Differentiation of Human Tonsil-Derived Mesenchymal Stem Cells into Schwann-Like Cells Improves Neuromuscular Function in a Mouse Model of Charcot-Marie-Tooth Disease Type 1A
Abstract
1. Introduction
2. Results
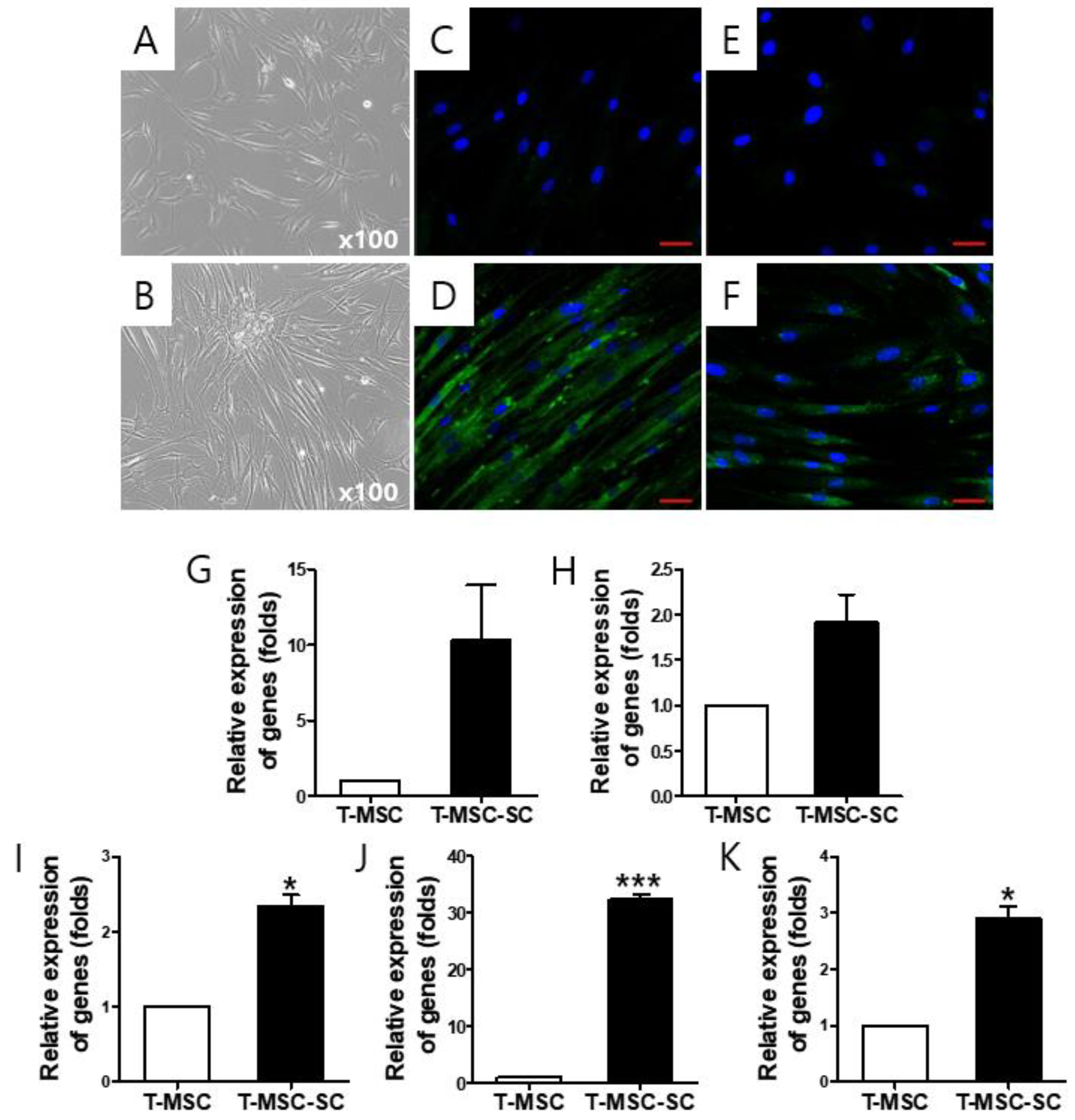
2.1. T-MSC-Derived SCs (T-MSC-SCs) Exhibit Schwann Cell and Neurotrophic Markers
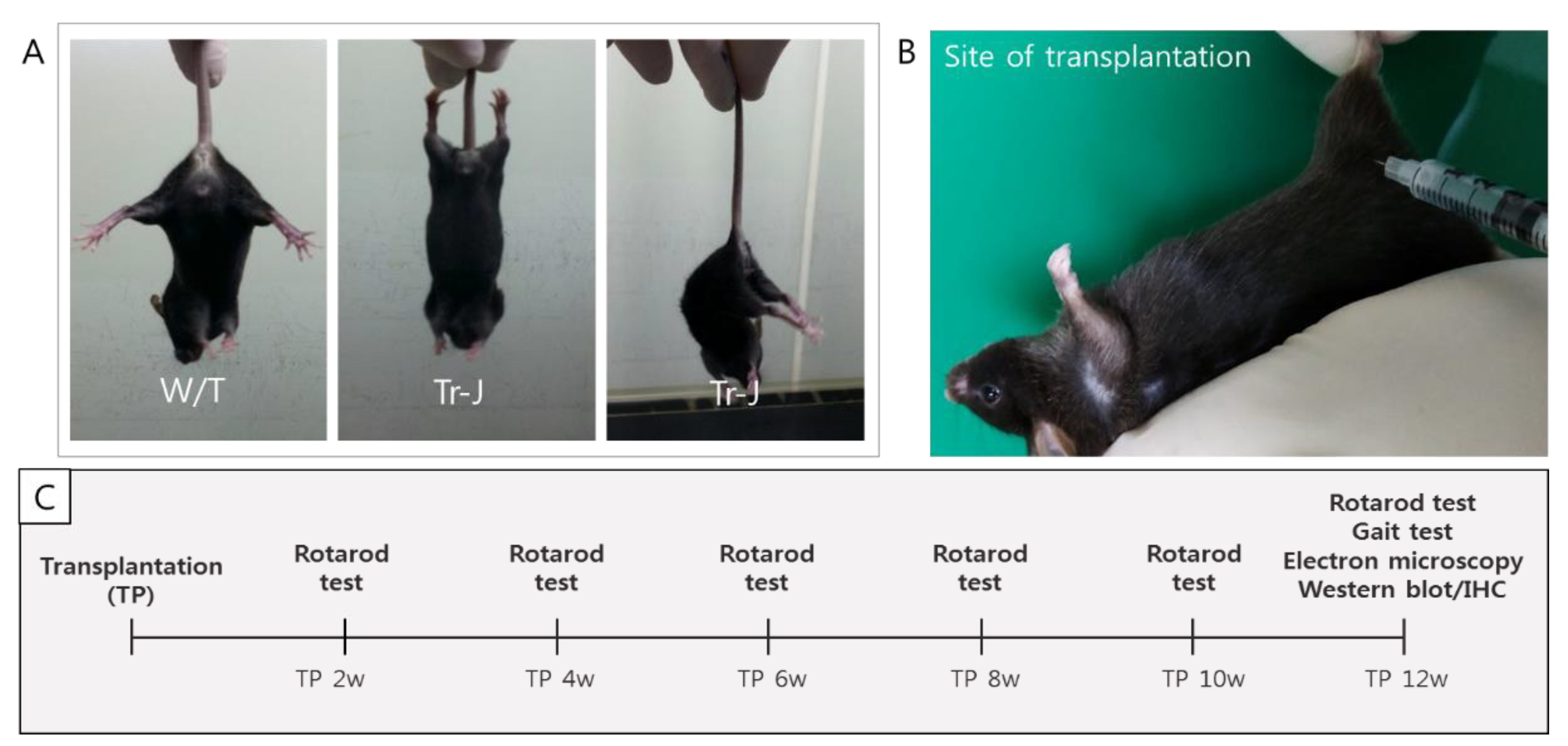
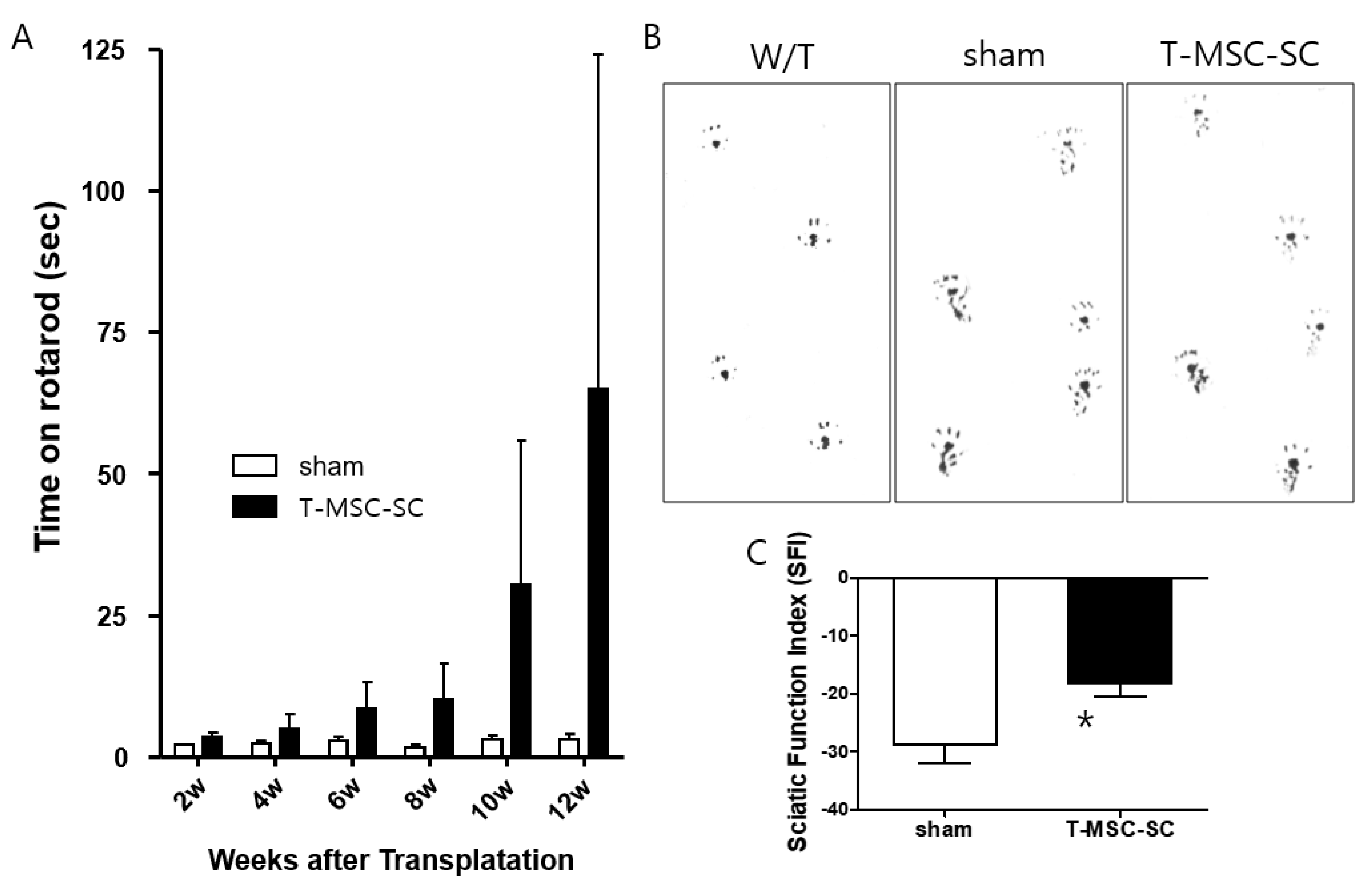
2.2. Motor Function after Transplanting T-MSC-SCs into the Tr-J Mice
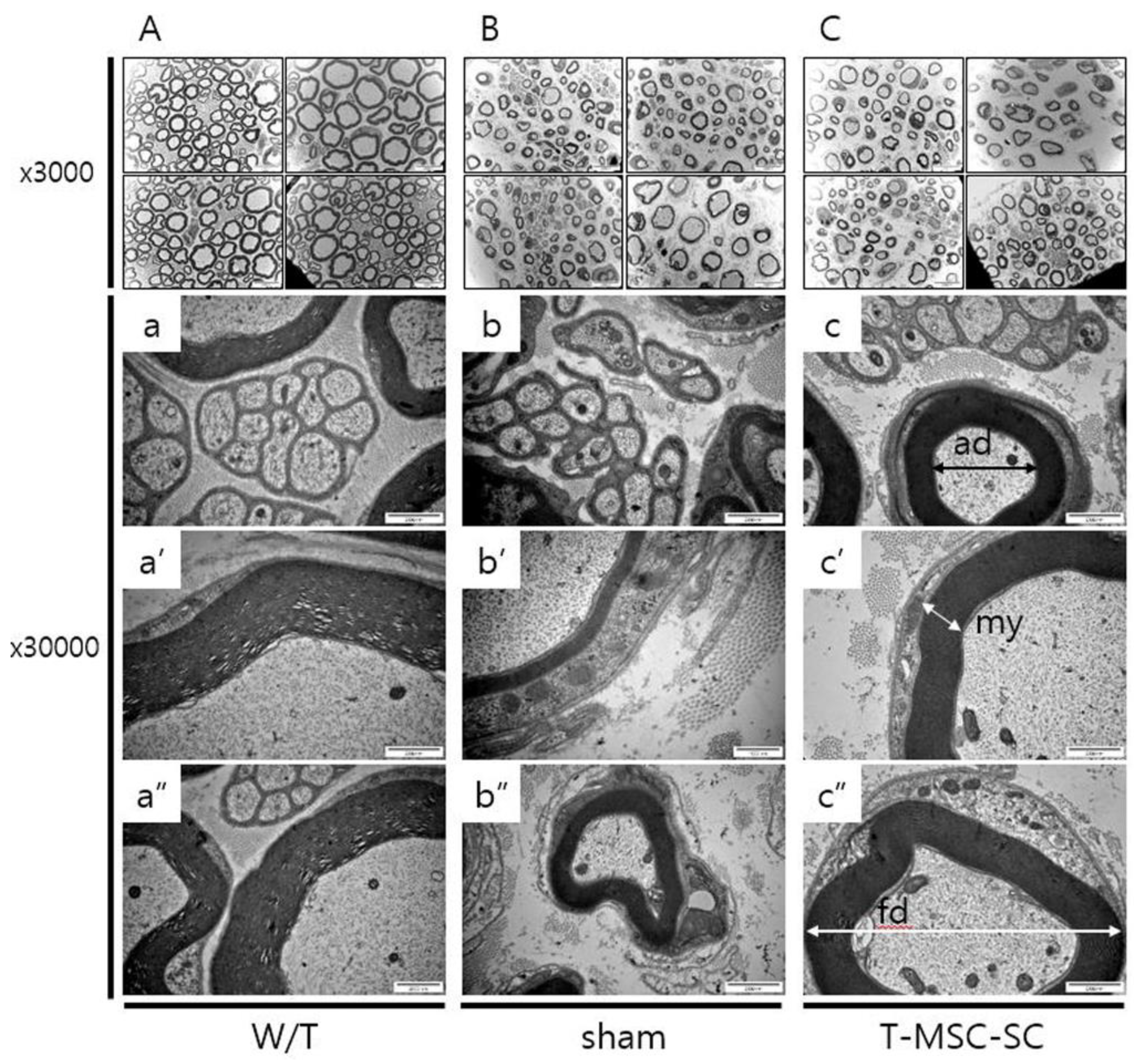
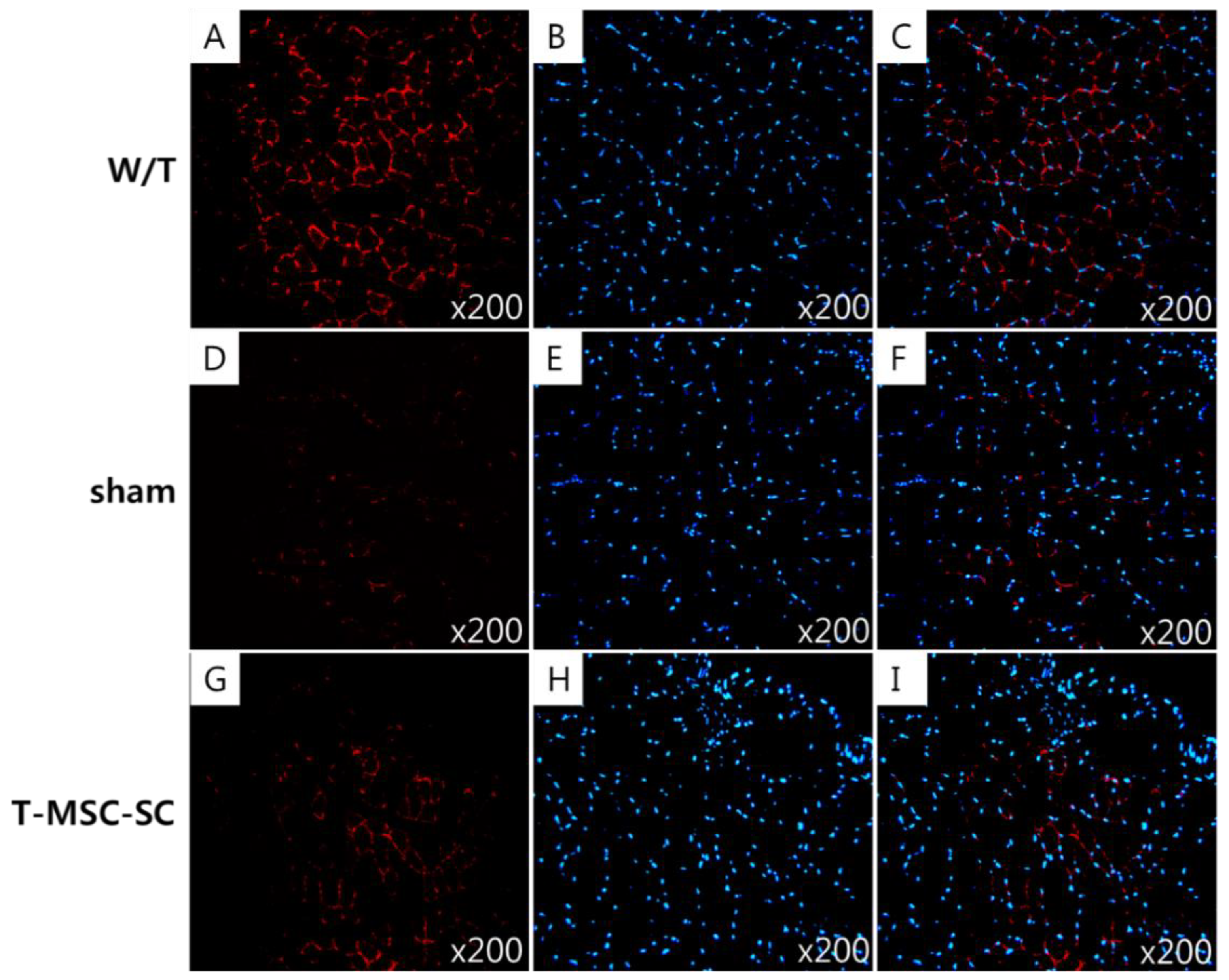
2.3. Ultrastructure of the Sciatic Nerve
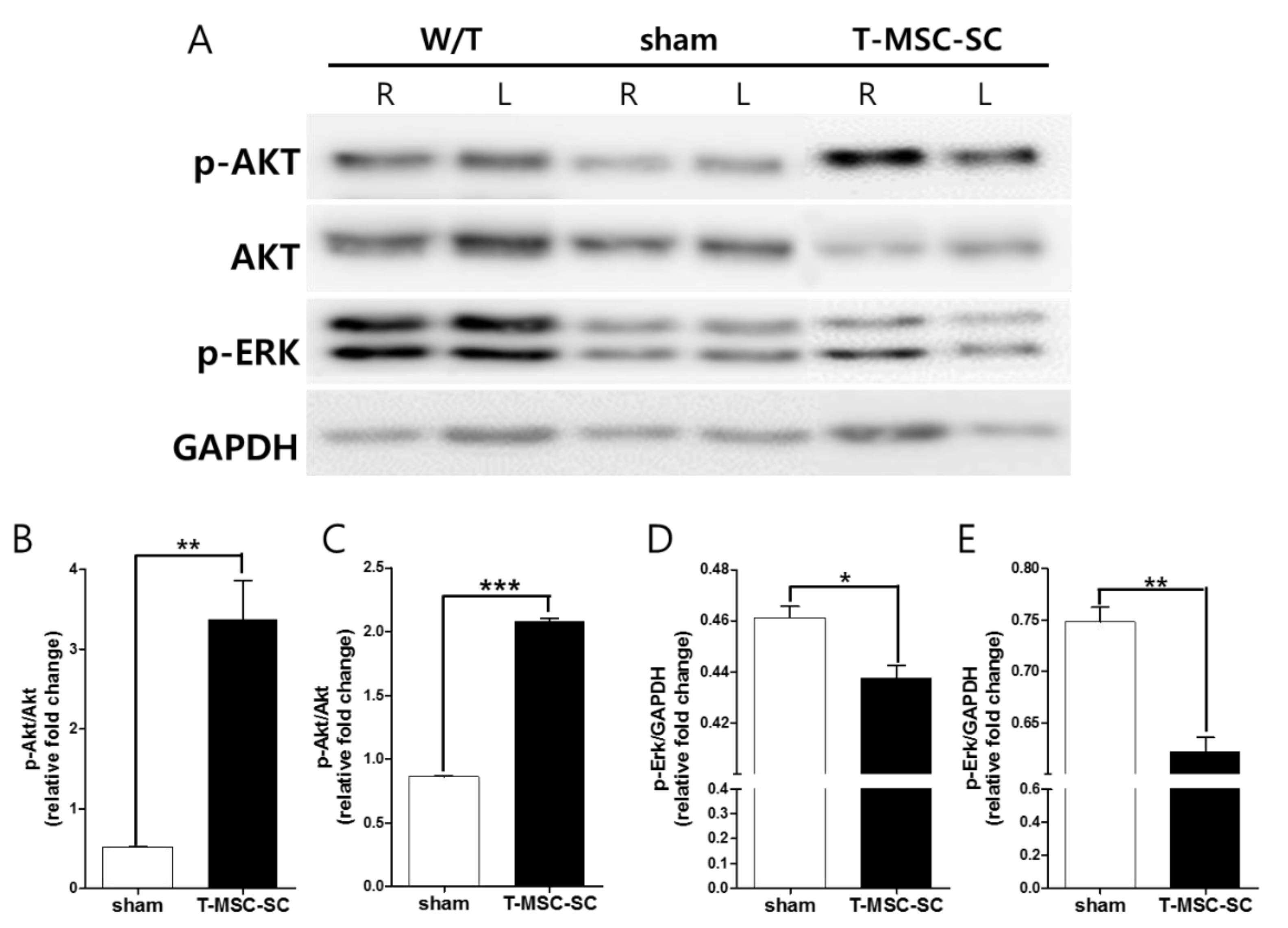
2.4. Western Blot Analysis of the Sciatic Nerve
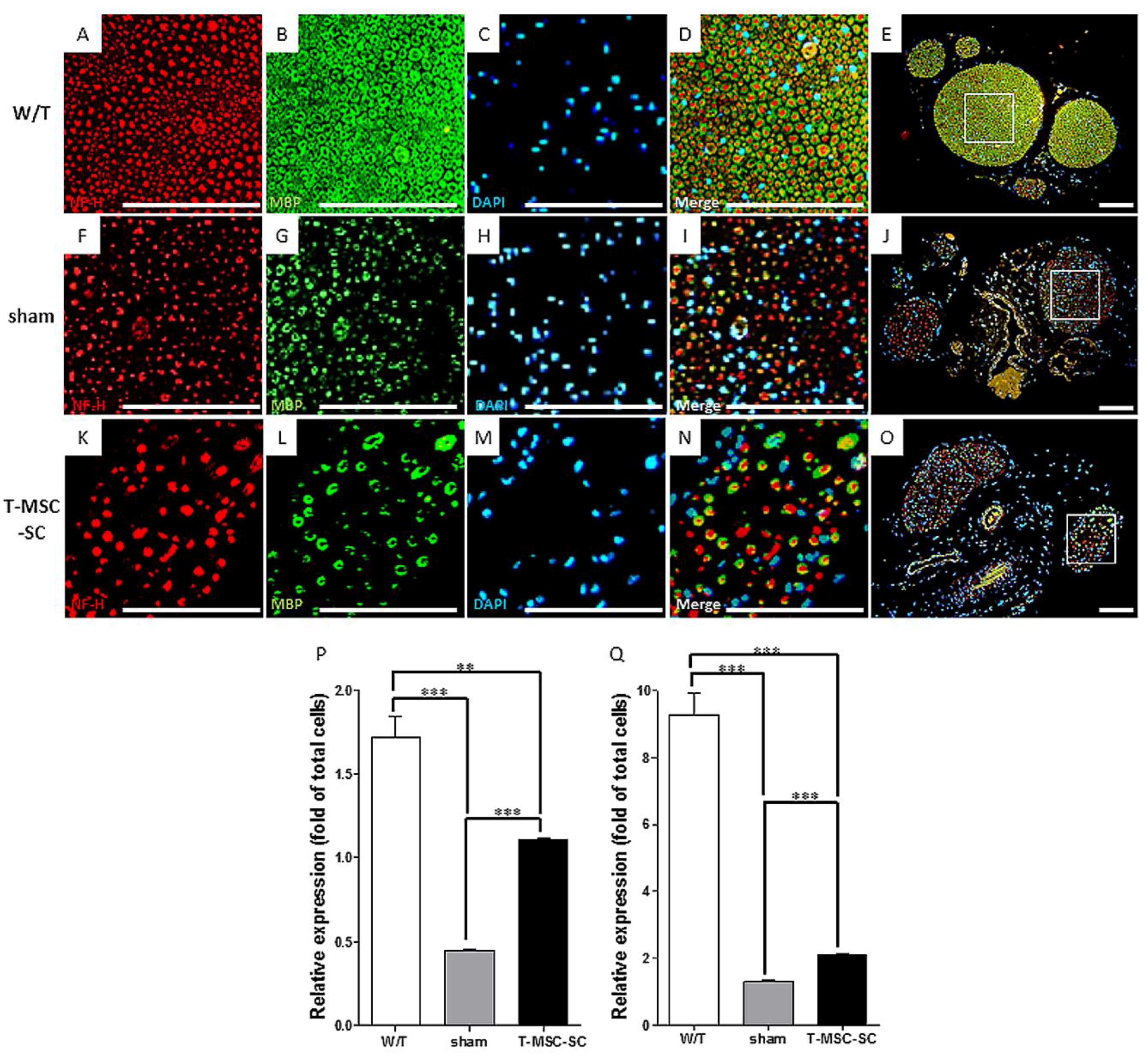
2.5. Expression of NF-H and MBP by Immunohistochemistry
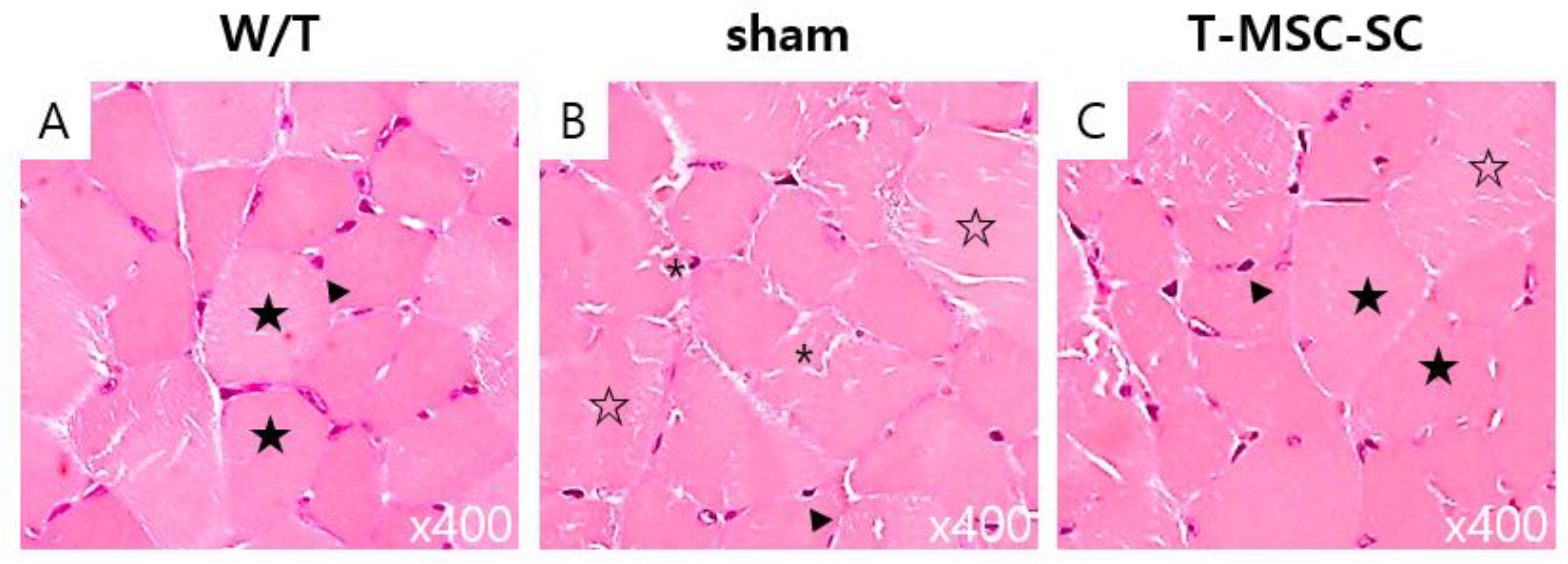
2.6. Skeletal Muscle Regeneration after T-MSC-SCs Transplantation
3. Discussion
4. Material and Methods
4.1. Ethics Statement
4.2. Animals
4.3. Preparation of T-MSCs and Differentiation into Schwann Cells
4.4. Immunocytochemistry
4.5. Real-Time Quantitative Polymerase Chain Reaction (Real-Time qPCR)
4.6. Transplantation
4.7. Rotarod Test
4.8. Sciatic Functional Index (SFI)
4.9. Electron Microscopy
4.10. Western Blotting
4.11. Immunohistochemistry and HE Staining
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| CMT1A | Charcot-Marie-Tooth type1A |
| T-MSC | tonsil-derived stem cell |
| Tr-J | trembler-J |
| SC | Schwann cell |
| T-MSC-SC | T-MSC differentiation toward the SC |
| PMP22 | peripheral myelin protein 22 |
| ESC | embryonic stem cell |
| iPSC | induced pluripotent stem cell |
| BM-MSCs | bone marrow-derived stem cells |
| Ad-MSC | adipose-derived Stem cells |
| UC-MSCs | umbilical cord-derived MSCs |
| SFI | sciatic function index |
| EM | electron microscopy |
| TST | tail suspension test |
References
- Saporta, M.A.; Shy, M.E. Inherited peripheral neuropathies. Neurol. Clin. 2013, 31, 597–619. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.I.; Lupski, J.R. Charcot-Marie-Tooth disease: A new paradigm for the mechanism of inherited disease. Trends Genet. 1994, 10, 128–133. [Google Scholar] [CrossRef]
- Roa, B.B.; Garcia, C.A.; Suter, U.; Kulpa, D.A.; Wise, C.A.; Mueller, J.; Welcher, A.A.; Snipes, G.J.; Shooter, E.M.; Patel, P.I.; et al. Charcot-Marie-Tooth disease type 1A: Association with a spontaneous point mutation in the PMP22 gene. N. Engl. J. Med. 1993, 329, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Meekins, G.D.; Emery, M.J.; Weiss, M.D. Nerve conduction abnormalities in the trembler-j mouse: A model for Charcot-Marie-Tooth disease type 1A? J. Peripher. Nerv. Syst. 2004, 9, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Scurry, A.N.; Heredia, D.J.; Feng, C.Y.; Gephart, G.B.; Hennig, G.W.; Gould, T.W. Structural and functional abnormalities of the neuromuscular junction in the Trembler-J homozygote mouse model of congenital hypomyelinating neuropathy. J. Neuropathol. Exp. Neurol. 2016, 75, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Jones, S.; Jia, X. Stem cell transplantation for peripheral nerve regeneration: Current options and opportunities. Int. J. Mol. Sci. 2017, 18, 94. [Google Scholar] [CrossRef] [PubMed]
- Lian, Q.; Lye, E.; Yeo, K.S.; Tan, E.K.W.; Salto-Tellez, M.; Liu, T.M.; Palanisamy, N.; El Oakley, R.M.; Lee, E.H.; Lim, B.; et al. Derivation of clinically compliant MSCs from CD105+, CD24− differentiated human ESCs. Stem Cells 2007, 25, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Xu, L.; Kim, G.H.; Kang, S.K.; Lee, S.W.; Park, S.H.; Kim, S.; Choi, T.H.; Kim, H.-S. Regeneration of peripheral nerves by transplanted sphere of human mesenchymal stem cells derived from embryonic stem cells. Biomaterials 2012, 33, 7039–7046. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Uemura, T.; Takamatsu, K.; Okada, M.; Kazuki, K.; Tabata, Y.; Ikada, Y.; Nakamura, H. Acceleration of peripheral nerve regeneration using nerve conduits in combination with induced pluripotent stem cell technology and a basic fibroblast growth factor drug delivery system. J. Biomed. Mater. Res. 2014, A 102, 1370–1378. [Google Scholar] [CrossRef]
- Heine, W.; Conant, K.; Griffin, J.; Hoke, A. Transplanted neural stem cells promote axonal regeneration through chronically denervated peripheral nerves. Exp. Neurol. 2004, 189, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Nijhuis, T.H.J.; Bodar, C.W.J.; van Neck, J.W.; Walbeehm, E.T.; Siemionow, M.; Madajka, M.; Cwykiel, J.; Blok, J.H.; Hovius, S.E.R. Natural conduits for bridging a 15-mm nerve defect: Comparison of the vein supported by muscle and bone marrow stromal cells with a nerve autograft. J. Plast. Reconstr. Aesthet. Surg. 2013, 66, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Z.; Luo, M.; Li, Y.; Zhang, K. Biological conduits combining bone marrow mesenchymal stem cells and extracellular matrix to treat long-segment sciatic nerve defects. Neural Regen. Res. 2015, 10, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Carlson, K.B.; Singh, P.; Feaster, M.M.; Ramnarain, A.; Pavlides, C.; Chen, Z.L.; Yu, W.M.; Feltri, M.L.; Strickland, S. Mesenchymal stem cells facilitate axon sorting, myelination, and functional recovery in paralyzed mice deficient in Schwann cell-derived laminin. Glia 2011, 59, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Marconi, S.; Castiglione, G.; Turano, E.; Bissolotti, G.; Angiari, S.; Farinazzo, A.; Constantin, G.; Bedogni, G.; Bedogni, A.; Bonetti, B. Human adipose-derived mesenchymal stem cells systemically injected promote peripheral nerve regeneration in the mouse model of sciatic crush. Tissue Eng. Part A 2012, 18, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, L.; Ahn, H.S.; Kim, M.H.; Kim, S.W. Amniotic mesenchymal stem cells display neurovascular tropism and aid in the recovery of injured peripheral nerves. J. Cell. Mol. Med. 2014, 18, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.C.; Cheng, F.C.; Chen, C.J.; Lai, S.Z.; Lee, C.W.; Yang, D.Y.; Chang, M.H.; Ho, S.P. Post-injury regeneration in rat sciatic nerve facilitated by neurotrophic factors secreted by amniotic fluid mesenchymal stem cells. J. Clin. Neurosci. 2007, 14, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Zarbakhsh, S.; Goudarzi, N.; Shirmohammadi, M.; Safari, M. Histological study of bone marrow and umbilical cord stromal cell transplantation in regenerating rat peripheral nerve. Cell J. 2016, 17, 10. [Google Scholar]
- Jung, N.; Park, S.; Choi, Y.; Park, J.W.; Hong, Y.; Park, H.; Yu, Y.; Kwak, G.; Kim, H.; Ryu, K.H.; et al. Tonsil-derived mesenchymal stem cells differentiate into a Schwann cell phenotype and promote peripheral nerve regeneration. Int. J. Mol. Sci. 2016, 17, 1867. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Choi, Y.; Jung, N.; Yu, Y.; Ryu, K.H.; Kim, H.S.; Jo, I.; Choi, B.O.; Jung, S.C. Myogenic differentiation potential of human tonsil-derived mesenchymal stem cells and their potential for use to promote skeletal muscle regeneration. Int. J. Mol. Med. 2016, 37, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.H.; Cho, K.A.; Park, H.S.; Kim, J.Y.; Woo, S.Y.; Jo, I.; Choi, Y.H.; Park, Y.M.; Jung, S.C.; Chung, S.M.; et al. Tonsil-derived mesenchymal stromal cells: Evaluation of biologic, immunologic and genetic factors for successful banking. Cytotherapy 2012, 14, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Djouad, F.; Jackson, W.M.; Bobick, B.E.; Janjanin, S.; Song, Y.; Huang, G.T.; Tuan, R.S. Activin A expression regulates multipotency of mesenchymal progenitor cells. Stem Cell Res. Ther. 2010, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, Y.R.; Park, W.J.; Kim, H.S.; Jung, S.C.; Woo, S.Y.; Jo, I.; Ryu, K.H.; Park, J.W. Characterisation of insulin-producing cells differentiated from tonsil derived mesenchymal stem cells. Differentiation 2015, 90, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Lee, B.J.; Park, H.Y.; Song, J.S.; Shin, S.C.; Lee, J.C.; Wang, S.G.; Jung, J.S. Effects of donor age, long-term passage culture, and cryopreservation on tonsil-derived mesenchymal stem cells. Cell. Physiol. Biochem. 2015, 36, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Hwang, S.; Jin, Y.M.; Yu, Y.; Jung, S.-A.; Jung, S.C.; Ryu, K.-H.; Kim, H.S.; Jo, I. CCN1 secreted by tonsil-derived mesenchymal stem cells promotes endothelial cell angiogenesis via integrin αvβ3 and AMPK. Cell. Physiol. 2015, 230, 140–149. [Google Scholar] [CrossRef] [PubMed]
- FitzGibbon, T.; Nestorovski, Z. Human intraretinal myelination: Axon diameters and axon/myelin thickness ratios. Indian J. Ophthalmol. 2013, 61, 567–575. [Google Scholar] [CrossRef] [PubMed]
- García-Pelagio, K.P.; Bloch, R.J.; Ortega, A.; González-Serratos, H. Biomechanics of the sarcolemma and costameres in single skeletal muscle fibers from normal and dystrophin-null mice. J. Muscle Res. Cell Motil. 2011, 31, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Kyurkchiev, D.; Bochev, I.; Ivanova-Todorova, E.; Mourdjeva, M.; Oreshkova, T.; Belemezova, K.; Kyurkchiev, S. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J Stem Cells. 2014, 26, 552–570. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Griffith, L.G.; Wells, A. Growth factor regulation of proliferation and survival of multipotential stromal cells. Stem Cell Res Ther. 2010, 26, 32. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G.; Pittenger, M.F. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Nave, K.A.; Sereda, M.W.; Ehrenreich, H. Mechanisms of disease: Inherited demyelinating neuropathies—From basic to clinical research. Nat. Clin. Pract. Neurol. 2007, 3, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Fledrich, R.; Stassart, R.M.; Klink, A.; Rasch, L.M.; Prukop, T.; Haag, L.; Czesnik, D.; Kungl, T.; Abdelaal, T.A.M.; Keric, N.; et al. Soluble neuregulin-1 modulates disease pathogenesis in rodent models of Charcot-Marie-Tooth disease 1A. Nat. Med. 2014, 20, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Martini, R. Neuregulin-1 alleviates Charcot-Marie-Tooth disease in rats. Nat. Med. 2014, 20, 984–985. [Google Scholar] [CrossRef] [PubMed]
- Suter, U.; Moskow, J.J.; Welcher, A.A.; Snipes, G.J.; Kosaras, B.; Sidman, R.L.; Buchberg, A.M.; Shooter, E.M. A leucine-to-proline mutation in the putative first transmembrane domain of the 22-kDa peripheral myelin protein in the trembler-J mouse. Proc. Natl. Acad. Sci. USA 1992, 89, 4382–4386. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.J.; Zhang, Y.; Zhao, J.; Jiang, B. Intramuscular injection of bone marrow mesenchymal stem cells with small gap neurorrhaphy for peripheral nerve repair. Neurosci. Lett. 2015, 585, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Schaakxs, D.; Kalbermatten, D.F.; Raffoul, W.; Wiberg, M.; Kingham, P.J. Regenerative cell injection in denervated muscle reduces atrophy and enhances recovery following nerve repair. Muscle Nerve 2013, 47, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Choi, Y.; Kwak, G.; Hong, Y.B.; Jung, N.; Kim, J.; Choi, B.; Jung, S.C. Application of differentiated human tonsil-derived stem cells to Trembler-J mice. Muscle Nerve 2018, 57, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Verhamme, C.; King, R.H.; ten Asbroek, A.L.; Muddle, J.R.; Nourallah, M.; Wolterman, R.; Baas, F.; van Schaik, I.N. Myelin and axon pathology in a long-term study of PMP22-overexpressing mice. J. Neuropathol. Exp. Neurol. 2011, 70, 386–398. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Institute for Laboratory Animal Research. Guide for the Care and Use of Laboratory Animals; National Academies Press (US): Washington, D.C., USA, 2011; Available online: https://www.ncbi.nlm.nih.gov/books/NBK54056/ (accessed on 11 August 2018).
- Notterpek, L.; Shooter, E.M.; Snipes, G.J. Upregulation of the endosomal-lysosomal pathway in the trembler-J neuropathy. J. Neurosci. 1997, 17, 4190. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Mombereau, C.; Vassout, A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci. Biobehav. Rev. 2005, 29, 571–625. [Google Scholar] [CrossRef] [PubMed]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology (Berl.) 1985, 85, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.A.; Kim, J.Y.; Kim, H.S.; Ryu, K.H.; Woo, S.Y. Tonsil-derived mesenchymal progenitor cells acquire a follicular dendritic cell phenotype under cytokine stimulation. Cytokine 2012, 59, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Inserra, M.M.; Bloch, D.A.; Terris, D.J. Functional indices for sciatic, peroneal, and posterior tibial nerve lesions in the mouse. Microsurgery 1998, 18, 119–124. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Jung, N.; Myung, S.; Choi, Y.; Chung, K.W.; Choi, B.-O.; Jung, S.-C. Differentiation of Human Tonsil-Derived Mesenchymal Stem Cells into Schwann-Like Cells Improves Neuromuscular Function in a Mouse Model of Charcot-Marie-Tooth Disease Type 1A. Int. J. Mol. Sci. 2018, 19, 2393. https://doi.org/10.3390/ijms19082393
Park S, Jung N, Myung S, Choi Y, Chung KW, Choi B-O, Jung S-C. Differentiation of Human Tonsil-Derived Mesenchymal Stem Cells into Schwann-Like Cells Improves Neuromuscular Function in a Mouse Model of Charcot-Marie-Tooth Disease Type 1A. International Journal of Molecular Sciences. 2018; 19(8):2393. https://doi.org/10.3390/ijms19082393
Chicago/Turabian StylePark, Saeyoung, Namhee Jung, Seoha Myung, Yoonyoung Choi, Ki Wha Chung, Byung-Ok Choi, and Sung-Chul Jung. 2018. "Differentiation of Human Tonsil-Derived Mesenchymal Stem Cells into Schwann-Like Cells Improves Neuromuscular Function in a Mouse Model of Charcot-Marie-Tooth Disease Type 1A" International Journal of Molecular Sciences 19, no. 8: 2393. https://doi.org/10.3390/ijms19082393
APA StylePark, S., Jung, N., Myung, S., Choi, Y., Chung, K. W., Choi, B.-O., & Jung, S.-C. (2018). Differentiation of Human Tonsil-Derived Mesenchymal Stem Cells into Schwann-Like Cells Improves Neuromuscular Function in a Mouse Model of Charcot-Marie-Tooth Disease Type 1A. International Journal of Molecular Sciences, 19(8), 2393. https://doi.org/10.3390/ijms19082393





