The Perturbation of Pulmonary Surfactant by Bacterial Lipopolysaccharide and Its Reversal by Polymyxin B: Function and Structure
Abstract
1. Introduction
2. Results
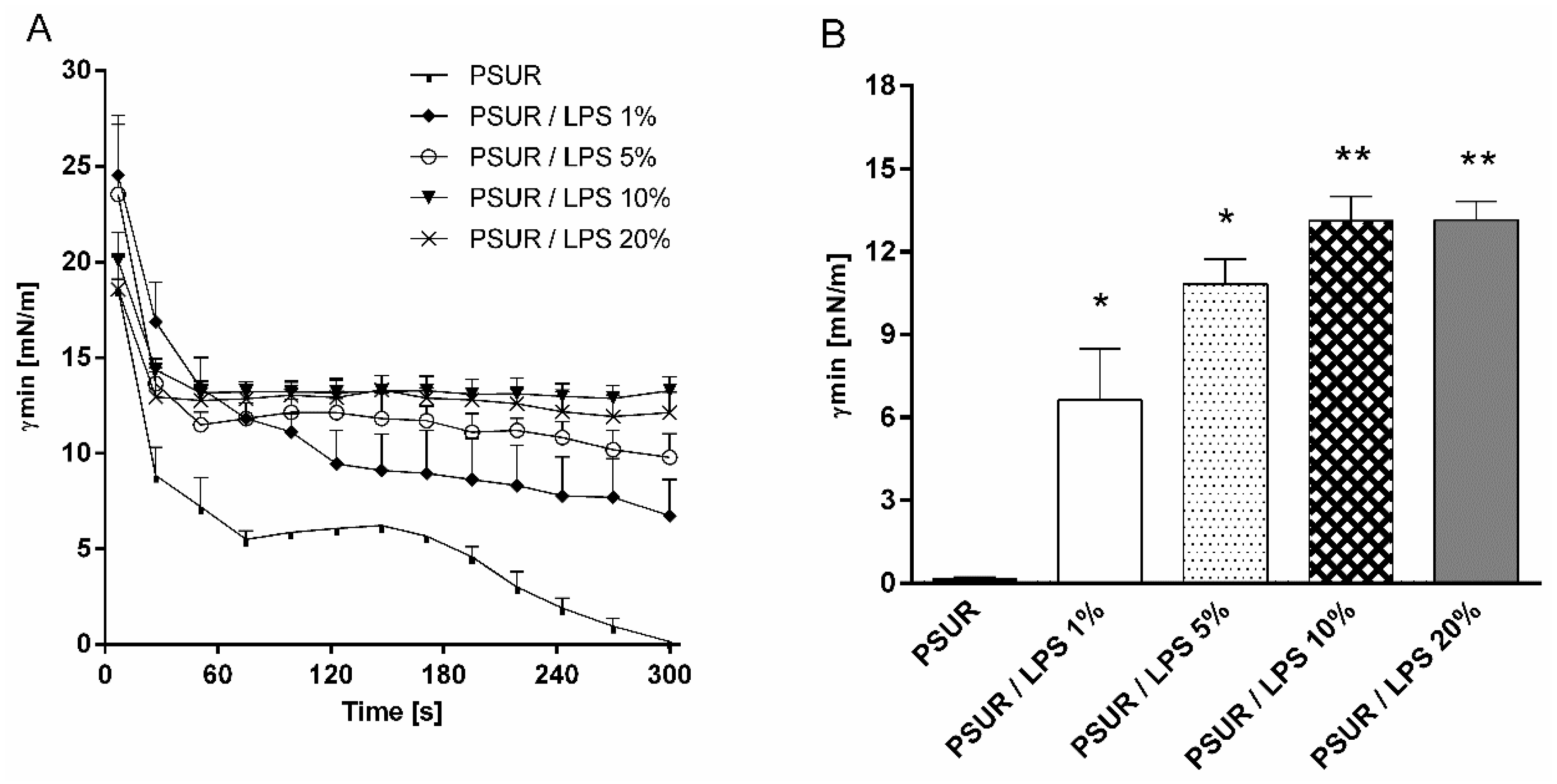
2.1. Surface Activity
2.1.1. Effect of Lipopolysaccharide
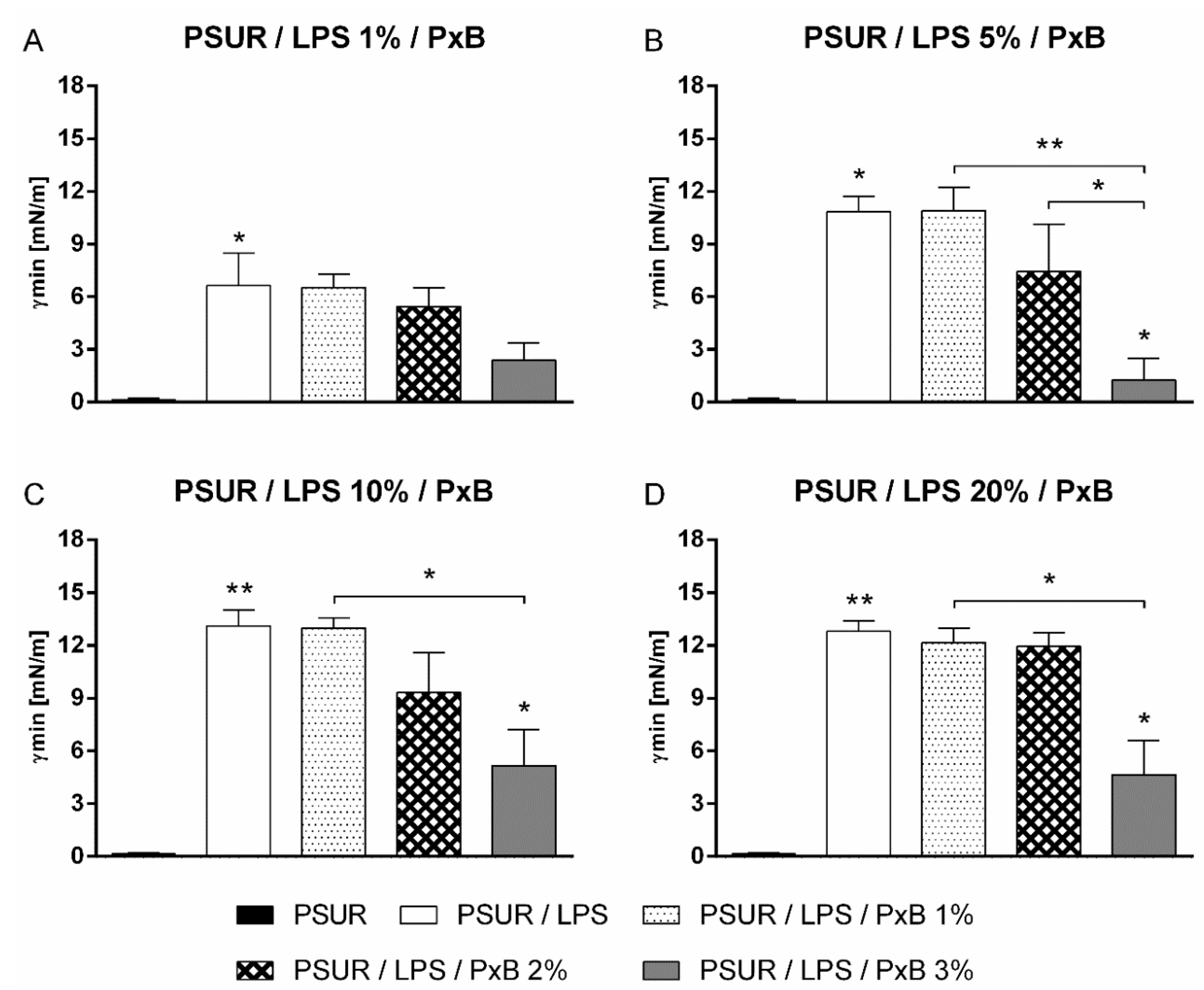
2.1.2. Effect of Polymyxin B
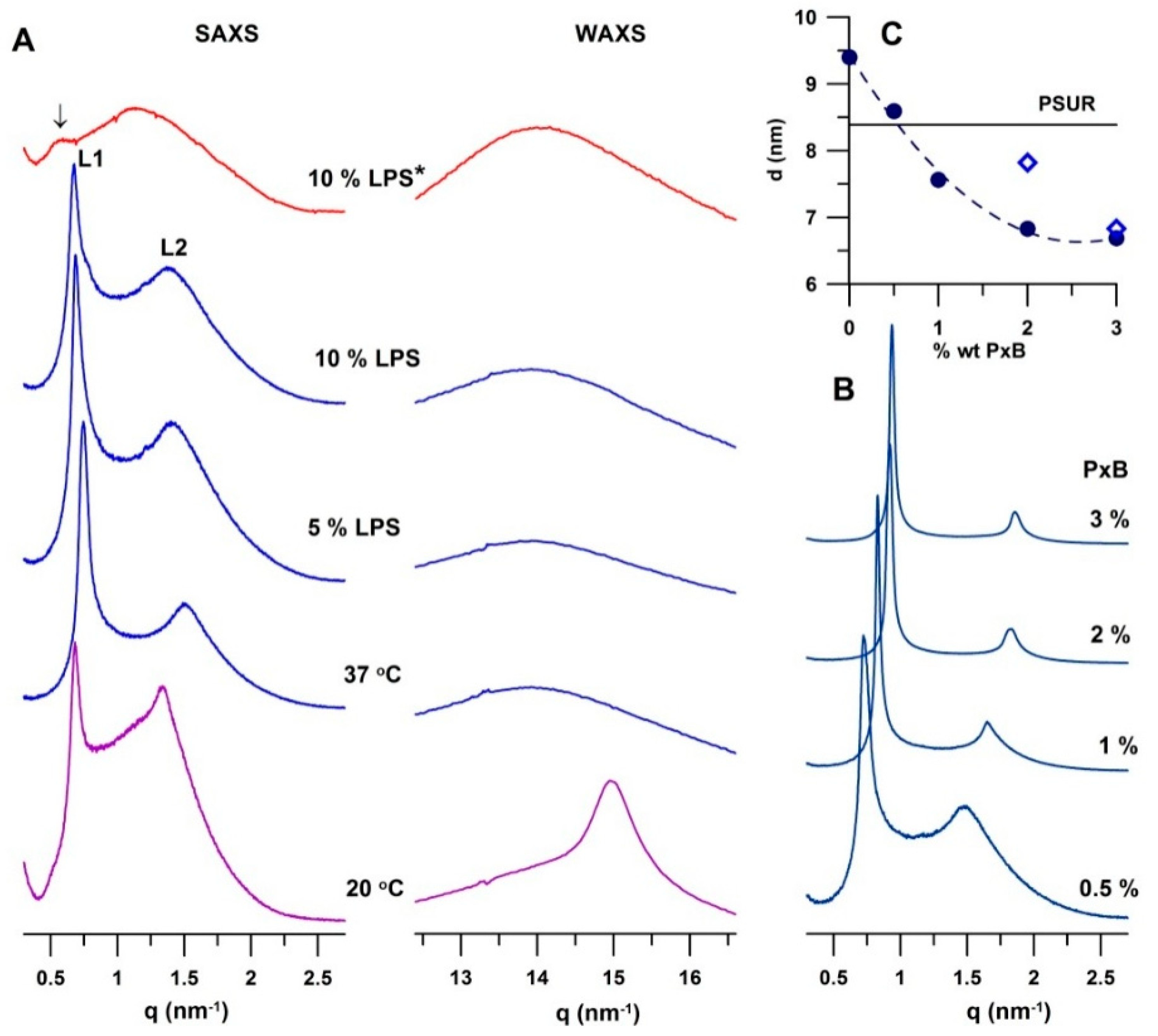
2.2. Structural Study
2.2.1. Modified Porcine Surfactant
2.2.2. Effect of Lipopolysaccharide
2.2.3. Effect of Polymyxin B
3. Discussion
3.1. Effect of Lipopolysaccharide
3.2. Effect of Polymyxin B
4. Materials and Methods
4.1. Chemicals
Mixtures
4.2. Evaluation of Surface Activity
4.3. SAXS and WAXS Experiments
4.4. Optical and Polarised Light Microscopy
4.5. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| LPS | Lipopolysaccharide |
| PxB | Polymyxin B |
| PSUR | Modified porcine pulmonary surfactant |
| SAXS | Small-angle X-ray scattering |
| WAXS | Wide-angle X-ray scattering |
| γmin | Minimum surface tension |
| γmax | Maximum surface tension |
| PL | Phospholipid |
References
- Alexander, C.; Rietschel, E.T. Bacterial lipopolysaccharides and innate immunity. J. Endotoxin Res. 2001, 7, 167–202. [Google Scholar] [CrossRef] [PubMed]
- Parra, E.; Pérez-Gil, J. Composition, structure and mechanical properties define performance of pulmonary surfactant membranes and films. Chem. Phys. Lipids 2015, 185, 153–175. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.Y.; Veldhuizen, R.A.; Neumann, A.W.; Petersen, N.O.; Possmayer, F. Current perspectives in pulmonary surfactant—Inhibition, enhancement and evaluation. Biochim. Biophys. Acta 2008, 1778, 1947–1977. [Google Scholar] [CrossRef] [PubMed]
- Augusto, L.A.; Li, J.; Synguelakis, M.; Johansson, J.; Chaby, R. Structural basis for interactions between lung surfactant protein C and bacterial lipopolysaccharide. J. Biol. Chem. 2002, 277, 23484–23492. [Google Scholar] [CrossRef] [PubMed]
- Augusto, L.A.; Synguelakis, M.; Johansson, J.; Pedron, T.; Girard, R.; Chaby, R. Interaction of pulmonary surfactant protein C with CD14 and lipopolysaccharide. Infect. Immun. 2003, 71, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Vento, G.; Tana, M.; Tirone, C.; Aurilia, C.; Lio, A.; Perelli, S.; Ricci, C.; Romagnoli, C. Effectiveness of treatment with surfactant in premature infants with respiratory failure and pulmonary infection. Acta Biomed. 2012, 83 (Suppl. 1), 33–36. [Google Scholar] [PubMed]
- DeLucca, A.J., 2nd; Brogden, K.A.; Engen, R. Enterobacter agglomerans lipopolysaccharide-induced changes in pulmonary surfactant as a factor in the pathogenesis of byssinosis. J. Clin. Microbiol. 1988, 26, 778–880. [Google Scholar] [PubMed]
- Brogden, K.A. Changes in pulmonary surfactant during bacterial pneumonia. Antonie Van Leeuwenhoek 1991, 59, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Storm, D.R.; Rosenthal, K.S.; Swanson, P.E. Polymyxin and related peptide antibiotics. Annu. Rev. Biochem. 1977, 46, 723–763. [Google Scholar] [CrossRef] [PubMed]
- Zaltash, S.; Palmblad, M.; Curstedt, T.; Johansson, J.; Persson, B. Pulmonary surfactant protein B: A structural model and a functional analogue. Biochim. Biophys. Acta 2000, 1466, 179–186. [Google Scholar] [CrossRef]
- Calkovska, A.; Some, M.; Linderholm, B.; Johansson, J.; Curstedt, T.; Robertson, B. Biophysical and physiological properties of porcine surfactant enriched with polymyxin B. Biol. Neonate 2005, 88, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Stichtenoth, G.; Jung, P.; Walter, G.; Johansson, J.; Robertson, B.; Curstedt, T.; Herting, E. Polymyxin B/pulmonary surfactant mixtures have increased resistance to inactivation by meconium and reduce growth of gram-negative bacteria in vitro. Pediatr. Res. 2006, 59, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Stichtenoth, G.; Haegerstrand-Bjorkman, M.; Walter, G.; Linderholm, B.; Herting, E.; Curstedt, T. Comparison of polymyxin E and polymyxin B as an additive to pulmonary surfactant in Escherichia coli pneumonia of ventilated neonatal rabbits. Biomed. Hub 2017, 2, 475877. [Google Scholar] [CrossRef]
- Cañadas, O.; García-Verdugo, I.; Keough, K.M.; Casals, C. SP-A permeabilizes lipopolysaccharide membranes by forming protein aggregates that extract lipids from the membrane. Biophys. J. 2008, 95, 3287–3294. [Google Scholar] [CrossRef] [PubMed]
- Cañadas, O.; Keough, K.M.; Casals, C. Bacterial lipopolysaccharide promotes destabilization of lung surfactant-like films. Biophys. J. 2011, 100, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Robertson, B.; Curstedt, T.; Johansson, J.; Jornvall, H.; Kobayashi, T. Structure and functional characterization of porcine surfactant isolated by liquid-gel chromatography. Prog. Respir. Res. 1990, 25, 237–246. [Google Scholar]
- Braun, A.; Stenger, P.C.; Warriner, H.E.; Zasadzinski, J.A.; Lu, K.W.; Taeusch, H.W. A freeze-fracture transmission electron microscopy and small angle X-ray diffraction study of the effects of albumin, serum, and polymers on clinical lung surfactant microstructure. Biophys. J. 2007, 93, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gil, J. Structure of pulmonary surfactants membranes and films: The role of proteins and lipid-protein interactions. Biochim. Biophys. Acta 2008, 1778, 1676–1695. [Google Scholar] [CrossRef] [PubMed]
- Bachofen, H.; Gerber, U.; Gehr, P.; Amrein, M.; Schurch, S. Structures of pulmonary surfactant films adsorbed to an air–liquid interface in vitro. Biochim. Biophys. Acta 2005, 1720, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Schürch, S.; Qanbar, R.; Bachofen, H.; Possmayer, F. The surface-associated surfactant reservoir in the alveolar lining. Biol. Neonate 1995, 67 (Suppl. 1), 61–76. [Google Scholar] [CrossRef] [PubMed]
- Ryder, M.P.; Wu, X.; McKelvey, G.R.; McGuire, J.; Schilke, K.F. Binding interactions of bacterial lipopolysaccharide and the cationic amphiphilic peptides polymyxin B and WLBU2. Colloids Surf. B Biointerfaces 2014, 120, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Larsson, M.; Haitsma, J.J.; Lachmann, B.; Larsson, K.; Nylander, T.; Wollmer, P. Enhanced efficacy of porcine lung surfactant extract by utilization of its aqueous swelling dynamics. Clin. Physiol. Funct. Imaging 2002, 22, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Ranck, J.L.; Mateu, L.; Sadler, D.M.; Tardieu, A.; Gulik-Krzywicki, T.; Luzzati, V. Order-disorder conformational transitions of the hydrocarbon chains of lipids. J. Mol. Biol. 1974, 85, 249–277. [Google Scholar] [CrossRef]
- Larsson, M.; Nylander, T.; Keough, K.M.; Nag, K. An X-ray diffraction study of alterations in bovine lung surfactant bilayer structures induced by albumin. Chem. Phys. Lipids 2006, 144, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Herting, E.; Rauprich, P.; Stichtenoth, G.; Walter, G.; Johansson, J.; Robertson, B. Resistance of different surfactant preparations to inactivation by meconium. Pediatr. Res. 2001, 50, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Kolomaznik, M.; Calkovska, A.; Herting, E.; Stichtenoth, G. Biophysical activity of animal-derived exogenous surfactants mixed with rifampicin. Adv. Exp. Med. Biol. 2015, 839, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Shido, A.; Nitta, K.; Inui, S.; Ganzuka, M.; Robertson, B. The critical concentration of surfactant in fetal lung liquid at birth. Respir. Physiol. 1990, 80, 181–192. [Google Scholar] [CrossRef]
- Brogden, K.A.; Cutlip, R.C.; Lehmkuhl, H.D. Complexing of bacterial lipopolysaccharide with lung surfactant. Infect. Immun. 1986, 52, 644–649. [Google Scholar] [PubMed]
- Sun, B.; Curstedt, T.; Lindgren, G.; Franzén, B.; Alaiya, A.A.; Calkovska, A.; Robertson, B. Biophysical and physiological properties of a modified porcine surfactant enriched with surfactant protein A. Eur. Respir. J. 1997, 10, 1967–1974. [Google Scholar] [CrossRef] [PubMed]
- García-Verdugo, I.; Cañadas, O.; Taneva, S.G.; Keough, K.M.; Casals, C. Surfactant protein A forms extensive lattice-like structures on 1,2-dipalmitoylphosphatidylcholine/rough-lipopolysaccharide-mixed monolayers. Biophys. J. 2007, 93, 3529–3540. [Google Scholar] [CrossRef] [PubMed]
- Stumpel, J.; Eibl, H.J.; Nicksch, A. X-ray analysis and calorimetry on phosphatidylcholine model membranes. Biochim. Biophys. Acta 1983, 727, 246–254. [Google Scholar] [CrossRef]
- Bernardino de la Serna, J.; Orädd, G.; Bagatolli, L.A.; Simonsen, A.C.; Marsh, D.; Lindblom, G.; Perez-Gil, J. Segregated phases in pulmonary surfactant membranes do not show coexistence of lipid populations with differentiated dynamic properties. Biophys. J. 2009, 97, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Larsson, M.; Larsson, K.; Nylander, T.; Wollmer, P. The bilayer melting transition in lung surfactant bilayers: The role of cholesterol. Eur. Biophys. J. 2003, 31, 633–636. [Google Scholar] [PubMed]
- Uhríková, D.; Lengyel, A.; Hanulová, M.; Funari, S.S.; Balgavý, P. The structural diversity of DNA-neutral phospholipids-divalent metal cations aggregates: A small-angle synchrotron X-ray diffraction study. Eur. Biophys. J. 2007, 36, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Nagle, J.F.; Tristram-Nagle, S. Structure of lipid bilayers. Biochim. Biophys. Acta 2000, 1469, 159–195. [Google Scholar] [CrossRef]
- Johansson, J.; Jörnvall, H.; Curstedt, T. Human surfactant polypeptide SP-B. Disulfide bridges, C-terminal end, and peptide analysis of the airway form. FEBS Lett. 1992, 301, 165–167. [Google Scholar] [CrossRef]
- Johansson, J.; Szyperski, T.; Curstedt, T.; Wüthrich, K. The NMR structure of the pulmonary surfactant-associated polypeptide SP-C in an apolar solvent contains a valyl-rich alpha-helix. Biochemistry 1994, 33, 6015–6023. [Google Scholar] [CrossRef] [PubMed]
- Mousseau, F.; Le Borgne, R.; Seyrek, E.; Berret, J.F. Biophysicochemical interaction of a clinical pulmonary surfactant with nanoalumina. Langmuir 2015, 31, 7346–7354. [Google Scholar] [CrossRef] [PubMed]
- Uhríková, D.; Kučerka, N.; Teixeira, J.; Gordeliy, V.; Balgavý, P. Structural changes in dipalmitoylphosphatidylcholine bilayer promoted by Ca2+ ions: A small-angle neutron scattering study. Chem. Phys. Lipids 2008, 155, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Schromm, A.B.; Brandenburg, K.; Rietschel, E.T.; Seydel, U. Do endotoxin aggregates intercalate into phospholipid membranes in a nonspecific, hydrophobic manner? J. Endotoxin Res. 1995, 2, 313–323. [Google Scholar] [CrossRef]
- Nomura, K.; Inaba, T.; Morigaki, K.; Brandenburg, K.; Seydel, U.; Kusumoto, S. Interaction of lipopolysaccharide and phospholipid in mixed membranes: Solid-state 31P-NMR spectroscopic and microscopic investigations. Biophys. J. 2008, 95, 1226–1238. [Google Scholar] [CrossRef] [PubMed]
- Seydel, U.; Brandenburg, K.; Koch, M.H.; Rietschel, E.T. Supramolecular structure of lipopolysaccharide and free lipid A under physiological conditions as determined by synchrotron small-angle X-ray diffraction. Eur. J. Biochem. 1989, 186, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, K.; Koch, M.H.J.; Seydel, U. Phase diagram of lipid A from Salmonella minnesota and Escherichia coli rough mutant lipopolysaccharide. J. Struct. Biol. 1990, 105, 11–21. [Google Scholar] [CrossRef]
- Brandenburg, K.; Koch, M.H.J.; Seydel, U. Phase diagram of deep rough mutant lipopolysaccharide from Salmonella minnesota R595. J. Struct. Biol. 1992, 108, 93–106. [Google Scholar] [CrossRef]
- Urbán, E.; Bóta, A.; Kocsis, B. Non-bilayer formation in the DPPE-DPPG vesicle system induced by deep rough mutant of Salmonella minnesota R595 lipopolysaccharide. Colloids Surf. B Biointerfaces 2006, 48, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Enhorning, G. Pulsating bubble technique for evaluating pulmonary surfactant. J. Appl. Physiol. 1977, 43, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.C.; Jacobs, D.M. Binding of polymyxin B to the lipid A porlion of bacterial lipopolysaccharides. Immunochemistry 1976, 13, 813–818. [Google Scholar] [CrossRef]
- Velkov, T.; Thompson, P.E.; Nation, R.L.; Li, J. Structure-activity relationships of polymyxin antibiotics. J. Med. Chem. 2010, 53, 1898–1916. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.E.; Feola, D.J.; Rapp, R.P. Polymyxin B sulfate and colistin: Old antibiotics for emerging multiresistant Gram-negative bacteria. Ann. Pharmacother. 1999, 33, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Kaconis, Y.; Kowalski, I.; Howe, J.; Brauser, A.; Richter, W.; Razquin-Olazarán, I.; Iñigo-Pestaña, M.; Garidel, P.; Rössle, M.; Martinez de Tejada, G.; et al. Biophysical mechanisms of endotoxin neutralization by cationic amphiphilic peptides. Biophys. J. 2011, 100, 2652–2661. [Google Scholar] [CrossRef] [PubMed]
- Uppu, D.S.; Haldar, J. Lipopolysaccharide neutralization by cationic-amphiphilic polymers through pseudoaggregate formation. Biomacromolecules 2016, 17, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Cajal, Y.; Rogers, J.; Berg, O.G.; Jain, M.K. Intermembrane molecular contacts by polymyxin B mediate exchange of phospholipids. Biochemistry 1996, 35, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Shai, Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim. Biophys. Acta 1999, 1462, 55–70. [Google Scholar] [CrossRef]
- Matsuzaki, K. Why and how are peptide-lipid interactions utilized for self-defense? Magainins and tachyplesins as archetypes. Biochim. Biophys. Acta 1999, 1462, 1–10. [Google Scholar] [CrossRef]
- Huang, H.W. Action of antimicrobial peptides: Two-state model. Biochemistry 2000, 39, 8347–8352. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.; Claro, B.; Silva, B.F.B.; Vale, N.; Gomes, P.; Gomes, M.S.; Funari, S.S.; Teixeira, J.; Uhríková, D.; Bastos, M. Unravelling a mechanism of action for a Cecropin A-Melittin hybrid antimicrobial peptide: The induced formation of multilamellar lipid stacks. Langmuir 2018, 34, 2158–2170. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolomaznik, M.; Liskayova, G.; Kanjakova, N.; Hubcik, L.; Uhrikova, D.; Calkovska, A. The Perturbation of Pulmonary Surfactant by Bacterial Lipopolysaccharide and Its Reversal by Polymyxin B: Function and Structure. Int. J. Mol. Sci. 2018, 19, 1964. https://doi.org/10.3390/ijms19071964
Kolomaznik M, Liskayova G, Kanjakova N, Hubcik L, Uhrikova D, Calkovska A. The Perturbation of Pulmonary Surfactant by Bacterial Lipopolysaccharide and Its Reversal by Polymyxin B: Function and Structure. International Journal of Molecular Sciences. 2018; 19(7):1964. https://doi.org/10.3390/ijms19071964
Chicago/Turabian StyleKolomaznik, Maros, Gilda Liskayova, Nina Kanjakova, Lukas Hubcik, Daniela Uhrikova, and Andrea Calkovska. 2018. "The Perturbation of Pulmonary Surfactant by Bacterial Lipopolysaccharide and Its Reversal by Polymyxin B: Function and Structure" International Journal of Molecular Sciences 19, no. 7: 1964. https://doi.org/10.3390/ijms19071964
APA StyleKolomaznik, M., Liskayova, G., Kanjakova, N., Hubcik, L., Uhrikova, D., & Calkovska, A. (2018). The Perturbation of Pulmonary Surfactant by Bacterial Lipopolysaccharide and Its Reversal by Polymyxin B: Function and Structure. International Journal of Molecular Sciences, 19(7), 1964. https://doi.org/10.3390/ijms19071964





