A Short Half-Life αIIbβ3 Antagonist ANTP266 Reduces Thrombus Formation
Abstract
1. Introduction
2. Results
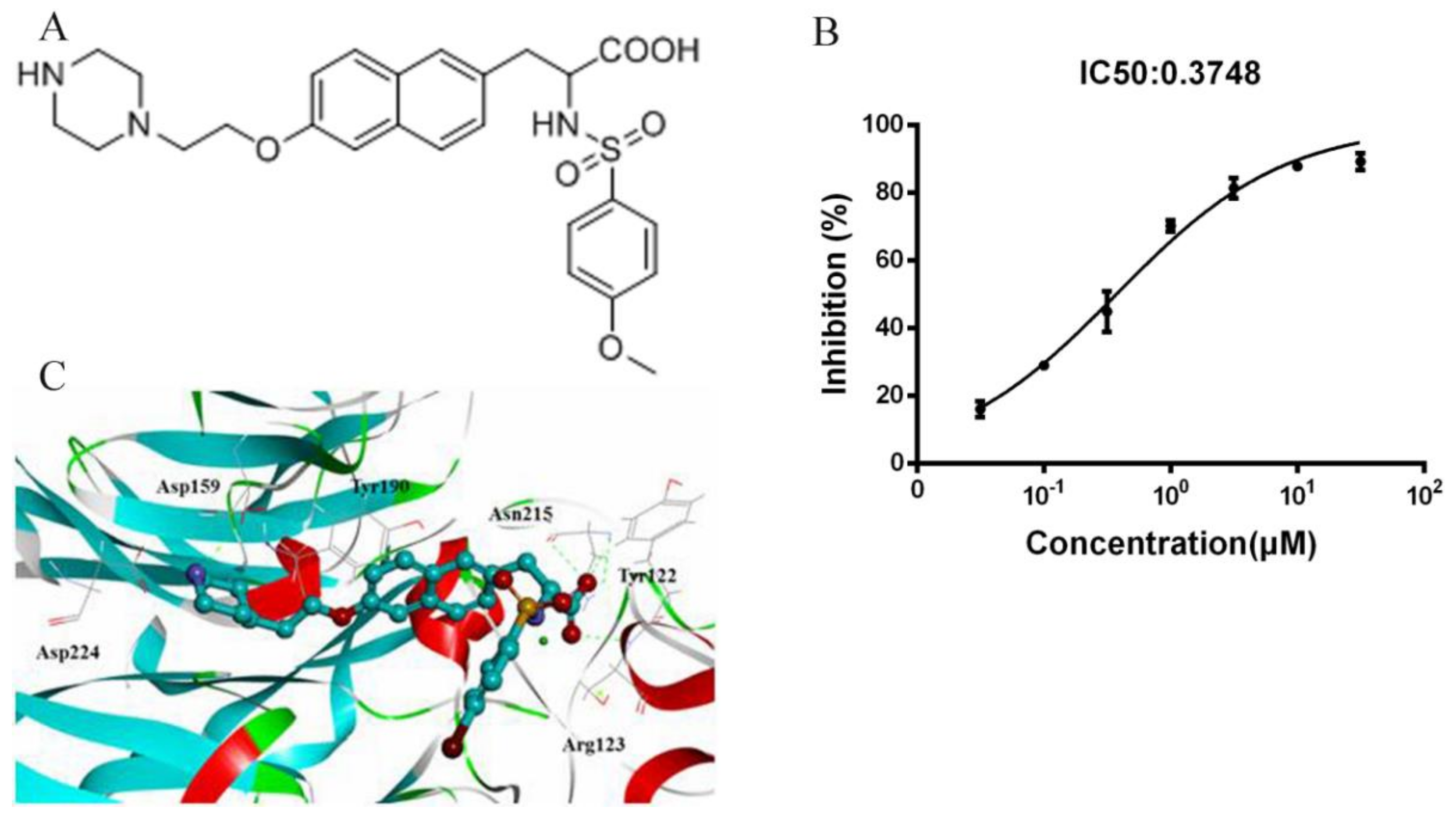
2.1. Fibrinogen/Integrin αIIbβ3 ELISA Screening Assay
2.2. Docking of ANTP266 to αIIbβ3
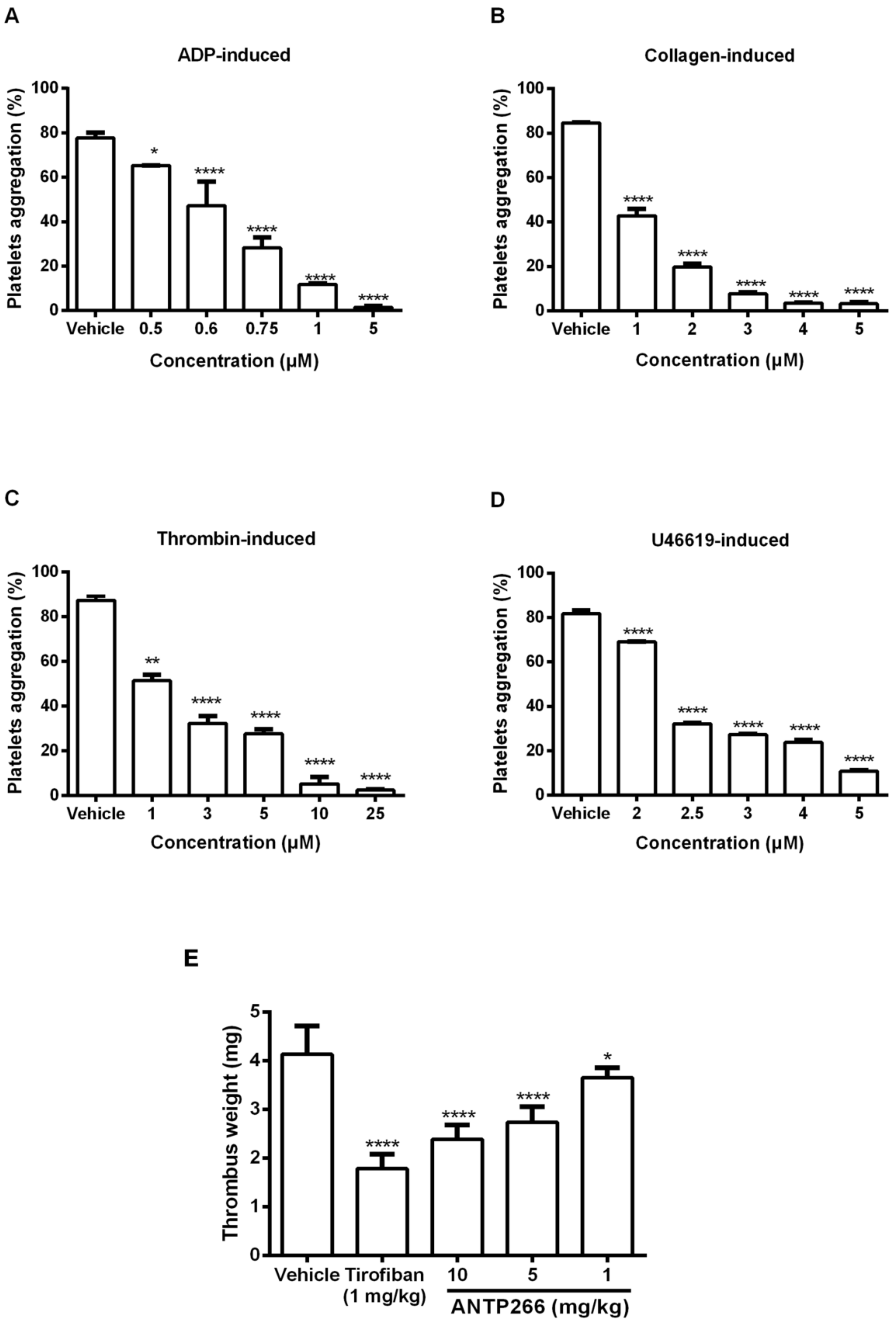
2.3. ANTP266 Inhibited Human Platelet Aggregation In Vitro
2.4. ANTP266 Inhibited Thrombotic Formation In Vivo
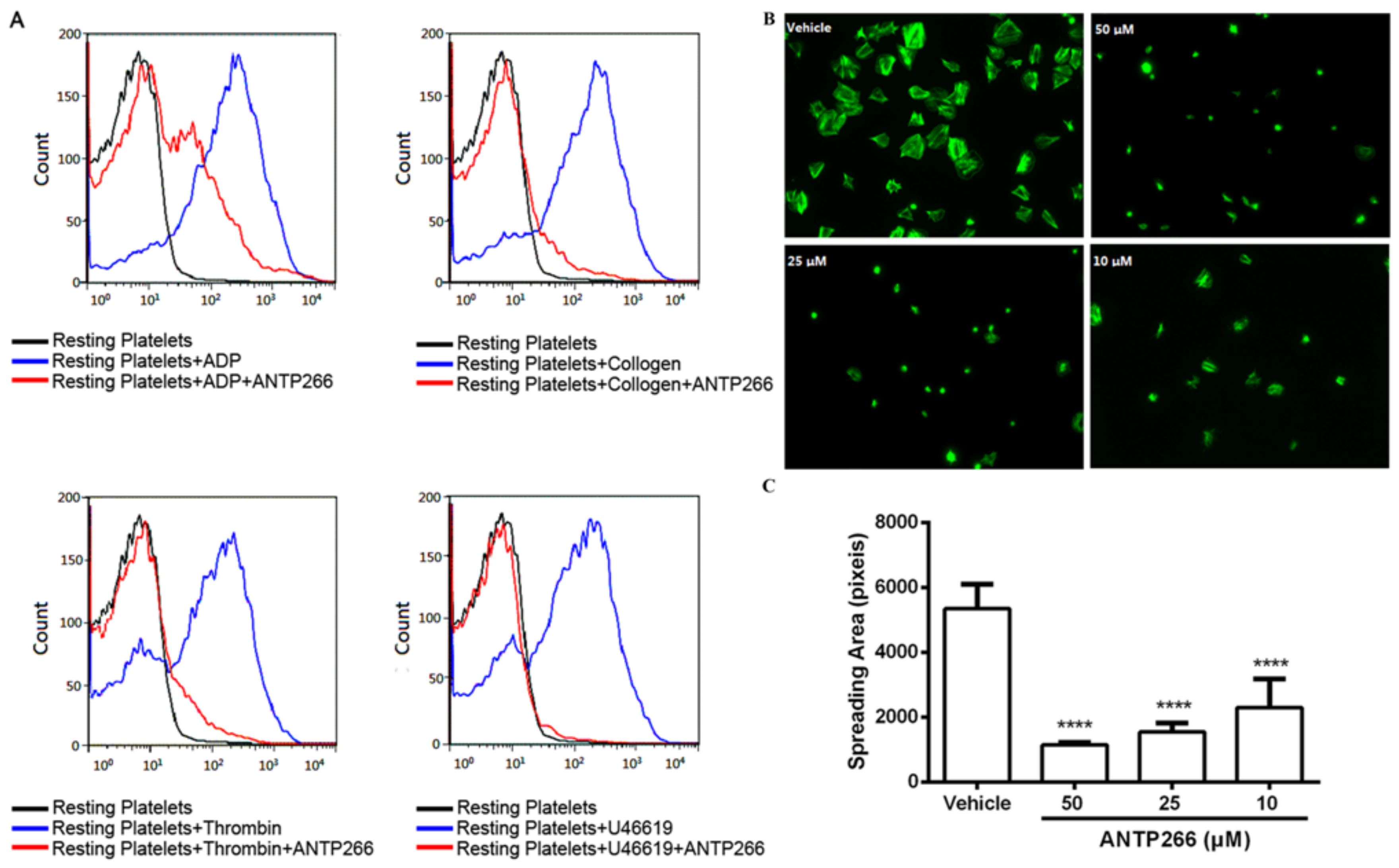
2.5. ANTP266 Inhibited Platelet Activation
2.6. ANTP266 Inhibited Platelet Spreading
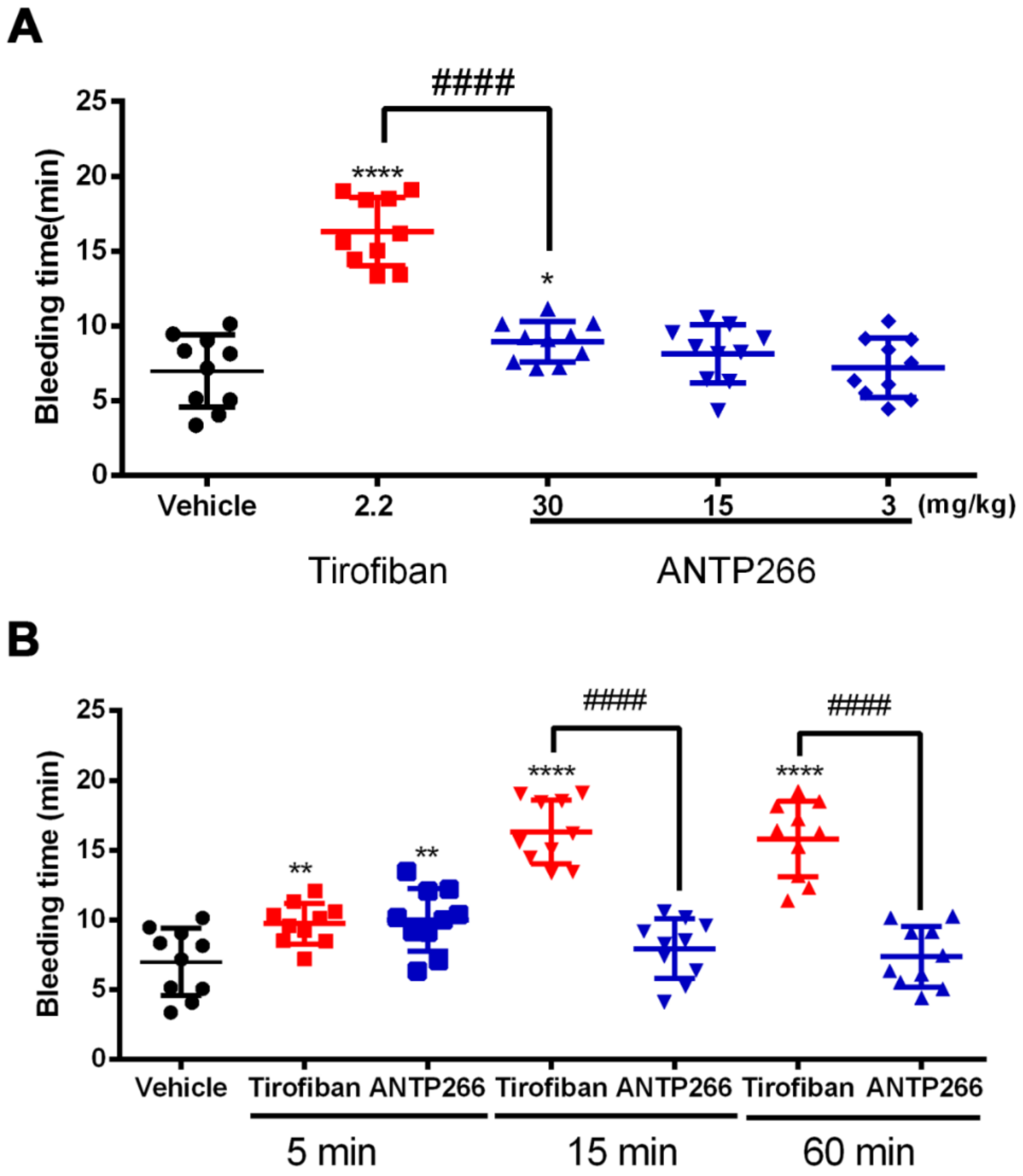
2.7. ANTP266 Exhibited a Low Bleeding Risk with a Short Plasm Half-Life
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animals
4.3. Interaction Assays between ANTP266 and Platelet αIIbβ3 by ELISA
4.4. Docking Studies
4.5. Platelet Aggregation in Aggregometer
4.6. P-Selectin Expression
4.7. Platelet Spreading on Fibrinogen-Coated Surface
4.8. In Vivo Arterio-Venous Shunt Thrombosis
4.9. Tail-Bleeding Assay
4.10. ADP-Induced Acute Pulmonary Thrombosis in Mice
4.11. Detection of ANTP266 in Plasma
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| FDA | Food and Drug Administration |
| CVDs | Cardiovascular diseases |
| ELIS | Enzyme linked immunosorbent assay |
| PGE1 | Prostaglandin E1 |
| HEPES | [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid] |
| PRP | Platelet-rich plasma |
| IgG | Immunoglobulin G |
| BSA | Albumin bovine serum |
| PE | P-phycoerythrin |
| FITC | Fluorescein isothiocyanate |
| HPLC | High performance liquid chromatography |
| ESI | Electrospray ionization |
| SRM | Selected reaction monitoring |
| VWF | Von Willebrand factor |
| PCI | Percutaneous coronary intervention |
References
- Goto, S.; Tomita, A. Antithrombotic Therapy for Prevention of Various Thrombotic Diseases. Drug Dev. Res. 2013, 74, 568–574. [Google Scholar] [CrossRef]
- Bledzka, K.; Smyth, S.S.; Plow, E.F. Integrin αIIbβ3: From discovery to efficacious therapeutic target. Circ. Res. 2013, 112, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Jonathan, M.G. Platelet adhesion signalling and the regulation of thrombus formation. J. Cell Sci. 2004, 117, 3415–3425. [Google Scholar]
- Ding, X.; Liu, T.D.; Xie, Z.L.; Zhao, Q.; Cao, Y.; Liu, X.D.; Wang, C.H.; Gamariel, R.R.; Ming, X.; Li, Z.Y.; et al. A Naphthalenic Derivative ND-1 Inhibits Thrombus Formation by Interfering the Binding of Fibrinogen to Integrin αIIbβ3. BioMed Res. Int. 2016, 2016, 8587164. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.L.; Cao, C.; Feng, S.; Huang, J.; Li, Z.Y. Progress in the research of GPIIb/IIIa antagonists. Future Med. Chem. 2015, 7, 1149–1171. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhu, J.; Negri, A.; Provasi, D.; Filizola, M.; Coller, B.S.; Springer, T.A. Closed headpiece of integrin αIIbβ3 and its complex with an αIIbβ3-specific antagonist that does not induce opening. Blood 2010, 116, 5050–5059. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Lee, J.Y.; Ahn, J.M.; Song, H.; Kim, W.J.; Yun, S.C.; Park, D.W.; Kang, S.J.; Lee, S.W.; Whan Lee, C.; et al. Impact of bleeding on subsequent early and late mortality after drug-eluting stent implantation. JACC Cardiovasc. Interv. 2011, 4, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Wang, Y.; Yang, W.; Xie, Z.; Li, Z. LX0702, a novel snake venom peptide derivative, inhibits thrombus formation via affecting the binding of fibrinogen with GPIIb/IIIa. J. Pharmacol. Sci. 2015, 127, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, P.C.; Peter, K. GPIIb/IIIa inhibitors: From bench to bedside and back to bench again. Thromb. Haemost. 2012, 107, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, H.S.; Ankit, A.P.; George, A.S. Optimal Use of Platelet Glycoprotein IIb/IIIa Receptor Antagonists in Patients Undergoing Percutaneous Coronary Interventions. Drugs 2011, 71, 2009–2030. [Google Scholar]
- Flevaris, P.; Stojanovic, A.; Gong, H.; Chishti, A.; Welch, E.; Du, X. A molecular switch that controls cell spreading and retraction. J. Cell Biol. 2007, 179, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Estevez, B.; Shen, B.; Du, X. Targeting integrin and integrin signaling in treating thrombosis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Delaney, M.K.; O'Kelly, K.A.; Du, X.P. Signaling during platelet adhesion and activation. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2341–2349. [Google Scholar] [CrossRef] [PubMed]
- Shattil, S.J.; Newman, P.J. Integrins: Dynamic scaffolds for adhesion and signaling in platelets. Blood 2004, 104, 1606–1615. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Delaney, M.K.; Du, X. Inside-out, outside-in, and inside-outside-in: G protein signaling in integrin-mediated cell adhesion, spreading, and retraction. Curr. Opin. Cell Biol. 2012, 24, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Qamar, A.; Bhatt, D.L. Current status of data on cangrelor. Pharmacol. Ther. 2016, 159, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Xu, X.; An, S.; Liu, H.; Yang, X.; Andersen, J.F.; Wang, Y.; Tokumasu, F.; Ribeiro, J.M.; Francischetti, I.M.; et al. A novel family of RGD-containing disintegrins (Tablysin-15) from the salivary gland of the horsefly Tabanus yao targets αIIbβ3 and αvβ3 and inhibits platelet aggregation and angiogenesis. Thromb. Haemost. 2011, 105, 1032–1045. [Google Scholar] [PubMed]
- Chen, M.; Ye, X.; Ming, X.; Chen, Y.; Wang, Y.; Su, X.; Su, W.; Kong, Y. A Novel Direct Factor Xa Inhibitory Peptide with Anti-Platelet Aggregation Activity from Agkistrodon acutus Venom Hydrolysates. Sci. Rep. 2015, 5, 10846. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, Y.; Zhou, J.S.; Syed, S.A.; Bulent, M.; Natalio, G.; Gunter, H.; Liu, J.L.; David, W.E. Platelet-derived ERp57 mediates platelet incorporation into a growing thrombus by regulation of the αIIbβ3 integrin. Blood 2013, 122, 3642–3650. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Y.; Hsieh, P.W.; Wu, Y.C.; Wu, C.C. Synthesis and pharmacological evaluation of novel β-nitrostyrene derivatives as tyrosine kinase inhibitors with potent antiplatelet activity. Biochem. Pharmacol. 2007, 74, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Chen, Y.; Wang, C.; Ding, X.; Rwibasira, G.; Kong, Y. Human cathelicidin LL-37 inhibits platelet aggregation and thrombosis via Src/PI3K/Akt signaling. Biochem. Biophys. Res. Commun. 2016, 473, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Shi, P.; Dai, J.; Lu, Y.; Chen, X.; Liu, X.; Zhang, K.; Wu, X.; Sun, Y.; Wang, K.; et al. Paired immunoglobulin-like receptor B regulates platelet activation. Blood 2014, 124, 2421–2430. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, X.; Zhi, H.Y.; Gao, J.; Katarzyna, B.; Tatiana, V.B.; Elzbieta, P.; Gilbert, C.W.; Liu, J.L.; Edward, F.P.; et al. Direct Interaction of Kindlin-3 with Integrin αIIbβ3 in Platelets Is Required for Supporting Arterial Thrombosis in Mice. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1961–1967. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Xu, C.; He, Z.L.; Zhou, Q.M.; Wang, J.B.; Li, Z.Y.; Ming, X. A novel peptide inhibitor of platelet aggregation from stiff silkworm, Bombyx batryticatus. Peptides 2014, 53, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Von Beckerath, N.; Taubert, D.; Pogatsa-Murray, G.; Schomig, E.; Kastrati, A.; Schomig, A. Absorption, metabolization, and antiplatelet effects of 300-, 600-, and 900-mg loading doses of clopidogrel: Results of the ISAR-CHOICE (Intracoronary Stenting and Antithrombotic Regimen: Choose between 3 High Oral Doses for Immediate Clopidogrel Effect) Trial. Circulation 2005, 112, 2946–2950. [Google Scholar] [PubMed]
| Dose (mg/kg) | Number of Deaths/Total | Number of Paralysis/Total | Protection Rate (%) | |
|---|---|---|---|---|
| Vehicle | - | 10/10 | 0/10 | 0 |
| Tirofiban | 2.2 | 1/10 | 1/10 | 80 |
| ANTP266 | 2.2 | 2/10 | 2/10 | 60 |
| 10 | 1/10 | 0/10 | 90 | |
| 5 | 2/10 | 1/10 | 70 | |
| 1 | 1/10 | 4/10 | 50 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.-D.; Ren, S.-H.; Ding, X.; Xie, Z.-L.; Kong, Y. A Short Half-Life αIIbβ3 Antagonist ANTP266 Reduces Thrombus Formation. Int. J. Mol. Sci. 2018, 19, 2306. https://doi.org/10.3390/ijms19082306
Liu T-D, Ren S-H, Ding X, Xie Z-L, Kong Y. A Short Half-Life αIIbβ3 Antagonist ANTP266 Reduces Thrombus Formation. International Journal of Molecular Sciences. 2018; 19(8):2306. https://doi.org/10.3390/ijms19082306
Chicago/Turabian StyleLiu, Tong-Dan, Shen-Hong Ren, Xue Ding, Zhou-Ling Xie, and Yi Kong. 2018. "A Short Half-Life αIIbβ3 Antagonist ANTP266 Reduces Thrombus Formation" International Journal of Molecular Sciences 19, no. 8: 2306. https://doi.org/10.3390/ijms19082306
APA StyleLiu, T.-D., Ren, S.-H., Ding, X., Xie, Z.-L., & Kong, Y. (2018). A Short Half-Life αIIbβ3 Antagonist ANTP266 Reduces Thrombus Formation. International Journal of Molecular Sciences, 19(8), 2306. https://doi.org/10.3390/ijms19082306