Boron: Functions and Approaches to Enhance Its Availability in Plants for Sustainable Agriculture
Abstract
1. Introduction
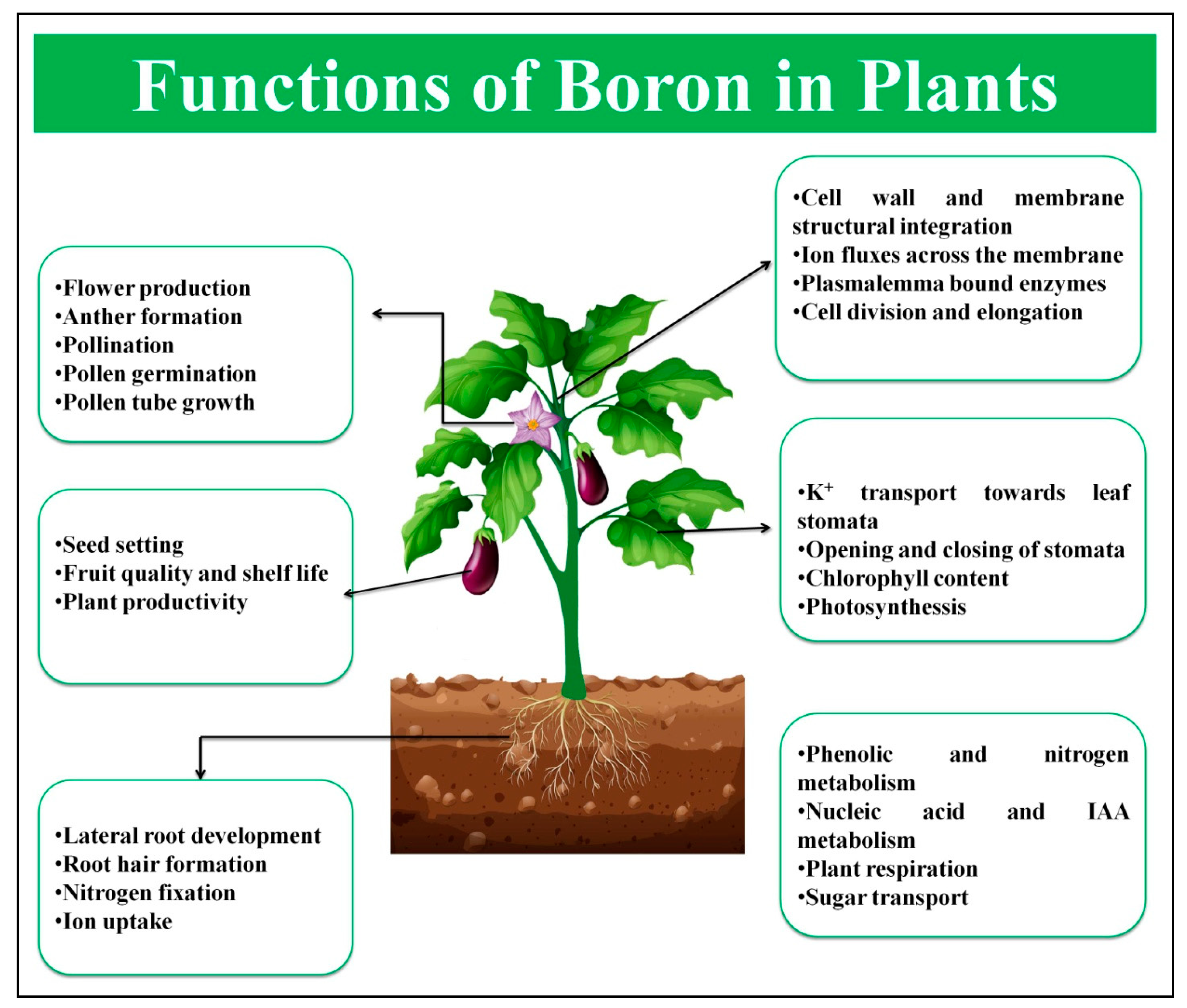
1.1. Functions of Boron (B)
1.2. Deficiency Symptoms
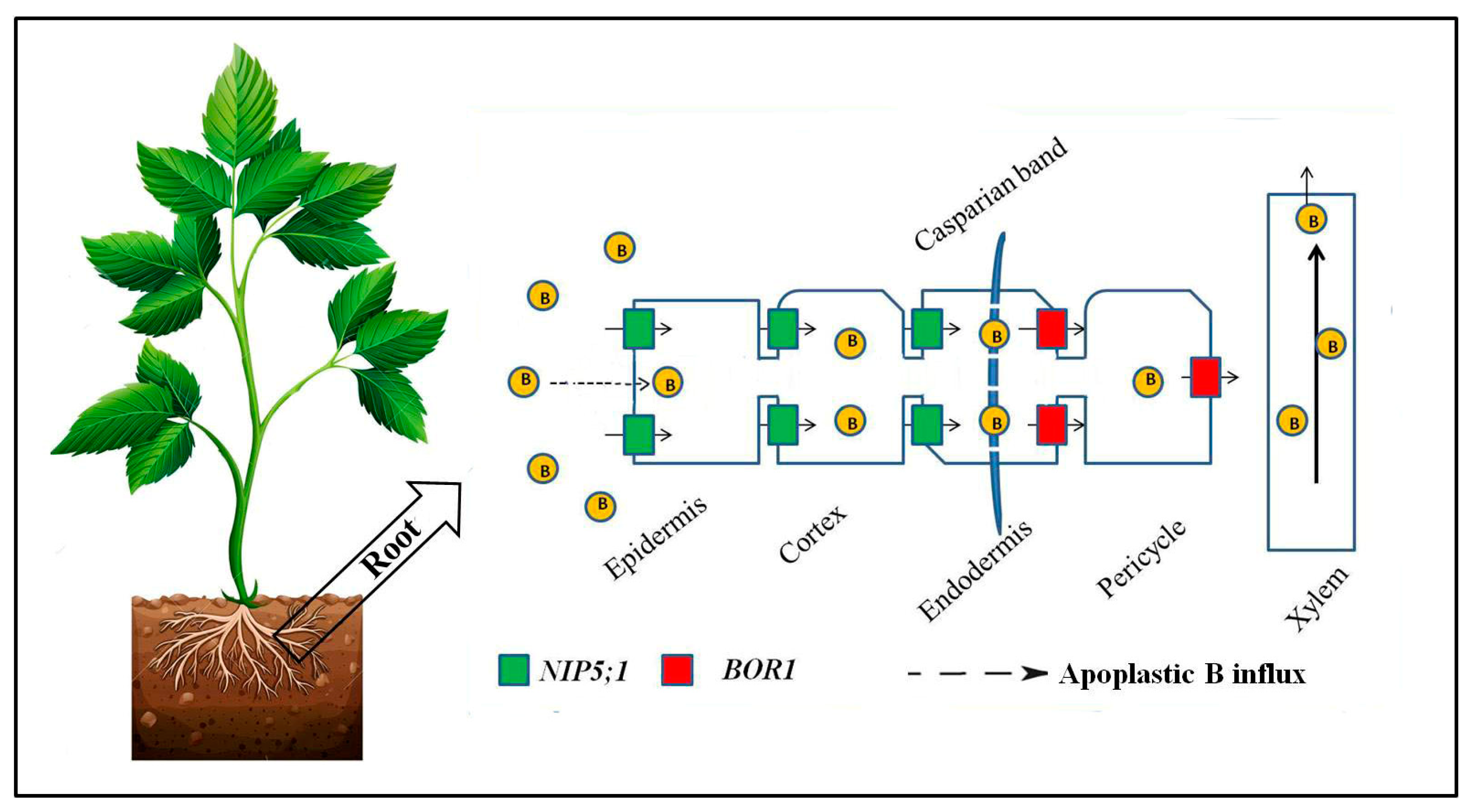
2. Mechanism of B Uptake and Long-Distance Transport under Limited Boron Supply
2.1. Facilitated Transport by Channels
2.2. Active Transport by Transporters
3. Approaches Utilized to Enhance B Uptake
3.1. Modification of Root Traits
3.2. Grafting
3.3. Biostimulators
3.3.1. Mycorrhizal Fungi (MF)
3.3.2. Plant-Growth-Promoting Rhizobacteria (PGPR)
3.4. Nanotechnology
4. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACA | Auto-inhibited Ca2+-ATPase |
| AMF | Arbuscular mycorrhizal fungi |
| CAX | Cation exchanger |
| CNGC | Cyclic nucleotide-gated ion channel |
| GA3 | Gibberellic acid |
| GIPC | Glycosylinositol phosphorylceramides |
| HS | Humic substances |
| IAA | Indolacetic acid |
| MF | Mycorrhizal fungi |
| MWCNTs | Multiwalled carbon nanotubes |
| NIP | NOD-26-like intrinsic protein |
| PGPR | Plant-growth-promoting rhizobacteria |
| RG-II | Rhamnoglacturonan-II |
References
- Brown, P.H.; Bellaloui, N.; Wimmer, M.A.; Bassil, E.S.; Ruiz, J.; Hu, H.; Pfeffer, H.; Dannel, F.; Romheld, V. Boron in plant biology. Plant Biol. 2002, 4, 205–223. [Google Scholar] [CrossRef]
- Hu, H.; Brown, P.H. Localization of boron in cell walls of squash and tobacco and its association with pectin- Evidence for a structural role of boron in the cell wall. Plant Physiol. 1994, 105, 681–689. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, M.A.; Ishii, T.; Albersheim, P.; Darvill, A.G. Rhamnogalacturonan II: Structure and function of a borate-linked cell wall pectic polysaccharide. Annu. Rev. Plant Biol. 2004, 55, 109–139. [Google Scholar] [CrossRef] [PubMed]
- Voxeur, A.; Fry, S.C. Glycosylinositol phosphorylceramides (GIPCs) from Rosa cell cultures are boron-bridged in the plasma membrane and form complexes with rhamnogalacturonan-II. Plant J. 2014, 79, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Ryden, P.; Sugimoto-Shirasu, K.; Smith, A.C.; Findlay, K.; Reiter, W.D.; McCann, M.C. Tensile properties of Arabidopsis cell walls depend on both a xyloglucan cross-linked microfibrillar network and rhamnogalacturonan II-borate complexes. Plant Physiol. 2003, 132, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, A.; O’Neill, M.A.; Ehwald, R. The pore size of nongraminaceous plant cell walls is rapidly decreased by borate ester cross-linking of the pectic polysaccharide rhamnogalacturonan II. Plant Physiol. 1999, 121, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Matsunaga, T.; Hayashi, N. Formation of rhamnogalacturonan II-borate dimer in pectin determines cell wall thickness of pumpkin tissue. Plant Physiol. 2001, 126, 1698–1705. [Google Scholar] [CrossRef] [PubMed]
- Goldbach, H.E.; Yu, Q.; Wingender, R.; Schulz, M.; Wimmer, M.; Findeklee, P.; Baluska, F. Rapid response reactions of roots to boron deprivation. J. Plant Nutr. Soil Sci. 2001, 164, 173–181. [Google Scholar] [CrossRef]
- Goldbach, H.E.; Wimmer, M.A. Boron in plants and animals: Is there a role beyond cell wall structure? J. Plant Nutr. Soil Sci. 2007, 170, 39–48. [Google Scholar] [CrossRef]
- Lawrence, K.; Bhalla, P.; Misra, P.C. Changes in (NADP)H-dependent redox activities in plasmalemma-enriched vesicles isolated from boron- and zinc-deficient chick pea roots. J. Plant Physiol. 1995, 146, 652–657. [Google Scholar] [CrossRef]
- Robertson, G.A.; Loughman, B.C. Rubidium uptake and boron deficiency in Vicia faba. J. Exp. Bot. 1973, 24, 1046–1052. [Google Scholar] [CrossRef]
- Dell, B.; Huang, L.B. Physiological response of plants to low boron. Plant Soil 1997, 193, 103–120. [Google Scholar] [CrossRef]
- Gupta, U.; Solanki, H. Impact of boron deficiency on plant growth. Int. J. Bioassay 2013, 2, 1048–1050. [Google Scholar]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2012; ISBN 9780123849069. [Google Scholar]
- Mengel, K.; Kirkby, E.A. Principles of Plant Nutrition, 5th ed.; Kluwcr Academic Publishers: Dordrecht, The Netherlands, 2001. [Google Scholar]
- Hajiboland, R.; Bahrami-Rad, S.; Bastani, S. Phenolics metabolism in boron-deficient tea [Camellia sinensis (L.) O. Kuntze] plants. Acta Biol. Hung. 2013, 64, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Chen, L.S.; Jiang, H.X.; Smith, B.R.; Yanga, L.T.; Xie, C.Y. Boron deficiency decreases growth and photosynthesis, and increases starch and hexoses in leaves of citrus seedlings. J. Plant Physiol. 2008, 165, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.G.; Liang, Y.C.; Shen, K. Effect of boron on the nitrate reductase activity in oilseed rape plants. J. Plant Nutr. 1993, 16, 1229–1239. [Google Scholar] [CrossRef]
- Bolanos, L.; Lukaszewski, K.; Bonilla, I.; Blevins, D. Why boron? Plant Physiol. Biochem. 2004, 42, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Yang, C.; Pan, Z.; Liu, Y.; Peng, S. Boron deficiency in woody plants: Various responses and tolerance mechanism. Front. Plant Sci. 2015. [Google Scholar] [CrossRef] [PubMed]
- El-Feky, S.S.; El-Shintinawy, F.; Shaker, E.M.; El-Din, H.A.S. Effect of elevated boron concentrations on the growth and yield of barley (Hordeum vulgare L.) and alleviation of its toxicity using different plant growth modulators. Aust. J. Crop Sci. 2012, 6, 1687–1695. [Google Scholar]
- Abdel-Motagally, F.M.F.; El-Zohri, M. Improvement of wheat yield grown under drought stress by boron foliar application at different growth stages. J. Saudi Soc. Agric. Sci. 2016. [Google Scholar] [CrossRef]
- Salam, M.A.; Siddique, M.A.; Rahim, M.A.; Rahman, M.A.; Goffar, M.A. Quality of tomato as influenced by boron and zinc in presence of different doses of cow dung. Bangladesh J. Agric. Res. 2011, 36, 151–163. [Google Scholar] [CrossRef]
- Miwa, K.; Takano, J.; Fujiwara, T. Improvement of seed yields under boron-limiting conditions through overexpression of BOR1, a boron transporter for xylem loading, in Arabidopsis thaliana. Plant J. 2006, 46, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Abid, M.; Ahmad, F.; Ullah, M.A.; Javaid, Q.; Ali, M.A. Impact of boron fertilization on dry matter production and mineral constitution of irrigated cotton. Pak. J. Bot. 2011, 43, 2903–2910. [Google Scholar]
- Camacho-Cristobal, J.J.; Gonzalez-Fontes, A. Boron deficiency decreases plasmalemma H+-ATPase expression and nitrate uptake, and promotes ammonium assimilation into asparagine in tobacco roots. Planta 2007, 226, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Quiles-Pando, C.; Rexach, J.; Navarro-Gochicoa, M.T.; Camacho-Cristóbal, J.J.; Herrera-Rodríguez, M.B.; González-Fontes, A. Boron deficiency increases the levels of cytosolic Ca2+ and expression of Ca2+ related genes in Arabidopsis thaliana roots. Plant Physiol. Biochem. 2013, 65, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Shorrocks, V.M. The occurrence and correction of boron deficiency. Plant Soil 1997, 193, 121–148. [Google Scholar] [CrossRef]
- Sheng, O.; Song, S.; Peng, S.; Deng, X. The effects of low boron on growth, gas exchange, boron concentration and distribution of ‘Newhall’ navel orange (Citrus sinensis Osb.) plants grafted on two rootstocks. Sci. Hortic. 2009, 121, 278–283. [Google Scholar] [CrossRef]
- Zhou, F.G.; Peng, S.A.; Liu, Y.Z.; Wei, Q.J.; Han, J.; Islam, M.Z. The physiological and nutritional responses of seven different citrus rootstock seedlings to boron deficiency. Trees 2014, 28, 295–307. [Google Scholar] [CrossRef]
- Bohnsack, C.W.; Albert, L.S. Early effects of boron deficiency on indoleacetic acid oxidase levels of squash root tips. Plant Physiol. 1977, 59, 1047–1050. [Google Scholar] [CrossRef] [PubMed]
- Miwa, K.; Fujiwara, T. Boron transport in plants: Co-ordinated regulation of transporters. Ann. Bot. 2010, 105, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Dordas, C.; Brown, P.H. Permeability of boric acid across lipid bilayers and factors affecting it. J. Membr. Biol. 2000, 175, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Dannel, F.; Pfeffer, H.; Römheld, V. Update on boron in higher plant-uptake, primary translocation and compartmentation. Plant Biol. 2002, 4, 193–204. [Google Scholar] [CrossRef]
- Takano, J.; Miwa, K.; Fujiwara, T. Boron transport mechanisms: Collaboration of channels and transporters. Trends Plant Sci. 2008, 13, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Takano, J.; Wada, M.; Ludewig, U.; Schaaf, G.; Von-Wiren, N.; Fujiwara, T. The Arabidopsis major intrinsic protein NIP5;1 is essential for efficient boron uptake and plant development under boron limitation. Plant Cell 2006, 18, 1498–1509. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Wallace, I.S.; Takano, J.; Roberts, D.M.; Fujiwara, T. NIP6;1 is a boric acid channel for preferential transport of boron to growing shoot tissues in Arabidopsis. Plant Cell 2008, 20, 2860–2875. [Google Scholar] [CrossRef] [PubMed]
- Hanaoka, H.; Uraguchi, S.; Takano, J.; Tanaka, M.; Fujiwara, T. OsNIP3;1, a rice boric acid channel, regulates boron distribution and is essential for growth under boron-deficient conditions. Plant J. 2014, 78, 890–902. [Google Scholar] [CrossRef] [PubMed]
- Takano, J.; Noguchi, K.; Yasumori, M.; Kobayashi, M.; Gajdos, Z.; Miwa, K.; Hayashi, H.; Yoneyama, T.; Fujiwara, T. Arabidopsis boron transporter for xylem loading. Nature 2002, 420, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Turtle-Schmidt, B.H.; Stroud, R.M. Structure of Bor1 supports an elevator transport mechanism for SLC4 anion exchangers. Proc. Natl. Acad. Sci. USA 2016, 113, 10542–10546. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Hanaoka, H.; Kobayashi, M.; Miyoshi, K.; Miwa, K.; Fujiwara, T. Cell type specificity of the expression of OsBOR1, a rice efflux boron transporter gene, is regulated in response to boron availability for efficient boron uptake and xylem loading. Plant Cell 2007, 19, 2624–2635. [Google Scholar] [CrossRef] [PubMed]
- Takano, J.; Miwa, K.; Yuan, L.; von Wirén, N.; Fujiwara, T. Endocytosis and degradation of BOR1, a boron transporter of Arabidopsis thaliana, regulated by boron availability. Proc. Natl. Acad. Sci. USA 2005, 102, 12276–12281. [Google Scholar] [CrossRef] [PubMed]
- Miwa, K.; Wakuta, S.; Takada, S.; Ide, K.; Takano, J.; Naito, S.; Omori, H.; Matsunaga, T.; Fujiwara, T. Roles of BOR2, a boron exporter, in crosslinking of rhamnogalacturonan II and root elongation under boron limitation in Arabidopsis thaliana. Plant Physiol. 2013, 163, 1699–1709. [Google Scholar] [CrossRef] [PubMed]
- Takada, S.; Miwa, K.; Omori, H.; Fujiwara, T.; Naito, S.; Takano, J. Improved tolerance to boron deficiency by enhanced expression of the boron transporter BOR2. Soil Sci. Plant Nutr. 2014, 60, 341–348. [Google Scholar] [CrossRef]
- Brown, P.H.; Hu, H. Phloem mobility of boron is species dependent: Evidence for phloem mobility in sorbitol-rich species. Ann. Bot. 1996, 77, 497–505. [Google Scholar] [CrossRef]
- Liakopoulos, G.; Stavrianakou, S.; Filippou, M.; Fasseas, C.; Tsadilas, C.; Drossopoulos, I.; Karabourniotis, G. Boron remobilization at low boron supply in olive (Olea europaea) in relation to leaf and phloem mannitol concentrations. Tree Physiol. 2005, 25, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.H.; Bellaloui, N.; Hu, H.; Dandekar, A. Transgenically enhanced sorbitol synthesis facilitates phloem boron transport and increases tolerance of tobacco to boron deficiency. Plant Physiol. 1999, 119, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Malamy, J.E. Intrinsic and environmental response pathways that regulate root system architecture. Plant Cell Environ. 2005, 28, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Wang, Q.L.; Li, Z.H.; Duan, L.S.; Tian, X.L. Effects of potassium deficiency on root growth of cotton seedlings and its physiological mechanisms. ACTA Agron. Sin. 2009, 35, 718–723. [Google Scholar] [CrossRef]
- Hodge, A.; Berta, G.; Doussan, C.; Merchan, F.; Crespi, M. Plant root growth, architecture and function. Plant Soil. 2009, 321, 153–187. [Google Scholar] [CrossRef]
- Martín-Rejano, E.M.; Camacho-Cristóbal, J.J.; Herrera-Rodríguez, M.B.; Rexach, J.; Navarro-Gochicoa, M.T.; González-Fontes, A. Auxin and ethylene are involved in the responses of root system architecture to low boron supply in Arabidopsis seedlings. Physiol. Plant. 2011, 142, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Neumann, G.; Martinoia, E. Cluster roots—An underground adaptation for survival in extreme environments. Trends Plant Sci. 2002, 7, 162–167. [Google Scholar] [CrossRef]
- Navara, J. Participation of individual root types in water uptake by maize seedlings. Biologia 1987, 42, 17–26. [Google Scholar]
- González-Fontes, A.; Herrera-Rodríguez, M.B.; Martín-Rejano, E.M.; Navarro-Gochicoa, M.T.; Rexach, J.; Camacho-Cristóbal, J.J. Root responses to boron deficiency mediated by ethylene. Front. Plant Sci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Bates, T.R.; Lynch, T.P. Stimulation of root hair elongation in Arabidopsis thailana by low phosphorus availability. Plant Cell Environ. 1996, 19, 529–538. [Google Scholar] [CrossRef]
- Perez-Torres, C.A.; Lopez-Bucio, J.; Cruz-Ramırez, A.; Ibarra-Laclette, E.; Dharmasiri, S.; Estelle, M.; Herrera-Estrella, L. Phosphate availability alters lateral root development in Arabidopsis by modulating auxin sensitivity via a mechanism involving the TIR1 auxin receptor. Plant Cell 2008, 20, 3258–3272. [Google Scholar] [CrossRef] [PubMed]
- Landsberg, E.C. Hormonal regulation of iron-stress response in sunflower roots: A morphological and cytological investigation. Protoplasma 1996, 194, 69–80. [Google Scholar] [CrossRef]
- Fazio, G.; Kviklys, D.; Grusak, M.A.; Robinson, T.L. Phenotypic diversity and QTL mapping of absorption and translocation of nutrients by apple rootstocks. Asp. Appl. Biol. 2013, 119, 37–50. [Google Scholar]
- Treeby, M.; Marschner, H.; Romheld, V. Mobilization of iron and other micronutrients cations from a calcerous soil by plant-borne, microbial, and synthetic metal chelators. Plant Soil 1989, 114, 217–226. [Google Scholar] [CrossRef]
- Bocanegra, M.P.; Lobartini, J.C.; Orioli, G.A. Plant uptake of iron chelated by humic acids of different molecular weights. Commun. Soil Sci. Plant Anal. 2006, 37, 239–248. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Huang, Y.; Mu, X.; Bie, Z. Melatonin application alters the root morphology and nitrogen uptake of watermelon. In Proceedings of the Second Asian Horticultural Congress, Chengdu, China, 26–28 September 2016; pp. 28–29. [Google Scholar]
- Nawaz, M.A.; Huang, Y.; Bie, Z.; Ahmed, W.; Reiter, R.J.; Niu, M.; Hameed, S. Melatonin: Current status and future perspectives in plant science. Front. Plant Sci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Sheng, O.; Peng, S.A.; Zhou, G.F.; Wei, Q.J.; Li, Q.H. Growth, root morphology and boron uptake by citrus rootstock seedlings differing in boron-deficiency responses. Sci. Hortic. 2011, 129, 426–432. [Google Scholar] [CrossRef]
- Bie, Z.; Nawaz, M.A.; Huang, Y.; Lee, J.M.; Colla, G. Introduction of vegetable grafting. In Vegetable Grafting, Principles and Practices, 1st ed.; Colla, G., Alfocea, F.P., Schwarz, D., Eds.; CABI Publishing: Wallingford, UK, 2017; pp. 1–21. [Google Scholar]
- Huang, Y.; Jiao, Y.; Nawaz, M.A.; Chen, C.; Liu, L.; Kong, Q.; Cheng, F.; Bie, Z. Improving magnesium uptake, photosynthesis and antioxidant enzyme activities of watermelon by grafting onto pumpkin rootstock under low magnesium. Plant Soil 2016, 409, 229–246. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Imtiaz, M.; Kong, Q.; Cheng, F.; Ahmad, W.; Huang, Y.; Bie, Z. Grafting: A technique to modify ion accumulation in horticultural crops. Front. Plant Sci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.A.; Shireen, F.; Huang, Y.; Zhilong, B.; Ahmed, W.; Saleem, B.A. Perspectives of vegetable grafting in Pakistan: Current status, challenges and opportunities. Int. J. Agric. Biol. 2017, 19, 1165–1174. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Wang, L.; Jiao, Y.; Chen, C.; Zhao, L.; Mei, M.; Yu, Y.; Bie, Z. Pumpkin rootstock improves nitrogen use efficiency of watermelon scion by enhancing nutrient uptake, cytokinin content, and expression of nitrate reductase genes. Plant Growth Reg. 2017, 82, 233–246. [Google Scholar] [CrossRef]
- Ahmed, W.; Nawaz, M.A.; Iqbal, M.A.; Khan, M.M. Effect of different rootstocks on plant nutrient status and yield in Kinnow mandarin (Citrus reticulata Blanco). Pak. J. Bot. 2007, 39, 1779–1786. [Google Scholar]
- Wojicik, P.; Wojcik, M.; Treder, W. Boron absorption and translocation in apple rootstock under conditions of low medium boron. J. Plant Nutr. 2003, 26, 961–968. [Google Scholar] [CrossRef]
- El-Motaium, R.; Hu, H.; Brown, P.H. The relation tolerance of six prunus rootstocks to boron and salinity. J. Am. Soc. Hort. Sci. 1994, 119, 1169–1175. [Google Scholar]
- Martínez-Ballesta, M.C.; Alcaraz-López, C.; Muries, B.; Mota-Cadenas, C.; Carvajal, M. Physiological aspects of rootstok-scion interactions. Sci. Hortic. 2010, 127, 112–118. [Google Scholar] [CrossRef]
- Tagliavini, M.; Scudellari, D.; Marangoni, B.; Bastianel, A.; Franzin, F.; Zamborlini, M. Leaf mineral composition of apple tree: Sampling date and effects of cultivar and rootstock. J. Plant Nutr. 1992, 15, 605–619. [Google Scholar] [CrossRef]
- Liu, G.; Jiang, C.; Wang, Y.; Peng, S.A.; Zhong, B.; Ceng, Q.; Yuan, S. Changes in mineral element contents of Newhall’ navel orange (Citrus sinensis Osb.) grafted on two different rootstocks under boron deficiency. Plant Nutr. Fertil. Sci. 2011, 1, 180–185. [Google Scholar]
- Liu, G.D.; Wang, R.D.; Wu, L.S.; Peng, S.A.; Wang, Y.H.; Jiang, C.C. Boron distribution and mobility in navel orange grafted on citrange and trifoliate orange. Plant Soil 2012, 360, 123–133. [Google Scholar] [CrossRef]
- Brown, P.H.; Zhang, Q.; Ferguson, L. Influence of rootstock on nutrient acquisition by pistachio. J. Plant Nutr. 1994, 17, 1137–1148. [Google Scholar] [CrossRef]
- Taylor, B.K.; Dimsey, R.T. Rootstock and scion effects on the leaf nutrient composition of citrus trees. Aus. J. Exp. Agric. 1993, 33, 363–371. [Google Scholar] [CrossRef]
- Hrotko, K.; Magyar, L.; Borsos, G.; Gyeviki, M. Rootstock effect on nutrient concentration of sweet cherry leaves. J. Plant Nutr. 2014, 37, 1395–1409. [Google Scholar] [CrossRef]
- Wang, N.; Yan, T.; Fu, L.; Zhou, G.; Liu, Y.Z.; Peng, S.A. Differences in boron distribution and forms in four citrus scion–rootstock combinations with contrasting boron efficiency under boron-deficient conditions. Trees 2014, 28, 1589–1598. [Google Scholar] [CrossRef]
- Wang, N.; Wei, Q.J.; Yan, T.; Pan, Z.; Liu, Y.Z.; Peng, S.A. Improving the boron uptake of boron-deficient navel orange plants under low boron conditions by inarching boron-efficient rootstock. Sci. Hortic. 2016, 199, 49–55. [Google Scholar] [CrossRef]
- Kuffaman, G.L.; Kneivel, D.P.; Watschke, T.L. Effect of a biostimulants on the heat tolerance associated with photosynthetic capacity, membrane stability and polyphenol production of perennial rye grass. Crop Sci. 2007, 47, 261–267. [Google Scholar] [CrossRef]
- Brown, P.; Saa, S. Biostimulants in agriculture. Front. Plant Sci. 2015, 6, 671. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.S.S.; Fleming, C.; Selby, C.; Rao, J.R.; Martin, T. Plant biostimulants: A review on the processing of macro algae and use of extracts for crop management to reduce abiotic stresses. J. Appl. Phycol. 2014, 26, 465–490. [Google Scholar] [CrossRef]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The use of biostimulants for enhancing nutrient uptake. In Advances in Agronomy, 1st ed.; Sparks, D.L., Ed.; Elsevier Inc.: New York, NY, USA, 2015; Volume 147, pp. 141–174. [Google Scholar]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.M. The influence of biostimulants on the growth and on the biochemical composition of Vicia faba CV. Giza 3 beans. Rom. Biotechnol. Lett. 2013, 18, 8061–8068. [Google Scholar]
- Pascual, I.; Azcona, I.; Aguirreolea, J.; Morales, F.; Corpas, F.J.; Palma, J.M.; Rellan-Alvarez, R.; Sanchez-Diaz, M. Growth, yield and fruit quality of pepper plants amended with two sanitized sewage sludges. J. Agric. Food Chem. 2010, 58, 6951–6959. [Google Scholar] [CrossRef] [PubMed]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acid as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Verkleij, F.N. Seaweed extract in agriculture and horticulture: A review. Biol. Agric. Hortic. 1992, 8, 309–324. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extract as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Turan, M.; Kose, C. Seaweed extracts improve copper uptake of grapewine. Acta Agric. Scand. Sect. B Soil Plant Sci. 2004, 54, 213–220. [Google Scholar]
- Johansson, J.F.; Paul, L.R.; Finlay, R.D. Microbial interactions in the mycorrhizosphere and their significance for sustainable agriculture. FEMS Microbiol. Ecol. 2004, 48, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Asaeda, T.; Wang, Q.; Rashid, M.H. Arbuscular mycorrhizal influences on growth, nutrient uptake, and use efficiency of Miscanthus sacchariflorus growing on nutrient-deficient river bank soil. Flora 2015, 212, 46–54. [Google Scholar] [CrossRef]
- Newman, E.I.; Reddell, P. The distribution of mycorrhizas among the families of vascular plants. New Phytol. 1987, 106, 745–751. [Google Scholar] [CrossRef]
- Lavola, A.; Aphalo, P.J.; Lehto, T. Boron and other elements in sporophores of ectomycorrhizal and saprotrophic fungi. Mycorrhiza 2011, 21, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Hobbie, J.E.; Hobbie, E.A. N-15 in symbiotic fungi and plants estimates nitrogen and carbon flux rates in Arctic tundra. Ecology 2006, 87, 816–822. [Google Scholar] [CrossRef]
- Hildebrandt, U.; Regvar, M.; Bothe, H. Arbuscular mycorrhiza and heavy metal tolerance. Phytochemistry 2007, 68, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.B.; Zobel, R.W.; Zeto, S.K. Effects of mycorrhizal fungus isolate on mineral acquisition by Panicum virgatum in acidic soil. Mycorrhiza 1999, 9, 167–176. [Google Scholar] [CrossRef]
- Lu, S.; Miller, H.M. The role of VA mycorrhizae in the absorption of P and Zn by maize in field and growth chamber experiments. Can. J. Soil Sci. 1989, 69, 97–109. [Google Scholar] [CrossRef]
- Kothari, S.K.; Marschner, H.; Römheld, V. Direct and indirect effects of VA mycorrhizal fungi and rhizosphere microorganisms on acquisition of mineral nutrients by maize (Zea mays L.) in a calcareous soil. New Phytol. 1990, 116, 637–645. [Google Scholar] [CrossRef]
- Lewis, D.H. Boron, lignification and the origin of vascular plants-a unified hypothesis. New Phytol. 1980, 84, 209–229. [Google Scholar] [CrossRef]
- Hu, H.; Brown, P.H. Absorption of boron by plant roots. Plant Soil 1997, 193, 49–58. [Google Scholar] [CrossRef]
- Lecourt, J.; Lauvergeat, V.; Ollat, N.; Vivin, P.; Cookson, S.J. Shoot and root ionome responses to nitrate supply in grafted grape vines are rootstock genotype dependent. Aust. J. Grape Wine Res. 2015, 21, 311–318. [Google Scholar] [CrossRef]
- Borgognone, D.; Colla, G.; Rouphael, Y.; Cardarelli, M.; Reac, E.; Schwarzd, D. Effect of nitrogen form and nutrient solution pH on growth and mineral composition of self-grafted and grafted tomatoes. Sci. Hortic. 2013, 149, 61–69. [Google Scholar] [CrossRef]
- Lehto, T.; Lavola, A.; Kallio, E.; Aphalo, P.J. Boron uptake by ectomycorrhizas of silver birch. Mycorrhiza 2004, 14, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Ruuhola, T.; Lehto, T. Do ectomycorrhizas affect boron uptake in Betula pendula? Can. J. For. Res. 2014, 44, 1013–1019. [Google Scholar] [CrossRef]
- Dixon, R.K.; Garrett, H.E.; Cox, G.S. Boron fertilization, vesicular-arbuscular mycorrhizal colonization and growth of Citrus jambhiri lush. J. Plant Nutr. 1989, 12, 687–700. [Google Scholar] [CrossRef]
- Clark, R.B.; Zeto, S.K. Mineral acquisition by mycorrhizal maize grown on acid and alkaline soil. Soil Biol. Biochem. 1996, 28, 1495–1503. [Google Scholar] [CrossRef]
- Mitchell, R.J.; Garrett, H.E.; Cox, G.S.; Atalay, A. Boron and ectomycorrhizal influences on mineral nutrition of container-grown Pinus ehinata mill. J. Plant Nutr. 1990, 13, 1555–1574. [Google Scholar] [CrossRef]
- Shin, F.; Cakmakci, R.; Kantar, F. Sugar beet and barley yields in relation to inoculation with N2-fixing and phosphate solubilizing bacteria. Plant Soil 2004, 265, 123–129. [Google Scholar] [CrossRef]
- Zahir, Z.A.; Munir, A.; Asghar, H.N.; Shahroona, B.; Arshad, M. Effectiveness of rhizobacteria containing ACC-deaminase for growth promotion of peas (Pisum sativum) under drought conditions. J. Microbiol. Biotechnol. 2008, 18, 958–963. [Google Scholar] [PubMed]
- Lucy, M.; Reed, E.; Glick, B.R. Application of free living plant growth-promoting rhizobacteria. Anton Leeuw 2004, 86, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.; Abbasi, M.K.; Khan, M.A.; Khaliq, A.; Sultan, T.; Aslam, M. Effect of plant growth-promoting rhizobacteria on growth, nodulation and nutrient accumulation of lentil under controlled conditions. Pedosphere 2012, 22, 848–859. [Google Scholar] [CrossRef]
- Patten, C.L.; Glick, B.R. Bacterial biosynthesis of indole-3-acetic acid. Can. J. Microbiol. 1996, 42, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Yildrim, E.; Donmez, M.F.; Turan, M. Use of bioinoculants in ameliorative effects on radish plants under salinity stress. J. Plant Nutr. 2008, 31, 2059–2074. [Google Scholar] [CrossRef]
- Kavinoa, M.; Harishb, S.; Kumara, N.; Saravanakumar, D.; Samiyappan, R. Effect of chitinolytic PGPR on growth, yield and physiological attributes of banana (Musa spp.) under field conditions. Appl. Soil Ecol. 2010, 45, 71–77. [Google Scholar] [CrossRef]
- Dawwam, G.E.; Elbeltagy-Emara, A.H.M.; Abbas, I.H.; Hassan, M.M. Beneficial effect of plant growth promoting bacteria isolated from the roots of potato plant. Ann. Agric. Sci. 2013, 58, 195–201. [Google Scholar] [CrossRef]
- Ahmed, I.; Yokota, A.; Fujiwara, T. A novel highly boron tolerant bacterium, Bacillus boroniphilus sp. nov., isolated from soil, that requires boron for its growth. Extremophiles 2007, 11, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Yokota, A.; Fujiwara, T. Gracilibacillus boraciitolerans sp. nov., a highly boron-tolerant and moderately halotolerant bacterium isolated from soil. Int. J. Syst. Evol. Microbiol. 2007, 57, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Miwa, H.; Fujiwara, T. Isolation and identification of boron-accumulating bacteria from contaminated soils and active sludge. Soil Sci. Plant Nutr. 2009, 55, 643–646. [Google Scholar] [CrossRef]
- Han, H.S.; Lee, K.D. Phosphate and potassium solubilizing bacteria effect on mineral uptake, soil availability and growth of eggplant. Res. J. Agric. Biol. Sci. 2005, 1, 176–180. [Google Scholar]
- Gul, A.; Ozaktan, H.; Kıdoglu, F.; Tuzel, Y. Rhizobacteria promoted yield of cucumber plants grown in perlite under Fusarium wilt stress. Sci. Hortic. 2013, 153, 22–25. [Google Scholar] [CrossRef]
- Ahirwar, N.K.; Gupta, G.; Singh, V.; Rawlley, R.K.; Ramana, S. Influence on growth and fruit yield of tomato (Lycopersicon esculentum Mill.) plants by inoculation with Pseudomonas fluorescence (SS5): Possible role of plant growth promotion. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 720–730. [Google Scholar]
- Siddiqui, A.R.; Shahzad, S.M.; Ashraf, M.; Nazeer, S.; Piracha, M.A.; Khalid, A.; Saleem, M.A.; Zaidi, S.S.A. Integrated use of p-solubilizing bacteria with and without acc-deaminase activity and compost for improving growth and yield of wheat. J. Environ. Agric. 2016, 1, 1–11. [Google Scholar]
- Arifa, M.S.; Riaza, M.; Shahzad, S.M.; Yasmeen, T.; Akhtar, M.J.; Riaz, M.A.; Jasseye, V.E.J.; Bragazzae, L.; Buttler, A. Associative interplay of plant growth promoting rhizobacteria (Pseudomonas aeruginosa QS40) with nitrogen fertilizers improves sunflower (Helianthus annuus L.) productivity and fertility of aridisol. Appl. Soil Ecol. 2016, 108, 238–247. [Google Scholar] [CrossRef]
- Kashyap, P.L.; Xiang, X.; Heiden, P. Chitosan nanoparticle based delivery systems for sustainable agriculture. Int. J. Biol. Macromol. 2015, 77, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Shabala, L.; Shabala, S.; Giraldo, J.P. Hydroxyl radical scavenging by cerium oxide nanoparticles improves Arabidopsis salinity tolerance by enhancing leaf mesophyll potassium retention. Environ. Sci. Nano 2018. [Google Scholar] [CrossRef]
- Ghormade, V.; Deshpande, M.V.; Paknikar, K.M. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol. Adv. 2011, 29, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Gómez, H.G.; Godina, F.R.; Ortiz, H.O.; Mendoza, A.B.; Torres, V.R.; De-la-Fuente, M.C. Use of chitosan-pva hydrogels with copper nanoparticles to improve the growth of grafted watermelon. Molecules 2017, 22, 1031. [Google Scholar] [CrossRef] [PubMed]
- Baruah, S.; Dutta, J. Nanotechnology applications in pollution sensing and degradation in agriculture: A review. Environ. Chem. Lett. 2009, 7, 191–204. [Google Scholar] [CrossRef]
- Davidson, D.; Gu, F.X. Materials for sustained and controlled release of nutrients and molecules to support plant growth. J. Agric. Food Chem. 2012, 60, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Peralta-Videa, J.R.; Rico, C.M.; Hernandez-Viezcas, J.A.; Sun, Y.; Niu, G.; Servin, A.; Nunez, J.E.; Duarte-Gardea, M.; Gardea-Torresdey, J.L. CeO2 and ZnO nanoparticles change the nutritional qualities of cucumber (Cucumis sativus). J. Agric. Food Chem. 2014, 62, 2752–2759. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, D.K.; Dasgupta-Schubert, N.; Villaseñor-Cendejas, L.M.; Villegas, J.; Carreto-Montoya, L.; Borjas-García, S.E. Interfacing carbon nanotubes (CNT) with plants: Enhancement of growth, water and ionic nutrient uptake in maize (Zea Mays) and implications for nanoagriculture. Appl. Nanosci. 2014, 4, 577–591. [Google Scholar] [CrossRef]
- Zheng, L.; Hong, F.; Lu, S.; Liu, C. Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol. Trace Elem. Res. 2005, 104, 84–91. [Google Scholar] [CrossRef]
- Waters, B.M.; Troupe, G.C. Natural variation in iron use efficiency and mineral remobilization in cucumber (Cucumis sativus). Plant Soil 2012, 352, 185–197. [Google Scholar] [CrossRef]
- Xu, F.S.; Wang, Y.H.; Meng, J. Mapping boron efficiency gene (s) in Brassica napus using RFLP and AFLP markers. Plant Breed. 2001, 120, 319–324. [Google Scholar] [CrossRef]
- Du, C.W.; Wang, Y.H.; Xu, F.S.; Yang, Y.H.; Wang, H.Y. Study on the physiological mechanism of boron utilization efficiency in rape cultivars. J. Plant Nutr. 2002, 25, 231–244. [Google Scholar] [CrossRef]
- Sarafi, E.; Tsouvaltzis, P.; Chatzissavvidis, C.; Siomos, A.; Therios, L. Melatonin and resveratrol reverse the toxic effect of high boron (B) and modulate biochemical parameters in pepper plants (Capsicum annuum L.). Plant Physiol. Biochem. 2017, 112, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Adesemoye, A.O.; Torbert, H.A.; Kloepper, J.W. Enhanced plant nutrient use efficiency with PGPR and AMF in an integrated nutrient management system. Can. J. Microbiol. 2008, 54, 876–886. [Google Scholar] [CrossRef] [PubMed]
| Grafting | ||||
| Crops | Rootstocks | Plant Parts Utilized | Increase in B Concentration (%) | References |
| Navel orange (Citrus sinensis Osb) | Carrizo Citrange [Citrus sinensis (L.) Osb. × Poncirus trifoliata (L.) Raf.] | New leaves Middle leaves Basal leaves Roots New twig Old twig | 24–51 53 66–149 63 25 24 | [29,73,79] |
| Trifoliate orange [Poncirus trifoliata (L.) Raf.] | New leaves Roots | 6 12 | [74] | |
| Troyer citrange (Citrus sinensis Washington × Poncirus trifoliata) | Leaves | 34 | [76] | |
| Cleoptra mandarin (Citrus reticulata) | Leaves | 29 | [76] | |
| Rough lemon (Citrus Jambhiri Lush) | Leaves | 29 | [76] | |
| Sweet Cherry (Prunus avium L.) cultivar Petrus | GiSelA 6 (Prunus cerasus × Prunus canescens, Gi 148/1) | Leaves | 20 | [77] |
| Sweet Cherry (Prunus avium) | Leaves | 15 | [77] | |
| Magyar | Leaves | 14 | [77] | |
| Sweet Cherry (Prunus avium L.), cultivar Rita | GiSelA 6 (Prunus cerasus × Prunus canescens, Gi 148/1) | Leaves | 13 | [77] |
| Sweet Cherry (Prunus avium) | Leaves | 8 | [77] | |
| Grapes (Vitis vinifera L.) | Riparia Gloirede Montpellier (RGM) and 1103 Paulsen (Vitis vinifera L.) | Leaves | 93 | [103] |
| Pistachio (Pistacia atlantica L.) cultivar Kerman | Pistacia atlantica | Leaves | 19 | [47] |
| Pistacia integerrima | Leaves | 3 | [47] | |
| UCBI (Pistacia atlantica× Pistacia integerrima) | Leaves | 68 | [47] | |
| Tomato (Solanum lycopersicum L.) | Maxifort (Solanum lycopersicum L. × Solanum habrochaites S. Knappand D.M. Spooner) | Leaves | 3 | [104] |
| Mycorrhizal Fungi | ||||
| Crop | AMF Species | Plant Part Utilized | Increase in B Concentration (%) | Reference |
| Silver Birch (Betula pendula) | Laccaria sp. | Roots Stem Leaves | 5–19 5 3 | [105,106] |
| Paxillus involutus | Roots Stem Leaves | 78 5–15 14 | [105,106] | |
| Rough lemon (Citrus Jambhiri Lush) | Glomus fasciculatum | Leaves | 11–18 * | [107] |
| Maize (Zea mays L.) | Glomus etunicatum WV579A | Shoot | 331–689 | [108] |
| Glomus diaphanum WV579B | Shoot | 261–510 | [108] | |
| Glomus intraradices WV894 | Shoot | 284–531 | [108] | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shireen, F.; Nawaz, M.A.; Chen, C.; Zhang, Q.; Zheng, Z.; Sohail, H.; Sun, J.; Cao, H.; Huang, Y.; Bie, Z. Boron: Functions and Approaches to Enhance Its Availability in Plants for Sustainable Agriculture. Int. J. Mol. Sci. 2018, 19, 1856. https://doi.org/10.3390/ijms19071856
Shireen F, Nawaz MA, Chen C, Zhang Q, Zheng Z, Sohail H, Sun J, Cao H, Huang Y, Bie Z. Boron: Functions and Approaches to Enhance Its Availability in Plants for Sustainable Agriculture. International Journal of Molecular Sciences. 2018; 19(7):1856. https://doi.org/10.3390/ijms19071856
Chicago/Turabian StyleShireen, Fareeha, Muhammad Azher Nawaz, Chen Chen, Qikai Zhang, Zuhua Zheng, Hamza Sohail, Jingyu Sun, Haishun Cao, Yuan Huang, and Zhilong Bie. 2018. "Boron: Functions and Approaches to Enhance Its Availability in Plants for Sustainable Agriculture" International Journal of Molecular Sciences 19, no. 7: 1856. https://doi.org/10.3390/ijms19071856
APA StyleShireen, F., Nawaz, M. A., Chen, C., Zhang, Q., Zheng, Z., Sohail, H., Sun, J., Cao, H., Huang, Y., & Bie, Z. (2018). Boron: Functions and Approaches to Enhance Its Availability in Plants for Sustainable Agriculture. International Journal of Molecular Sciences, 19(7), 1856. https://doi.org/10.3390/ijms19071856





