Decellularized Tissue for Muscle Regeneration
Abstract
1. Introduction
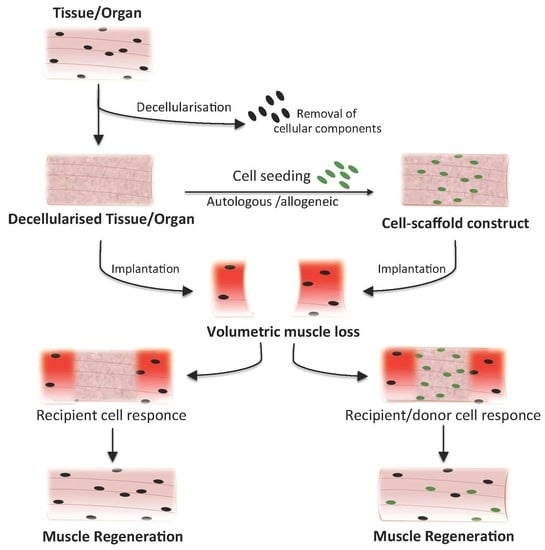
2. Acellular Tissues and Biomaterials for VML Treatment: Types and Methods
3. VML Models for Testing Acellular Tissues
4. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | tridimensional |
| ECM | extracellular matrix |
| FGF | fibroblast growth factor |
| HGF | hepatocyte growth factor |
| IGF1 | insulin-like growth factor 1 |
| PCL | polycaprolactone |
| PEG | poly(ethylene glycolic) |
| PGA | polyglycolic acid |
| PLGA | poly(lactic-co-glycolic acid) |
| PLLA | poly(l-lactic acid) |
| SC | satellite cells |
| SDS | sodium dodecil sulfate |
| SIS | Small intestine submucosa matrix |
| UBM | urinary bladder matrix |
| VEGF | vascular endothelial growth factor |
| VML | volumetric muscle loss |
References
- Gattazzo, F.; Urciuolo, A.; Bonaldo, P. Extracellular matrix: A dynamic microenvironment for stem cell niche. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 2506–2519. [Google Scholar] [CrossRef] [PubMed]
- Qazi, T.H.; Mooney, D.J.; Pumberger, M.; Geissler, S.; Duda, G.N. Biomaterials based strategies for skeletal muscle tissue engineering: Existing technologies and future trends. Biomaterials 2015, 53, 502–521. [Google Scholar] [CrossRef] [PubMed]
- Muiznieks, L.D.; Keeley, F.W. Molecular assembly and mechanical properties of the extracellular matrix: A fibrous protein perspective. Biochim. Biophys. Acta Mol. Basis Dis. 2013, 1832, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Juhas, M.; Bursac, N. Engineering skeletal muscle repair. Curr. Opin. Biotechnol. 2013, 24, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Moran, E.C.; Dhal, A.; Vyas, D.; Lanas, A.; Soker, S.; Baptista, P.M. Whole-organ bioengineering: Current tales of modern alchemy. Transl. Res. 2014, 163, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.J.; De Coppi, P.; Speggiorin, S.; Roebuck, D.; Butler, C.R.; Samuel, E.; Crowley, C.; McLaren, C.; Fierens, A.; Vondrys, D.; et al. Stem-cell-based, tissue engineered tracheal replacement in a child: A 2-year follow-up study. Lancet 2012, 380, 994–1000. [Google Scholar] [CrossRef]
- Kwee, B.J.; Mooney, D.J. Biomaterials for skeletal muscle tissue engineering. Curr. Opin. Biotechnol. 2017, 47, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Hinds, S.; Bian, W.; Dennis, R.G.; Bursac, N. The role of extracellular matrix composition in structure and function of bioengineered skeletal muscle. Biomaterials 2011, 32, 3575–3583. [Google Scholar] [CrossRef] [PubMed]
- Gillies, A.R.; Smith, L.R.; Lieber, R.L.; Varghese, S. Method for Decellularizing Skeletal Muscle without Detergents or Proteolytic Enzymes. Tissue Eng. Part C Methods 2011, 17. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, M. Skeletal muscle formation in vertebrates. Curr. Opin. Genet. Dev. 2001, 11, 440–448. [Google Scholar] [CrossRef]
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef] [PubMed]
- Grzelkowska-Kowalczyk, K. The Importance of Extracellular Matrix in Skeletal Muscle Development and Function; World’ s Largest Science, Technology & Medicine Open Access Book Publisher: London, UK, 2016. [Google Scholar]
- Rosenblatt, J.D.; Lunt, A.I.; Parry, D.J.; Partridge, T.A. Culturing satellite cells from living single muscle fiber explants. In Vitro Cell. Dev. Biol. Anim. 1995, 31, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Baghdadi, M.B.; Castel, D.; Machado, L.; Fukada, S.-I.; Birk, D.E.; Relaix, F.; Tajbakhsh, S.; Mourikis, P. Reciprocal signalling by Notch-Collagen V-CALCR retains muscle stem cells in their niche. Nature 2018, 557, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Bentzinger, C.F.; Wang, Y.X.; Von Maltzahn, J.; Soleimani, V.D.; Yin, H.; Rudnicki, M.A. Fibronectin regulates Wnt7a signaling and satellite cell expansion. Cell Stem Cell 2013, 12, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Urciuolo, A.; Quarta, M.; Morbidoni, V.; Gattazzo, F.; Molon, S.; Grumati, P.; Montemurro, F.; Tedesco, F.S.; Blaauw, B.; Cossu, G.; et al. Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Quarta, M.; Brett, J.O.; DiMarco, R.; De Morree, A.; Boutet, S.C.; Chacon, R.; Gibbons, M.C.; Garcia, V.A.; Su, J.; Shrager, J.B.; et al. An artificial niche preserves the quiescence of muscle stem cells and enhances their therapeutic efficacy. Nat. Biotechnol. 2016, 34, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Rayagiri, S.S.; Ranaldi, D.; Raven, A.; Mohamad Azhar, N.I.F.; Lefebvre, O.; Zammit, P.S.; Borycki, A.G. Basal lamina remodeling at the skeletal muscle stem cell niche mediates stem cell self-renewal. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Ngan, C.G.Y.; Quigley, A.; Kapsa, R.M.I.; Choong, P.F.M. Engineering skeletal muscle—From two to three dimensions. J. Tissue Eng. Regen. Med. 2017, 12, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.T.; Daly, K.A.; Reing, J.E.; Badylak, S.F. Biologic scaffold composed of skeletal muscle extracellular matrix. Biomaterials 2012, 33, 2916–2925. [Google Scholar] [CrossRef] [PubMed]
- Perniconi, B.; Costa, A.; Aulino, P.; Teodori, L.; Adamo, S.; Coletti, D. The pro-myogenic environment provided by whole organ scale acellular scaffolds from skeletal muscle. Biomaterials 2011, 32, 7870–7882. [Google Scholar] [CrossRef] [PubMed]
- Coppi, P.D.E.; Bellini, S.; Conconi, M.T.; Sabatti, M.; Simonato, E.; Gamba, P.G.; Nussdorfer, G.G.; Parnigotto, P.P. Myoblast-Acellular Skeletal Muscle Matrix Constructs Full-Thickness Abdominal Wall Defects. Tissue Eng. 2006, 12, 1929–1936. [Google Scholar] [CrossRef] [PubMed]
- Merritt, E.K.; Hammers, D.W.; Tierney, M.; Suggs, L.J.; Walters, T.J.; Farrar, R.P. Functional Assessment of Skeletal Muscle Regeneration Utilizing Homologous Extracellular Matrix as Scaffolding. Tissue Eng. Part A 2010, 16, 1395–1405. [Google Scholar] [CrossRef] [PubMed]
- Fishman, J.M.; Lowdell, M.W.; Urbani, L.; Ansari, T.; Burns, A.J.; Turmaine, M. Immunomodulatory effect of a decellularized skeletal muscle scaffold in a discordant xenotransplantation model. Proc. Natl. Acad. Sci. USA 2013, 110. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.M.; Henderson, B.E.P.; Walters, T.J.; Corona, B.T. Co-delivery of a laminin-111 supplemented hyaluronic acid based hydrogel with minced muscle graft in the treatment of volumetric muscle loss injury. PLoS ONE 2018, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sicari, B.M.; Agrawal, V.; Siu, B.F.; Medberry, C.J.; Dearth, C.L.; Turner, N.J.; Badylak, S.F. A Murine Model of Volumetric Muscle Loss and a Regenerative Medicine Approach for Tissue Replacement. Tissue Eng. Part A 2012, 18, 1941–1948. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.J.; Badylak, J.S.; Weber, D.J.; Badylak, S.F. Biologic scaffold remodeling in a dog model of complex musculoskeletal injury. J. Surg. Res. 2012, 176, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, Z.Q.; Turner, N.J.; Teng, S.F.; Cheng, W.Y.; Zhou, H.Y.; Zhang, L.; Hu, H.W.; Wang, Q.; Badylak, S.F. Perfusion-decellularized skeletal muscle as a three-dimensional scaffold with a vascular network template. Biomaterials 2016, 89, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Valentin, J.E.; Turner, N.J.; Gilbert, T.W.; Badylak, S.F. Functional skeletal muscle formation with a biologic scaffold. Biomaterials 2010, 31, 7475–7484. [Google Scholar] [CrossRef] [PubMed]
- Greising, S.M.; Rivera, J.C.; Goldman, S.M.; Watts, A.; Aguilar, C.A.; Corona, B.T. Unwavering Pathobiology of Volumetric Muscle Loss Injury. Sci. Rep. 2017, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Quarta, M.; Cromie, M.; Chacon, R.; Blonigan, J.; Garcia, V.; Akimenko, I.; Hamer, M.; Paine, P.; Stok, M.; Shrager, J.B.; et al. Bioengineered constructs combined with exercise enhance stem cell-mediated treatment of volumetric muscle loss. Nat. Commun. 2017. [Google Scholar] [CrossRef] [PubMed]
- McClure, M.J.; Cohen, D.J.; Ramey, A.N.; Bivens, C.B.; Mallu, S.; Isaacs, J.E.; Imming, E.; Huang, Y.-C.; Sunwoo, M.; Schwartz, Z.; et al. Decellularized muscle supports new muscle fibers and improves function following volumetric injury. Tissue Eng. Part A 2018. [Google Scholar] [CrossRef] [PubMed]
- Urciuolo, A.; Urbani, L.; Perin, S.; Maghsoudlou, P.; Scottoni, F.; Gjinovci, A.; Collins-Hooper, H.; Loukogeorgakis, S.; Tyraskis, A.; Torelli, S.; et al. Decellularised skeletal muscles allow functional muscle regeneration by promoting host cell migration. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Kasukonis, B.; Kim, J.; Brown, L.; Jones, J.; Ahmadi, S.; Washington, T.; Wolchok, J. Codelivery of Infusion Decellularized Skeletal Muscle with Minced Muscle Autografts Improved Recovery from Volumetric Muscle Loss Injury in a Rat Model. Tissue Eng. Part A 2016, 22, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Fisher, L.E.; Sicari, B.M.; Rubin, J.P.; Dearth, C.L.; Wolf, M.T.; Ambrosio, F.; Boninger, M.; Turner, N.J.; Weber, D.J.; Simpson, T.W.; et al. An Acellular Biologic Scaffold Promotes Skeletal Muscle Formation in Mice and Humans with Volumetric Muscle Loss an Acellular Biologic Scaffold Promotes Skeletal Muscle Formation in Mice and Humans with Volumetric Muscle Loss. Sci. Transl. Med. 2014. [Google Scholar] [CrossRef]
- Dziki, J.; Badylak, S.; Yabroudi, M.; Sicari, B.; Ambrosio, F.; Stearns, K.; Turner, N.; Wyse, A.; Boninger, M.L.; Brown, E.H.P.; et al. An acellular biologic scaffold treatment for volumetric muscle loss: Results of a 13-patient cohort study. NPJ Regen. Med. 2016, 1, 16008. [Google Scholar] [CrossRef] [PubMed]
- Hussein, K.H.; Park, K.M.; Kang, K.S.; Woo, H.M. Biocompatibility Evaluation of Tissue-Engineered Decellularized Scaffolds for Biomedical Application; Elsevier B.V.: Amsterdam, The Netherlands, 2016; Volume 67, ISBN 8233244236. [Google Scholar]
- Yu, Y.; Alkhawaji, A.; Ding, Y.; Mei, J. Decellularized scaffolds in regenerative medicine. Oncotarget 2016, 7, 58671–58683. [Google Scholar] [CrossRef] [PubMed]
- Rana, D.; Zreiqat, H.; Benkirane-Jessel, N.; Ramakrishna, S.; Ramalingam, M. Development of decellularized scaffolds for stem cell-driven tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 942–965. [Google Scholar] [CrossRef] [PubMed]
- Ott, H.C.; Matthiesen, T.S.; Goh, S.K.; Black, L.D.; Kren, S.M.; Netoff, T.I.; Taylor, D.A. Perfusion-decellularized matrix: Using nature’s platform to engineer a bioartificial heart. Nat. Med. 2008, 14, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Guyette, J.P.; Gilpin, S.E.; Charest, J.M.; Tapias, L.F.; Ren, X.; Ott, H.C. Perfusion decellularization of whole organs. Nat. Protoc. 2014, 9, 1451–1468. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Mishra, N.C. Decellularization Methods for Scaffold Fabrication. Methods Mol. Biol. 2017. [Google Scholar] [CrossRef]
- Jank, B.J.; Xiong, L.; Moser, P.T.; Guyette, J.P.; Ren, X.; Cetrulo, C.L.; Leonard, D.A.; Fernandez, L.; Fagan, S.P.; Ott, H.C. Engineered composite tissue as a bioartificial limb graft. Biomaterials 2015, 61, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Gerli, M.F.M.; Guyette, J.P.; Evangelista-Leite, D.; Ghoshhajra, B.B.; Ott, H.C. Perfusion decellularization of a human limb: A novel platform for composite tissue engineering and reconstructive surgery. PLoS ONE 2018, 13, e0191497. [Google Scholar] [CrossRef] [PubMed]
- Porzionato, A.; Sfriso, M.M.; Pontini, A.; Macchi, V.; Petrelli, L.; Pavan, P.G.; Natali, A.N.; Bassetto, F.; Vindigni, V. Decellularized Human Skeletal Muscle as Biologic Scaffold for Reconstructive Surgery. Int. J. Mol. Sci. 2015, 14808–14831. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, M.; Trevisan, C.; Maghin, E.; Franzin, C.; Pozzobon, M. Mouse Skeletal Muscle Decellularization. Methods Mol. Biol. 2017. [Google Scholar] [CrossRef]
- Fuoco, C.; Petrilli, L.L.; Cannata, S.; Gargioli, C. Matrix scaffolding for stem cell guidance toward skeletal muscle tissue engineering. J. Orthop. Surg. Res. 2016, 11, 86. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saxena, A.K.; Willital, G.H.; Vacanti, J.P. Vascularized three-dimensional skeletal muscle tissue-engineering. Biomed. Mater. Eng. 2001, 11, 275–281. [Google Scholar] [PubMed]
- Saxena, A.K.; Marler, J.; Benvenuto, M.; Willital, G.H.; Vacanti, J.P. Skeletal muscle tissue engineering using isolated myoblasts on synthetic biodegradable polymers: Preliminary studies. Tissue Eng. 1999, 5, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Boldrin, L.; Malerba, A.; Vitiello, L.; Cimetta, E.; Piccoli, M.; Messina, C.; Gamba, P.G.; Elvassore, N.; De Coppi, P. Efficient delivery of human single fiber-derived muscle precursor cells via biocompatible scaffold. Cell Transplant. 2008, 17, 576–584. [Google Scholar] [CrossRef]
- Borselli, C.; Storrie, H.; Benesch-Lee, F.; Shvartsman, D.; Cezar, C.; Lichtman, J.W.; Vandenburgh, H.H.; Mooney, D.J. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc. Natl. Acad. Sci. USA 2010, 107, 3287–3292. [Google Scholar] [CrossRef] [PubMed]
- De Coppi, P.; Delo, D.; Farrugia, L.; Udompanyanan, K.; Yoo, J.J.; Nomi, M.; Atala, A.; Soker, S. Angiogenic gene-modified muscle cells for enhancement of tissue formation. Tissue Eng. 2005, 11, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.; Boontheekul, T.; Mooney, D.J. Regulating activation of transplanted cells controls tissue regeneration. Proc. Natl. Acad. Sci. USA 2006, 103, 2494–2499. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Pareta, R.A.; Wu, R.; Shi, Y.; Zhou, X.; Liu, H.; Deng, C.; Sun, X.; Atala, A.; Opara, E.C.; et al. Skeletal myogenic differentiation of urine-derived stem cells and angiogenesis using microbeads loaded with growth factors. Biomaterials 2013, 34, 1311–1326. [Google Scholar] [CrossRef] [PubMed]
- Page, R.L.; Malcuit, C.; Vilner, L.; Vojtic, I.; Shaw, S.; Hedblom, E.; Hu, J.; Pins, G.D.; Rolle, M.W.; Dominko, T. Restoration of Skeletal Muscle Defects with Adult Human Cells Delivered on Fibrin Microthreads. Tissue Eng. Part A 2011, 17, 2629–2640. [Google Scholar] [CrossRef] [PubMed]
- Beier, J.P.; Stern-Straeter, J.; Foerster, V.T.; Kneser, U.; Stark, G.B.; Bach, A.D. Tissue engineering of injectable muscle: Three-dimensional myoblast-fibrin injection in the syngeneic rat animal model. Plast. Reconstr. Surg. 2006, 118, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.A.; Flaibani, M.; Blaauw, B.; Pozzobon, M.; Figallo, E.; Reggiani, C.; Vitiello, L.; Elvassore, N.; De Coppi, P. In vivo tissue engineering of functional skeletal muscle by freshly isolated satellite cells embedded in a photopolymerizable hydrogel. FASEB J. 2011, 25, 2296–2304. [Google Scholar] [CrossRef] [PubMed]
- Lesman, A.; Koffler, J.; Atlas, R.; Blinder, Y.J.; Kam, Z.; Levenberg, S. Engineering vessel-like networks within multicellular fibrin-based constructs. Biomaterials 2011, 32, 7856–7869. [Google Scholar] [CrossRef] [PubMed]
- Cronin, E.M.; Thurmond, F.A.; Bassel-Duby, R.; Williams, R.S.; Wright, W.E.; Nelson, K.D.; Garner, H.R. Protein-coated poly(l-lactic acid) fibers provide a substrate for differentiation of human skeletal muscle cells. J. Biomed. Mater. Res. 2004, 69A, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Lee, S.J.; Christ, G.J.; Atala, A.; Yoo, J.J. The influence of electrospun aligned poly(ε-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes. Biomaterials 2008, 29, 2899–2906. [Google Scholar] [CrossRef] [PubMed]
- Fuoco, C.; Rizzi, R.; Biondo, A.; Longa, E.; Mascaro, A.; Shapira-Schweitzer, K.; Kossovar, O.; Benedetti, S.; Salvatori, M.L.; Santoleri, S.; et al. In vivo generation of a mature and functional artificial skeletal muscle. EMBO Mol. Med. 2015, 7, 411–422. [Google Scholar] [CrossRef] [PubMed]
| Decellularized Tissue | Method of Decellularization | Seeded Cells | Mplanted Species | In Vivo Outcome | Ref. |
|---|---|---|---|---|---|
| Porcine small intestine submucosa (SIS); Canine skeletal muscle | Immersion | – | Rat | Remodeling and partial skeletal muscle regeneration. Comparable results between SIS and skeletal muscle scaffolds | [21] |
| Murine skeletal muscle | Immersion | – | Mouse | Remodeling and partial skeletal muscle regeneration | [22] |
| Rat skeletal muscle | Immersion | Rat SC–derived myoblasts | Rat | Partial skeletal muscle regeneration | [23] |
| Rat skeletal muscle | Immersion | – | Rat | Partial skeletal muscle regeneration. No force restoration | [24] |
| Rat skeletal muscle | Immersion | Murine myoblasts | Rat | Improvement of donor cells survival | [25] |
| Porcine urinary bladder matrix (UBM) | Immersion | Minced muscle | Rat | Fibrosis and scarce skeletal muscle regeneration | [26] |
| Porcine SIS | Immersion | – | Mouse | Remodeling and partial skeletal muscle regeneration | [27] |
| Porcine SIS | Immersion | – | Dog | Remodeling and partial skeletal muscle regeneration. No functional recovery | [28] |
| Porcine SIS; Porcine skeletal muscle | Perfusion | – | Rat | Skeletal muscle regeneration with partial functional recovery. Improved results for skeletal muscle scaffolds | [29] |
| Porcine SIS; Carbodiimide-crosslinked porcine SIS | Immersion | – | Rat | Skeletal muscle regeneration with partial functional recovery. Improved results for SIS scaffolds | [30] |
| Porcine SIS; Porcine UBM | Immersion | – | Pig | Remodeling and fibrosis. No functional recovery | [31] |
| Murine skeletal muscle | Immersion | Co-culture of adult murine or human muscle stem cells and muscle resident cells | Mouse | Functional skeletal muscle regeneration improved after exercise regimen | [32] |
| Rat skeletal muscle | Patent | – | Rat | Functional skeletal muscle regeneration | [33] |
| Rat skeletal muscle | Perfusion | – | Mouse | Functional skeletal muscle regeneration | [34] |
| Rat skeletal muscle | Infusion | Minced muscle vs no cells | Rat | Functional skeletal muscle regeneration improved when cell seeded scaffolds are used | [35] |
| Porcine UBM | Immersion | – | Mouse | Skeletal muscle regeneration with partial functional recovery | [36] |
| Rat and primate forearm | Perfusion | Co-culture of C2C12 cells, fibroblasts and HUVEC | Rat | Reperfused vascular tree | [44] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urciuolo, A.; De Coppi, P. Decellularized Tissue for Muscle Regeneration. Int. J. Mol. Sci. 2018, 19, 2392. https://doi.org/10.3390/ijms19082392
Urciuolo A, De Coppi P. Decellularized Tissue for Muscle Regeneration. International Journal of Molecular Sciences. 2018; 19(8):2392. https://doi.org/10.3390/ijms19082392
Chicago/Turabian StyleUrciuolo, Anna, and Paolo De Coppi. 2018. "Decellularized Tissue for Muscle Regeneration" International Journal of Molecular Sciences 19, no. 8: 2392. https://doi.org/10.3390/ijms19082392
APA StyleUrciuolo, A., & De Coppi, P. (2018). Decellularized Tissue for Muscle Regeneration. International Journal of Molecular Sciences, 19(8), 2392. https://doi.org/10.3390/ijms19082392