Below versus above Ground Plant Sources of Abscisic Acid (ABA) at the Heart of Tropical Forest Response to Warming
Abstract
1. Introduction
1.1. Global Increase of Atmospheric CO2 and Surface Warming Trends
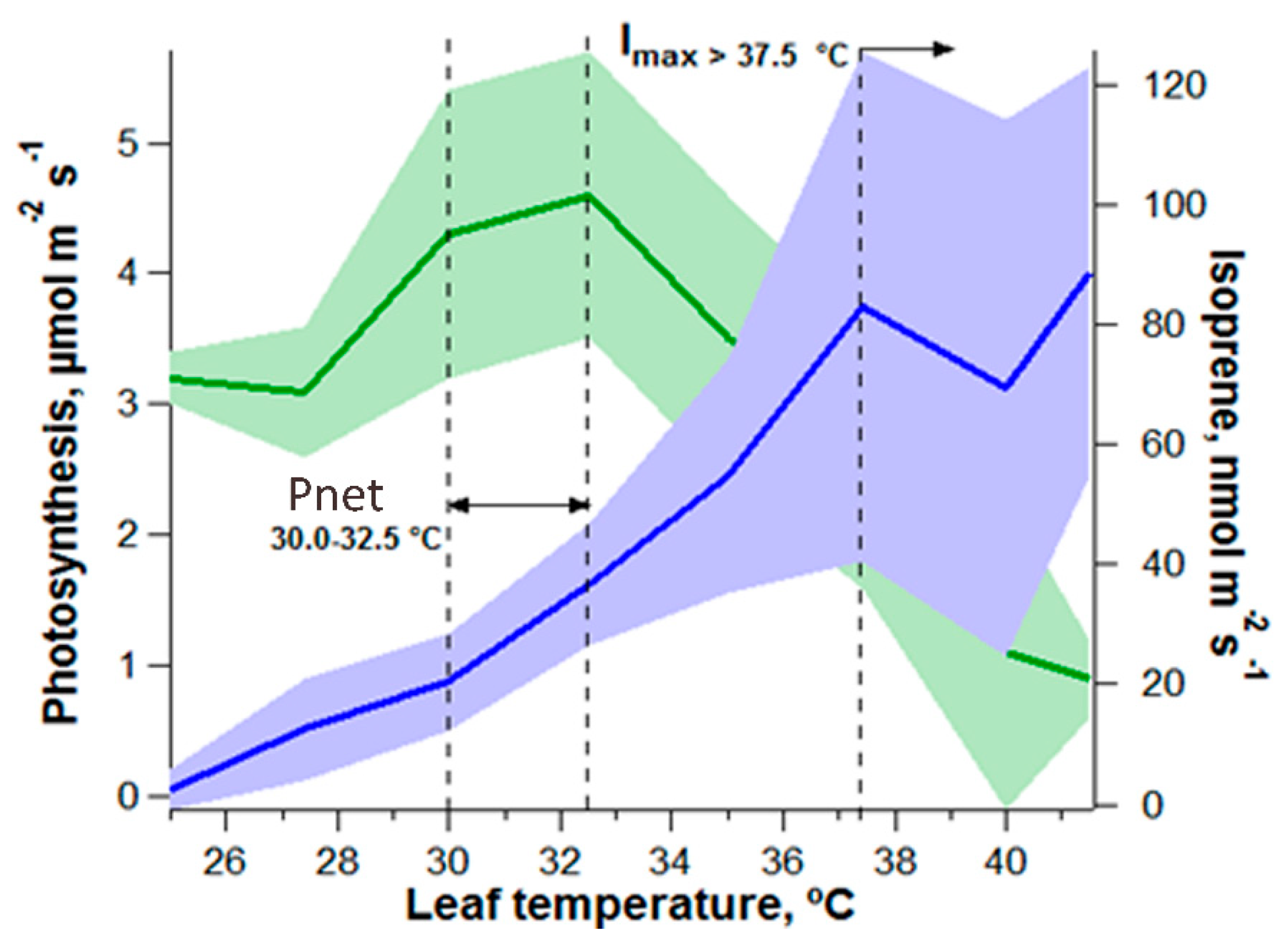
1.2. Tropical Forest CO2 and Water Fluxes during Warming and Drought Conditions
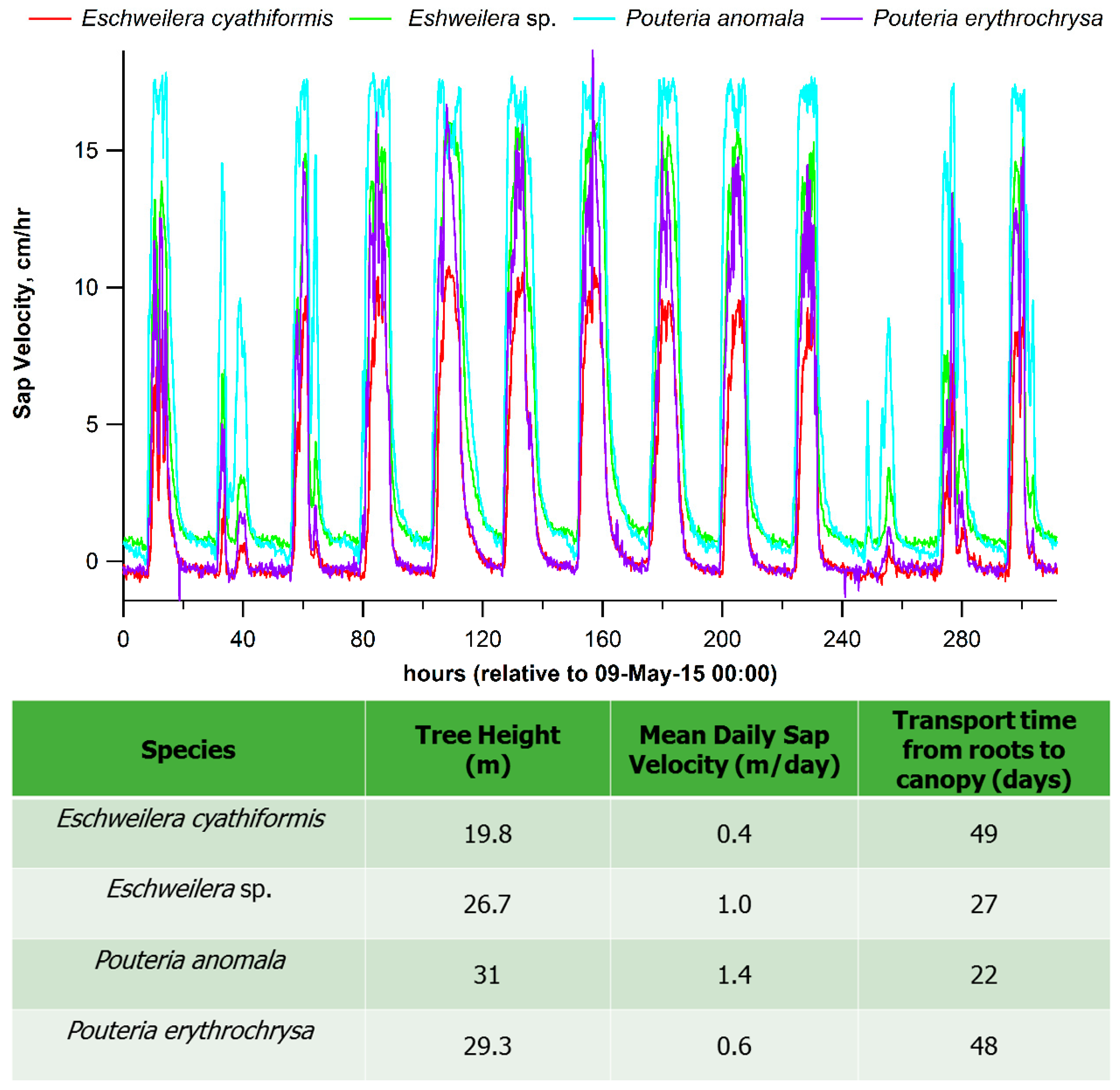
1.3. Plants Hydraulic Strategies in Response to Warming and Water Deficit
1.4. Abscisic Acid (ABA) and Reactive Oxygen Species (ROS) Signaling during Warming and Water Deficit
1.5. Biochemical Mechanisms of Isohydric and Anisohydric Strategies
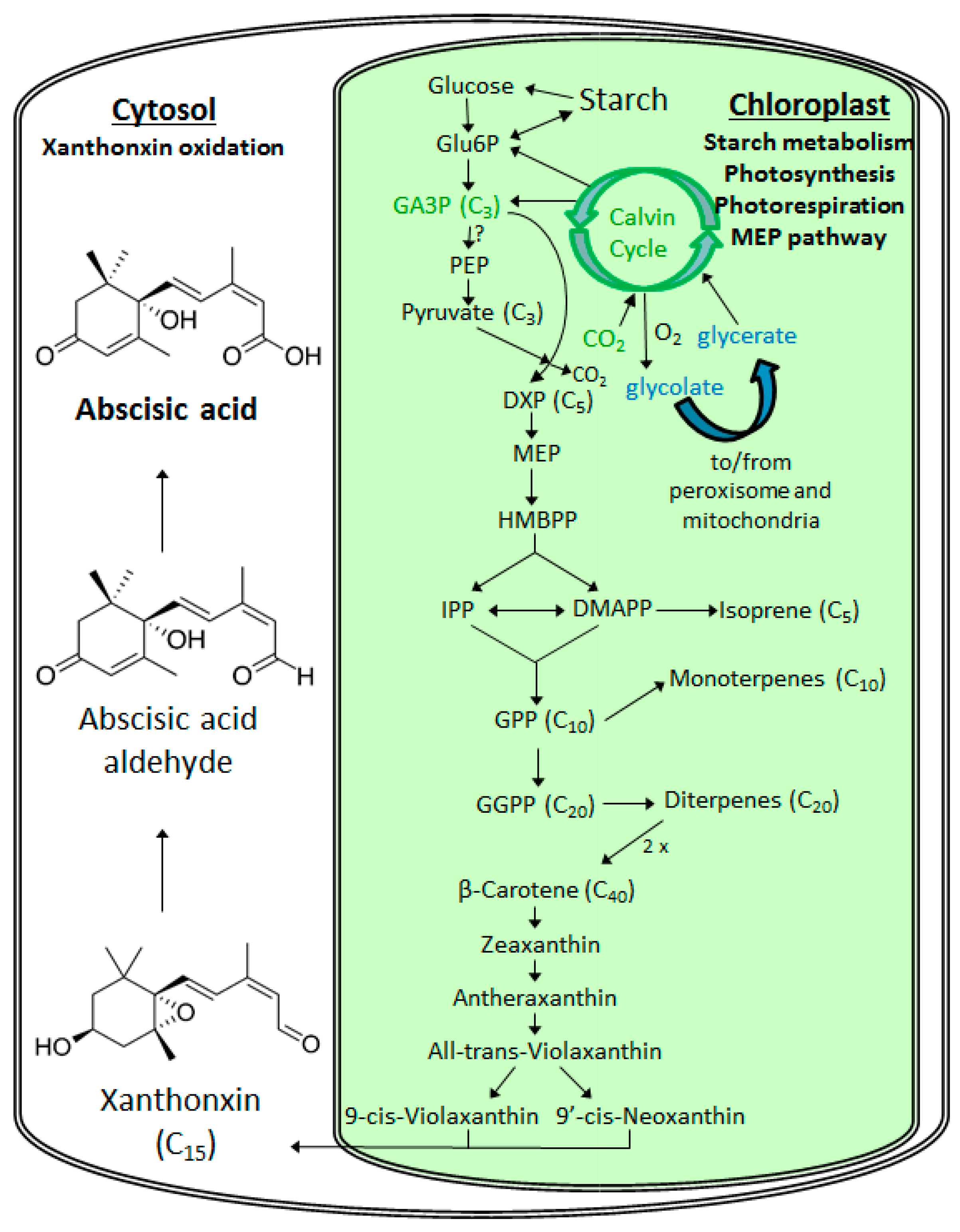
2. Metabolism of Abscisic Acid (ABA)
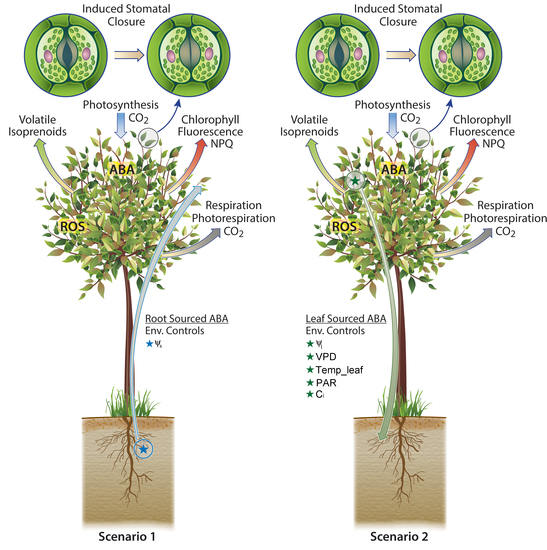
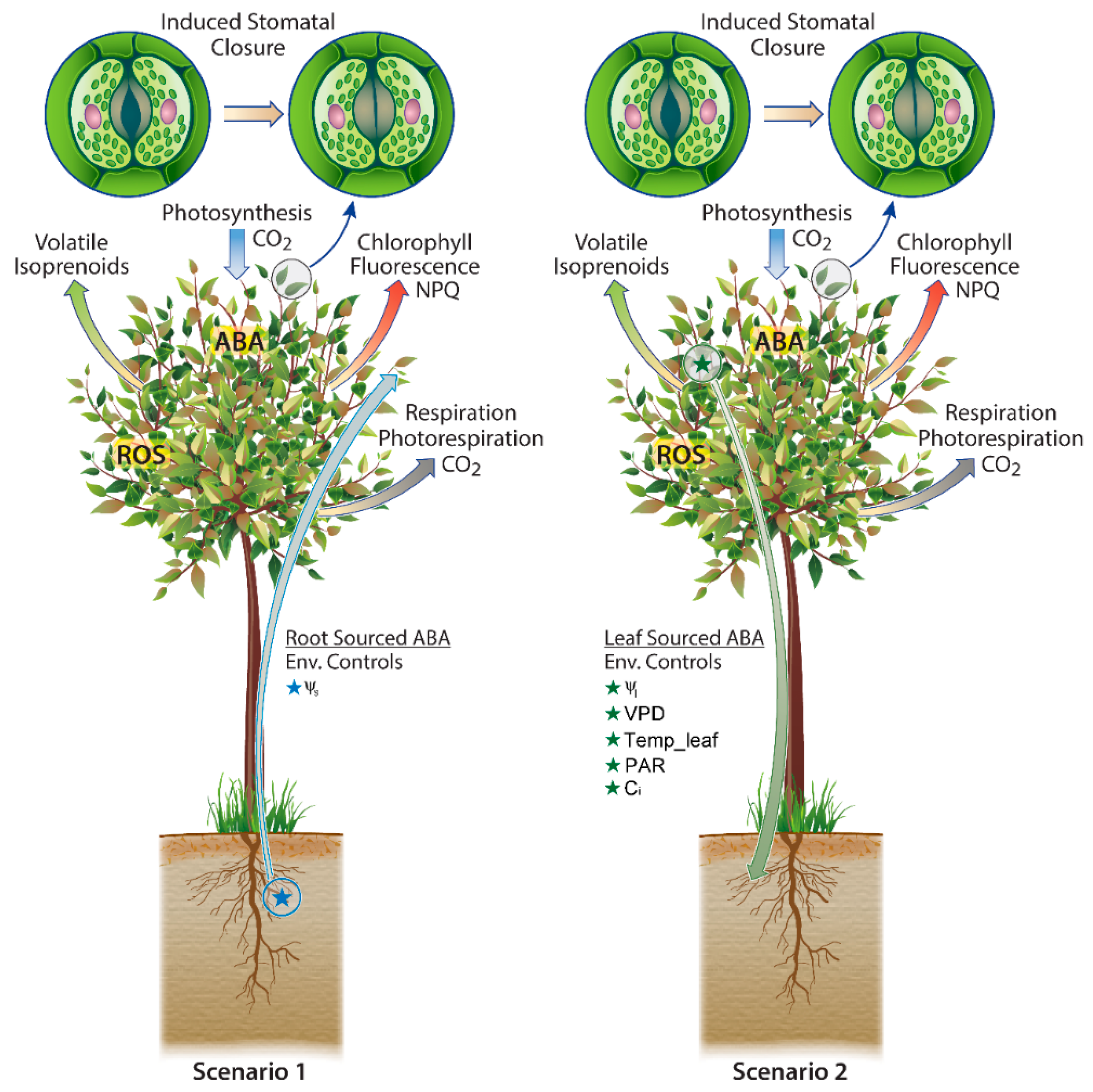
Two Scenarios of ABA Biosynthesis with Distinct Environmental Controls
3. Modeling of Leaf and Canopy Conductance to Water and CO2 in a Changing World
4. Future Experiments and Studies
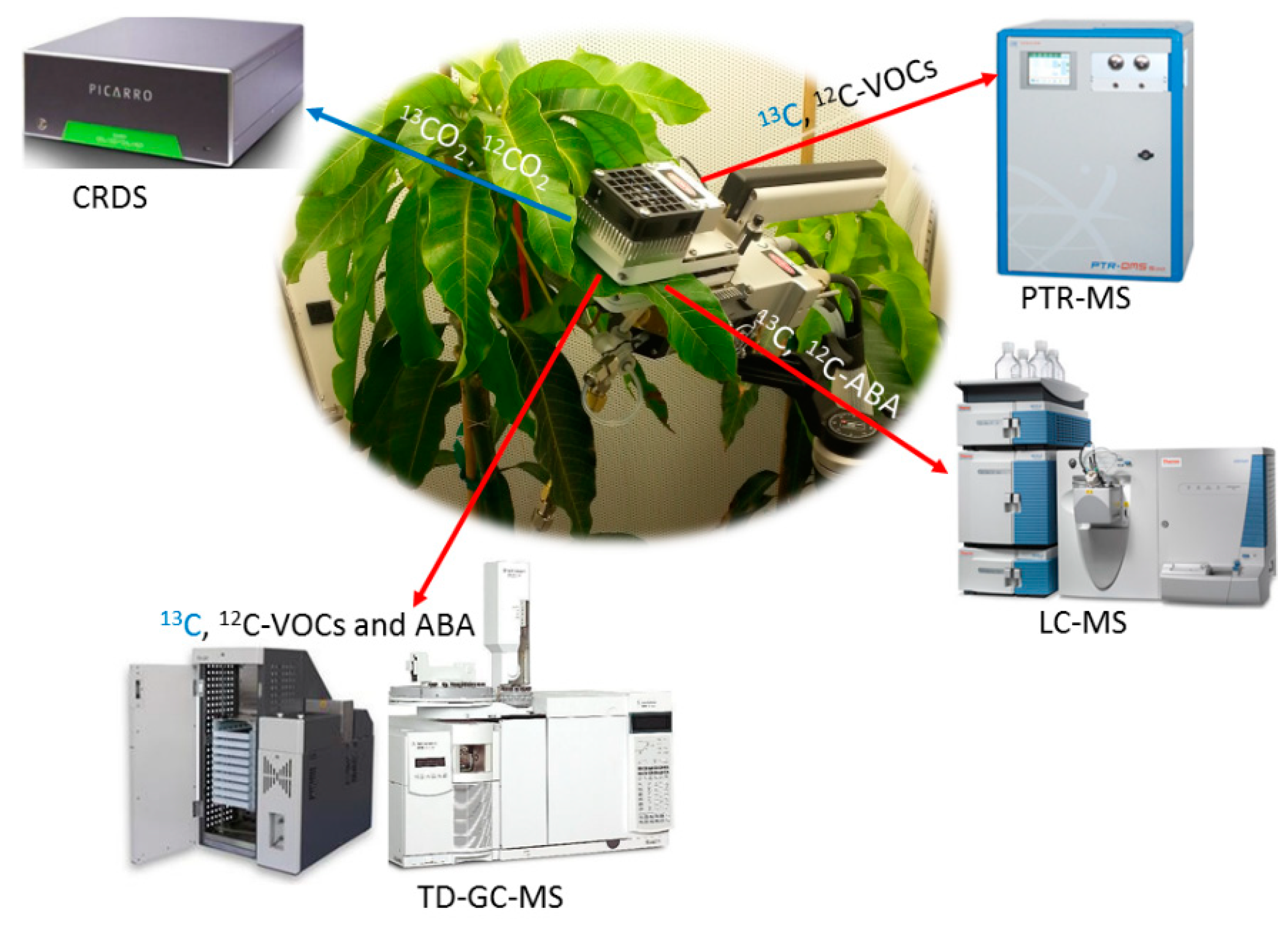
4.1. Demonstration of Recent Photosynthesis in Leaves as a Principal Carbon Source for ABA
4.2. Leaf ABA Biosynthesis as a Function of Leaf Temperature (VPD Constant) and VPD (Leaf Temperature Constant)
4.3. Evaluating the Role of ABA Biosynthesis on Stomatal Control in Distinct Plant Functional Types
4.4. Quantification Tissue Concentration of ABA, ROS, Antioxidant Capacity, Membrane Peroxidation Biomarkers
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Feldman, D.; Collins, W.; Gero, P.; Torn, M.; Mlawer, E.; Shippert, T. Observational determination of surface radiative forcing by CO2 from 2000 to 2010. Nature 2015, 519, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.; Yoon, J.-H.; Marengo, J.A.; Subramaniam, A.; Nobre, C.A.; Mariotti, A.; Neelin, J.D. Causes and impacts of the 2005 Amazon drought. Environ. Res. Lett. 2008, 3, 014002. [Google Scholar] [CrossRef]
- Schimel, D.S. Terrestrial ecosystems and the carbon cycle. Glob. Chang. Biol. 1995, 1, 77–91. [Google Scholar] [CrossRef]
- Beer, C.; Reichstein, M.; Tomelleri, E.; Ciais, P.; Jung, M.; Carvalhais, N.; Roedenbeck, C.; Arain, M.A.; Baldocchi, D.; Bonan, G.B.; et al. Terrestrial gross carbon dioxide uptake: Global distribution and covariation with climate. Science 2010, 329, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Birdsey, R.A.; Phillips, O.L.; Jackson, R.B. The structure, distribution, and biomass of the world’s forests. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 593–622. [Google Scholar] [CrossRef]
- Fernandez-Martinez, M.; Vicca, S.; Janssens, I.A.; Luyssaert, S.; Campioli, M.; Sardans, J.; Estiarte, M.; Penuelas, J. Spatial variability and controls over biomass stocks, carbon fluxes, and resource-use efficiencies across forest ecosystems. Trees-Struct. Funct. 2014, 28, 597–611. [Google Scholar] [CrossRef]
- Boden, T.; Marland, G.; Andres, R.J. Global, Regional, and National Fossil-Fuel CO2 Emissions; Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory (ORNL): Oak Ridge, TN, USA, 2010.
- Le Quere, C.; Moriarty, R.; Andrew, R.M.; Canadell, J.G.; Sitch, S.; Korsbakken, J.I.; Friedlingstein, P.; Peters, G.P.; Andres, R.J.; Boden, T.A.; et al. Global carbon budget 2015. Earth Syst. Sci. Data 2015, 7, 349–396. [Google Scholar] [CrossRef]
- Phillips, O.L.; Aragão, L.E.; Lewis, S.L.; Fisher, J.B.; Lloyd, J.; López-González, G.; Malhi, Y.; Monteagudo, A.; Peacock, J.; Quesada, C.A. Drought sensitivity of the Amazon rainforest. Science 2009, 323, 1344–1347. [Google Scholar] [CrossRef] [PubMed]
- Malhi, Y.; Roberts, J.T.; Betts, R.A.; Killeen, T.J.; Li, W.; Nobre, C.A. Climate change, deforestation, and the fate of the Amazon. Science 2008, 319, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Malhi, Y.; Grace, J. Tropical forests and atmospheric carbon dioxide. Trends Ecol. Evol. 2000, 15, 332–337. [Google Scholar] [CrossRef]
- Laan-Luijkx, I.; Velde, I.; Krol, M.; Gatti, L.; Domingues, L.; Correia, C.; Miller, J.; Gloor, M.; Leeuwen, T.; Kaiser, J. Response of the Amazon carbon balance to the 2010 drought derived with carbontracker South America. Glob. Biogeochem. Cycles 2015, 29, 1092–1108. [Google Scholar] [CrossRef]
- Lewis, S.L.; Brando, P.M.; Phillips, O.L.; van der Heijden, G.M.; Nepstad, D. The 2010 Amazon drought. Science 2011, 331, 554. [Google Scholar] [CrossRef] [PubMed]
- Jardine, K.J.; Jardine, A.B.; Holm, J.A.; Lombardozzi, D.L.; Negron-Juarez, R.I.; Martin, S.T.; Beller, H.R.; Gimenez, B.O.; Higuchi, N.; Chambers, J.Q. Monoterpene ‘thermometer’of tropical forest-atmosphere response to climate warming. Plant Cell Environ. 2017, 40, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Olivares, I.; Svenning, J.-C.; van Bodegom, P.M.; Balslev, H. Effects of warming and drought on the vegetation and plant diversity in the Amazon basin. Bot. Rev. 2015, 81, 42–69. [Google Scholar] [CrossRef]
- Nobre, C.A.; Borma, L.D.S. ‘Tipping points’ for the Amazon forest. Curr. Opin. Environ. Sustain. 2009, 1, 28–36. [Google Scholar] [CrossRef]
- Brestic, M.; Zivcak, M.; Kalaji, H.M.; Carpentier, R.; Allakhverdiev, S.I. Photosystem II thermostability in situ: Environmentally induced acclimation and genotype-specific reactions in Triticum aestivum L. Plant Physiol. Biochem. 2012, 57, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Brestic, M.; Zivcak, M. PSII fluorescence techniques for measurement of drought and high temperature stress signal in crop plants: Protocols and applications. In Molecular Stress Physiology of Plants; Springer: Berlin, Germnay, 2013; pp. 87–131. [Google Scholar]
- Chen, D.; Neumann, K.; Friedel, S.; Kilian, B.; Chen, M.; Altmann, T.; Klukas, C. Dissecting the phenotypic components of crop plant growth and drought responses based on high-throughput image analysis. Plant Cell 2014. [Google Scholar] [CrossRef] [PubMed]
- Overdieck, D.; Ziche, D.; Böttcher-Jungclaus, K. Temperature responses of growth and wood anatomy in European beech saplings grown in different carbon dioxide concentrations. Tree Physiol. 2007, 27, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Jardine, K.J.; Chambers, J.Q.; Holm, J.; Jardine, A.B.; Fontes, C.G.; Zorzanelli, R.F.; Meyers, K.T.; de Souza, V.F.; Garcia, S.; Gimenez, B.O. Green leaf volatile emissions during high temperature and drought stress in a central Amazon rainforest. Plants 2015, 4, 678–690. [Google Scholar] [CrossRef] [PubMed]
- Pšidová, E.; Živčák, M.; Stojnić, S.; Orlović, S.; Gömöry, D.; Kučerová, J.; Ditmarová, Ľ.; Střelcová, K.; Brestič, M.; Kalaji, H.M. Altitude of origin influences the responses of PSII photochemistry to heat waves in European beech (Fagus sylvatica L.). Environ. Exp. Bot. 2018, 152, 97–106. [Google Scholar] [CrossRef]
- Lambers, H.; Chapin, F.S.; Pons, T.L. Plant water relations. In Plant Physiological Ecology; Springer: New York, NY, USA, 2008; pp. 163–223. [Google Scholar]
- Higuchi, N.; Santos, J.D.; Lima, A.J.N.; Higuchi, F.G.; Chambers, J.Q. A floresta Amazônica e a água da chuva. Floresta 2011, 41, 427–434. [Google Scholar] [CrossRef]
- Petrov, P.; Petrova, A.; Dimitrov, I.; Tashev, T.; Olsovska, K.; Brestic, M.; Misheva, S. Relationships between leaf morpho-anatomy, water status and cell membrane stability in leaves of wheat seedlings subjected to severe soil drought. J. Agron. Crop Sci. 2018, 204, 219–227. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Cochard, H. Hydraulic failure defines the recovery and point of death in water-stressed conifers. Plant Physiol. 2009, 149, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Klein, T. The variability of stomatal sensitivity to leaf water potential across tree species indicates a continuum between isohydric and anisohydric behaviours. Funct. Ecol. 2014, 28, 1313–1320. [Google Scholar] [CrossRef]
- Gimenez, B.O.; Jardine, K.J.; Higuchi, N.; Negron-Juarez, R.; Sampaio-Filho, I.; Cobello, L.; Fontes, C.G.; Dawson, T.E.; Varadharajan, C.; Christianson, D.S.; et al. Hysteresis patterns as a proxy for leaf temperature and ecophysiological interactions during the 2015–2016 El Niño event in the Amazon forest. Funct. Ecol. 2018. in review. [Google Scholar]
- Koch, G.W.; Amthor, J.S.; Goulden, M.L. Diurnal patterns of leaf photosynthesis, conductance and water potential at the top of a lowland rain forest canopy in Cameroon: Measurements from the Radeau des Cimes. Tree Physiol. 1994, 14, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.Q.; Silver, W.L. Some aspects of ecophysiological and biogeochemical responses of tropical forests to atmospheric change. Philos. Trans. R. Soc. B Biol. Sci. 2004, 359, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Piedade, M.T.F.; Long, S.; Junk, W.J. Leaf and canopy photosynthetic CO2 uptake of a stand of Echinochloa polystachya on the central Amazon floodplain. Oecologia 1994, 97, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Goulden, M.L.; Miller, S.D.; Da Rocha, H.R.; Menton, M.C.; de Freitas, H.C.; e Silva Figueira, A.M.; de Sousa, C.A.D. Diel and seasonal patterns of tropical forest CO2 exchange. Ecol. Appl. 2004, 14, 42–54. [Google Scholar] [CrossRef]
- Bright, J.; Desikan, R.; Hancock, J.T.; Weir, I.S.; Neill, S.J. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J. 2006, 45, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Downton, W.; Loveys, B.; Grant, W. Stomatal closure fully accounts for the inhibition of photosynthesis by abscisic acid. New Phytol. 1988, 108, 263–266. [Google Scholar] [CrossRef]
- Hose, E.; Steudle, E.; Hartung, W. Abscisic acid and hydraulic conductivity of maize roots: A study using cell-and root-pressure probes. Planta 2000, 211, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Parent, B.; Hachez, C.; Redondo, E.; Simonneau, T.; Chaumont, F.; Tardieu, F. Drought and abscisic acid effects on aquaporin content translate into changes in hydraulic conductivity and leaf growth rate: A trans-scale approach. Plant Physiol. 2009, 149, 2000–2012. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Jiang, M.; Zhang, J.; Tan, M.; Hu, X. Mitogen-activated protein kinase is involved in abscisic acid-induced antioxidant defense and acts downstream of reactive oxygen species production in leaves of maize plants. Plant Physiol. 2006, 141, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Hauser, F.; Li, Z.; Waadt, R.; Schroeder, J.I. Snapshot: Abscisic acid signaling. Cell 2017, 171, 1708. [Google Scholar] [CrossRef] [PubMed]
- Mou, W.; Li, D.; Luo, Z.; Mao, L.; Ying, T. Transcriptomic analysis reveals possible influences of ABA on secondary metabolism of pigments, flavonoids and antioxidants in tomato fruit during ripening. PLoS ONE 2015, 10, e0129598. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, T.; Nakashima, K.; Miyakawa, T.; Kuromori, T.; Tanokura, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Molecular basis of the core regulatory network in ABA responses: Sensing, signaling and transport. Plant Cell Physiol. 2010, 51, 1821–1839. [Google Scholar] [CrossRef] [PubMed]
- Jammes, F.; Song, C.; Shin, D.; Munemasa, S.; Takeda, K.; Gu, D.; Cho, D.; Lee, S.; Giordo, R.; Sritubtim, S. Map kinases MPK9 and MPK12 are preferentially expressed in guard cells and positively regulate ROS-mediated ABA signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 20520–20525. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.M.; Mori, I.C.; Pei, Z.M.; Leonhardt, N.; Torres, M.A.; Dangl, J.L.; Bloom, R.E.; Bodde, S.; Jones, J.D.; Schroeder, J.I. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003, 22, 2623–2633. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, K.E.; Nishimura, N.; Hitomi, K.; Getzoff, E.D.; Schroeder, J.I. Early abscisic acid signal transduction mechanisms: Newly discovered components and newly emerging questions. Genes Dev. 2010, 24, 1695–1708. [Google Scholar] [CrossRef] [PubMed]
- Jardine, A.; Jardine, K.; Fuentes, J.; Martin, S.; Martins, G.; Durgante, F.; Carneiro, V.; Higuchi, N.; Manzi, A.; Chambers, J. Highly reactive light-dependent monoterpenes in the Amazon. Geophys. Res. Lett. 2015, 42, 1576–1583. [Google Scholar] [CrossRef]
- Jardine, K.J.; Jardine, A.B.; Souza, V.F.; Carneiro, V.; Ceron, J.V.; Gimenez, B.O.; Soares, C.P.; Durgante, F.M.; Higuchi, N.; Manzi, A.O.; et al. Methanol and isoprene emissions from the fast growing tropical pioneer species Vismia guianensis (aubl.) pers. (Hypericaceae) in the central Amazon forest. Atmos. Chem. Phys. 2016, 16, 6441–6452. [Google Scholar] [CrossRef]
- Jardine, K.J.; Abrell, L.; Jardine, A.; Huxman, T.; Saleska, S.; Arneth, A.; Monson, R.; Karl, T.; Fares, S.; Loreto, F.; et al. Within-plant isoprene oxidation confirmed by direct emissions of oxidation products methyl vinyl ketone and methacrolein. Glob. Chang. Biol. 2012, 18, 973–984. [Google Scholar] [CrossRef]
- Jardine, K.J.; Meyers, K.; Abrell, L.; Alves, E.G.; Serrano, A.M.; Kesselmeier, J.; Karl, T.; Guenther, A.; Chambers, J.Q.; Vickers, C. Emissions of putative isoprene oxidation products from mango branches under abiotic stress. J. Exp. Bot. 2013, 64, 3697–3709. [Google Scholar] [CrossRef] [PubMed]
- Karl, T.; Harley, P.; Emmons, L.; Thornton, B.; Guenther, A.; Basu, C.; Turnipseed, A.; Jardine, K. Efficient atmospheric cleansing of oxidized organic trace gases by vegetation. Science 2010, 330, 816–819. [Google Scholar] [CrossRef] [PubMed]
- Voss, I.; Sunil, B.; Scheibe, R.; Raghavendra, A. Emerging concept for the role of photorespiration as an important part of abiotic stress response. Plant Biol. 2013, 15, 713–722. [Google Scholar] [CrossRef] [PubMed]
- McAdam, S.A.; Brodribb, T.J. Linking turgor with ABA biosynthesis: Implications for stomatal responses to vapour pressure deficit across land plants. Plant Physiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.Q.; Robertson, A.L.; Carneiro, V.M.; Lima, A.J.; Smith, M.-L.; Plourde, L.C.; Higuchi, N. Hyperspectral remote detection of niche partitioning among canopy trees driven by blowdown gap disturbances in the central Amazon. Oecologia 2009, 160, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Uhl, C.; Buschbacher, R.; Serrao, E. Abandoned pastures in eastern Amazonia. I. Patterns of plant succession. J. Ecol. 1988, 76, 663–681. [Google Scholar] [CrossRef]
- Vieira, I.C.G.; de Almeida, A.S.; Davidson, E.A.; Stone, T.A.; de Carvalho, C.J.R.; Guerrero, J.B. Classifying successional forests using Landsat spectral properties and ecological characteristics in eastern Amazonia. Remote Sens. Environ. 2003, 87, 470–481. [Google Scholar] [CrossRef]
- Zalamea, P.-C.; Stevenson, P.R.; Madriñán, S.; Aubert, P.-M.; Heuret, P. Growth pattern and age determination for Cecropia sciadophylla (Urticaceae). Am. J. Bot. 2008, 95, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, R.C.; Ickes, K.; Ganade, G.; Williamson, G.B. Alternative successional pathways in the Amazon basin. J. Ecol. 2001, 89, 528–537. [Google Scholar] [CrossRef]
- McCulloh, K.A.; Johnson, D.M.; Meinzer, F.C.; Voelker, S.L.; Lachenbruch, B.; Domec, J.C. Hydraulic architecture of two species differing in wood density: Opposing strategies in co-occurring tropical pioneer trees. Plant Cell Environ. 2012, 35, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Poorter, L.; McDonald, I.; Alarcón, A.; Fichtler, E.; Licona, J.C.; Peña-Claros, M.; Sterck, F.; Villegas, Z.; Sass-Klaassen, U. The importance of wood traits and hydraulic conductance for the performance and life history strategies of 42 rainforest tree species. New Phytol. 2010, 185, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, D.; Särkinen, T.; Alexander, S.; Amorim, A.M.; Bittrich, V.; Celis, M.; Daly, D.C.; Fiaschi, P.; Funk, V.A.; Giacomin, L.L. Amazon plant diversity revealed by a taxonomically verified species list. Proc. Natl. Acad. Sci. USA 2017, 114, 10695–10700. [Google Scholar] [CrossRef] [PubMed]
- Cosme, L.H.; Schietti, J.; Costa, F.R.; Oliveira, R.S. The importance of hydraulic architecture to the distribution patterns of trees in a central Amazonian forest. New Phytol. 2017, 215, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Nobre, A.D.; Cuartas, L.A.; Hodnett, M.; Rennó, C.D.; Rodrigues, G.; Silveira, A.; Waterloo, M.; Saleska, S. Height above the nearest drainage—A hydrologically relevant new terrain model. J. Hydrol. 2011, 404, 13–29. [Google Scholar] [CrossRef]
- Oliveira, R.S.; Dawson, T.E.; Burgess, S.S.; Nepstad, D.C. Hydraulic redistribution in three Amazonian trees. Oecologia 2005, 145, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Suwa, R.; Sakai, T.; dos Santos, J.; da Silva, R.P.; Kajimoto, T.; Ishizuka, M.; Higuchi, N. Significance of topographic gradient in stem diameter-height allometry for precise biomass estimation of a tropical moist forest in the central Amazon. Jpn. Agric. Res. Q. 2013, 47, 109–114. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Yeh, S.; Wiberley, A.E.; Falbel, T.G.; Gong, D.M.; Fernandez, D.E. Evolution of the isoprene biosynthetic pathway in Kudzu. Plant Physiol. 2005, 137, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Parry, A.D.; Horgan, R. Carotenoids and abscisic acid (ABA) biosynthesis in higher plants. Physiol. Plant. 1991, 82, 320–326. [Google Scholar] [CrossRef]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.-P.; Sussmilch, F.; Nichols, D.S.; Cardoso, A.A.; Brodribb, T.J.; McAdam, S.A. Leaves, not roots or floral tissue, are the main site of rapid, external pressure-induced ABA biosynthesis in Angiosperms. J. Exp. Bot. 2018, 69, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Davies, W.J.; Zhang, J. Root signals and the regulation of growth and development of plants in drying soil. Annu. Rev. Plant Biol. 1991, 42, 55–76. [Google Scholar] [CrossRef]
- Boursiac, Y.; Léran, S.; Corratgé-Faillie, C.; Gojon, A.; Krouk, G.; Lacombe, B. ABA transport and transporters. Trends Plant Sci. 2013, 18, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Jackson, A.C.; Symonds, R.C.; Mulholland, B.J.; Dadswell, A.R.; Blake, P.S.; Burbidge, A.; Taylor, I.B. Ectopic expression of a tomato 9-cis-epoxycarotenoid dioxygenase gene causes over-production of abscisic acid. Plant J. 2000, 23, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Yang, S.H.; Han, K.H. Upregulation of an arabidopsis RING-H2 gene, XERICO, confers drought tolerance through increased abscisic acid biosynthesis. Plant J. 2006, 47, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.; Pereira, J.; Chaves, M.; Rodrigues, M.; Pacheco, C. ABA xylem concentrations determine maximum daily leaf conductance of field-grown Vitis vinifera L. Plants. Plant Cell Environ. 1995, 18, 511–521. [Google Scholar] [CrossRef]
- Tardieu, F.; Katerji, N.; Bethenod, O.; Zhang, J.; Davies, W. Maize stomatal conductance in the field: Its relationship with soil and plant water potentials, mechanical constraints and ABA concentration in the xylem sap. Plant Cell Environ. 1991, 14, 121–126. [Google Scholar] [CrossRef]
- Soar, C.J.; Speirs, J.; Maffei, S.M.; Loveys, B.R. Gradients in stomatal conductance, xylem sap ABA and bulk leaf ABA along canes of vitis vinifera cv. Shiraz: Molecular and physiological studies investigating their source. Funct. Plant Biol. 2004, 31, 659–669. [Google Scholar] [CrossRef]
- McAdam, S.A.; Manzi, M.; Ross, J.J.; Brodribb, T.J.; Gómez-Cadenas, A. Uprooting an abscisic acid paradigm: Shoots are the primary source. Plant Signal. Behav. 2016, 11, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Schurr, U.; Davies, W. Control of stomatal behaviour by abscisic acid which apparently originates in the roots. J. Exp. Bot. 1987, 38, 1174–1181. [Google Scholar] [CrossRef]
- Zhang, J.; Davies, W. Abscisic acid produced in dehydrating roots may enable the plant to measure the water status of the soil. Plant Cell Environ. 1989, 12, 73–81. [Google Scholar] [CrossRef]
- Tardieu, F.; Davies, W. Integration of hydraulic and chemical signalling in the control of stomatal conductance and water status of droughted plants. Plant Cell Environ. 1993, 16, 341–349. [Google Scholar] [CrossRef]
- McAdam, S.A.; Brodribb, T.J.; Ross, J.J. Shoot-derived abscisic acid promotes root growth. Plant Cell Environ. 2016, 39, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Manzi, M.; Lado, J.; Rodrigo, M.J.; Zacarías, L.; Arbona, V.; Gómez-Cadenas, A. Root ABA accumulation in long-term water-stressed plants is sustained by hormone transport from aerial organs. Plant Cell Physiol. 2015, 56, 2457–2466. [Google Scholar] [CrossRef] [PubMed]
- Christmann, A.; Weiler, E.W.; Steudle, E.; Grill, E. A hydraulic signal in root-to-shoot signalling of water shortage. Plant J. 2007, 52, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Schulze, E.-D.; Hall, A. Stomatal responses, water loss and CO2 assimilation rates of plants in contrasting environments. In Physiological Plant Ecology II; Springer: Berlin, Gremany, 1982; pp. 181–230. [Google Scholar]
- Jardine, K.J.; Chambers, J.; Alves, E.G.; Teixeira, A.; Garcia, S.; Holm, J.; Higuchi, N.; Manzi, A.; Abrell, L.; Fuentes, J.D.; et al. Dynamic balancing of isoprene carbon sources reflects photosynthetic and photorespiratory responses to temperature stress. Plant Physiol. 2014, 166, 2051–2064. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, J.; Farquhar, G.D. Effects of rising temperatures and [CO2] on the physiology of tropical forest trees. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 1811–1817. [Google Scholar] [CrossRef] [PubMed]
- Jardine, K.; Abrell, L.; Kurc, S.A.; Huxman, T.; Ortega, J.; Guenther, A. Volatile organic compound emissions from Larrea tridentata (Creosotebush). Atmos. Chem. Phys. 2010, 10, 12191–12206. [Google Scholar] [CrossRef]
- Jardine, K.; Abrell, L.; Yanez Serrano, A.M.; Arneth, A.; Alves, E.; Kesselmeier, J.; Huxman, T.; Saleska, S.; Jardine, A.; Taylor, T.; et al. Ecosystem-scale compensation points of formic and acetic acid in the central Amazon. Biogeosciences 2011, 8, 3709–3720. [Google Scholar] [CrossRef]
- Alves, E.G.; Harley, P.; Gonçalves, J.F.D.C.; Moura, C.E.D.S.; Jardine, K. Effects of light and temperature on isoprene emission at different leaf developmental stages of Eschweilera coriacea in central Amazon. Acta Amazon. 2014, 44, 9–18. [Google Scholar] [CrossRef]
- Tardieu, F.; Tuberosa, R. Dissection and modelling of abiotic stress tolerance in plants. Curr. Opin. Plant Biol. 2010, 13, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Tardieu, F.; Simonneau, T.; Parent, B. Modelling the coordination of the controls of stomatal aperture, transpiration, leaf growth, and abscisic acid: Update and extension of the Tardieu–Davies model. J. Exp. Bot. 2015, 66, 2227–2237. [Google Scholar] [CrossRef] [PubMed]
- Huntingford, C.; Smith, D.M.; Davies, W.J.; Falk, R.; Sitch, S.; Mercado, L.M. Combining the [ABA] and net photosynthesis-based model equations of stomatal conductance. Ecol. Model. 2015, 300, 81–88. [Google Scholar] [CrossRef]
- Sauter, A.; Davies, W.J.; Hartung, W. The long-distance abscisic acid signal in the droughted plant: The fate of the hormone on its way from root to shoot. J. Exp. Bot. 2001, 52, 1991–1997. [Google Scholar] [CrossRef] [PubMed]
- Dewar, R.C. The Ball–Berry–Leuning and Tardieu–Davies stomatal models: Synthesis and extension within a spatially aggregated picture of guard cell function. Plant Cell Environ. 2002, 25, 1383–1398. [Google Scholar] [CrossRef]
- Ball, J.T.; Woodrow, I.E.; Berry, J.A. A model predicting stomatal conductance and its contribution to the control of photosynthesis under different environmental conditions. In Progress in Photosynthesis Research; Springer: Berlin, Germany, 1987; pp. 221–224. [Google Scholar]
- Farquhar, G.; von Caemmerer, S.V.; Berry, J. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Ju, W.; Wang, S.; Yu, G.; Zhou, Y.; Wang, H. Modeling the impact of drought on canopy carbon and water fluxes for a subtropical evergreen coniferous plantation in southern China through parameter optimization using an ensemble Kalman filter. Biogeosciences 2010, 7, 845–857. [Google Scholar] [CrossRef]
- Schmelz, E.A.; Engelberth, J.; Alborn, H.T.; O’donnell, P.; Sammons, M.; Toshima, H.; Tumlinson, J.H. Simultaneous analysis of phytohormones, phytotoxins, and volatile organic compounds in plants. Proc. Natl. Acad. Sci. USA 2003, 100, 10552–10557. [Google Scholar] [CrossRef] [PubMed]
- Perin, E.C.; Crizel, R.L.; Galli, V.; da Silva Messias, R.; Rombaldi, C.V.; Chaves, F.C. Extraction and quantification of abscisic acid and derivatives in Strawberry by lC-MS. Food Anal. Methods 2018, 1–6. [Google Scholar] [CrossRef]
- Souza, F.C.D. Dinâmica de Uma Floresta de Terra Firme na Estação Experimental de Silvicultura Tropical, Manaus, Amazonas. Ph.D. Thesis, Instituto Nacional de Pesquisas da Amazônia, Manaus, Brazil, 2011. [Google Scholar]
- Von Arx, G.; Archer, S.R.; Hughes, M.K. Long-term functional plasticity in plant hydraulic architecture in response to supplemental moisture. Ann. Bot. 2012, 109, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Daie, J.; Wyse, R. Adaptation of the enzyme-linked immunosorbent assay (ELISA) to the quantitative analysis of abscisic acid. Anal. Biochem. 1982, 119, 365–371. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sampaio Filho, I.D.J.; Jardine, K.J.; De Oliveira, R.C.A.; Gimenez, B.O.; Cobello, L.O.; Piva, L.R.d.O.; Candido, L.A.; Higuchi, N.; Chambers, J.Q. Below versus above Ground Plant Sources of Abscisic Acid (ABA) at the Heart of Tropical Forest Response to Warming. Int. J. Mol. Sci. 2018, 19, 2023. https://doi.org/10.3390/ijms19072023
Sampaio Filho IDJ, Jardine KJ, De Oliveira RCA, Gimenez BO, Cobello LO, Piva LRdO, Candido LA, Higuchi N, Chambers JQ. Below versus above Ground Plant Sources of Abscisic Acid (ABA) at the Heart of Tropical Forest Response to Warming. International Journal of Molecular Sciences. 2018; 19(7):2023. https://doi.org/10.3390/ijms19072023
Chicago/Turabian StyleSampaio Filho, Israel De Jesus, Kolby Jeremiah Jardine, Rosilena Conceição Azevedo De Oliveira, Bruno Oliva Gimenez, Leticia Oliveira Cobello, Luani Rosa de Oliveira Piva, Luiz Antonio Candido, Niro Higuchi, and Jeffrey Quintin Chambers. 2018. "Below versus above Ground Plant Sources of Abscisic Acid (ABA) at the Heart of Tropical Forest Response to Warming" International Journal of Molecular Sciences 19, no. 7: 2023. https://doi.org/10.3390/ijms19072023
APA StyleSampaio Filho, I. D. J., Jardine, K. J., De Oliveira, R. C. A., Gimenez, B. O., Cobello, L. O., Piva, L. R. d. O., Candido, L. A., Higuchi, N., & Chambers, J. Q. (2018). Below versus above Ground Plant Sources of Abscisic Acid (ABA) at the Heart of Tropical Forest Response to Warming. International Journal of Molecular Sciences, 19(7), 2023. https://doi.org/10.3390/ijms19072023