UDP-Glucose 4-Epimerase and β-1,4-Galactosyltransferase from the Oyster Magallana gigas as Valuable Biocatalysts for the Production of Galactosylated Products
Abstract
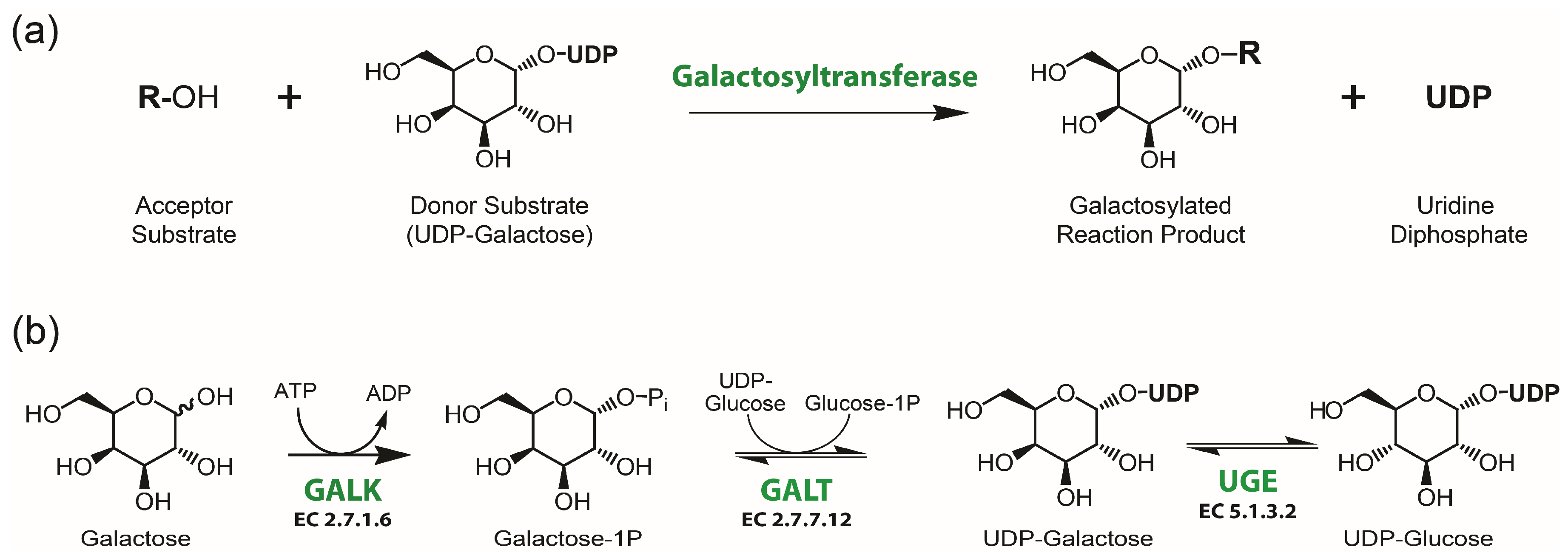
1. Introduction
2. Results
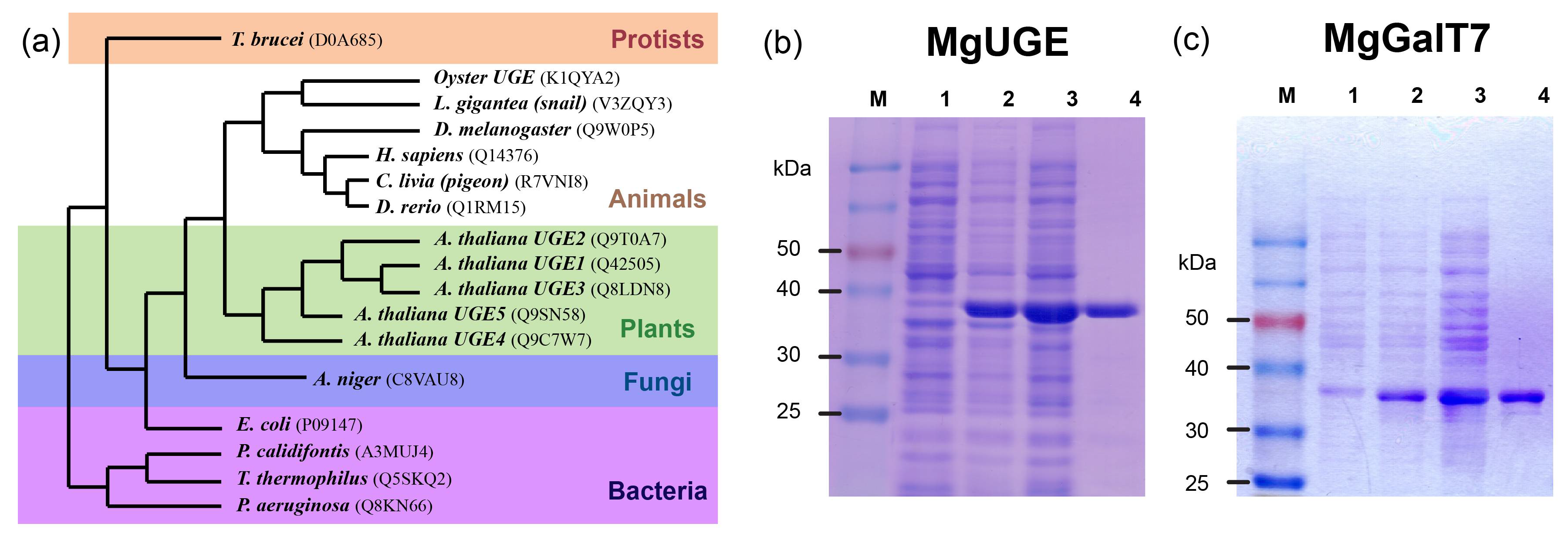
2.1. Cloning and Homology Analysis of the Oyster UGE Gene
2.2. Protein Expression and Purification
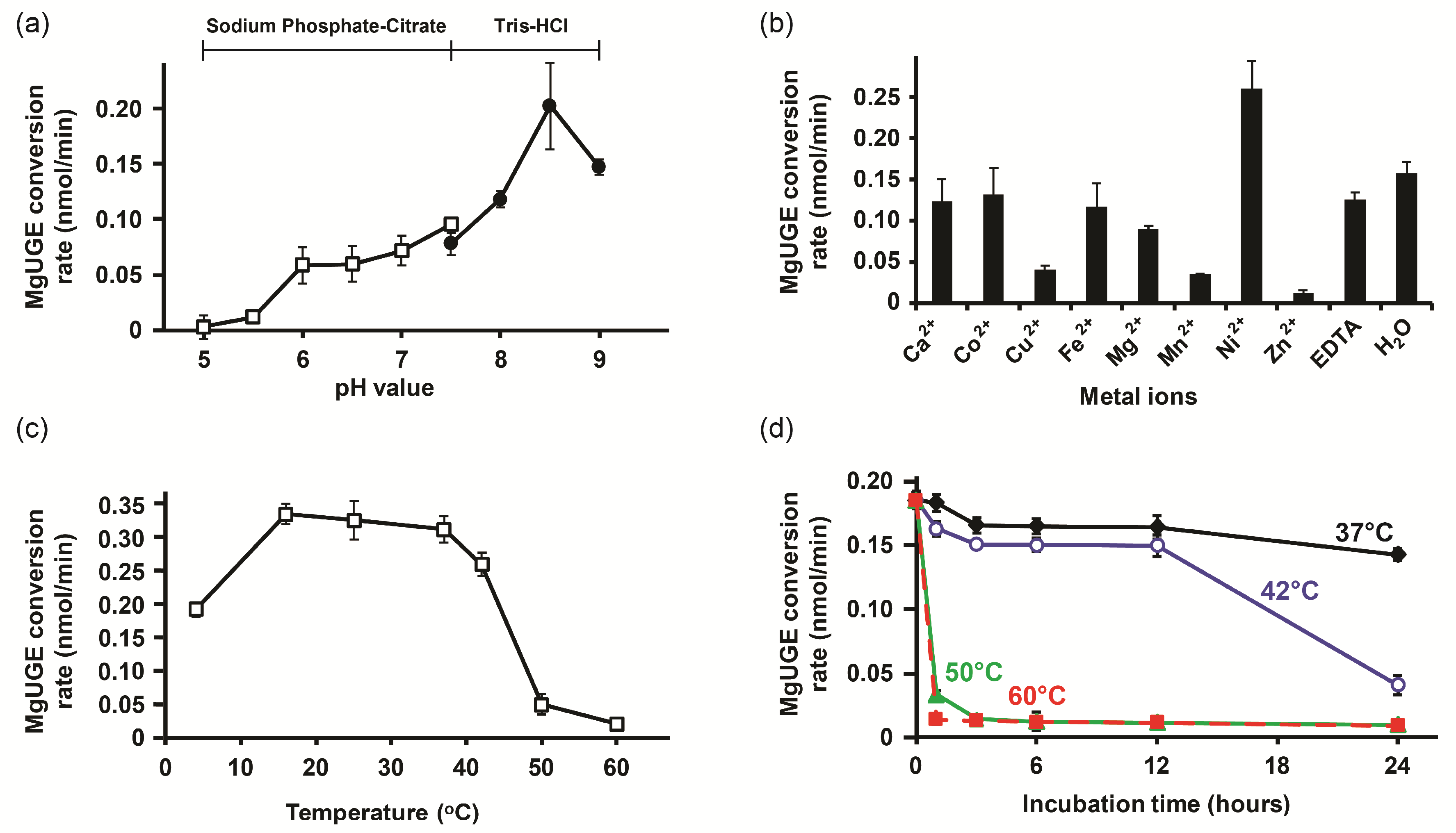
2.3. Biochemical Characterization
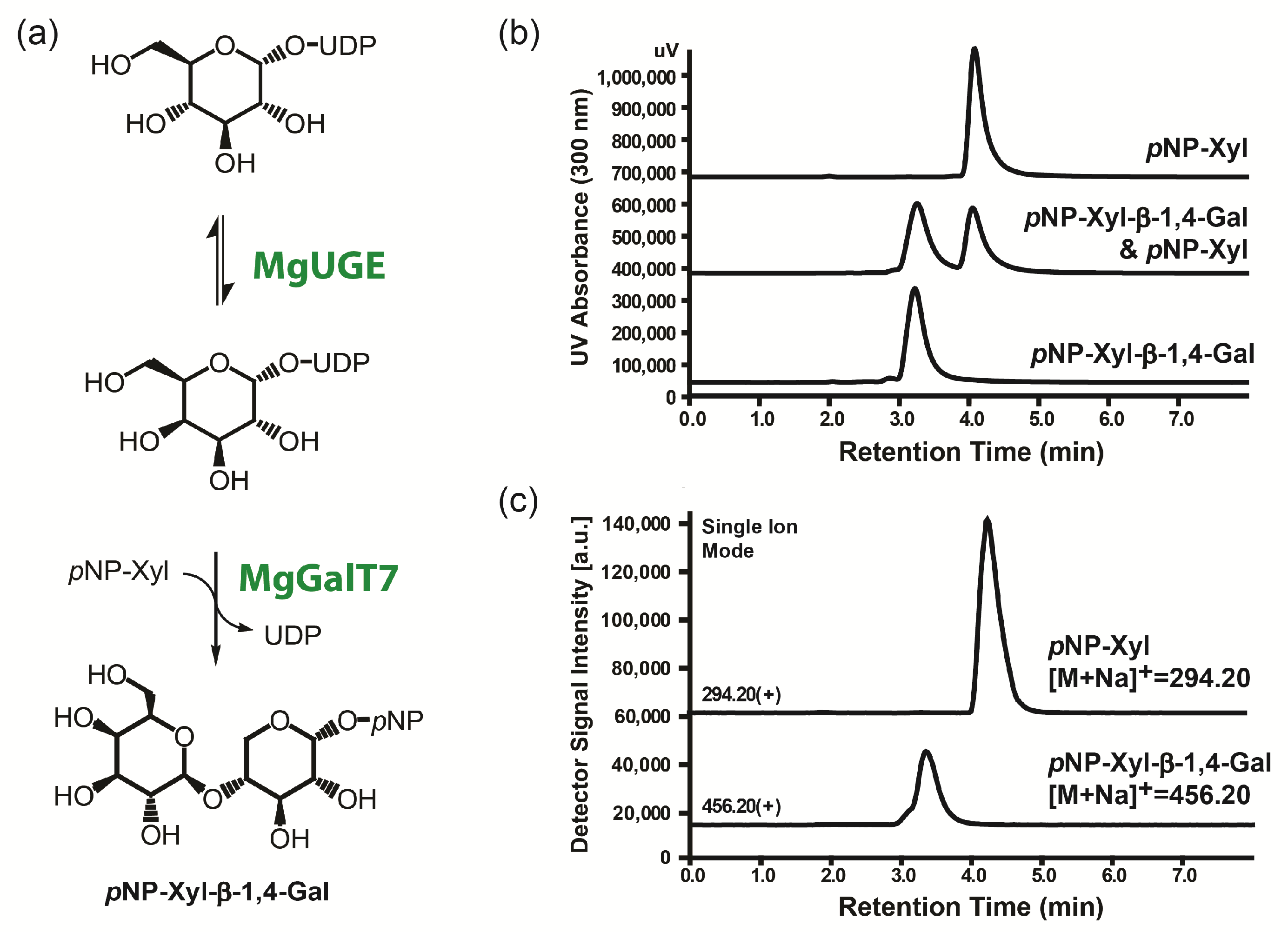
2.4. Enzymatic Galactosylation Reaction of Para-Nitrophenol Xylose
3. Discussion
3.1. Characterization of MgUGE
3.2. Potential of MgUGE in Galactosylation Reactions
3.3. Molecular Mechanism of MgUGE
4. Materials and Methods
4.1. General
4.2. Gene Amplification and Construction of the Expression Vectors
4.3. Expression and Purification of Recombinant MgUGE and MgGalT7
4.4. Enzymatic Assay
4.5. Biochemical Characterization of MgUGE
4.6. Enzymatic Galactosylation Reaction
4.7. Homology Modeling and Phylogenetic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| cDNA | Complementary deoxyribonucleic acid |
| DEPC | Diethylpyrocarbonate |
| EDTA | Ethylenediaminetetraacetic acid |
| ESI-MS | Electrospray ionization mass spectrometry |
| GALK | Galactokinase |
| GALT | Galactose-1-phosphate uridylyltransferase |
| HPLC | High-performance liquid chromatography |
| ID | Identifier |
| IPTG | Isopropyl-β-D-thiogalactopyranoside |
| LCMS | Liquid chromatography-mass spectrometry |
| MgGalT7 | β-1,4-Galactosyltransferase derived from Magallana gigas |
| MUSCLE | Multiple sequence comparison by log-expectation |
| NAD+ | Oxidized form of nicotinamide adenine dinucleotide |
| NADH | Reduced form of nicotinamide adenine dinucleotide |
| PAGE | Polyacrylamide gel electrophoresis |
| PCR | Polymerase chain reaction |
| PDB | Protein data bank |
| PhyML | Phylogenies maximum likelihood |
| pNP | para-Nitrophenol |
| ODS | Octadecylsilyl |
| RNA | Ribonucleic acid |
| SDS | Sodium dodecyl sulfate |
| SIM | Single ion mode |
| StUGD | UDP-glucose dehydrogenase derived from Sphaerobacter thermophilus |
| UDP | Uridine diphosphate |
| UGE | UDP-glucose 4-epimerase |
References
- Babad, H.; Hassid, W.Z. Soluble uridine diphosphate d-galactose: d-Glucose β-4-d-galactosyltransferase from bovine milk. J. Biol. Chem. 1966, 241, 2672–2678. [Google Scholar] [PubMed]
- Derensy-Dron, D.; Krzewinski, F.; Brassart, C.; Bouquelet, S. β-1,3-Galactosyl-N-acetylhexosamine phosphorylase from Bifidobacterium bifidum DSM 20082: Characterization, partial purification and relation to mucin degradation. Biotechnol. Appl. Biochem. 1999, 29, 3–10. [Google Scholar] [PubMed]
- Sussman, M.; Osborn, M.J. UDP-galactose polysaccharide transferase in the cellular slime mold, Dictyostelium discoideum: Appearance and disappearance of activity during cell differentiation. Proc. Natl. Acad. Sci. USA 1964, 52, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, C.; Baenziger, J.; Kornfeld, S. Deficient uridine diphosphate-N-acetylglucosamine:glycoprotein N-acetylglucosaminyltransferase activity in a clone of Chinese hamster ovary cells with altered surface glycoproteins. J. Biol. Chem. 1975, 250, 3303–3309. [Google Scholar] [PubMed]
- Basu, S.; Kaufman, B.; Roseman, S. Conversion of Tay-Sachs ganglioside to monosialoganglioside by brain uridine diphosphate d-galactose: Glycolipid galactosyltransferase. J. Biol. Chem. 1965, 240, 4115–4117. [Google Scholar] [PubMed]
- Kadam, S.K.; Peppler, M.S.; Sanderson, K.E. Temperature-sensitive mutants in rfaI and rfaJ, genes for galactosyltransferase I and glucosyltransferase II, for synthesis of lipopolysaccharide in Salmonella typhimurium. Can. J. Microbiol. 1985, 31, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Bretting, H.; Whittaker, N.F.; Kabat, E.A.; Königsmann-Lange, K.; Thiem, H.-J. Chemical and immunochemical studies on the structure of four snail galactans. Carbohydr. Res. 1981, 98, 213–236. [Google Scholar] [CrossRef]
- Frey, P.A. The Leloir pathway: A mechanistic imperative for three enzymes to change the stereochemical configuration of a single carbon in galactose. FASEB J. 1996, 10, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Ballard, F. Kinetic studies with liver galactokinase. Biochem. J. 1966, 101, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Wedekind, J.E.; Frey, P.A.; Rayment, I. Three-Dimensional Structure of Galactose-1-phosphate Uridylyltransferase from Escherichia coli at 1.8A Resolution. Biochemistry 1995, 34, 11049–11061. [Google Scholar] [CrossRef] [PubMed]
- Schulz, J.M.; Watson, A.L.; Sanders, R.; Ross, K.L.; Thoden, J.B.; Holden, H.M.; Fridovich-Keil, J.L. Determinants of function and substrate specificity in human UDP-galactose 4′-epimerase. J. Biol. Chem. 2004, 279, 32796–32803. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.E.; Nolan, L.D.; Frey, P.A. UMP-dependent reduction of UDP-galactose 4-epimerase-NAD+ complex by sodium cyanoborohydride. Biochim. Biophys. Acta-Enzymol. 1974, 334, 442–447. [Google Scholar] [CrossRef]
- Wee, T.G.; Davis, J.; Frey, P.A. Studies on the mechanism of action of uridine diphosphate-galactose-4-epimerase: I. An ambiguity in the chemical trapping of a proposed keto-intermediate by NaB3H4. J. Biol. Chem. 1972, 247, 1339–1342. [Google Scholar] [PubMed]
- Timson, D.J. The molecular basis of galactosemia—Past, present and future. Gene 2016, 589, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Aldairi, A.; Ogundipe, O.; Pye, D. Antiproliferative activity of glycosaminoglycan-like polysaccharides derived from marine molluscs. Mar. Drugs 2018, 16, 63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Fang, X.; Guo, X.; Li, L.; Luo, R.; Xu, F.; Yang, P.; Zhang, L.; Wang, X.; Qi, H.; et al. The oyster genome reveals stress adaptation and complexity of shell formation. Nature 2012, 490, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hrmova, M.; Shirley, N.J.; Lahnstein, J.; Fincher, G.B. Gene expression patterns and catalytic properties of UDP-d-glucose 4-epimerases from barley (Hordeum vulgare L.). Biochem. J. 2006, 394, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Kotake, T.; Takata, R.; Verma, R.; Takaba, M.; Yamaguchi, D.; Orita, T.; Kaneko, S.; Matsuoka, K.; Koyama, T.; Reiter, W.D.; et al. Bifunctional cytosolic UDP-glucose 4-epimerases catalyse the interconversion between UDP-d-xylose and UDP-l-arabinose in plants. Biochem. J. 2009, 424, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Dalrymple, S.A.; Ko, J.; Sheoran, I.; Kaminskyj, S.G.; Sanders, D.A. Elucidation of substrate specificity in Aspergillus nidulans UDP-galactose-4-epimerase. PLoS ONE 2013, 8, e76803. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.B.; Hogness, D.S. The enzymes of the galactose operon in Escherichia coli. I. Purification and characterization of uridine diphosphogalactose 4-epimerase. J. Biol. Chem. 1964, 239, 2469–2481. [Google Scholar] [PubMed]
- Dey, P.M. UDP-galactose 4′-epimerase from Vicia faba seeds. Phytochemistry 1984, 23, 729–732. [Google Scholar] [CrossRef]
- Prodan-Zitnik, I.; Karas-Kuzelicki, N.; Lukac-Bajalo, J. Positive correlation between galactose-1-phosphate uridyltransferase (GALT) and UDP-galactose-4′-epimerase (GALE) activities. Clin. Biochem. 2009, 42, 1561–1564. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Hofmann, E.E.; Powell, E.N.; Klinck, J.M.; Kusaka, K. A population dynamics model for the Japanese oyster, Crassostrea gigas. Aquaculture 1997, 149, 285–321. [Google Scholar] [CrossRef]
- Barber, C.; Rosti, J.; Rawat, A.; Findlay, K.; Roberts, K.; Seifert, G.J. Distinct properties of the five UDP-d-glucose/UDP-d-galactose 4-epimerase isoforms of Arabidopsis thaliana. J. Biol. Chem. 2006, 281, 17276–17285. [Google Scholar] [CrossRef] [PubMed]
- Geren, C.R.; Ebner, K.E. Purification and characterization of UDP-galactose-4-epimerase from bovine tissues. J. Biol. Chem. 1977, 252, 2082–2088. [Google Scholar] [PubMed]
- Lee, L.-J.; Kimura, A.; Tochikura, T. Purification and properties of UDP-galactose 4-epimerase form Bifidobacterium bifidum. Agric. Biol. Chem. Tokyo 1978, 42, 731–737. [Google Scholar]
- Liu, Y.; Thoden, J.B.; Kim, J.; Berger, E.; Gulick, A.M.; Ruzicka, F.J.; Holden, H.M.; Frey, P.A. Mechanistic roles of tyrosine 149 and serine 124 in UDP-galactose 4-epimerase from Escherichia coli. Biochemistry 1997, 36, 10675–10684. [Google Scholar] [CrossRef] [PubMed]
- Thoden, J.B.; Wohlers, T.M.; Fridovich-Keil, J.L.; Holden, H.M. Human UDP-galactose 4-epimerase: Accommodation of UDP-N-acetylglucosamine within the active site. J. Biol. Chem. 2001, 276, 15131–15136. [Google Scholar] [CrossRef] [PubMed]
- Thoden, J.B.; Gulick, A.M.; Holden, H.M. Molecular structures of the S124A, S124T, and S124V site-directed mutants of UDP-galactose 4-epimerase from Escherichia coli. Biochemistry 1997, 36, 10685–10695. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Franke, J.; Sussman, M. Synthesis of uridine diphosphate glucose pyrophosphorylase during the development of Dictyostelium discoideum. J. Biol. Chem. 1971, 246, 6381–6388. [Google Scholar] [PubMed]
- Gu, B.; Laborda, P.; Wei, S.; Duan, X.C.; Song, H.B.; Liu, L.; Voglmeir, J. Discovery and biochemical characterization of the UDP-xylose biosynthesis pathway in Sphaerobacter thermophilus. Protein Pept. Lett. 2016, 23, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, J.F.; Corrales, G.; Casas, J.; Fernandez-Mayoralas, A.; Garcia-Junceda, E. Synthesis and evaluation of xylopyranoside derivatives as “decoy acceptors” of human β-1,4-galactosyltransferase 7. Mol. Biosyst. 2011, 7, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Y.; Laborda, P.; Lu, A.-M.; Duan, X.-C.; Ma, H.-Y.; Liu, L.; Voglmeir, J. N-Acetylglucosamine 2-epimerase from Pedobacter heparinus: First experimental evidence of a deprotonation/reprotonation mechanism. Catalysts 2016, 6, 212. [Google Scholar] [CrossRef]
- Webb, B.; Sali, A. Comparative Protein Structure Modeling Using MODELLER. In Current Protocols in Bioinformatics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002. [Google Scholar]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple Ligand–Protein Interaction Diagrams for Drug Discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
- Dereeper, A.; Guignon, V.; Blanc, G.; Audic, S.; Buffet, S.; Chevenet, F.; Dufayard, J.F.; Guindon, S.; Lefort, V.; Lescot, M.; et al. Phylogeny.fr: Robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008, 36, W465–W469. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, H.-B.; He, M.; Cai, Z.-P.; Huang, K.; Flitsch, S.L.; Liu, L.; Voglmeir, J. UDP-Glucose 4-Epimerase and β-1,4-Galactosyltransferase from the Oyster Magallana gigas as Valuable Biocatalysts for the Production of Galactosylated Products. Int. J. Mol. Sci. 2018, 19, 1600. https://doi.org/10.3390/ijms19061600
Song H-B, He M, Cai Z-P, Huang K, Flitsch SL, Liu L, Voglmeir J. UDP-Glucose 4-Epimerase and β-1,4-Galactosyltransferase from the Oyster Magallana gigas as Valuable Biocatalysts for the Production of Galactosylated Products. International Journal of Molecular Sciences. 2018; 19(6):1600. https://doi.org/10.3390/ijms19061600
Chicago/Turabian StyleSong, Hui-Bo, Meng He, Zhi-Peng Cai, Kun Huang, Sabine L. Flitsch, Li Liu, and Josef Voglmeir. 2018. "UDP-Glucose 4-Epimerase and β-1,4-Galactosyltransferase from the Oyster Magallana gigas as Valuable Biocatalysts for the Production of Galactosylated Products" International Journal of Molecular Sciences 19, no. 6: 1600. https://doi.org/10.3390/ijms19061600
APA StyleSong, H.-B., He, M., Cai, Z.-P., Huang, K., Flitsch, S. L., Liu, L., & Voglmeir, J. (2018). UDP-Glucose 4-Epimerase and β-1,4-Galactosyltransferase from the Oyster Magallana gigas as Valuable Biocatalysts for the Production of Galactosylated Products. International Journal of Molecular Sciences, 19(6), 1600. https://doi.org/10.3390/ijms19061600





