.; Wong, T.Y.; Ong, F.S. Genetic risk, ethnic variations and pharmacogenetic biomarkers in amd and polypoidal choroidal vasculopathy. Expert Rev. Ophthalmol. 2013, 8, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Laude, A.; Cackett, P.D.; Vithana, E.N.; Yeo, I.Y.; Wong, D.; Koh, A.H.; Wong, T.Y.; Aung, T. Polypoidal choroidal vasculopathy and neovascular age-related macular degeneration: Same or different disease? Prog. Retin. Eye Res. 2010, 29, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.S.; Cheung, C.M.G.; Wong, T.Y. Asian age-related macular degeneration: Current concepts and gaps in knowledge. Asia-Pac. J. Ophthalmol. 2013, 2, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.W.; Wong, T.Y.; Cheung, C.M.G. Polypoidal choroidal vasculopathy in asians. J. Clin. Med. 2015, 4, 782–821. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.W.; Yanagi, Y.; Lee, W.-K.; Ogura, Y.; Yeo, I.; Wong, T.Y.; Cheung, C.M.G. Age-related macular degeneration and polypoidal choroidal vasculopathy in asians. Prog. Retin. Eye Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Coppens, G.; Spielberg, L.; Leys, A. Polypoidal choroidal vasculopathy, diagnosis and management. Bull. Soc. Belge Ophtalmol. 2011, 317, 39–44. [Google Scholar]
- Yadav, S.; Parry, D.G.; Beare, N.A.V.; Pearce, I.A. Polypoidal choroidal vasculopathy: A common type of neovascular age-related macular degeneration in caucasians. Br. J. Ophthalmol. 2017, 101, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Yannuzzi, L.A.; Wong, D.W.; Sforzolini, B.S.; Goldbaum, M.; Tang, K.C.; Spaide, R.F.; Freund, K.B.; Slakter, J.S.; Guyer, D.R.; Sorenson, J.A.; et al. Polypoidal choroidal vasculopathy and neovascularized age-related macular degeneration. Arch. Ophthalmol. 1999, 117, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Lorentzen, T.D.; Subhi, Y.; Sørensen, T.L. Prevalence of polypoidal choroidal vasculopathy in white patients with exudative age-related macular degeneration: Systematic review and meta-analysis. Retina 2017. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.M.; Lai, T.Y.; Chen, S.J.; Chong, V.; Lee, W.K.; Htoon, H.; Ng, W.Y.; Ogura, Y.; Wong, T.Y. Understanding indocyanine green angiography in polypoidal choroidal vasculopathy: The group experience with digital fundus photography and confocal scanning laser ophthalmoscopy. Retina 2014, 34, 2397–2406. [Google Scholar] [CrossRef] [PubMed]
- Imamura, Y.; Engelbert, M.; Iida, T.; Freund, K.B.; Yannuzzi, L.A. Polypoidal choroidal vasculopathy: A review. Surv. Ophthalmol. 2010, 55, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.; Chen, C.; Wu, D.; Li, H. Polypoidal choroidal vasculopathy in elderly chinese patients. Graefes Arch. Clin. Exp. Ophthalmol. 2004, 242, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Wu, W.C. Polypoidal choroidal vasculopathy in taiwanese patients. Ophthalmic Surg. Lasers Imaging 2009, 40, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.M.; Li, X.; Mathur, R.; Lee, S.Y.; Chan, C.M.; Yeo, I.; Loh, B.K.; Williams, R.; Wong, E.Y.; Wong, D.; et al. A prospective study of treatment patterns and 1-year outcome of asian age-related macular degeneration and polypoidal choroidal vasculopathy. PLoS ONE 2014, 9, e101057. [Google Scholar] [CrossRef] [PubMed]
- Scassellati-Sforzolini, B.; Mariotti, C.; Bryan, R.; Yannuzzi, L.A.; Giuliani, M.; Giovannini, A. Polypoidal choroidal vasculopathy in italy. Retina 2001, 21, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Ladas, I.; Rouvas, A.; Moschos, M.; Synodinos, E.; Karagiannis, D.; Koutsandrea, C. Polypoidal choroidal vasculopathy and exudative age-related macular degeneration in greek population. Eye 2004, 18, 455. [Google Scholar] [CrossRef] [PubMed]
- Ilginis, T.; Ottosen, S.; Harbo Bundsgaard, K.; Uggerhøj Andersen, C.; Vorum, H. Polypoidal choroidal vasculopathy in patients diagnosed with neovascular age-related macular degeneration in denmark. Acta Ophthalmol. 2012, 90, e487–e488. [Google Scholar] [CrossRef] [PubMed]
- Lorentzen, T.D.; Subhi, Y.; Sørensen, T.L. Presenting characteristics and prevalence of polypoidal choroidal vasculopathy in scandinavian patients with treatment-naïve exudative age-related macular degeneration. Acta Ophthalmol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Cackett, P.; Yeo, I.; Cheung, C.M.; Vithana, E.N.; Wong, D.; Tay, W.T.; Tai, E.S.; Aung, T.; Wong, T.Y. Relationship of smoking and cardiovascular risk factors with polypoidal choroidal vasculopathy and age-related macular degeneration in chinese persons. Ophthalmology 2011, 118, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.J.; Heo, J.W.; Kim, T.W.; Ahn, J.; Chung, H. Prevalence and risk factors of age-related macular degeneration in korea: The korea national health and nutrition examination survey 2010–2011. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Liang, Y.B.; Gao, L.Q.; Peng, Y.; Shen, R.; Duan, X.R.; Friedman, D.S.; Sun, L.P.; Mitchell, P.; Wang, N.L.; et al. Prevalence of age-related macular degeneration in a rural chinese population: The handan eye study. Ophthalmology 2011, 118, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, L.G.; Chen, W.; Schu, M.; Yaspan, B.L.; Yu, Y.; Thorleifsson, G.; Zack, D.J.; Arakawa, S.; Cipriani, V.; Ripke, S.; et al. Seven new loci associated with age-related macular degeneration. Nat. Genet. 2013, 45, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Nakata, I.; Yamashiro, K.; Kawaguchi, T.; Gotoh, N.; Nakanishi, H.; Akagi-Kurashige, Y.; Miyake, M.; Tsujikawa, A.; Oishi, A.; Saito, M.; et al. Association between the cholesteryl ester transfer protein gene and polypoidal choroidal vasculopathy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6068–6073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, M.; Wen, F.; Zuo, C.; Chen, H.; Wu, K.; Zeng, R. Different impact of high-density lipoprotein-related genetic variants on polypoidal choroidal vasculopathy and neovascular age-related macular degeneration in a chinese han population. Exp. Eye Res. 2013, 108, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Yannuzzi, L.A.; Ciardella, A.; Spaide, R.F.; Rabb, M.; Freund, K.B.; Orlock, D.A. The expanding clinical spectrum of idiopathic polypoidal choroidal vasculopathy. Arch. Ophthalmol. 1997, 115, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Uyama, M.; Wada, M.; Nagai, Y.; Matsubara, T.; Matsunaga, H.; Fukushima, I.; Takahashi, K.; Matsumura, M. Polypoidal choroidal vasculopathy: Natural history. Am. J. Ophthalmol. 2002, 133, 639–648. [Google Scholar] [CrossRef]
- Koh, A.; Lee, W.K.; Chen, L.-J.; Chen, S.-J.; Hashad, Y.; Kim, H.; Lai, T.Y.; Pilz, S.; Ruamviboonsuk, P.; Tokaji, E.; et al. Everest study: Efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina 2012, 32, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, A.; Amato, G.P.; D’Altobrando, E.; Giuliani, M. Optical coherence tomography (oct) in idiopathic polypoidal choroidal vasculopathy (ipcv). Doc. Ophthalmol. 1999, 97, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Srour, M.; Querques, G.; Semoun, O.; El Ameen, A.; Miere, A.; Sikorav, A.; Zambrowski, O.; Souied, E.H. Optical coherence tomography angiography characteristics of polypoidal choroidal vasculopathy. Br. J. Ophthalmol. 2016, 100, 1489–1493. [Google Scholar] [CrossRef] [PubMed]
- Iijima, H.; Imai, M.; Gohdo, T.; Tsukahara, S. Optical coherence tomography of idiopathic polypoidal choroidal vasculopathy. Am. J. Ophthalmol. 1999, 127, 301–305. [Google Scholar] [CrossRef]
- Lee, W.K.; Baek, J.; Dansingani, K.K.; Lee, J.H.; Freund, K.B. Choroidal morphology in eyes with polypoidal choroidal vasculopathy and normal or subnormal subfoveal choroidal thickness. Retina 2016, 36, S73–S82. [Google Scholar] [CrossRef] [PubMed]
- Alshahrani, S.T.; Al Shamsi, H.N.; Kahtani, E.S.; Ghazi, N.G. Spectral-domain optical coherence tomography findings in polypoidal choroidal vasculopathy suggest a type 1 neovascular growth pattern. Clin. Ophthalmol. 2014, 8, 1689–1695. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.E.; Kang, S.W.; Lee, J.H.; Kim, Y.T. Choroidal thickness in polypoidal choroidal vasculopathy and exudative age-related macular degeneration. Ophthalmology 2011, 118, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Oh, J.; Kwon, S.S.; Yoo, J.; Huh, K. Comparison of choroidal thickness among patients with healthy eyes, early age-related maculopathy, neovascular age-related macular degeneration, central serous chorioretinopathy, and polypoidal choroidal vasculopathy. Retina 2011, 31, 1904–1911. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, H.; Yamagishi, T.; Yamazaki, T.; Kawasaki, R.; Kinoshita, S. Subfoveal choroidal thickness in typical age-related macular degeneration and polypoidal choroidal vasculopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2011, 249, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Pe’er, J.; Folberg, R.; Itin, A.; Gnessin, H.; Hemo, I.; Keshet, E. Upregulated expression of vascular endothelial growth factor in proliferative diabetic retinopathy. Br. J. Ophthalmol. 1996, 80, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Kliffen, M.; Sharma, H.S.; Mooy, C.M.; Kerkvliet, S.; de Jong, P.T. Increased expression of angiogenic growth factors in age-related maculopathy. Br. J. Ophthalmol. 1997, 81, 154–162. [Google Scholar] [CrossRef] [PubMed]
- VEGF Inhibition Study in Ocular Neovascularization (VISION) Clinical Trial Group; Chakravarthy, U.; Adamis, A.P.; Cunningham, E.T., Jr.; Goldbaum, M.; Guyer, D.R.; Katz, B.; Patel, M. Year 2 efficacy results of 2 randomized controlled clinical trials of pegaptanib for neovascular age-related macular degeneration. Ophthalmology 2006, 113, 1508. [Google Scholar]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.K.; Chung, C.Y.; Kim, R.Y.; for the MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Heier, J.S.; Antoszyk, A.N.; Pavan, P.R.; Leff, S.R.; Rosenfeld, P.J.; Ciulla, T.A.; Dreyer, R.F.; Gentile, R.C.; Sy, J.P.; Hantsbarger, G.; et al. Ranibizumab for treatment of neovascular age-related macular degeneration: A phase i/ii multicenter, controlled, multidose study. Ophthalmology 2006, 113, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.M.; Wong, T.Y. Ranibizumab and bevacizumab for amd. N. Engl. J. Med. 2011, 365, 2237. [Google Scholar] [PubMed]
- CATT Research Group; Martin, D.F.; Maguire, M.G.; Ying, G.S.; Grunwald, J.E.; Fine, S.L.; Jaffe, G.J. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2011, 364, 1897–1908. [Google Scholar]
- Heier, J.S.; Brown, D.M.; Chong, V.; Korobelnik, J.F.; Kaiser, P.K.; Nguyen, Q.D.; Kirchhof, B.; Ho, A.; Ogura, Y.; Yancopoulos, G.D.; et al. Intravitreal aflibercept (vegf trap-eye) in wet age-related macular degeneration. Ophthalmology 2012, 119, 2537–2548. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Erfurth, U.; Kaiser, P.K.; Korobelnik, J.F.; Brown, D.M.; Chong, V.; Nguyen, Q.D.; Ho, A.C.; Ogura, Y.; Simader, C.; Jaffe, G.J.; et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: Ninety-six-week results of the view studies. Ophthalmology 2014, 121, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, U.; Harding, S.P.; Rogers, C.A.; Downes, S.M.; Lotery, A.J.; Culliford, L.A.; Reeves, B.C.; IVAN Study Investigators. Alternative treatments to inhibit vegf in age-related choroidal neovascularisation: 2-year findings of the ivan randomised controlled trial. Lancet 2013, 382, 1258–1267. [Google Scholar] [CrossRef]
- Kodjikian, L.; Souied, E.H.; Mimoun, G.; Mauget-Faysse, M.; Behar-Cohen, F.; Decullier, E.; Huot, L.; Aulagner, G.; GEFAL Study Group. Ranibizumab versus bevacizumab for neovascular age-related macular degeneration: Results from the gefal noninferiority randomized trial. Ophthalmology 2013, 120, 2300–2309. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Lee, W.K.; Baek, J.; Kwon, O.W.; Lee, J.H. Photodynamic therapy versus combination therapy in polypoidal choroidal vasculopathy: Changes of aqueous vascular endothelial growth factor. Am. J. Ophthalmol. 2013, 156, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.P.; Chan, W.M.; Liu, D.T.; Lai, T.Y.; Choy, K.W.; Pang, C.P.; Lam, D.S. Aqueous humor levels of vascular endothelial growth factor and pigment epithelium-derived factor in polypoidal choroidal vasculopathy and choroidal neovascularization. Am. J. Ophthalmol. 2006, 141, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Gomi, F.; Sawa, M.; Sakaguchi, H.; Tsujikawa, M.; Oshima, Y.; Kamei, M.; Tano, Y. Efficacy of intravitreal bevacizumab for polypoidal choroidal vasculopathy. Br. J. Ophthalmol. 2008, 92, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.Y.; Lee, G.K.; Luk, F.O.; Lam, D.S. Intravitreal ranibizumab with or without photodynamic therapy for the treatment of symptomatic polypoidal choroidal vasculopathy. Retina 2011, 31, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Ijiri, S.; Sugiyama, K. Short-term efficacy of intravitreal aflibercept for patients with treatment-naive polypoidal choroidal vasculopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Oishi, A.; Kojima, H.; Mandai, M.; Honda, S.; Matsuoka, T.; Oh, H.; Kita, M.; Nagai, T.; Fujihara, M.; Bessho, N.; et al. Comparison of the effect of ranibizumab and verteporfin for polypoidal choroidal vasculopathy: 12-month laptop study results. Am. J. Ophthalmol. 2013, 156, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Kokame, G.T.; Yeung, L.; Lai, J.C. Continuous anti-vegf treatment with ranibizumab for polypoidal choroidal vasculopathy: 6-month results. Br. J. Ophthalmol. 2010, 94, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Oishi, A.; Miyamoto, N.; Mandai, M.; Honda, S.; Matsuoka, T.; Oh, H.; Kita, M.; Nagai, T.; Bessho, N.; Uenishi, M.; et al. Laptop study: A 24-month trial of verteporfin versus ranibizumab for polypoidal choroidal vasculopathy. Ophthalmology 2014, 121, 1151–1152. [Google Scholar] [CrossRef] [PubMed]
- Hikichi, T.; Higuchi, M.; Matsushita, T.; Kosaka, S.; Matsushita, R.; Takami, K.; Ohtsuka, H.; Ariga, H. One-year results of three monthly ranibizumab injections and as-needed reinjections for polypoidal choroidal vasculopathy in japanese patients. Am. J. Ophthalmol. 2012, 154, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.M.; Koh, H.J. Long-term visual outcome and prognostic factors after intravitreal ranibizumab injections for polypoidal choroidal vasculopathy. Am. J. Ophthalmol. 2013, 156, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Kokame, G.T.; Yeung, L.; Teramoto, K.; Lai, J.C.; Wee, R. Polypoidal choroidal vasculopathy exudation and hemorrhage: Results of monthly ranibizumab therapy at one year. Ophthalmologica 2014, 231, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, A.; Egger, A. Ranibizumab 0.5 mg in patients with polypoidal choroidal vasculopathy: Results from the DRAGON study. In Proceedings of the American Academy of Ophthalmology Annual Meeting, Chicago, IL, USA, 15–18 October 2016. [Google Scholar]
- Saito, M.; Kano, M.; Itagaki, K.; Sekiryu, T. Efficacy of intravitreal aflibercept in japanese patients with exudative age-related macular degeneration. Jpn. J. Ophthalmol. 2017, 61, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Okada, A.A.; Kano, M.; Koizumi, H.; Saito, M.; Maruko, I.; Sekiryu, T.; Iida, T. One-year results of intravitreal aflibercept for polypoidal choroidal vasculopathy. Ophthalmology 2015, 122, 1866–1872. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, M.; Morizane, Y.; Hirano, M.; Kimura, S.; Kumase, F.; Shiode, Y.; Doi, S.; Toshima, S.; Hosogi, M.; Fujiwara, A.; et al. One-year outcomes of a treat-and-extend regimen of intravitreal aflibercept for polypoidal choroidal vasculopathy. Jpn. J. Ophthalmol. 2017, 61, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Shin, J.P.; Kim, H.W.; Chang, W.; Kim, Y.C.; Lee, S.J.; Chung, I.Y.; Lee, J.E.; VAULT Study Group. Efficacy of fixed-dosing aflibercept for treating polypoidal choroidal vasculopathy: 1-year results of the vault study. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Kokame, G.T.; Lai, J.C.; Wee, R.; Yanagihara, R.; Shantha, J.G.; Ayabe, J.; Hirai, K. Prospective clinical trial of intravitreal aflibercept treatment for polypoidal choroidal vasculopathy with hemorrhage or exudation (epic study): 6 month results. BMC Ophthalmol. 2016, 16, 127. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Yamane, S.; Taoka, R.; Arakawa, A.; Kadonosono, K. Aflibercept for polypoidal choroidal vasculopathy: As needed versus fixed interval dosing. Retina 2016, 36, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Ogura, Y.; Terasaki, H.; Gomi, F.; Yuzawa, M.; Iida, T.; Honda, M.; Nishijo, K.; Sowade, O.; Komori, T.; Schmidt-Erfurth, U.; et al. Efficacy and safety of intravitreal aflibercept injection in wet age-related macular degeneration: Outcomes in the japanese subgroup of the view 2 study. Br. J. Ophthalmol. 2015, 99, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.Y.; Chan, W.M.; Liu, D.T.; Luk, F.O.; Lam, D.S. Intravitreal bevacizumab (avastin) with or without photodynamic therapy for the treatment of polypoidal choroidal vasculopathy. Br. J. Ophthalmol. 2008, 92, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.K.; Peng, C.H.; Chang, C.K.; Hu, C.C.; Chen, L.J. One-year outcomes of intravitreal bevacizumab (avastin) therapy for polypoidal choroidal vasculopathy. Retina 2011, 31, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Baek, J.S.; Lee, D.W.; Kim, C.G.; Kim, J.W. Short-term effectiveness of intravitreal bevacizumab vs. Ranibizumab injections for patients with polypoidal choroidal vasculopathy. Korean J. Ophthalmol. 2012, 26, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.W.; Walsh, A.W.; Kramer, M.; Hasan, T.; Michaud, N.; Flotte, T.J.; Haimovici, R.; Gragoudas, E.S. Photodynamic therapy of experimental choroidal neovascularization using lipoprotein-delivered benzoporphyrin. Arch. Ophthalmol. 1995, 113, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.; Miller, J.W.; Michaud, N.; Moulton, R.S.; Hasan, T.; Flotte, T.J.; Gragoudas, E.S. Liposomal benzoporphyrin derivative verteporfin photodynamic therapy. Selective treatment of choroidal neovascularization in monkeys. Ophthalmology 1996, 103, 427–438. [Google Scholar] [CrossRef]
- Bressler, N.M. Treatment of Age-Related Macular Degeneration with Photodynamic Therapy Study, G. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: Two-year results of 2 randomized clinical trials-tap report 2. Arch. Ophthalmol. 2001, 119, 198–207. [Google Scholar] [PubMed]
- Verteporfin in Photodynamic Therapy Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: Two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization—verteporfin in photodynamic therapy report 2. Am. J. Ophthalmol. 2001, 131, 541–560. [Google Scholar]
- Silva, R.M.; Figueira, J.; Cachulo, M.L.; Duarte, L.; De Abreu, J.R.F.; Cunha-Vaz, J. Polypoidal choroidal vasculopathy and photodynamic therapy with verteporfin. Graefes Arch. Clin. Exp. Ophthalmol. 2005, 243, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Gomi, F.; Ohji, M.; Sayanagi, K.; Sawa, M.; Sakaguchi, H.; Oshima, Y.; Ikuno, Y.; Tano, Y. One-year outcomes of photodynamic therapy in age-related macular degeneration and polypoidal choroidal vasculopathy in Japanese patients. Ophthalmology 2008, 115, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Honda, S.; Kurimoto, Y.; Kagotani, Y.; Yamamoto, H.; Takagi, H.; Uenishi, M.; for the Hyogo Macular Disease Study Group. Photodynamic therapy for typical age-related macular degeneration and polypoidal choroidal vasculopathy: A 30-month multicenter study in Hyogo, Japan. Jpn. J. Ophthalmol. 2009, 53, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Sliwinska, P.; van den Bergh, H.; Sickenberg, M.; Koh, A.H. Photodynamic therapy for polypoidal choroidal vasculopathy. Prog. Retin. Eye Res. 2013, 37, 182–199. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, D.; Yamamoto, T.; Kawasaki, R.; Yamashita, H. Two-year visual outcomes after photodynamic therapy in age-related macular degeneration patients with or without polypoidal choroidal vasculopathy lesions. Retina 2009, 29, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Oh, J.; Oh, I.K.; Huh, K. Retinal pigment epithelial tear after half fluence pdt for serous pigment epithelial detachment in central serous chorioretinopathy. Ophthalmic Surg. Lasers Imaging 2009, 40, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Klais, C.M.; Ober, M.D.; Freund, K.B.; Ginsburg, L.H.; Luckie, A.; Mauget-Faysse, M.; Coscas, G.; Gross, N.E.; Yannuzzi, L.A. Choroidal infarction following photodynamic therapy with verteporfin. Arch. Ophthalmol. 2005, 123, 1149–1153. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, T.; Gomi, F.; Sawa, M.; Tsujikawa, M.; Tano, Y. Marked vascular changes of polypoidal choroidal vasculopathy after photodynamic therapy. Br. J. Ophthalmol. 2008, 92, 936–940. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Shiraga, F.; Shiragami, C.; Shirakata, Y.; Fujiwara, A. Two-year results of reduced-fluence photodynamic therapy for polypoidal choroidal vasculopathy. Am. J. Ophthalmol. 2013, 155, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; Lai, T.Y.Y.; Takahashi, K.; Wong, T.Y.; Chen, L.J.; Ruamviboonsuk, P.; Tan, C.S.; Feller, C.; Margaron, P.; Lim, T.H.; et al. Efficacy and safety of ranibizumab with or without verteporfin photodynamic therapy for polypoidal choroidal vasculopathy: A randomized clinical trial. JAMA Ophthalmol. 2017, 135, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Gomi, F.; Oshima, Y.; Mori, R.; Kano, M.; Saito, M.; Yamashita, A.; Iwata, E.; Maruko, R.; Fujisan Study Group. Initial versus delayed photodynamic therapy in combination with ranibizumab for treatment of polypoidal choroidal vasculopathy: The fujisan study. Retina 2015, 35, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.K.; Ogura, Y.; Iida, T.; Chen, S.-J.; Wong, T.Y.; Mitchell, P.; Ishibashi, T.; Leal, S. Efficacy and safety of intravitreal aflibercept in polypoidal choroidal vasculopathy: 12-month results of the planet study. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1199. [Google Scholar]
- Gillies, M.C.; Campain, A.; Barthelmes, D.; Simpson, J.M.; Arnold, J.J.; Guymer, R.H.; McAllister, I.L.; Essex, R.W.; Morlet, N.; Hunyor, A.P.; et al. Long-term outcomes of treatment of neovascular age-related macular degeneration: Data from an observational study. Ophthalmology 2015, 122, 1837–1845. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Kim, J.W.; Lee, D.W.; Cho, S.W.; Kim, C.G. Intravitreal bevacizumab and ranibizumab injections for patients with polypoidal choroidal vasculopathy. Eye 2012, 26, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.K.; Kim, K.S.; Kim, W.; Lee, S.B.; Jeon, S. Responses to photodynamic therapy in patients with polypoidal choroidal vasculopathy consisting of polyps resembling grape clusters. Am. J. Ophthalmol. 2012, 154, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.W.; Cheung, C.M.G.; Mathur, R.; Li, X.; Chan, C.M.; Yeo, I.; Wong, E.; Lee, S.Y.; Wong, D.; Wong, T.Y. Three-year results of polypoidal choroidal vasculopathy treated with photodynamic therapy: Retrospective study and systematic review. Retina 2015, 35, 1577–1593. [Google Scholar] [CrossRef] [PubMed]
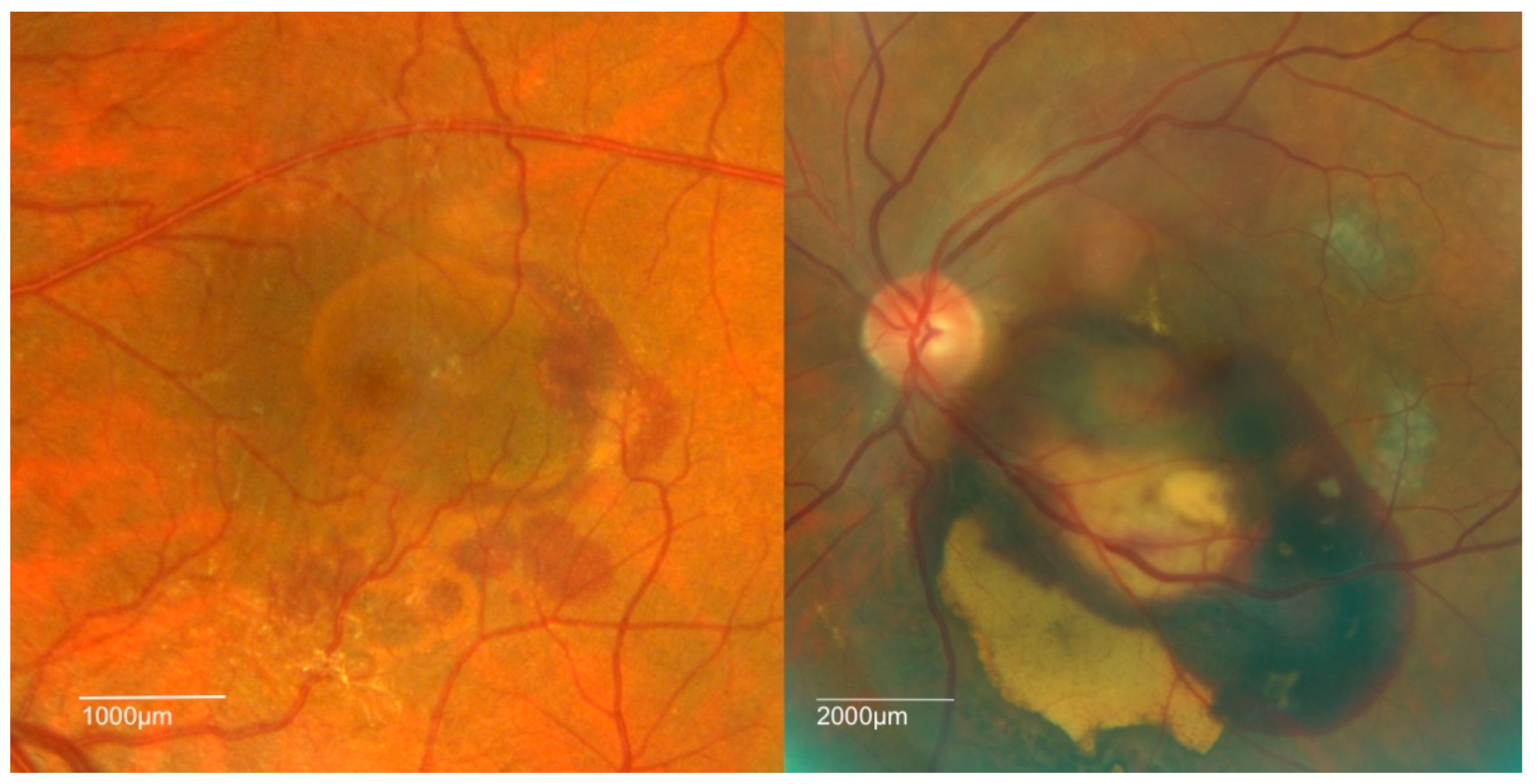
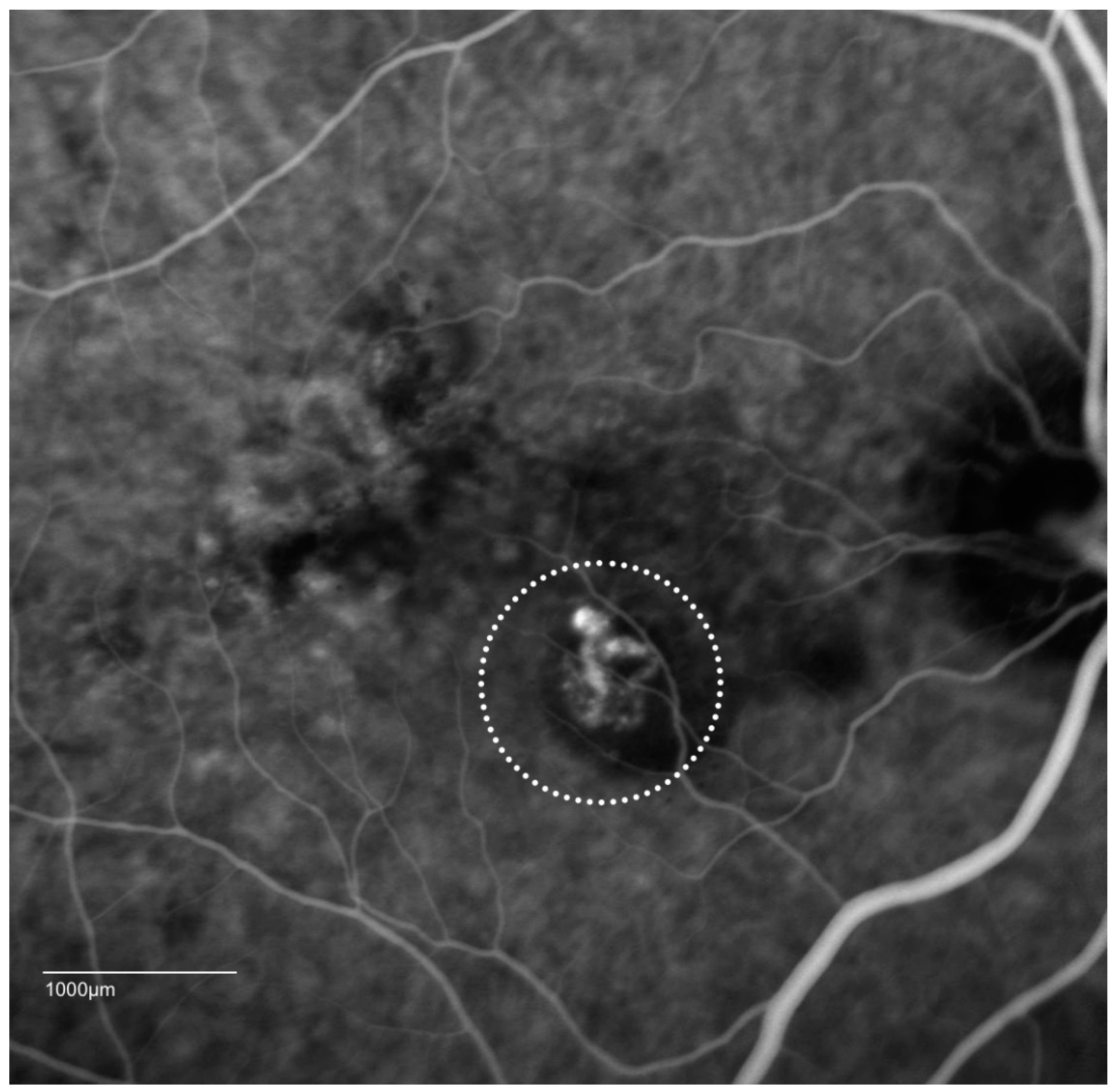
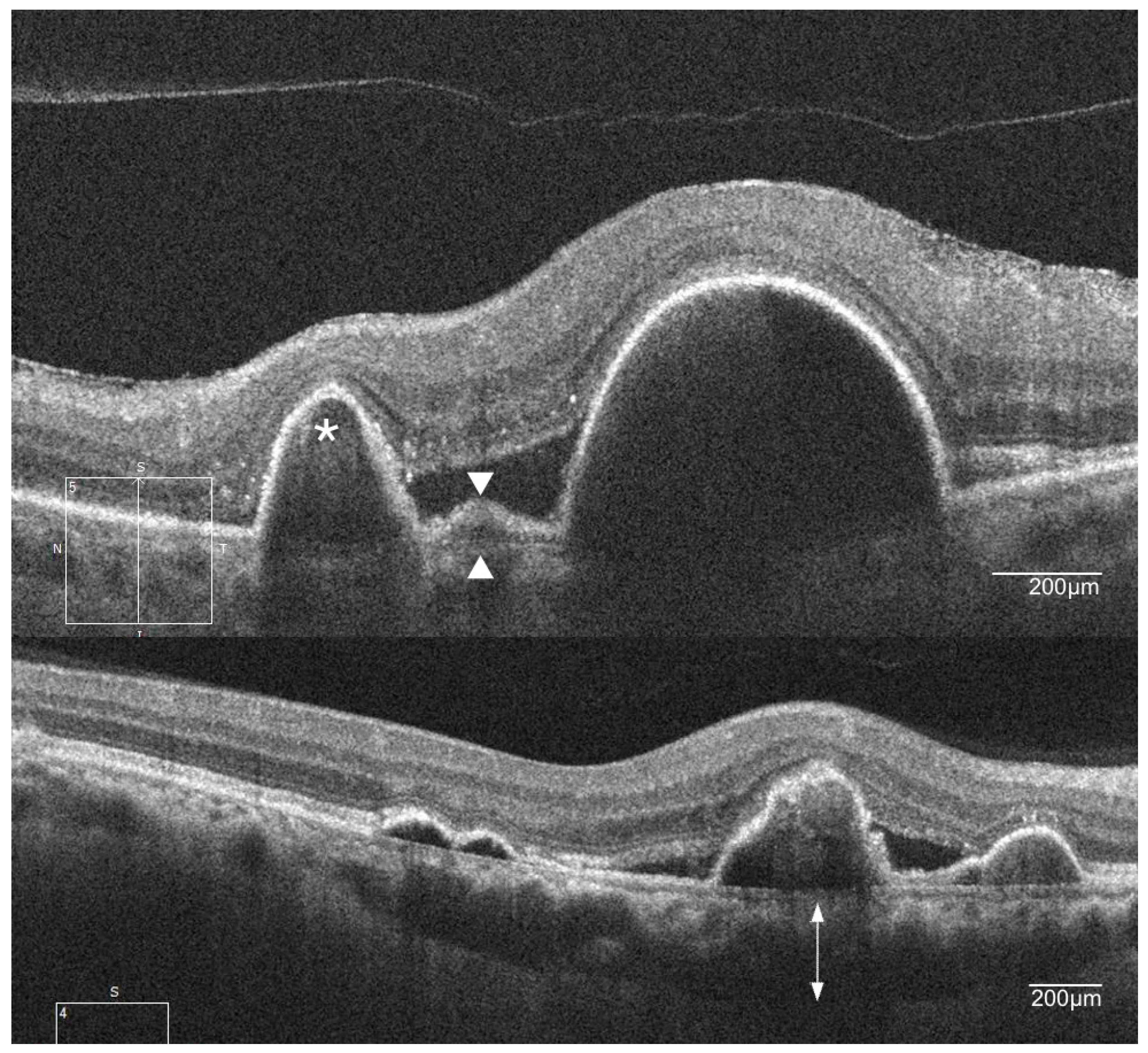
| Study | Follow-Up, Months | Treatment | Sample Size | Number of Injections | Number of PDT | Polyp Regression Rate, % | Baseline Vision, Letters | Mean Vision Change, Letters |
|---|---|---|---|---|---|---|---|---|
| LAPTOP (2014) | 12 | Ranibizumab 3 × monthly + PRN | 47 | 5.8 | - | Not reported | 88.0 | 4.0 |
| PDT | 46 | 5.2 | 1.5 | Not reported | 84.0 | −2.0 | ||
| FUJISAN (2015) | 12 | Initial PDT + ranibizumab 3 × monthly + PRN | 37 | 4.5 | 1.1 | 62.1 | 54.3 | 8.1 |
| Ranibizumab 3 × monthly + PRN + deferred PRN PDT | 35 | 6.8 | 1.4 | 54.8 | 54.9 | 8.8 | ||
| EVEREST I (2012) | 6 | Ranibizumab 3 × monthly + PRN | 21 | 5.2 | 1.9 (sham) | 28.6 | 49.0 | 9.2 |
| PDT + ranibizumab PRN | 19 | 3.9 | 1.7 | 77.8 | 57.2 | 10.9 | ||
| PDT | 21 | 4.2 | 1.4 | 71.4 | 56.6 | 7.5 | ||
| EVEREST II (2017) | 12 | Ranibizumab 3 × monthly + PRN | 168 | 7.3 | 2.3 (sham) | 34.7 | 61.1 | 5.1 |
| PDT + ranibizumab PRN | 154 | 5.2 | 1.5 | 69.3 | 61.2 | 8.3 | ||
| PLANET (2017) | 12 | Aflibercept 3 × monthly + 8-weekly | 318 | 8.1 | - | 38.9 | 57.7 | 10.7 |
| Aflibercept 3 × monthly + 8-weekly + rescue PDT | 8.0 | 0.2 | 44.8 | 59.0 | 10.9 | |||
| LAPTOP (2014) | 12 | Ranibizumab 3 × monthly + PRN | 47 | 5.8 | - | Not reported | 88.0 | 4.0 |
| PDT | 46 | 5.2 | 1.5 | Not reported | 84.0 | −2.0 | ||
| FUJISAN (2015) | 12 | Initial PDT + ranibizumab 3 × monthly + PRN | 37 | 4.5 | 1.1 | 62.1 | 54.3 | 8.1 |
| Ranibizumab 3 × monthly + PRN + deferred PRN PDT | 35 | 6.8 | 1.4 | 54.8 | 54.9 | 8.8 | ||
| EVEREST I (2012) | 6 | Ranibizumab 3 × monthly + PRN | 21 | 5.2 | 1.9 (sham) | 28.6 | 49.0 | 9.2 |
| PDT + ranibizumab PRN | 19 | 3.9 | 1.7 | 77.8 | 57.2 | 10.9 | ||
| PDT | 21 | 4.2 | 1.4 | 71.4 | 56.6 | 7.5 | ||
| EVEREST II (2017) | 12 | Ranibizumab 3 × monthly + PRN | 168 | 7.3 | 2.3 (sham) | 34.7 | 61.1 | 5.1 |
| PDT + ranibizumab PRN | 154 | 5.2 | 1.5 | 69.0 | 61.2 | 8.3 | ||
| PLANET (2017) | 12 | Aflibercept 3 × monthly + 8-weekly | 318 | 8.1 | - | 38.9 | 57.7 | 10.7 |
| Aflibercept 3 × monthly + 8-weekly + rescue PDT | 8.0 | 0.2 | 44.8 | 59.0 | 10.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teo, K.Y.C.; Gillies, M.; Fraser-Bell, S. The Use of Vascular Endothelial Growth Factor Inhibitors and Complementary Treatment Options in Polypoidal Choroidal Vasculopathy: A Subtype of Neovascular Age-Related Macular Degeneration. Int. J. Mol. Sci. 2018, 19, 2611. https://doi.org/10.3390/ijms19092611
Teo KYC, Gillies M, Fraser-Bell S. The Use of Vascular Endothelial Growth Factor Inhibitors and Complementary Treatment Options in Polypoidal Choroidal Vasculopathy: A Subtype of Neovascular Age-Related Macular Degeneration. International Journal of Molecular Sciences. 2018; 19(9):2611. https://doi.org/10.3390/ijms19092611
Chicago/Turabian StyleTeo, Kelvin Yi Chong, Mark Gillies, and Samantha Fraser-Bell. 2018. "The Use of Vascular Endothelial Growth Factor Inhibitors and Complementary Treatment Options in Polypoidal Choroidal Vasculopathy: A Subtype of Neovascular Age-Related Macular Degeneration" International Journal of Molecular Sciences 19, no. 9: 2611. https://doi.org/10.3390/ijms19092611
APA StyleTeo, K. Y. C., Gillies, M., & Fraser-Bell, S. (2018). The Use of Vascular Endothelial Growth Factor Inhibitors and Complementary Treatment Options in Polypoidal Choroidal Vasculopathy: A Subtype of Neovascular Age-Related Macular Degeneration. International Journal of Molecular Sciences, 19(9), 2611. https://doi.org/10.3390/ijms19092611





