The Effect of GPRC5a on the Proliferation, Migration Ability, Chemotherapy Resistance, and Phosphorylation of GSK-3β in Pancreatic Cancer
Abstract
1. Introduction
2. Results
2.1. The Data-Mining Analysis for GPRC5a in PaCa
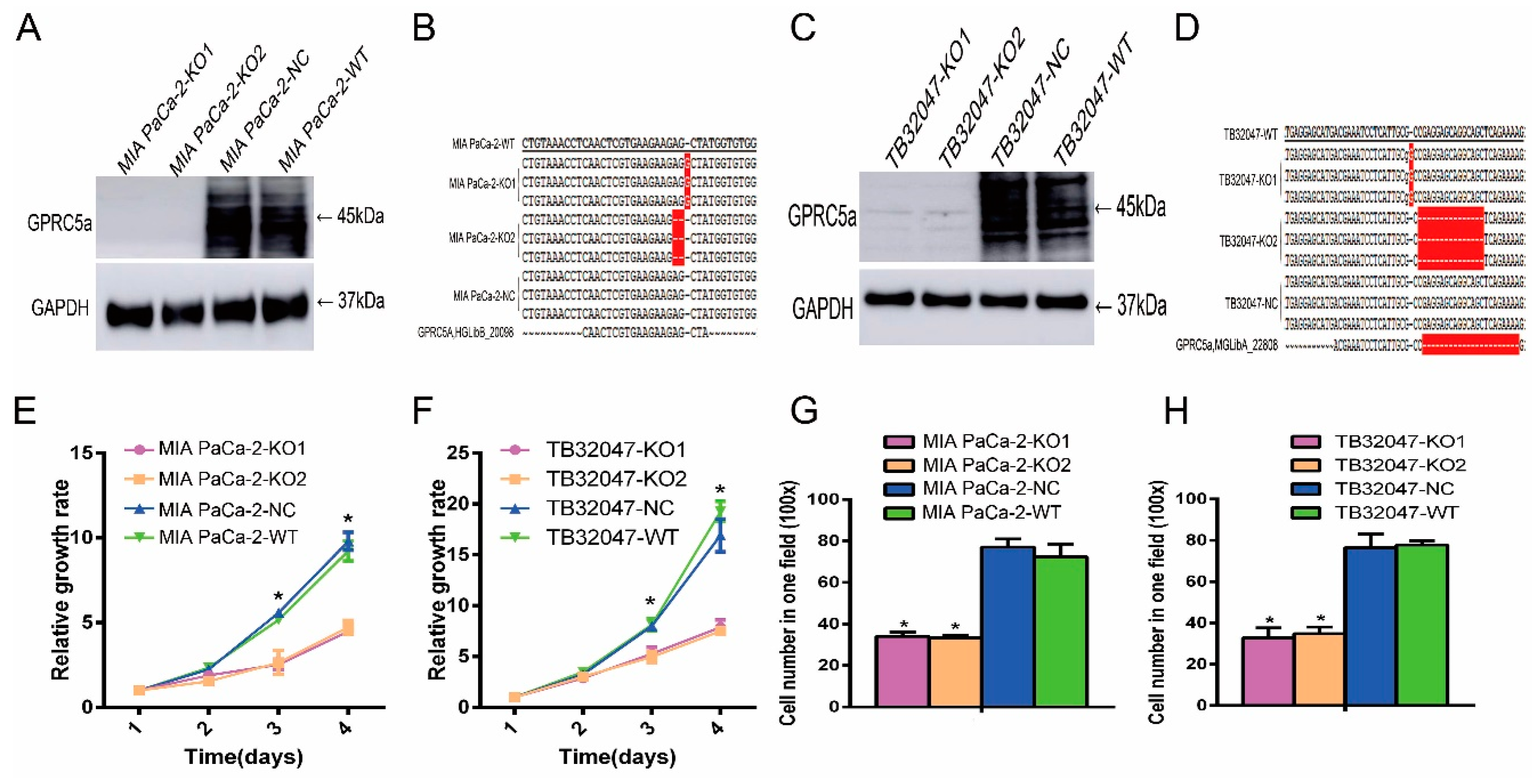
2.2. Expression of GPRC5a in PaCa Cell Lines
2.3. Inhibition of the Proliferation and Migration Ability of TB32047 and MIA PaCa-2 Cells by Knockout of GPRC5a
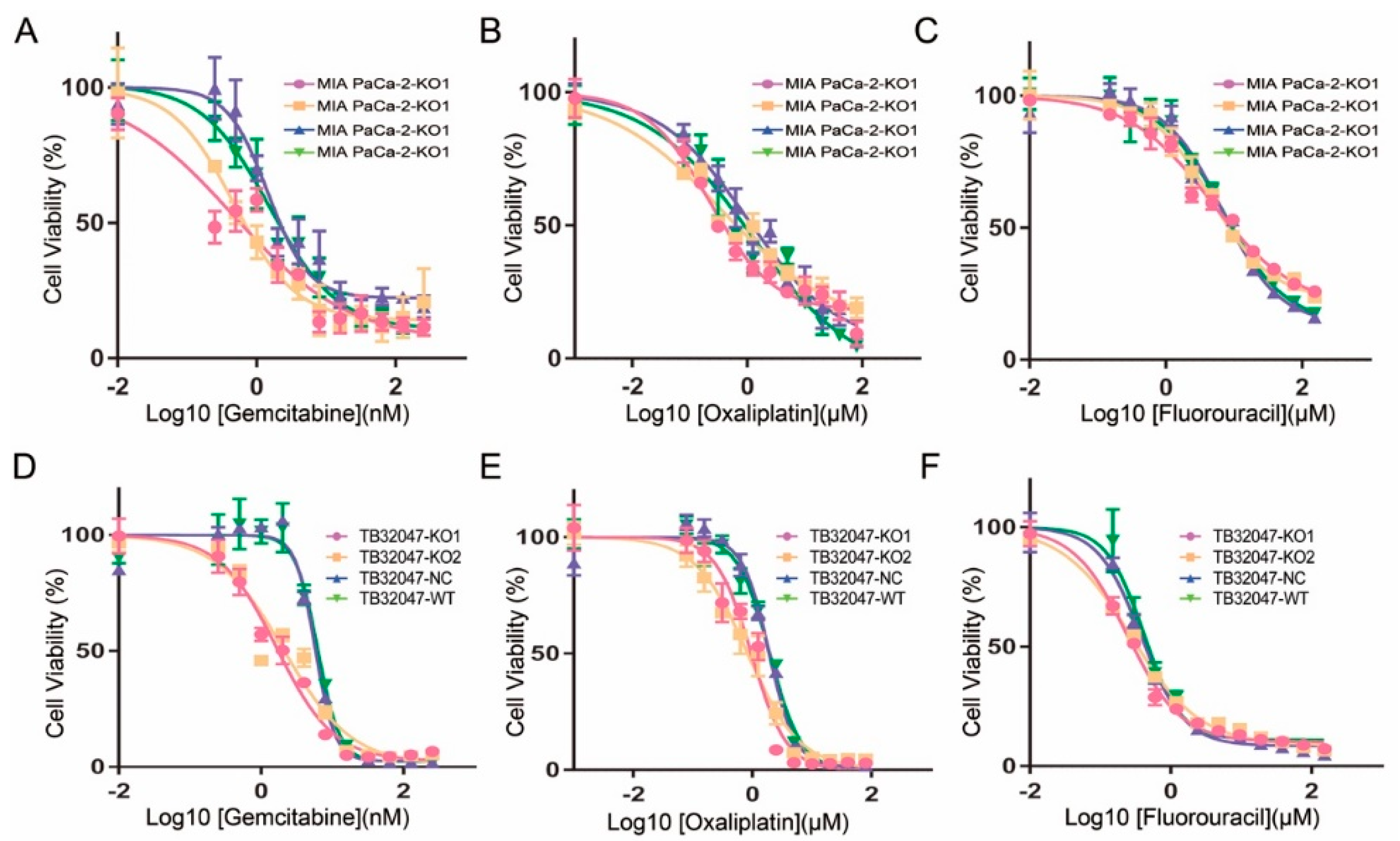
2.4. Inhibition of the Chemotherapy Drug Resistance in TB32047 and MIA PaCa-2 Cells by Knockout of GPRC5a
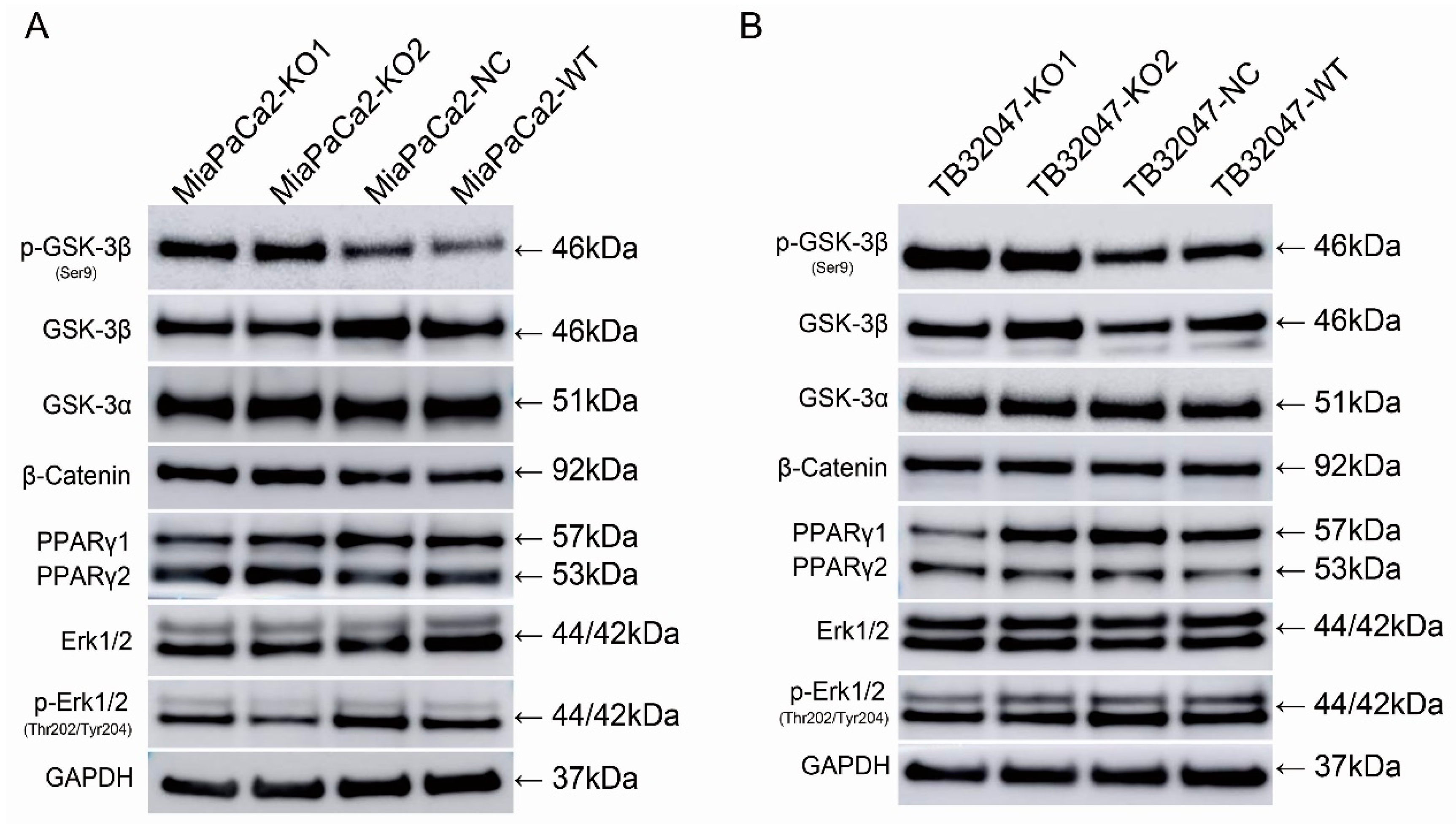
2.5. Knockout of GPRC5a Promotes the Phosphorylation of GSK-3β at Ser9 in TB32047 and MIA PaCa-2 Cells
3. Discussion
4. Methods
4.1. Data-Mining Analysis
4.2. Cell Culture
4.3. Western Blot
4.4. Quantitative RT-PCR
4.5. The CRISPR/Cas9 Gene Editing Knockout System
4.6. Proliferation Assay
4.7. Migration Assay
4.8. Chemotherapy Drugs Resistance Assay
4.9. Immunofluorescence Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- New, M.; van Acker, T.; Long, J.S.; Sakamaki, J.I.; Ryan, K.M.; Tooze, S.A. Molecular Pathways Controlling Autophagy in Pancreatic Cancer. Front. Oncol. 2017, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, R.; Sant, M.; Coleman, M.P.; Francisci, S.; Baili, P.; Pierannunzio, D.; Trama, A.; Visser, O.; Brenner, H.; Ardanaz, E.; et al. Cancer survival in Europe 1999-2007 by country and age: Results of EUROCARE-5—A population-based study. Lancet Oncol. 2014, 15, 23–34. [Google Scholar] [CrossRef]
- Lianos, G.D.; Christodoulou, D.K.; Katsanos, K.H.; Katsios, C.; Glantzounis, G.K. Minimally Invasive Surgical Approaches for Pancreatic Adenocarcinoma: Recent Trends. J. Gastrointest. Cancer 2017, 48, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.F.; Poruk, K.E.; Wolfgang, C.L. Pancreatic cancer surgery: Past, present, and future. Chin. J. Cancer Res. 2015, 27, 332–348. [Google Scholar] [PubMed]
- Halbrook, C.J.; Lyssiotis, C.A. Employing Metabolism to Improve the Diagnosis and Treatment of Pancreatic Cancer. Cancer Cell 2017, 31, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Frank, T.S.; Sun, X.; Zhang, Y.; Yang, J.; Fisher, W.E.; Gingras, M.C.; Li, M. Genomic profiling guides the choice of molecular targeted therapy of pancreatic cancer. Cancer Lett. 2015, 363, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kiyotani, K.; Chan, H.T.; Nakamura, Y. Immunopharmacogenomics towards personalized cancer immunotherapy targeting neoantigens. Cancer Sci. 2018, 109, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Venkatakrishnan, A.J.; Deupi, X.; Lebon, G.; Tate, C.G.; Schertler, G.F.; Babu, M.M. Molecular signatures of G-protein-coupled receptors. Nature 2013, 494, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Rigoutsos, I. The emerging roles of GPRC5A in diseases. Oncoscience 2014, 1, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Jahny, E.; Yang, H.; Liu, B.; Jahnke, B.; Lademann, F.; Knosel, T.; Rummele, P.; Grutzmann, R.; Aust, D.E.; Pilarsky, C.; et al. The G Protein-Coupled Receptor RAI3 Is an Independent Prognostic Factor for Pancreatic Cancer Survival and Regulates Proliferation via STAT3 Phosphorylation. PLoS ONE 2017, 12, e0170390. [Google Scholar] [CrossRef] [PubMed]
- Solinski, H.J.; Gudermann, T.; Breit, A. Pharmacology and signaling of MAS-related G protein-coupled receptors. Pharmacol. Rev. 2014, 66, 570–597. [Google Scholar] [CrossRef] [PubMed]
- Gentry, P.R.; Sexton, P.M.; Christopoulos, A. Novel Allosteric Modulators of G Protein-coupled Receptors. J. Biol. Chem. 2015, 290, 19478–19488. [Google Scholar] [CrossRef] [PubMed]
- Scholz, N.; Gehring, J.; Guan, C.; Ljaschenko, D.; Fischer, R.; Lakshmanan, V.; Kittel, R.J.; Langenhan, T. The adhesion GPCR latrophilin/CIRL shapes mechanosensation. Cell Rep. 2015, 11, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Ferre, S.; Casado, V.; Devi, L.A.; Filizola, M.; Jockers, R.; Lohse, M.J.; Milligan, G.; Pin, J.P.; Guitart, X. G protein-coupled receptor oligomerization revisited: Functional and pharmacological perspectives. Pharmacol. Rev. 2014, 66, 413–434. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Ghosh, E.; Shukla, A.K. Emerging Approaches to GPCR Ligand Screening for Drug Discovery. Trends Mol. Med. 2015, 21, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Kadara, H.; Fujimoto, J.; Men, T.; Ye, X.; Lotan, D.; Lee, J.S.; Lotan, R. A Gprc5a tumor suppressor loss of expression signature is conserved, prevalent, and associated with survival in human lung adenocarcinomas. Neoplasia 2010, 12, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Fujimoto, J.; Men, T.; Ye, X.; Deng, J.; Lacroix, L.; Clifford, J.L.; Mao, L.; Van Pelt, C.S.; Lee, J.J.; et al. Identification of the retinoic acid-inducible Gprc5a as a new lung tumor suppressor gene. J. Natl. Cancer Inst. 2007, 99, 1668–1682. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ye, D.; Wang, T.; Guo, W.; Song, H.; Liao, Y.; Xu, D.; Zhu, H.; Zhang, Z.; Deng, J. Repression of GPRC5A is associated with activated STAT3, which contributes to tumor progression of head and neck squamous cell carcinoma. Cancer Cell Int. 2017, 17, 34. [Google Scholar] [CrossRef] [PubMed]
- Chester, C.; Maecker, H.T. Algorithmic Tools for Mining High-Dimensional Cytometry Data. J. Immunol. 2015, 195, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yang, H.; Taher, L.; Denz, A.; Grutzmann, R.; Pilarsky, C.; Weber, G.F. Identification of Prognostic Biomarkers by Combined mRNA and miRNA Expression Microarray Analysis in Pancreatic Cancer. Transl. Oncol. 2018, 11, 700–714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wen, Y.; Guo, X. CRISPR/Cas9 for genome editing: Progress, implications and challenges. Hum. Mol. Genet. 2014, 23, R40–R46. [Google Scholar] [CrossRef] [PubMed]
- Binenbaum, Y.; Na’ara, S.; Gil, Z. Gemcitabine resistance in pancreatic ductal adenocarcinoma. Drug Resist. Updat. 2015, 23, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Laguna, I.; Hidalgo, M. Pancreatic cancer: From state-of-the-art treatments to promising novel therapies. Nat. Rev. Clin. Oncol. 2015, 12, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Domoto, T.; Pyko, I.V.; Furuta, T.; Miyashita, K.; Uehara, M.; Shimasaki, T.; Nakada, M.; Minamoto, T. Glycogen synthase kinase-3beta is a pivotal mediator of cancer invasion and resistance to therapy. Cancer Sci. 2016, 107, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.A.; Nebert, D.W.; Hickman, J.A.; Richburg, J.H.; Goldsworthy, T.L. Perturbation of the mitosis/apoptosis balance: A fundamental mechanism in toxicology. Toxicol. Soc. 1997, 38, 107–115. [Google Scholar] [CrossRef]
- Beurel, E.; Grieco, S.F.; Jope, R.S. Glycogen synthase kinase-3 (GSK3): Regulation, actions, and diseases. Pharmacol. Ther. 2015, 148, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Tejeda-Munoz, N.; Robles-Flores, M. Glycogen synthase kinase 3 in Wnt signaling pathway and cancer. IUBMB Life 2015, 67, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Ougolkov, A.V.; Fernandez-Zapico, M.E.; Savoy, D.N.; Urrutia, R.A.; Billadeau, D.D. Glycogen synthase kinase-3beta participates in nuclear factor kappaB-mediated gene transcription and cell survival in pancreatic cancer cells. Cancer Res. 2005, 65, 2076–2081. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W., 3rd; Baldwin, A.S. Maintenance of constitutive IkappaB kinase activity by glycogen synthase kinase-3alpha/beta in pancreatic cancer. Cancer Res. 2008, 68, 8156–8163. [Google Scholar] [CrossRef] [PubMed]
- Shimasaki, T.; Ishigaki, Y.; Nakamura, Y.; Takata, T.; Nakaya, N.; Nakajima, H.; Sato, I.; Zhao, X.; Kitano, A.; Kawakami, K.; et al. Glycogen synthase kinase 3beta inhibition sensitizes pancreatic cancer cells to gemcitabine. J. Gastroenterol. 2012, 47, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Kitano, A.; Shimasaki, T.; Chikano, Y.; Nakada, M.; Hirose, M.; Higashi, T.; Ishigaki, Y.; Endo, Y.; Takino, T.; Sato, H.; et al. Aberrant glycogen synthase kinase 3beta is involved in pancreatic cancer cell invasion and resistance to therapy. PLoS ONE 2013, 8, e55289. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; Li, L.; Fridley, B.L.; Jenkins, G.D.; Kalari, K.R.; Lingle, W.; Petersen, G.; Lou, Z.; Wang, L. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer Cell 2009, 16, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Badea, L.; Herlea, V.; Dima, S.O.; Dumitrascu, T.; Popescu, I. Combined gene expression analysis of whole-tissue and microdissected pancreatic ductal adenocarcinoma identifies genes specifically overexpressed in tumor epithelia. Hepato Gastroenterol. 2008, 55, 2016–2027. [Google Scholar]
- Zhang, G.; He, P.; Tan, H.; Budhu, A.; Gaedcke, J.; Ghadimi, B.M.; Ried, T.; Yfantis, H.G.; Lee, D.H.; Maitra, A.; et al. Integration of metabolomics and transcriptomics revealed a fatty acid network exerting growth inhibitory effects in human pancreatic cancer. Clin. Cancer Res. 2013, 19, 4983–4993. [Google Scholar] [CrossRef] [PubMed]
- Barry, S.; Chelala, C.; Lines, K.; Sunamura, M.; Wang, A.; Marelli-Berg, F.M.; Brennan, C.; Lemoine, N.R.; Crnogorac-Jurcevic, T. S100P is a metastasis-associated gene that facilitates transendothelial migration of pancreatic cancer cells. Clin. Exp. Metastasis 2013, 30, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Paris, P.L.; Chen, J.; Ngo, V.; Yao, H.; Frazier, M.L.; Killary, A.M.; Liu, C.G.; Liang, H.; Mathy, C.; et al. Next generation sequencing of pancreatic cyst fluid microRNAs from low grade-benign and high grade-invasive lesions. Cancer Lett. 2015, 356, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Donahue, T.R.; Tran, L.M.; Hill, R.; Li, Y.; Kovochich, A.; Calvopina, J.H.; Patel, S.G.; Wu, N.; Hindoyan, A.; Farrell, J.J.; et al. Integrative survival-based molecular profiling of human pancreatic cancer. Clin. Cancer Res. 2012, 18, 1352–1363. [Google Scholar] [CrossRef] [PubMed]
- Frampton, A.E.; Castellano, L.; Colombo, T.; Giovannetti, E.; Krell, J.; Jacob, J.; Pellegrino, L.; Roca-Alonso, L.; Funel, N.; Gall, T.M.; et al. MicroRNAs cooperatively inhibit a network of tumor suppressor genes to promote pancreatic tumor growth and progression. Gastroenterology 2014, 146, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Thul, P.J.; Akesson, L.; Wiking, M.; Mahdessian, D.; Geladaki, A.; Ait Blal, H.; Alm, T.; Asplund, A.; Bjork, L.; Breckels, L.M.; et al. A subcellular map of the human proteome. Science 2017, 356, eaal3321. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.; Varambally, S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef] [PubMed]
- Sanjana, N.E.; Shalem, O.; Zhang, F. Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods 2014, 11, 783–784. [Google Scholar] [CrossRef] [PubMed]
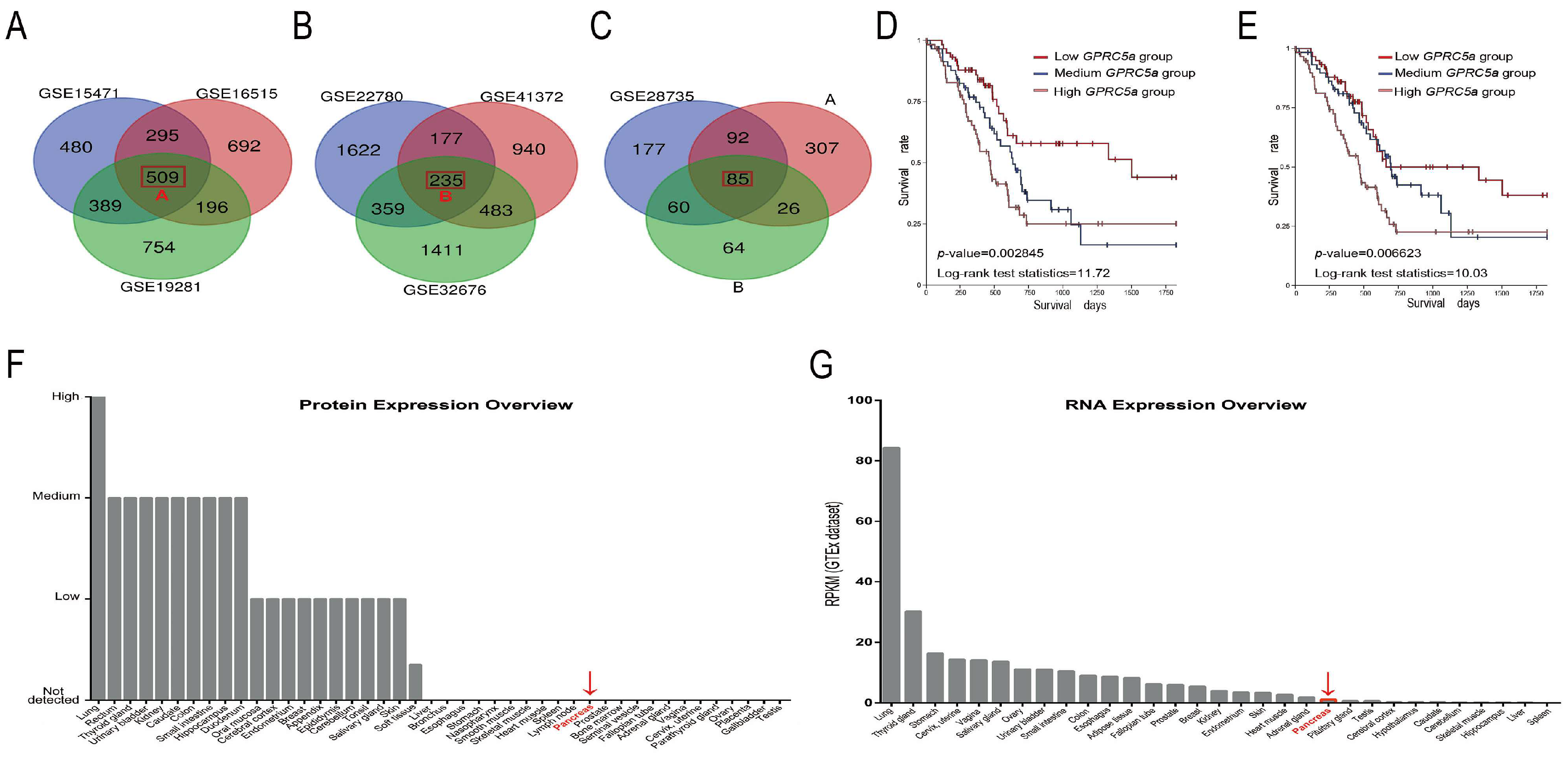

| Microarray | p-Value | Student’s t-Value | logFC |
|---|---|---|---|
| GSE15471 | 1.12 × 10−12 | 8.689697 | 2.5051499 |
| GSE16515 | 1.08 × 10−10 | 8.048017 | 4.3196152 |
| GSE19281 | 5.56 × 10−3 | 3.6910787 | 2.28094556 |
| GSE22780 | 0.0186679 | 2.584605 | 2.44569319 |
| GSE28735 | 2.89 × 10−12 | 8.059183 | 1.1751238 |
| GSE32676 | 1.68 × 10−3 | 3.45 | 2.23 |
| GSE41372 | 2.34 × 10−6 | 7.93 | 3.35 |
| Cells | Gemcitabine (nM) | Oxaliplatin (µM) | Fluorouracil (µM) | |||
|---|---|---|---|---|---|---|
| EC50 | 95% CI | EC50 | 95% CI | EC50 | 95% CI | |
| MIA PaCa-2-KO1 | 0.455 | 0.305–0.605 | 0.224 | 0.169–0.300 | 4.642 | 3.577–6.340 |
| MIA PaCa-2-KO2 | 0.476 | 0.322–0.665 | 0.357 | 0.210–0.661 | 4.502 | 3.720–5.525 |
| MIA PaCa-2-NC | 1.458 | 1.128–1.938 | 1.015 | 0.628–2.011 | 6.675 | 5.406–8.424 |
| MIA PaCa-2-WT | 1.546 | 1.122–2.146 | 1.171 | 0.614–4.312 | 6.777 | 5.321–9.061 |
| TB32047-KO1 | 0.240 | 0.212–0.268 | 1.661 | 1.378–2.000 | 0.962 | 0.772–1.179 |
| TB32047-KO2 | 0.274 | 0.242–0.306 | 2.084 | 1.487–2.931 | 0.828 | 0.666–1.024 |
| TB32047-NC | 0.384 | 0.345–4.266 | 5.774 | 5.309–6.281 | 1.894 | 1.713–2.092 |
| TB32047-WT | 0.413 | 0.353–0.486 | 6.178 | 5.589–6.830 | 1.947 | 1.743–2.168 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Yang, H.; Pilarsky, C.; Weber, G.F. The Effect of GPRC5a on the Proliferation, Migration Ability, Chemotherapy Resistance, and Phosphorylation of GSK-3β in Pancreatic Cancer. Int. J. Mol. Sci. 2018, 19, 1870. https://doi.org/10.3390/ijms19071870
Liu B, Yang H, Pilarsky C, Weber GF. The Effect of GPRC5a on the Proliferation, Migration Ability, Chemotherapy Resistance, and Phosphorylation of GSK-3β in Pancreatic Cancer. International Journal of Molecular Sciences. 2018; 19(7):1870. https://doi.org/10.3390/ijms19071870
Chicago/Turabian StyleLiu, Bin, Hai Yang, Christian Pilarsky, and Georg F. Weber. 2018. "The Effect of GPRC5a on the Proliferation, Migration Ability, Chemotherapy Resistance, and Phosphorylation of GSK-3β in Pancreatic Cancer" International Journal of Molecular Sciences 19, no. 7: 1870. https://doi.org/10.3390/ijms19071870
APA StyleLiu, B., Yang, H., Pilarsky, C., & Weber, G. F. (2018). The Effect of GPRC5a on the Proliferation, Migration Ability, Chemotherapy Resistance, and Phosphorylation of GSK-3β in Pancreatic Cancer. International Journal of Molecular Sciences, 19(7), 1870. https://doi.org/10.3390/ijms19071870





