Selenium Improves Physiological Parameters and Alleviates Oxidative Stress in Strawberry Seedlings under Low-Temperature Stress
Abstract
1. Introduction
2. Results and Analysis
2.1. The Photosynthetic Parameters Were Significantly Affected by Se Applications
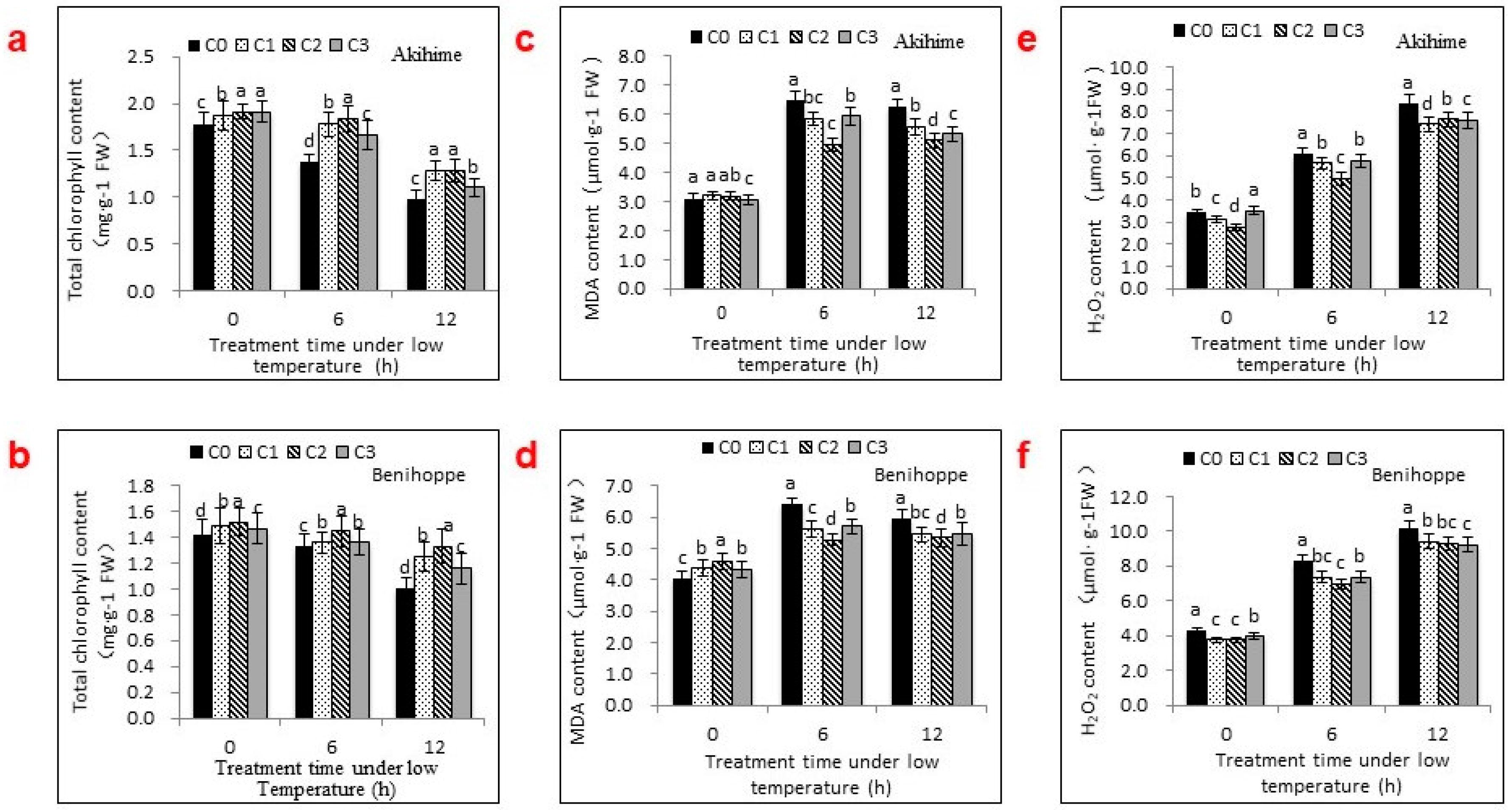
2.2. The Effects of Se Applications on Chlorophyll, MDA, and H2O2 Contents
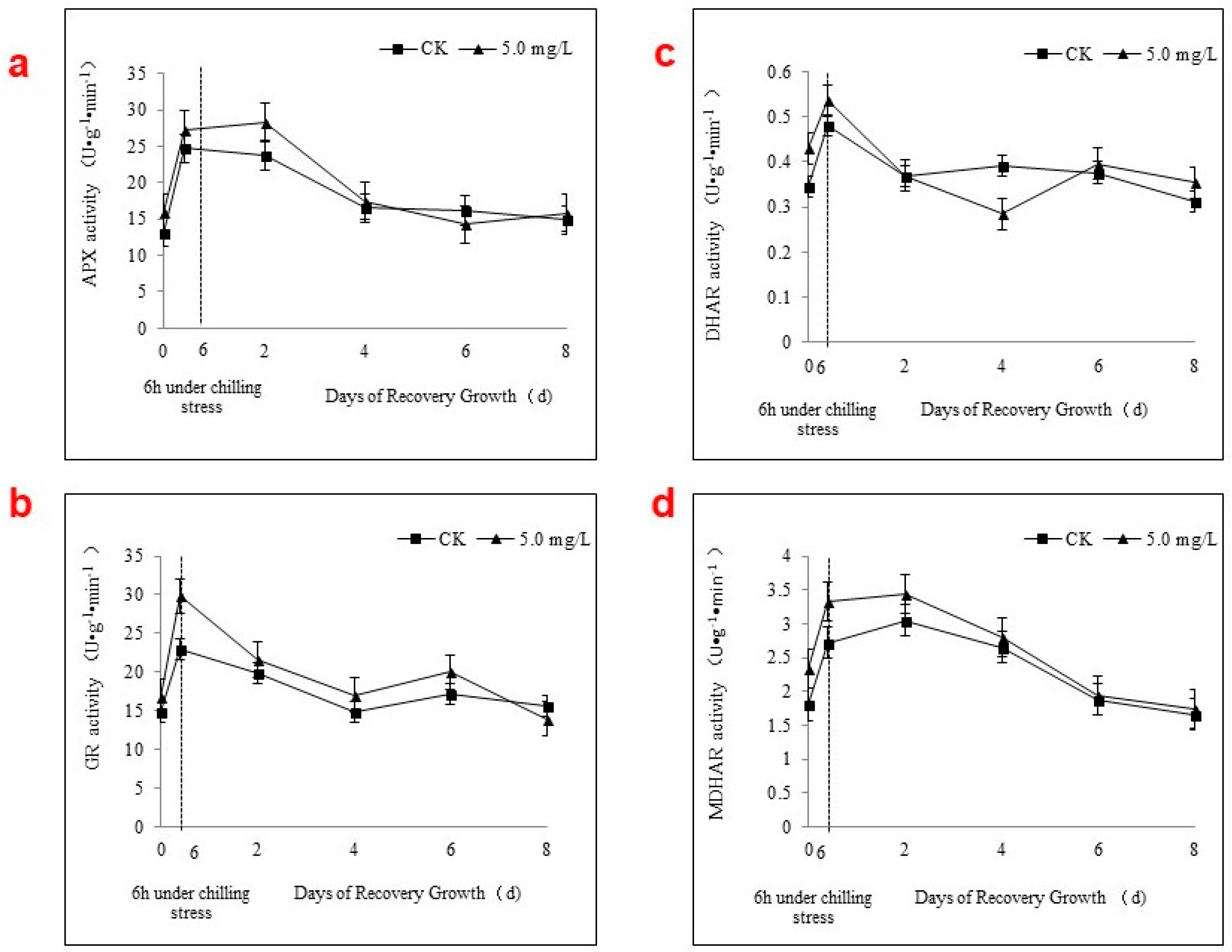
2.3. Antioxidant Enzyme Activities
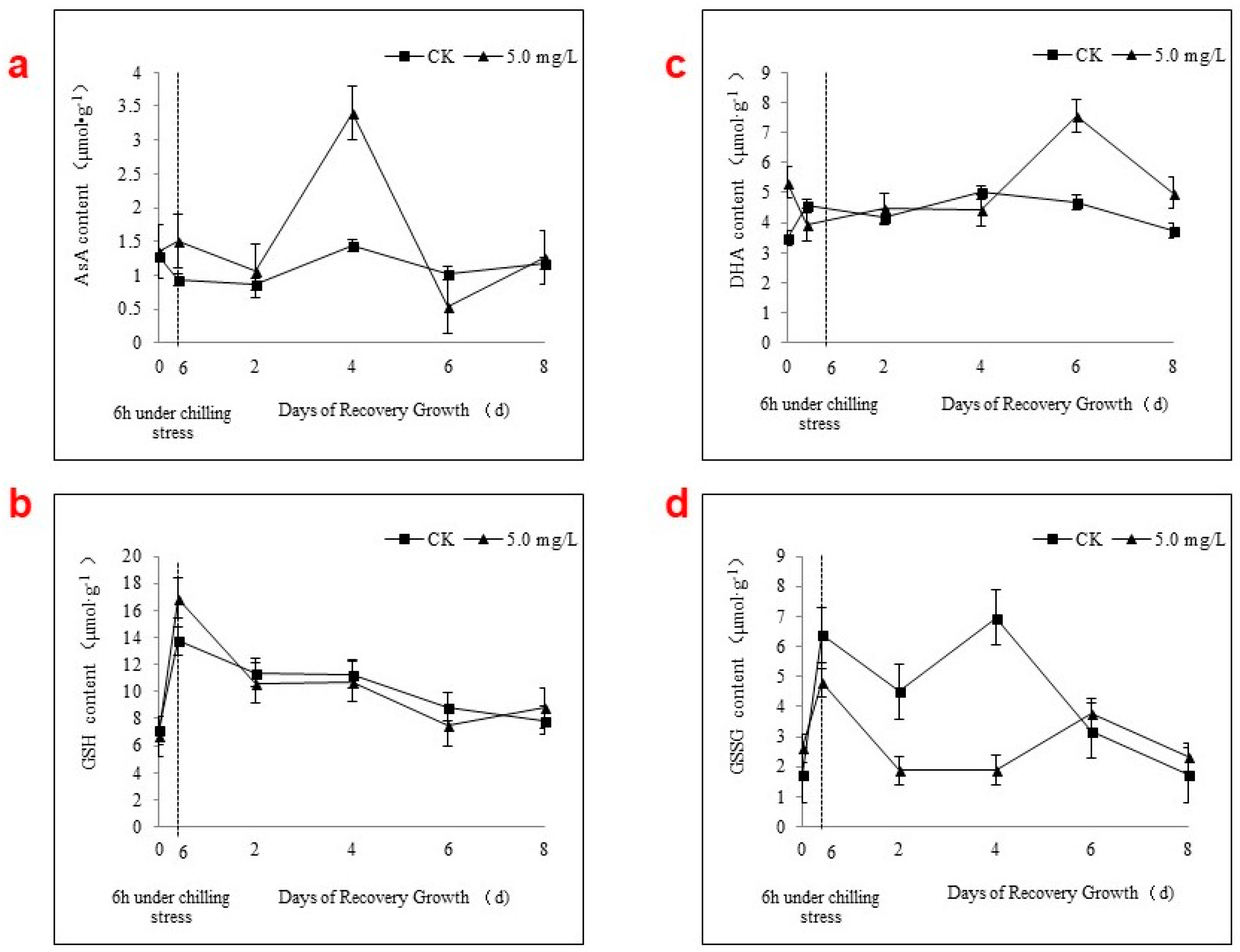
2.4. APX Activity and the AsA-GSH Cycle during Chilling Stress and Recovery Growth
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Se Treatment and Chilling Stress
4.3. Determination of Chlorophyll Content and Photosynthetic Parameters
4.4. Determination of Malondialdehyde (MDA) and H2O2 Contents
4.5. Determination of Antioxidant Enzyme Activities
4.6. Determination of APX Enzyme Activity and the AsA-GSH Cycle
4.7. Statistical Analyses
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AES-ZJU | Agricultural Experiment Station of Zhejiang University |
| AsA | Ascorbic acid |
| APX | Ascorbate peroxidase |
| CAT | Catalase |
| DHAR | Dehydroascorbate reductase |
| GR | Glutathione reductase |
| GSH | Glutathione |
| GSSG | Oxidized glutathione |
| H2O2 | Hydrogen peroxide |
| MDA | Malondialdehyde |
| MDHAR | Monodehydroascorbate reductase |
| POD | Peroxide enzyme |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
References
- Maughan, T.L.; Black, B.L.; Drost, D. Critical temperature for sub-lethal cold injury of strawberry leaves. Sci. Hortic. 2015, 183, 8–12. [Google Scholar] [CrossRef]
- Luo, Y.; Tang, H.R.; Zhang, Y. Effect of low temperature stress on activities of SOD and enzymes of Ascorbate-Glutathione Cycle. Acta Hortic. Sin. 2007, 34, 1405–1410. (In Chinese) [Google Scholar]
- Gupta, S.; Gupta, M. Alleviation of selenium toxicity in Brassica juncea L.: Salicylic acid-mediated modulation in toxicity indicators, stress modulators, and sulfur-related gene transcripts. Protoplasma 2016, 253, 1515–1528. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Hong, Y.H.; Xia, W. Effect of selenium on the photosynthesis and yield of early rice (Oryza sativa L.). J. Hunan Agric. Univ. 1997, 23, 432–434. (In Chinese) [Google Scholar]
- Djanaguiraman, M.; Prasad, P.V.V.; Seppanen, M. Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant. Physiol. Biochem. 2010, 48, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.Z.; Yao, X.Q.; Zhang, Z.N. Responses of wheat seedlings to exogenous selenium supply under cold stress. Biol. Trace Element Res. 2010, 136, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Chen, D.; Tan, Y.L.; Wang, D.; Shen, W.Q.; Zhou, J.H.; Huang, C.P. Alleviating effects of exogenous selenium on Dendrobium officinale seedlings under low temperature stress and the change of its antioxidative physiology characteristics. Acta Bot. Boreali-Occident. Sin. 2013, 33, 747–754. (In Chinese) [Google Scholar]
- Proietti, P.; Nasini, L.; Buono, D.D.; D’Amato, R.; Tedeschini, E.; Businelli, D. Selenium protects olive (Olea europaea L.) from drought stress. Sci. Hortic. 2013, 164, 165–171. [Google Scholar] [CrossRef]
- Saidi, I.; Chtourou, Y.; Djebali, W. Selenium alleviates cadmium toxicity by preventing oxidative stress in sunflower (Helianthus annuus) seedlings. J. Plant Physiol. 2014, 171, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Hawrylak-Nowak, B.; Dresler, S.; Wójcik, M. Selenium affects physiological parameters and phytochelatins accumulation in cucumber (Cucumis sativus L.) plants grown under cadmium exposure. Sci. Hortic. 2014, 172, 10–18. [Google Scholar] [CrossRef]
- Mozafariyan, M.; Shekari, L.; Hawrylak-Nowak, B.; Kamelmanesh, M.M. Protective role of selenium on pepper exposed to cadmium stress during reproductive stage. Biol. Trace Element Res. 2014, 160, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.Z.; Wang, R.G.; Guo, J.K.; Wu, F.C.; Xu, Y.M.; Feng, R.W. The effect of selenium on the subcellular distribution of antimony to regulate the toxicity of antimony in paddy rice. Environ. Sci. Pollut. Res. 2015, 22, 5111–5123. [Google Scholar] [CrossRef] [PubMed]
- Rajashekar, C.B.; Panda, M. Water stress is a component of cold acclimation process essential for inducing full freezing tolerance in strawberry. Sci. Hortic. 2014, 174, 54–59. [Google Scholar] [CrossRef]
- Huang, C.P.; Wang, D.; Sun, L.; Wei, L. Effects of exogenous salicylic acid on the physiological characteristics of Dendrobium officinale under chilling stress. Plant Growth Regul. 2016, 79, 199–208. [Google Scholar] [CrossRef]
- Dai, H.P.; Jia, G.L.; Shan, C.J. Jasmonic acid-induced hydrogen peroxide activates MEK1/2 in upregulating the redox states of ascorbate and glutathione in wheat leaves. Acta Physiol. Plant. 2015, 37, 200. [Google Scholar] [CrossRef]
- Wang, S.C.; Liang, D.; Li, C.; Hao, Y.L.; Ma, F.W.; Shu, H.R. Influence of drought stress on the cellular ultrastructure and antioxidant system in leaves of drought-tolerant and drought-sensitive apple rootstocks. Plant Physiol. Biochem. 2012, 51, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Diao, M.; Ma, L.; Wang, J.W.; Cui, J.X.; Fu, A.F.; Liu, H.Y. Selenium promotes the growth and photosynthesis of tomato seedlings under salt stress by enhancing chloroplast antioxidant defense system. J. Plant. Growth Regul. 2014, 33, 671–682. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Sharkey, T.D. Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 74–79. [Google Scholar] [CrossRef]
- Foyer, C.H. Free radical processes in plants. Biochem. Soc. Trans. 1996, 24, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Sugimoto, Y. Effect of protein modification by malondialdehyde on the interaction between the oxygen-evolving complex 33 kDa protein and photosystem II core proteins. Planta 2010, 231, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhu, C.; Li, L.P.; Sun, Z.Y.; Pan, X.B. Effects of exogenous salicylic acid on growth and H2O2-metabolizing enzymes in rice seedlings under lead stress. J. Environ. Sci. 2007, 19, 44–49. [Google Scholar] [CrossRef]
- Han, D.; Li, X.H.; Xiong, S.L.; Tu, S.X.; Chen, Z.G.; Li, J.P.; Xie, Z.J. Selenium uptake, speciation and stressed response of Nicotiana tabacum L. Environ. Exp. Bot. 2013, 95, 6–14. [Google Scholar] [CrossRef]
- Feng, R.W.; Wei, C.Y.; Tu, S.X. The roles of selenium in protecting plants against abiotic stresses. Environ. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, S. An overview of selenium uptake, metabolism, and toxicity in plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef] [PubMed]
- Habibi, G. Physiological, photochemical and ionic responses of sunflower seedlings to exogenous selenium supply under salt stress. Acta Physiol. Plant. 2017, 39, 213. [Google Scholar] [CrossRef]
- Garg, N.; Chandel, S. Role of arbuscular mycorrhiza in arresting reactive oxygenspecies (ROS) and strengthening antioxidant defense in Cajanus cajan (L.) Millsp. nodules under salinity (NaCl) and cadmium (Cd) stress. Plant Growth Regul. 2015, 75, 521–534. [Google Scholar] [CrossRef]
- Wiciarz, M.; Niewiadomska, E.; Kruk, J. Effects of salt stress on low molecular antioxidants and redox state of plastoquinone and P700 in Arabidopsis thaliana (glycophyte) and Eutrema salsugineum (halophyte). Photosynthetica 2018, 56, 811–819. [Google Scholar] [CrossRef]
- Kang, G.Z.; Li, G.Z.; Liu, G.Q.; Xu, W.; Peng, X.Q.; Wang, C.Y.; Zhu, Y.J.; Guo, T.C. Exogenous salicylic acid enhances wheat drought tolerance by influence on the expression of genes related to ascorbate-glutathione cycle. Biol. Plant. 2013, 57, 718–724. [Google Scholar] [CrossRef]
- Singh, V.P.; Singh, S.; Kumar, J.; Prasad, S.M. Investigating the roles of ascorbate-glutathione cycle and thiol metabolism in arsenate tolerance in ridged Luffa seedlings. Protoplasma 2015, 252, 1217–1229. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.J.; Zhou, Y.; Liu, M.J. Nitric oxide participates in the regulation of the ascorbate-glutathione cycle by exogenous jasmonic acid in the leaves of wheat seedlings under drought stress. Protoplasma 2015, 252, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Mroczek-Zdyrska, M.; Strubiṅska, J.; Hanaka, A. Selenium improves physiological parameters and alleviates oxidative stress in shoots of lead-exposed Vicia faba L. minor plants grown under phosphorus-deficient conditions. J. Plant Growth Regul. 2017, 36, 186–199. [Google Scholar] [CrossRef]
- Ellis, D.R.; Salt, D.E. Plant, selenium and human health. Curr. Opin. Plant Biol. 2003, 6, 273–279. [Google Scholar] [CrossRef]
- Yang, X.; Dong, W.B. Research progress of Se-enriched food. J. Food Saf. Qual. 2017, 8, 2091–2096. (In Chinese) [Google Scholar]
- Wang, X.F.; Luo, Z.; Wan, Y.N.; Wang, Q.; Sun, H.J.; Guo, Y.B.; Li, H.F. Effects of foliar-applied selenite and selenate on selenium accumulation in strawberry. J. Agric. Resour. Environ. 2016, 33, 334–339. (In Chinese) [Google Scholar]
- Fontanella, M.C.; D’Amato, R.; Regni, L.; Proietti, P.; Beone, G.M.; Businelli, D. Selenium speciation profiles in biofortified sangiovese wine. J. Trace Element Med. Biol. 2017, 43, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Wu, F.B.; Zhang, G.P.; Dominy, P. Four barley genotypes respond differently to cadmium: Lipid peroxidation and activities of antioxidant capacity. Environ. Exp. Bot. 2003, 50, 67–78. [Google Scholar] [CrossRef]
- Lin, Z.F.; Li, S.S.; Lin, G.Z.; Guo, J.Y. The accumulation of hydrogen peroxide in senescing leaves and chloroplasts in relation to lipid peroxidation. Acta Photophysiol. Sin. 1988, 14, 16–22. (In Chinese) [Google Scholar]
- Prochazkova, D.; Sairam, R.K.; Srivastava, G.C.; Singh, D.V. Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant. Sci. 2001, 161, 765–771. [Google Scholar] [CrossRef]
- Ali, S.; Bai, P.; Zeng, F.R.; Cai, S.G.; Shamsi, I.H.; Qiu, B.Y.; Wu, F.B.; Zhang, G.P. The ecotoxicological and interactive effects of chromium and aluminum on growth, oxidative damage and antioxidant enzymes on two barley genotypes differing in Al tolerance. Environ. Exp. Bot. 2011, 70, 185–191. [Google Scholar] [CrossRef]
- Putter, J. Peroxidases. In Methods of Enzymatic Analysis: II; Bergmeyer, H.U., Ed.; Academic Press: New York, NY, USA, 1974; pp. 685–690. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Garcia-Limones, C.; Hervas, A.; Navas-Cortes, J.A.; Jimenez-Diaz, R.M.; Tena, M. Induction of an antioxidant enzyme system and other oxidative stress markers associated with compatible and incompatible interactions between chickpea (Cicer arietinum L.) and Fusarium oxysporum f. sp. Ciceris. Physiol. Mol. Plant Pathol. 2002, 61, 325–337. [Google Scholar] [CrossRef]
- Aravind, P.; Prasad, M.N.V. Modulation of cadmium-induced oxidative stress in Ceratophyllum demersum by zinc involves ascorbate—Glutathione cycle and glutathione metabolism. Plant. Physiol. Biochem. 2005, 43, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Dalton, D.A.; Russell, S.A.; Hanus, F.J.; Pascoe, G.A.; Evans, H.J. Enzymatic reactions of ascorbate and glutathione that prevent peroxide damage in soybean root nodules. Proc. Natl. Acad. Sci. USA 1986, 83, 3811–3815. [Google Scholar] [CrossRef] [PubMed]
- Hodges, D.M.; Andrews, C.J.; Johnson, D.A.; Hamilton, R.I.; Hodges, D.M.; Andrews, C.J.; Johnson, D.A.; Hamilton, R.I. Antioxidant compound responses to chilling stress in differentially sensitive inbred maize lines. Physiol. Plant. 1996, 98, 685–692. [Google Scholar] [CrossRef]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem 1980, 106, 207–212. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Qin, N.; Sun, L.; Yu, M.; Hu, W.; Qi, Z. Selenium Improves Physiological Parameters and Alleviates Oxidative Stress in Strawberry Seedlings under Low-Temperature Stress. Int. J. Mol. Sci. 2018, 19, 1913. https://doi.org/10.3390/ijms19071913
Huang C, Qin N, Sun L, Yu M, Hu W, Qi Z. Selenium Improves Physiological Parameters and Alleviates Oxidative Stress in Strawberry Seedlings under Low-Temperature Stress. International Journal of Molecular Sciences. 2018; 19(7):1913. https://doi.org/10.3390/ijms19071913
Chicago/Turabian StyleHuang, Chongping, Nannan Qin, Li Sun, Mingyan Yu, Weizhen Hu, and Zhenyu Qi. 2018. "Selenium Improves Physiological Parameters and Alleviates Oxidative Stress in Strawberry Seedlings under Low-Temperature Stress" International Journal of Molecular Sciences 19, no. 7: 1913. https://doi.org/10.3390/ijms19071913
APA StyleHuang, C., Qin, N., Sun, L., Yu, M., Hu, W., & Qi, Z. (2018). Selenium Improves Physiological Parameters and Alleviates Oxidative Stress in Strawberry Seedlings under Low-Temperature Stress. International Journal of Molecular Sciences, 19(7), 1913. https://doi.org/10.3390/ijms19071913



