Treatment with Growth Hormone (GH) Increased the Metabolic Activity of the Brain in an Elder Patient, Not GH-Deficient, Who Suffered Mild Cognitive Alterations and Had an ApoE 4/3 Genotype
Abstract
1. Introduction
2. Results
2.1. Cognitive Test: TAVEC Test
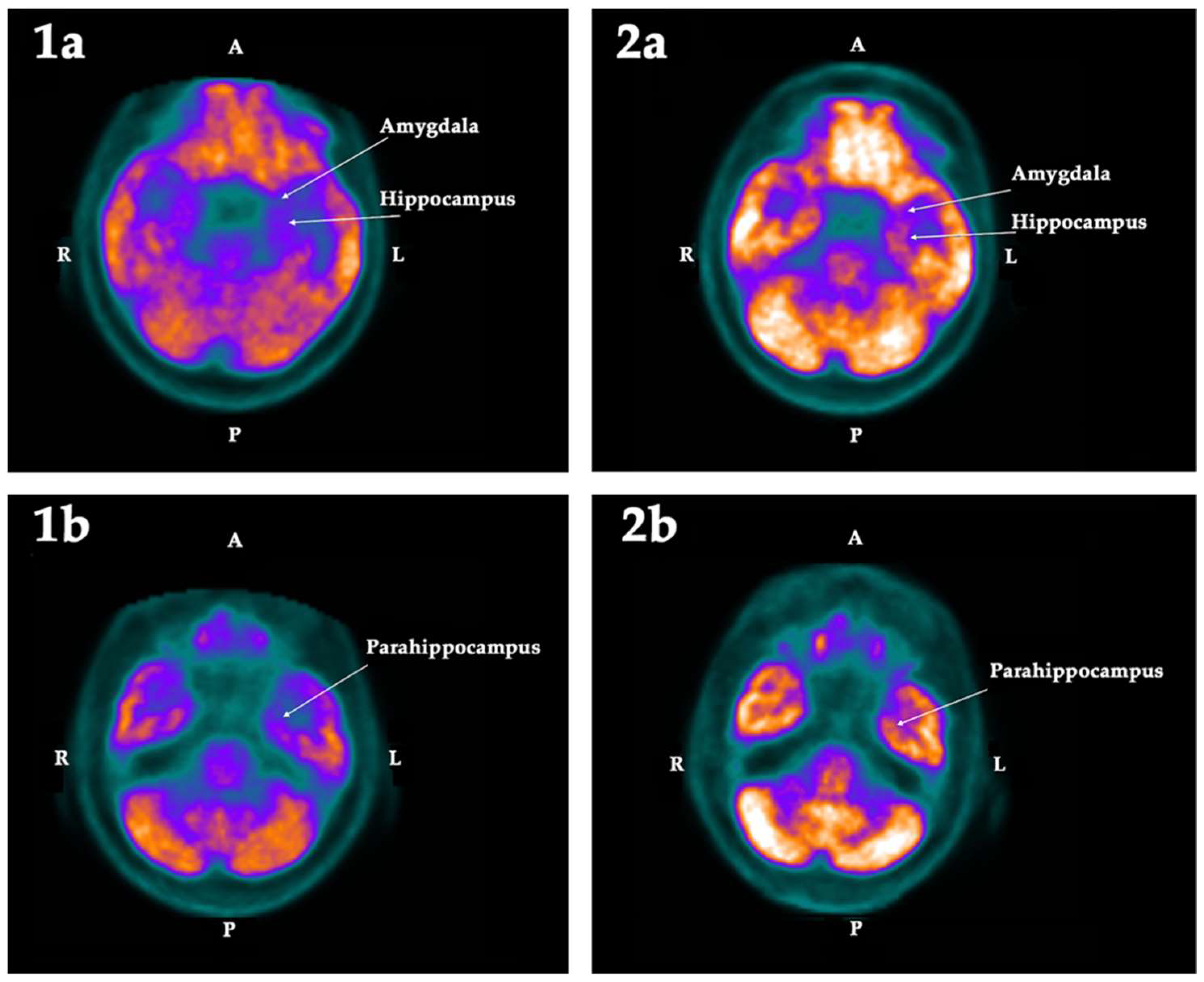
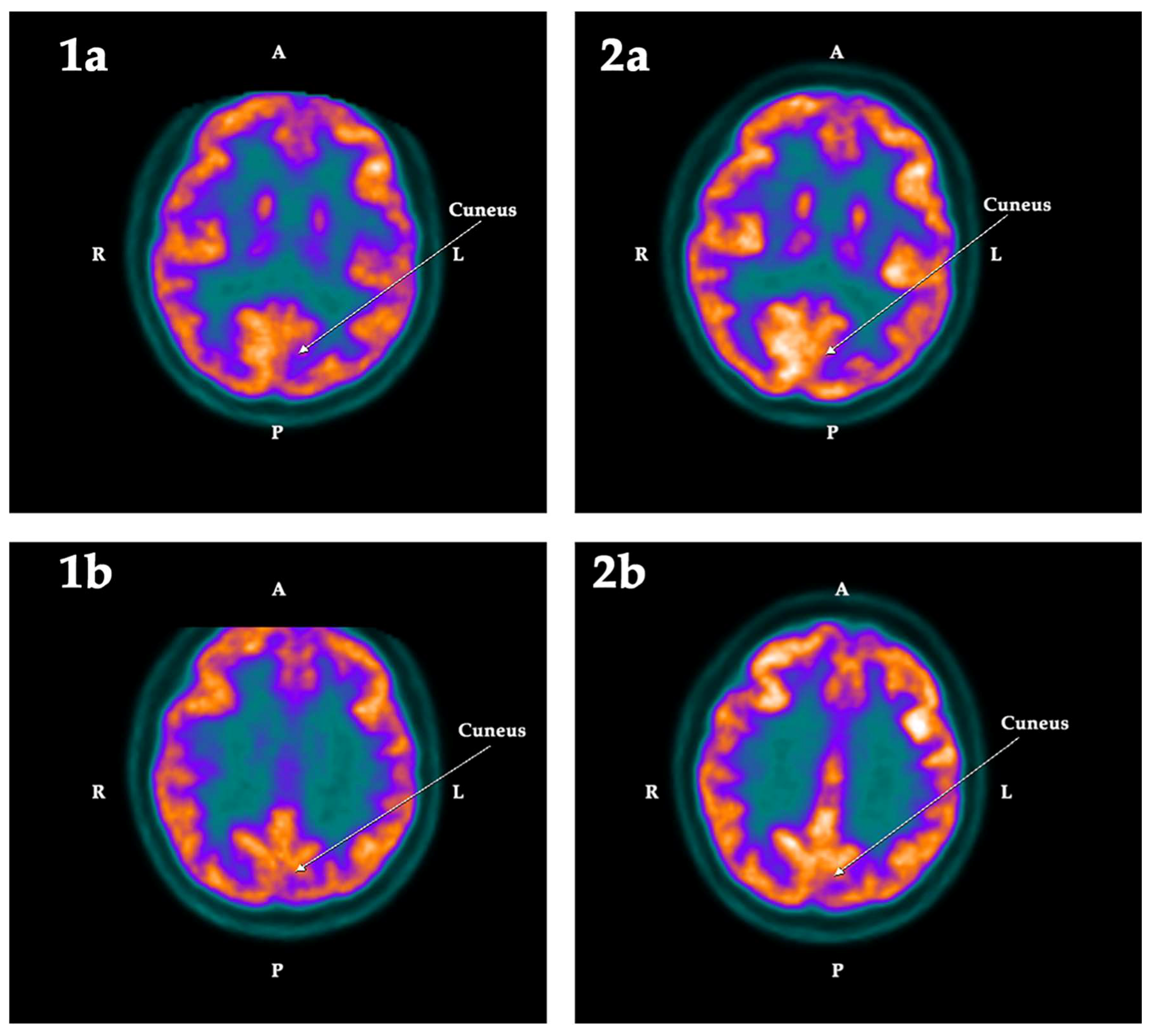
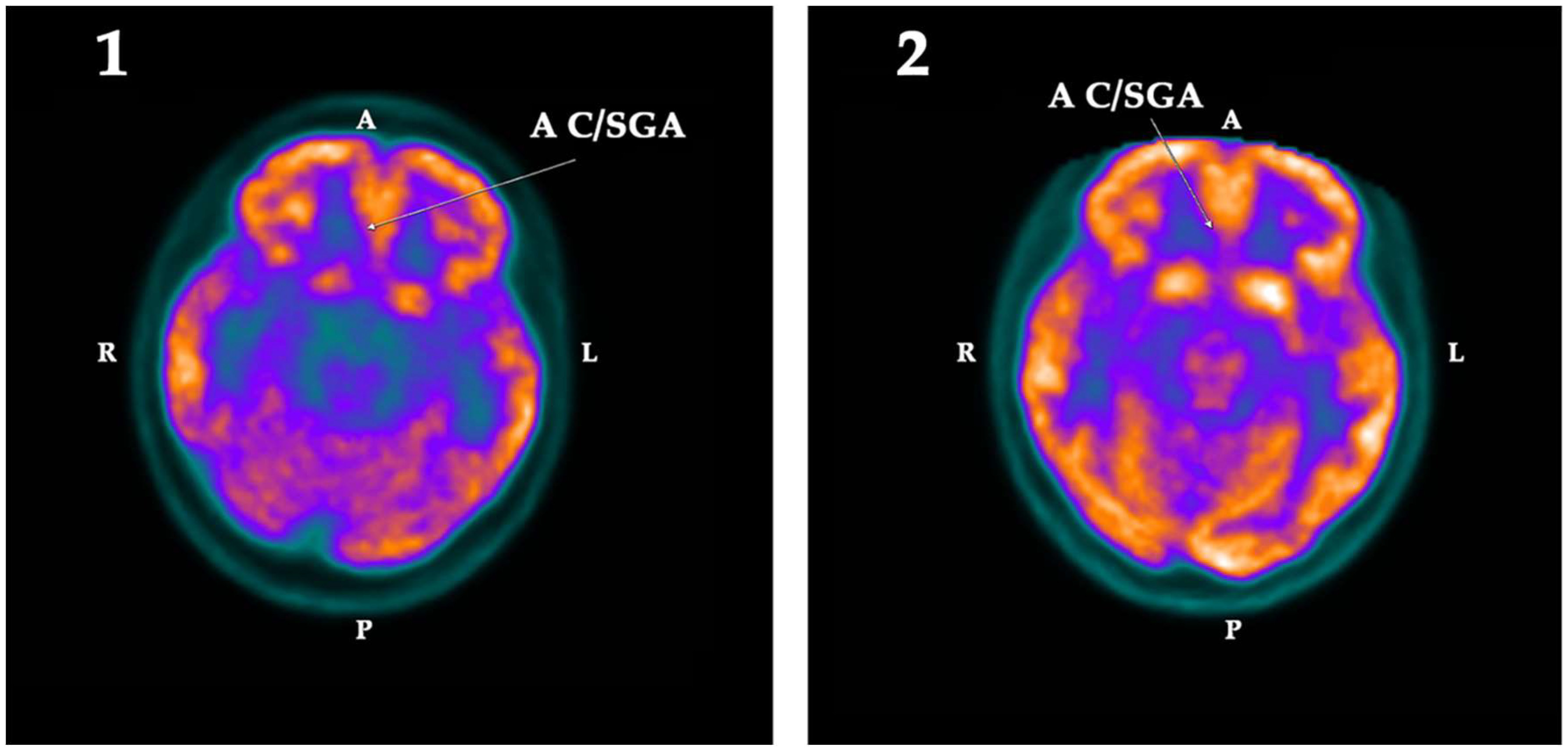
2.2. PET-SCAN Studies
2.3. Blood Analysis
3. Discussion
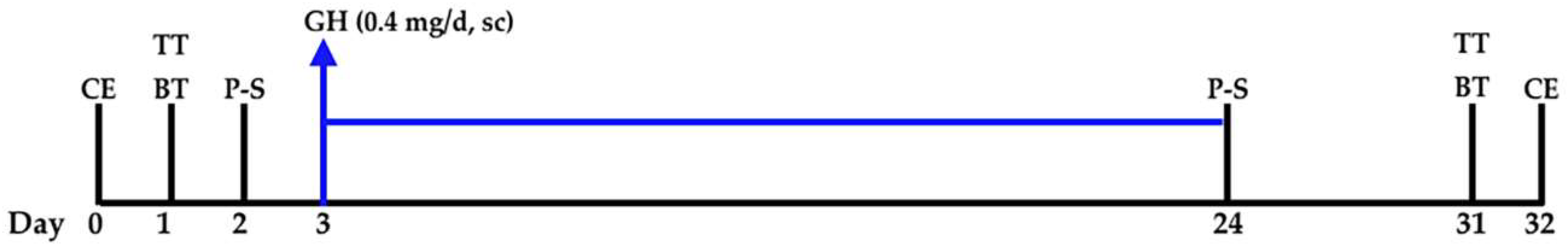
4. Materials and Methods
4.1. Cognitive Tests
4.2. PET-SCANs
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lobie, P.E.; García-Aragón, J.; Lincoln, D.T.; Barnard, R.; Wilcox, J.N.; Waters, M.J. Localization and ontogeny of growth hormone receptor gene expression in the central nervous system. Brain Dev. Brain Res. 1993, 74, 225–233. [Google Scholar] [CrossRef]
- Nyberg, F.; Burman, P. Growth hormone and its receptors in the central nervous system—Location and functional significance. Horm. Res. 1996, 45, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Arámburo, C.; Alba-Betancourt, C.; Luna, M.; Harvey, S. Expression and function of growth hormone in the nervous system: A brief review. Gen. Comp. Endocrinol. 2014, 203, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Devesa, J.; Almengló, C.; Devesa, P. Multiple Effects of Growth Hormone in the Body: Is it Really the Hormone for Growth? Clin. Med. Insights Endocrinol. Diabetes 2016, 9, 47–71. [Google Scholar] [CrossRef] [PubMed]
- Lobie, P.E.; Zhu, T.; Graichen, R.; Goh, E.L. Growth hormone, insulin-like growth factor I and the CNS: Localization, function and mechanism of action. Growth Horm. IGF Res. 2000, 10, S51–S56. [Google Scholar] [CrossRef]
- Webb, E.A.; O’Reilly, M.A.; Clayden, J.D.; Seunarine, K.K.; Chong, W.K.; Dale, N.; Salt, A.; Clark, C.A.; Dattani, M.T. Effect of growth hormone deficiency on brain structure, motor function and cognition. Brain 2012, 135, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Auyeung, B.; Lombardo, M.V.; Baron-Cohen, S. Prenatal and postnatal hormone effects on the human brain and cognition. Pflügers Arch. 2013, 465, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Donahue, C.P.; Jensen, R.V.; Ochiishi, T.; Eisenstein, I.; Zhao, M.; Shors, T.; Kosik, K.S. Transcriptional profiling reveals regulated genes in the hippocampus during memory formation. Hippocampus 2002, 12, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Donahue, C.P.; Kosik, K.S.; Shors, T.J. Growth hormone is produced within the hippocampus where it responds to age, sex, and stress. Proc. Natl. Acad. Sci. USA 2006, 103, 6031–6036. [Google Scholar] [CrossRef] [PubMed]
- David Aberg, N.; Lind, J.; Isgaard, J.; Georg, K.H. Peripheral growth hormone induces cell proliferation in the intact adult rat brain. Growth Horm. IGF Res. 2010, 20, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Devesa, P.; Reimunde, P.; Gallego, R.; Devesa, J.; Arce, V. Growth hormone (GH) treatment may cooperate with locally-produced GH in increasing the proliferative response of hippocampal progenitors to kainate-induced injury. Brain Inj. 2011, 25, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Waters, M.J.; Blackmore, D.G. Growth hormone (GH), brain development and neural stem cells. Pediatr. Endocrinol. Rev. 2011, 9, 549–553. [Google Scholar] [PubMed]
- Deijen, J.B.; de Boer, H.; van der Veen, E.A. Cognitive changes during growth hormone replacement in adult men. Psychoneuroendocrinology 1998, 23, 45–55. [Google Scholar] [CrossRef]
- Jørgensen, J.O.; Vahl, N.; Hansen, T.B.; Thuesen, L.; Hagen, C.; Christiansen, J.S. Growth hormone versus placebo treatment for one year in growth hormone deficient adults: Increase in exercise capacity and normalization of body composition. Clin. Endocrinol. 1996, 45, 681–688. [Google Scholar] [CrossRef]
- Wass, J.A.; Reddy, R. Growth hormone and memory. J. Endocrinol. 2010, 207, 125–126. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, F.; Hallberg, M. Growth hormone and cognitive function. Nat. Rev. Endocrinol. 2013, 9, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, W.E.; Ramsey, M.; Carter, C.S. Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Ageing Res. Rev. 2005, 4, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Aberg, N.D.; Brywe, K.G.; Isgaard, J. Aspects of growth hormone and insulin-like growth factor-I related to neuroprotection, regeneration, and functional plasticity in the adult brain. Sci. World J. 2006, 6, 53–80. [Google Scholar] [CrossRef] [PubMed]
- Pathipati, P.; Gorba, T.; Scheepens, A.; Goffin, V.; Sun, Y.; Fraser, M. Growth hormone and prolactin regulate human neural stem cell regenerative activity. Neuroscience 2011, 190, 409–427. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-C.; Kanekiyo, T.; Xu, H.; Bu, G. Apolipoprotein E and Alzheimer disease: Risk, mechanisms, and therapy. Nat. Rev. Neurol. 2013, 9, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Arwert, L.I.; Veltman, D.J.; Deijen, J.B.; van Dam, P.S. Effects of growth hormone substitution therapy on cognitive functioning in growth hormone deficient patients: A functional MRI study. Neuroendocrinology 2006, 83, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Rhodin, A.; von Ehren, M.; Skottheim, B.; Grönbladh, A.; Ortiz-Nieto, F.; Raininko, R.; Gordh, T.; Nyberg, F. Recombinant growth hormone improves cognitive capacity in a patient exposed to chronic opioids. Acta Anaesthesiol. Scand. 2014, 58, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Park, K.; Lee, H.; Kim, M. The effect of recombinant human growth hormone therapy in patients with completed stroke: A pilot trial. Ann. Rehabil. Med. 2012, 36, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Devesa, J.; Reimunde, P.; Devesa, P.; Barberá, M.; Arce, V. Growth hormone (GH) and brain trauma. Horm. Behav. 2013, 63, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Devesa, J.; Díaz-Getino, G.; Rey, P.; García-Cancela, J.; Loures, I.; Nogueiras, S.; de Mendoza, A.H.; Salgado, L.; González, M.; Pablos, T.; et al. Brain Recovery after a Plane Crash: Treatment with Growth Hormone (GH) and Neurorehabilitation: A Case Report. Int. J. Mol. Sci. 2015, 16, 30470–30482. [Google Scholar] [CrossRef] [PubMed]
- Nylander, E.; Grönbladh, A.; Zelleroth, S.; Diwakarla, S.; Nyberg, F.; Hallberg, M. Growth hormone is protective against acute methadone-induced toxicity by modulating the NMDA receptor complex. Neuroscience 2016, 339, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Devesa, J.; Lema, H.; Zas, E.; Munín, B.; Taboada, P.; Devesa, P. Learning and Memory Recoveries in a Young Girl Treated with Growth Hormone and Neurorehabilitation. J. Clin. Med. 2016, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Quintana, A.; Agra, C.; Outeiral, L.; Devesa, A.; Llorente, D.; Devesa, J. Cognitive Evolution of a Patient Who Suffered a Subarachnoid Haemorrhage Eight Years Ago, after Being Treated with Growth Hormone, Melatonin and Neurorehabilitation. Reports 2018, 1, 2. [Google Scholar] [CrossRef]
- Li, R.C.; Guo, S.Z.; Raccurt, M.; Moudilou, E.; Morel, G.; Brittian, K.R.; Gozal, D. Exogenous growth hormone attenuates cognitive deficits induced by intermittent hypoxia in rats. Neuroscience 2011, 196, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Enhamre-Brolin, E.; Carlsson, A.; Hallberg, M.; Nyberg, F. Growth hormone reverses streptozotocin-induced cognitive impairments in male mice. Behav. Brain Res. 2013, 238, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Srimontri, P.; Hirota, H.; Kanno, H.; Okada, T.; Hirabayashi, Y.; Kato, K. Infusion of growth hormone into the hippocampus induces molecular and behavioral responses in mice. Exp. Brain Res. 2014, 232, 2957–2966. [Google Scholar] [CrossRef] [PubMed]
- Molina, D.P.; Ariwodola, O.J.; Weiner, J.L.; Brunso-Bechtold, J.K.; Adams, M.M. Growth hormone and insulin-like growth factor-I alter hippocampal excitatory synaptic transmission in young and old rats. Age 2013, 35, 1575–1587. [Google Scholar] [CrossRef] [PubMed]
- Ong, L.K.; Chow, W.Z.; TeBay, C.; Kluge, M.; Pietrogrande, G.; Zalewska, K.; Crock, P.; Åberg, N.D.; Bivard, A.; Johnson, S.J.; et al. Growth Hormone Improves Cognitive Function after Experimental Stroke. Stroke 2018, 49, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, P.S.; Perfilieva, E.; Björk-Eriksson, T.; Alborn, A.M.; Nordborg, C.; Peterson, D.A.; Gage, F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998, 4, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Driessen, M.; Herrmann, J.; Stahl, K.; Zwaan, M.; Meier, S.; Hill, A.; Osterheider, M.; Petersen, D. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Arch. Gen. Psychiatry 2000, 57, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Gould, E.; McEwen, B.S.; Tanapat, P.; Galea, L.A.; Fuchs, E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J. Neurosci. 1997, 17, 2492–2498. [Google Scholar] [CrossRef] [PubMed]
- Sorrells, S.F.; Paredes, M.F.; Cebrian-Silla, A.; Sandoval, K.; Qi, D.; Kelley, K.W.; James, D.; Mayer, S.; Chang, J.; Auguste, K.I.; et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 2018, 555, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Thuret, S. Adult Human Hippocampal Neurogenesis: Controversy and Evidence. Trends Mol. Med. 2018, 24, 521–522. [Google Scholar] [CrossRef] [PubMed]
- Kempermann, G.; Gage, F.H.; Aigner, L.; Song, H.; Curtis, M.A.; Thuret, S.; Kuhn, H.G.; Jessberger, S.; Frankland, P.W.; Cameron, H.A.; et al. Human Adult Neurogenesis: Evidence and Remaining Questions. Cell Stem Cell 2018, 23, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Boldrini, M.; Fulmore, C.A.; Tartt, A.N.; Simeon, L.R.; Pavlova, I.; Poposka, V.; Rosoklija, G.B.; Stankov, A.; Arango, V.; Dwork, A.J.; et al. Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell 2018, 22, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Toda, T.; Parylak, S.L.; Linker, S.B.; Gage, F.H. The role of adult hippocampal neurogenesis in brain health and disease. Mol. Psychiatry 2018. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Hernández, J.D.; García-García, F.; Camacho-Abrego, I.; Flores, G.; Juárez-Aguilar, E. Intracerebroventricular administration of growth hormone induces morphological changes in pyramidal neurons of the hippocampus and prefrontal cortex in adult rats. Synapse 2018. Epub ahead of print. [Google Scholar]
- Tamura, Y.; Kataoka, Y. PET imaging of neurogenic activity in the adult brain: Toward in vivo imaging of human neurogenesis. Neurogenesis 2017, 4, e1281861. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Jack, C.R., Jr.; Xu, Y.C.; Waring, S.C.; O’Brien, P.C.; Smith, G.E.; Ivnik, R.J.; Tangalos, E.G.; Boeve, B.F.; Kokmen, E. Memory and MRI-based hippocampal volumes in aging and AD. Neurology 2000, 54, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Son, Y.D.; Noh, Y.; Lee, H.; Kim, Y.B.; Park, K.H. Glucose Hypometabolism in Hippocampal Subdivisions in Alzheimer’s Disease: A Pilot Study Using High-Resolution ¹⁸F-FDG PET and 7.0-T MRI. J. Clin. Neurol. 2018, 14, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Sáez, J.M. Possible usefulness of growth hormone/insulin-like growth factor-I axis in Alzheimer’s disease treatment. Endocr. Metab. Immune Disord. Drug Targets 2012, 12, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, V.E.; Locatelli, V.; Rizzi, L. Neurotrophic and Neuroregenerative Effects of GH/IGF1. Int. J. Mol. Sci. 2017, 18, 2441. [Google Scholar] [CrossRef] [PubMed]
- Vetere, G.; Kenney, J.W.; Tran, L.M.; Xia, F.; Steadman, P.E.; Parkinson, J.; Josselyn, S.A.; Frankland, P.W. Chemogenetic Interrogation of a Brain-wide Fear Memory Network in Mice. Neuron 2017, 94, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, D.J.; Tedoldi, A.; Hunt, S.; Sullivan, R.; Watts, N.R.; Power, J.M.; Bartlett, P.F.; Sah, P. Evidence for newly generated interneurons in the basolateral amygdala of adult mice. Mol. Psychiatry 2018, 23, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Baumann, O.; Mattingley, J.B. Functional Organization of the Parahippocampal Cortex: Dissociable Roles for Context Representations and the Perception of Visual Scenes. J. Neurosci. 2016, 36, 2536–2542. [Google Scholar] [CrossRef] [PubMed]
- Niida, R.; Mimura, R. The Center of Sadness, Pain, and Recognition. Brain Nerve 2017, 69, 417–426. [Google Scholar] [PubMed]
- Joyce, M.K.P.; Barbas, H. Cortical connections position primate Area 25 as a Keystone for Interoception, Emotion, and Memory. J. Neurosci. 2018, 38, 1677–1698. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Cano, E.; Sarró, S.; Monté, G.C.; Maristany, T.; Salvador, R.; McKenna, P.J.; Pomarol-Clotet, E. Evidence for structural and functional abnormality in the subgenual anterior cingulate cortex in major depressive disorder. Psychol. Med. 2014, 44, 3263–3273. [Google Scholar] [CrossRef] [PubMed]
- Diepenbroek, C.; Serlie, M.J.; Fliers, E.; Kalsbeek, A.; la Fleur, S.E. Brain areas and pathways in the regulation of glucose metabolism. Biofactors 2013, 39, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Stender, J.; Kupers, R.; Rodell, A.; Thibaut, A.; Chatelle, C.; Bruno, M.-A.; Gejl, M.; Claire, B.; Laureys, S.; Hustinx, R.; et al. Quantitative rates of brain glucose metabolism distinguish minimally conscious from vegetative state patients. J. Cereb. Blood Flow Metab. 2015, 35, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, W.E.; Bennett, C.; Ingram, R.; Donahue, A.; Ingraham, J.; Chen, H.; Moore, T.; Brunso-Bechtold, J.K.; Riddle, D. Growth hormone and IGF-I modulate local cerebral glucose utilization and ATP levels in a model of adult-onset growth hormone deficiency. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E604–E610. [Google Scholar] [CrossRef] [PubMed]
- Devesa, J.; Lima, L.; Tresguerres, J.A. Neuroendocrine control of growth hormone secretion in humans. Trends Endocrinol. Metab. 1992, 3, 175–183. [Google Scholar] [CrossRef]
- Markowska, A.L.; Mooney, M.; Sonntag, W.E. Insulin-like growth factor-1 ameliorates age-related behavioral deficits. Neuroscience 1998, 87, 559–569. [Google Scholar] [CrossRef]
- Ramsey, M.M.; Weiner, J.L.; Moore, T.P.; Carter, C.S.; Sonntag, W.E. Growth hormone treatment attenuates age-related changes in hippocampal short-term plasticity. Neuroscience 2004, 129, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Deijen, J.B.; Arwert, L.I.; Drent, M.L. The GH/IGF-I Axis and Cognitive Changes across a 4-Year Period in Healthy Adults. ISRN Endocrinol. 2011, 2011, 249421. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, L.; Sorbi, S.; de Leon, M.J.; Li, Y.; Nacmias, B.; Myoung, P.S.; Tsui, W.; Ginestroni, A.; Bessi, V.; Fayyazz, M.; et al. Hypometabolism exceeds atrophy in presymptomatic early-onset familial Alzheimer’s disease. J. Nucl. Med. 2006, 47, 1778–1786. [Google Scholar] [PubMed]
- Liu, Y.; Yu, J.T.; Wang, H.F.; Han, P.R.; Tan, C.C.; Wang, C.; Meng, X.; Risacher, S.L.; Saykin, A.J.; Tan, L. APOE genotype and neuroimaging markers of Alzheimer’s disease: Systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2015, 86, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Keeney, J.T.-R.; Ibrahimi, S.; Zhao, L. Human ApoE isoforms differentially modulate glucose and amyloid metabolic pathways in female brain: Evidence of the mechanism of neuroprotection by ApoE2 and implications for Alzheimer’s prevention and early intervention. J. Alzheimers Dis. 2015, 48, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Fuenzalida, K.; Quintanilla, R.; Ramos, P.; Piderit, D.; Fuentealba, R.A.; Martinez, G.; Inestrosa, N.C.; Bronfman, M. Peroxisome proliferator-activated receptor gamma up-regulates the Bcl-2 anti-apoptotic protein in neurons and induces mitochondrial stabilization and protection against oxidative stress and apoptosis. J. Biol. Chem. 2007, 282, 37006–37015. [Google Scholar] [CrossRef] [PubMed]
- Miglio, G.; Rosa, A.C.; Rattazzi, L.; Collino, M.; Lombardi, G.; Fantozzi, R. PPARgamma stimulation promotes mitochondrial biogenesis and prevents glucose deprivation-induced neuronal cell loss. Neurochem. Int. 2009, 55, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Bevan, P. Insulin signaling. J. Cell Sci. 2001, 114, 1429–1430. [Google Scholar] [PubMed]
- Costoya, J.A.; Finidori, J.; Moutoussamy, S.; Señarís, R.; Devesa, J.; Arce, V.M. Activation of growth hormone receptor delivers an antiapoptotic signal: Evidence for a role of Akt in this pathway. Endocrinology 1999, 140, 5937–5943. [Google Scholar] [CrossRef] [PubMed]
- Devesa, P.; Agasse, F.; Xapelli, S.; Almengló, C.; Devesa, J.; Malva, J.O.; Arce, V.M. Growth hormone pathways signaling for cell proliferation and survival in hippocampal neural precursors from postnatal mice. BMC Neurosci. 2014, 15, 100. [Google Scholar] [CrossRef] [PubMed]
- Caicedo, D.; Díaz, O.; Devesa, P.; Devesa, J. Growth Hormone (GH) and Cardiovascular System. Int. J. Mol. Sci. 2018, 19, 290. [Google Scholar] [CrossRef] [PubMed]
- Keane, J.; Tajouri, L.; Gray, B. The effect of growth hormone administration on the regulation of mitochondrial apoptosis in-vivo. Int. J. Mol. Sci. 2015, 16, 12753–12772. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.D.; Barsness, S.M.; Borson, S.; Merriam, G.R.; Friedman, S.D.; Craft, S.; Vitiello, M.V. Effects of growth hormone–releasing hormone on cognitive function in adults with mild cognitive impairment and healthy older adults: Results of a controlled trial. Arch. Neurol. 2012, 69, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.D.; Baker, L.D.; Borson, S.; Jensen, J.E.; Barsness, S.M.; Craft, S.; Merriam, G.R.; Otto, R.K.; Novotny, E.J.; Vitiello, M.V. Growth hormone-releasing hormone effects on brain γ-aminobutyric acid levels in mild cognitive impairment and healthy aging. JAMA Neurol. 2013, 70, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Grönbladh, A.; Johansson, J.; Nyberg, F.; Hallberg, M. Recombinant human growth hormone affects the density and functionality of GABAB receptors in the male rat brain. Neuroendocrinology 2013, 97, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S. Tesamorelin: A review of its use in the management of HIV-associated lipodystrophy. Drugs 2011, 71, 1071–1091. [Google Scholar] [CrossRef] [PubMed]
- Hammers, A.; Allom, R.; Koepp, M.J.; Free, S.L.; Myers, R.; Lemieux, L.; Mitchell, T.N.; Brooks, D.J.; Duncan, J.S. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum. Brain Mapp. 2003, 19, 224–247. [Google Scholar] [CrossRef] [PubMed]
| ASSAY | ASSAY | Pre | 1 Month |
|---|---|---|---|
| 1. RI-A1 | Immediate recall of the first learning assay | −1 | 0 |
| 2. RI-A5 | Immediate recall of the fifth learning assay | −2 | 0 |
| 3. RI-AT | Total words remembered in the whole of the 5 assays | −2 | 0 |
| 4. RI-B | Immediate recall of the list of interference | −1 | 1 |
| 5. RG-Pr | Percentage of words in the region of primacy, over the total number of words remembered in the total of the 5 tests | −2 | 0 |
| 6. Rg-Rc | Percentage of words from the middle region, about the total number of words remembered in the 5 essays | 0 | 0 |
| 7. Rg-Rc | Percentage of words from the region of recency, on the total number of words remembered in the 5 assays | −1 | 0 |
| 8. RL-CP | Short-term free memory | −1 | 1 |
| 9. RC-CP | Long-term free memory | −1 | 0 |
| 10. RL-LP | Memory with short-term keys | −1 | 0 |
| 11. RC-LP | Memory with long-term keys | −2 | 0 |
| 12. Esem-RI-A | Use of the serial strategy in the immediate recall of list A | −1 | −1 |
| 13. Esem-RI-S | Use of the serial strategy in the immediate recall of list B | −1 | −1 |
| 14. Esem-RL-CP | Use of serial strategy in short-term free recall | −1 | 0 |
| 15. Esem-RL-LP | Use of serial strategy in long-term free recall | −1 | −1 |
| 16. Eser-RI-A | Use of the semantic strategy in the immediate recall of list A | −1 | −1 |
| 17. Eser-RI-B | Use of the semantic strategy in the immediate recall of list B | −1 | 0 |
| 18. Eser-RL-CP | Use of the semantic strategy in short-term free recall | 0 | 0 |
| 19. Eser-RL-LP | Use of the semantic strategy in long-term free recall | 1 | 0 |
| 20. P | Total number of perseverations | 1 | 1 |
| 21. I-RL | Number of intrusions in the whole of free recall tests | 0 | 1 |
| 22. I-RL | Number of intrusions in the whole of memory tests with keys | −1 | 0 |
| 23. Recon-Ac | Number of success in the recognition test | −2 | 1 |
| 24. FP | Number of false positives in the recognition test | 1 | 0 |
| 25. Discriminability | Discrimination index | −2 | 0 |
| 26. Bias | Response bias index | 0 | 0 |
| 27. RI-S versus R1-A1 | Comparison between the memory of list B and the memory of the first learning test in list A | 0 | 1 |
| 28. RL-CP versus RI-A5 | Comparison between short-term free recall and the immediate recall of the fifth learning test in list A | −1 | 0 |
| 29. RC-CP versus RO-LP | Comparison between remembering with short-term keys and remembering with long-term keys | 1 | 0 |
| 30. RL-LP versus RL-CP | Comparison between long-term free memory and short-term free memory | 1 | 0 |
| 31. RC-LP versus RL-LP | Comparison between memory with long-term keys and long-term free recall | 0 | 1 |
| 32. Recon-Ac versus RL-Lp | Comparison between recognition and long-term free recall | −1 | 0 |
| 33. Recon-Ac versus Rcl-LP | Comparison between recognition and recall with long-term keys | −1 | 0 |
| ROI | First PET-SCAN | p < | Second PET-SCAN | ||||||
|---|---|---|---|---|---|---|---|---|---|
| L | R | Asym | Hypom | L | R | Asym | Hypom | ||
| Hippocampus | −12.49 | −6.55 | −5.94 | Left | 0.025 | −6.49 | −2.84 | −3.65 | |
| Amygdala | −13.38 | −7.20 | −6.18 | Left | 0.025 | −9.47 | −1.52 | −7.96 | |
| Parahippocampus | −11.93 | −7.24 | −4.69 | Left | 0.025 | −10.86 | −8.19 | −2.67 | Bilat |
| Cuneus | −5.42 | 4.06 | −9.48 | Left | 0.025 | −0.90 | 6.41 | −7.31 | Left |
| AC/SGA | −2.43 | −11.16 | 8.73 | Right | 0.025 | 3.08 | 0.91 | −2.17 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devesa, J.; Núñez, I.; Agra, C.; Bejarano, A.; Devesa, P. Treatment with Growth Hormone (GH) Increased the Metabolic Activity of the Brain in an Elder Patient, Not GH-Deficient, Who Suffered Mild Cognitive Alterations and Had an ApoE 4/3 Genotype. Int. J. Mol. Sci. 2018, 19, 2294. https://doi.org/10.3390/ijms19082294
Devesa J, Núñez I, Agra C, Bejarano A, Devesa P. Treatment with Growth Hormone (GH) Increased the Metabolic Activity of the Brain in an Elder Patient, Not GH-Deficient, Who Suffered Mild Cognitive Alterations and Had an ApoE 4/3 Genotype. International Journal of Molecular Sciences. 2018; 19(8):2294. https://doi.org/10.3390/ijms19082294
Chicago/Turabian StyleDevesa, Jesús, Iria Núñez, Carlos Agra, Alejandro Bejarano, and Pablo Devesa. 2018. "Treatment with Growth Hormone (GH) Increased the Metabolic Activity of the Brain in an Elder Patient, Not GH-Deficient, Who Suffered Mild Cognitive Alterations and Had an ApoE 4/3 Genotype" International Journal of Molecular Sciences 19, no. 8: 2294. https://doi.org/10.3390/ijms19082294
APA StyleDevesa, J., Núñez, I., Agra, C., Bejarano, A., & Devesa, P. (2018). Treatment with Growth Hormone (GH) Increased the Metabolic Activity of the Brain in an Elder Patient, Not GH-Deficient, Who Suffered Mild Cognitive Alterations and Had an ApoE 4/3 Genotype. International Journal of Molecular Sciences, 19(8), 2294. https://doi.org/10.3390/ijms19082294







