Effects of Periostracum Cicadae on Cytokines and Apoptosis Regulatory Proteins in an IgA Nephropathy Rat Model
Abstract
1. Introduction
2. Results
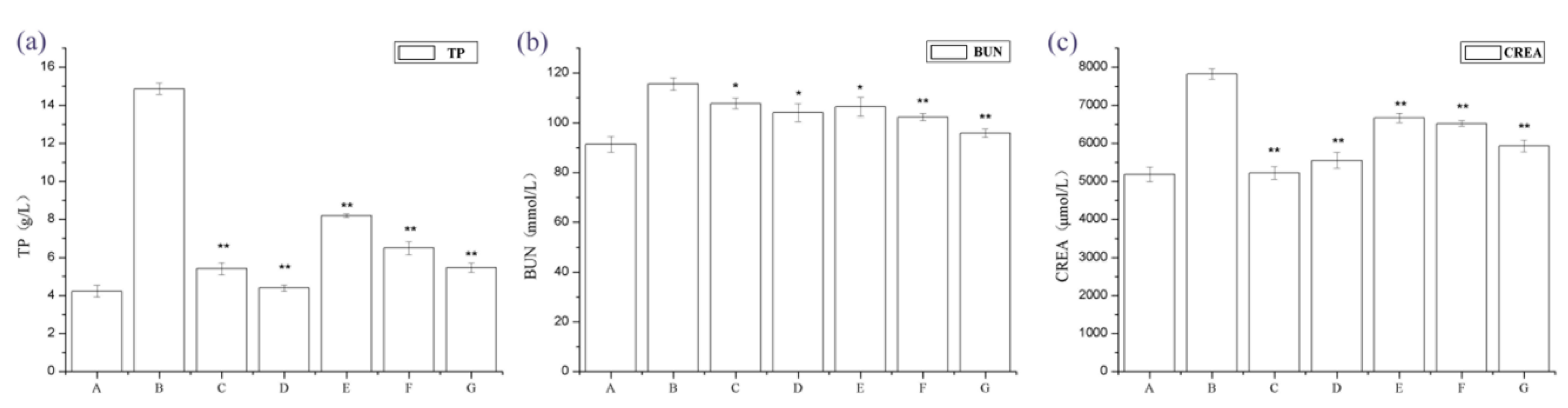
2.1. The Effect of Periostracum Cicadae on Proteinuria and Other Biochemical Parameters
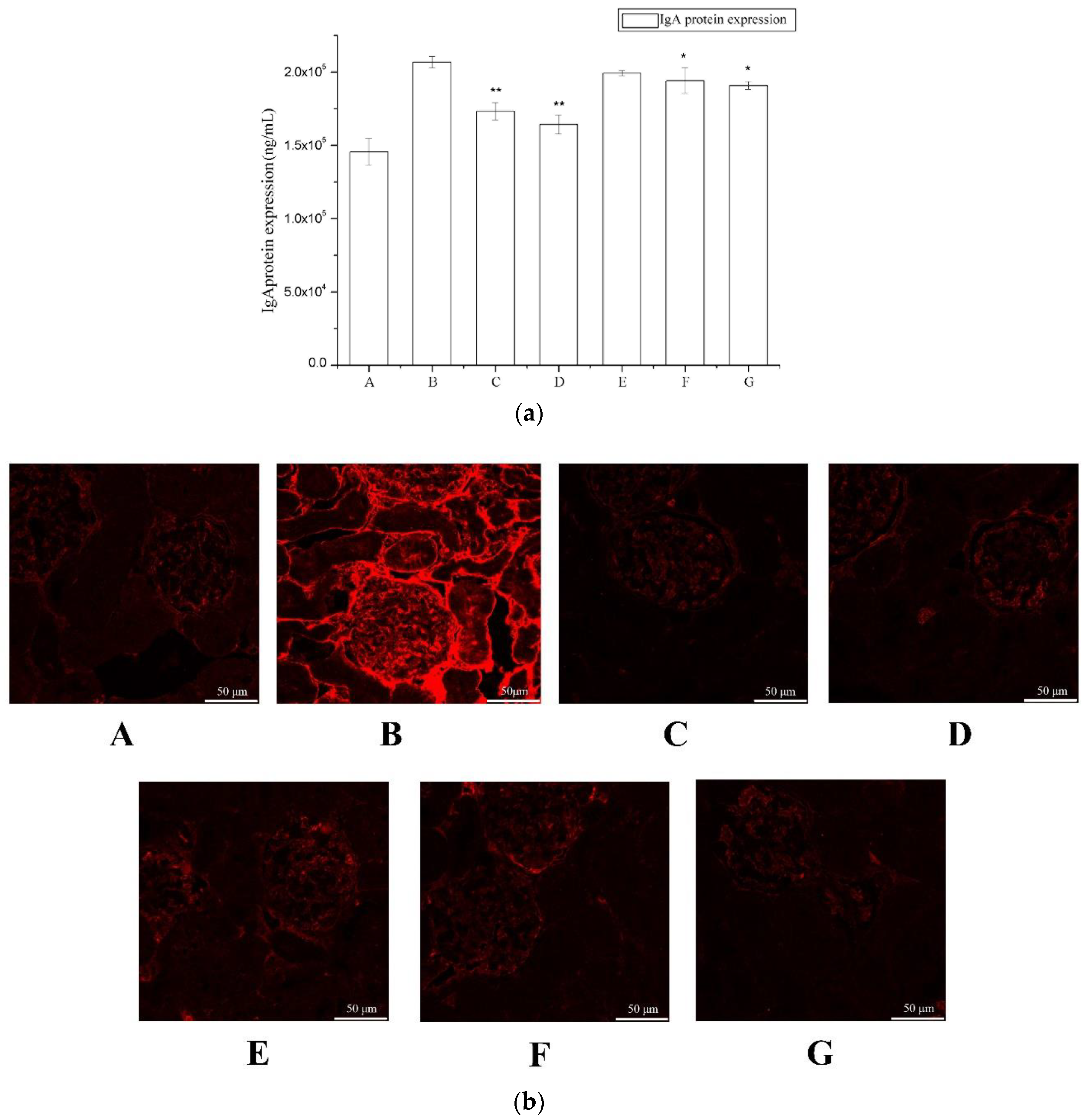
2.2. The Effect of Periostracum Cicadae on IgA
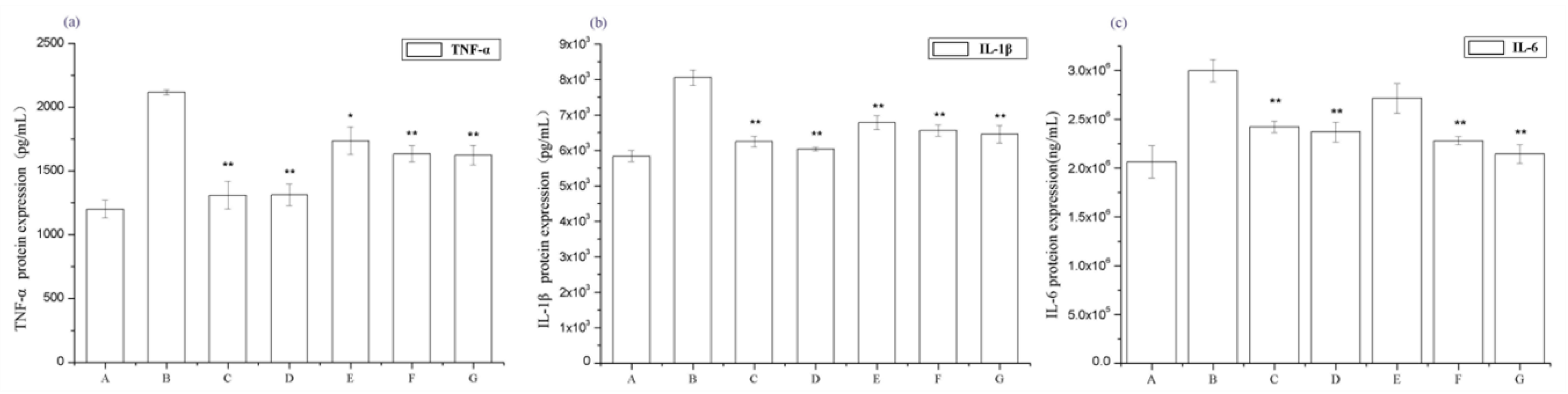
2.3. Periostracum Cicadae Alleviates Inflammation in Rats with IgAN
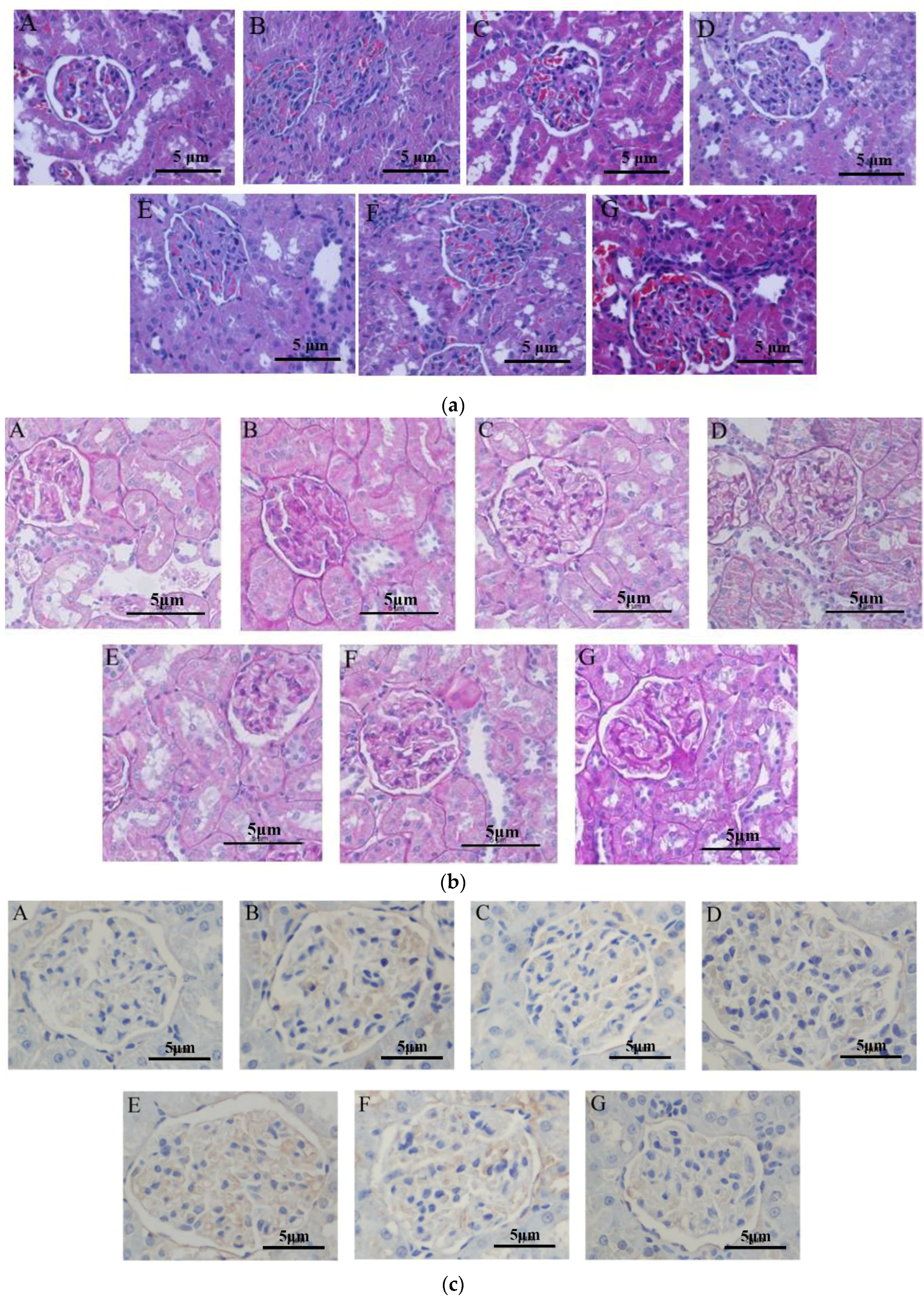
2.4. The Effect of Periostracum Cicadae on Inflammatory Cell Infiltration
2.5. Periostracum Cicadae Induces Cellular Apoptosis in the IgAN Rat Model
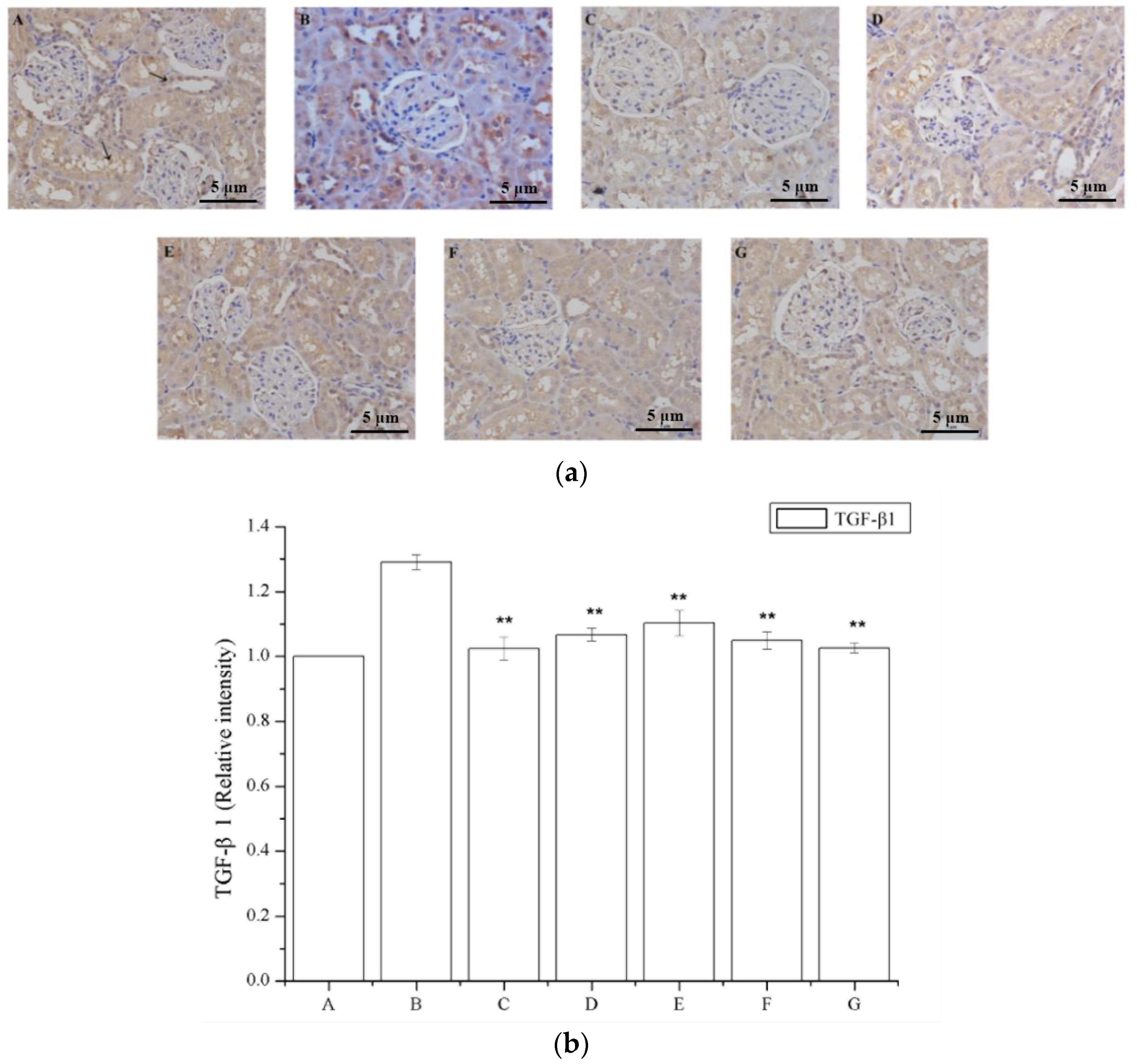
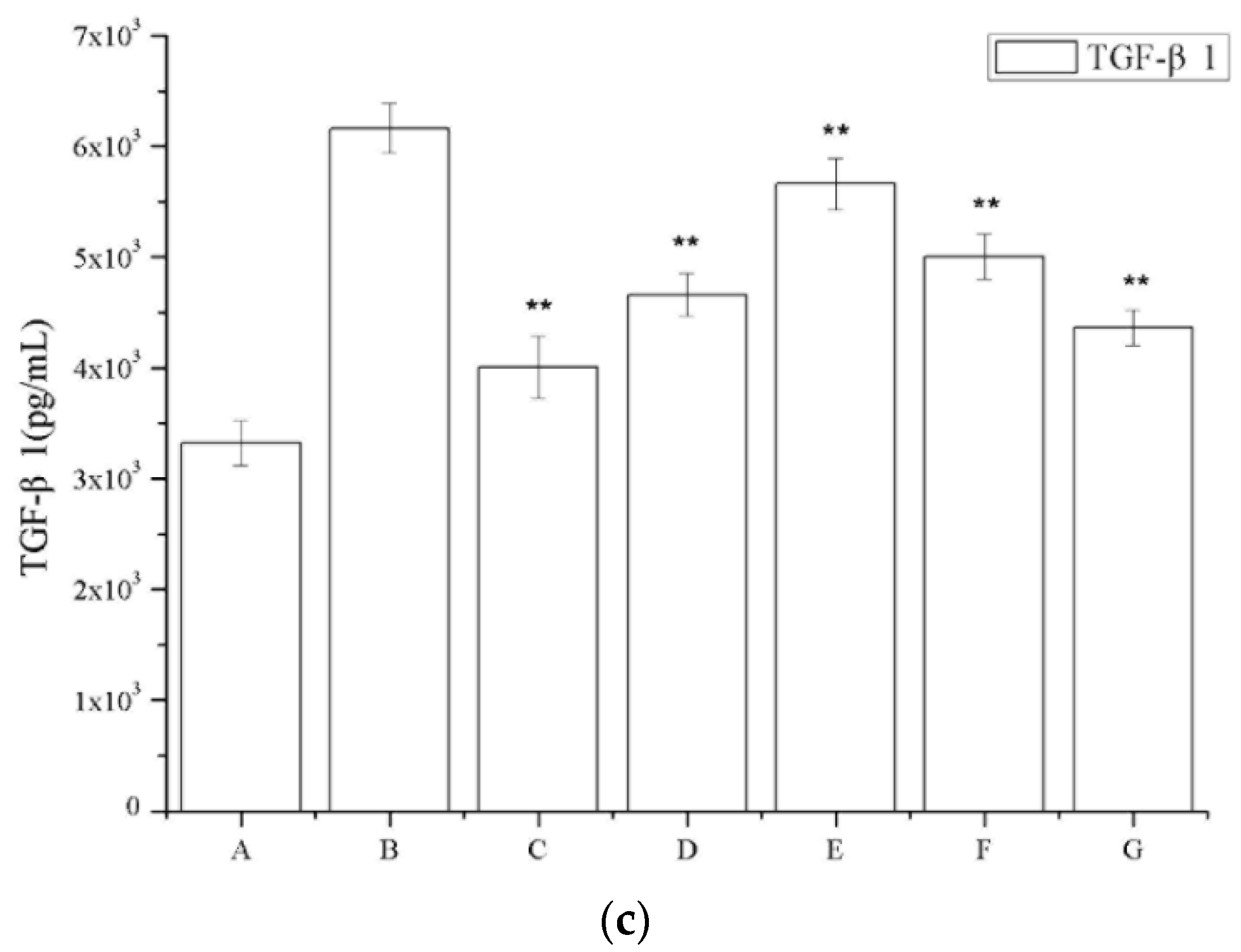
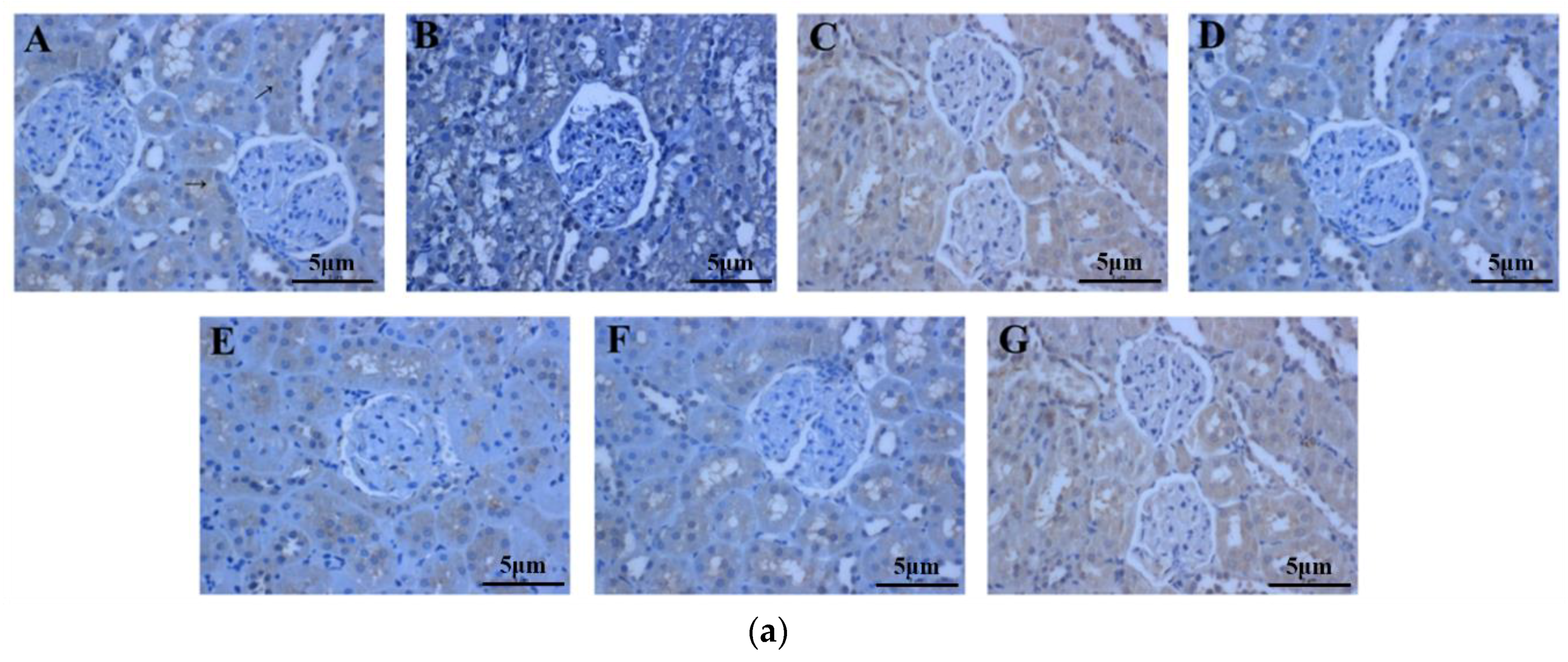
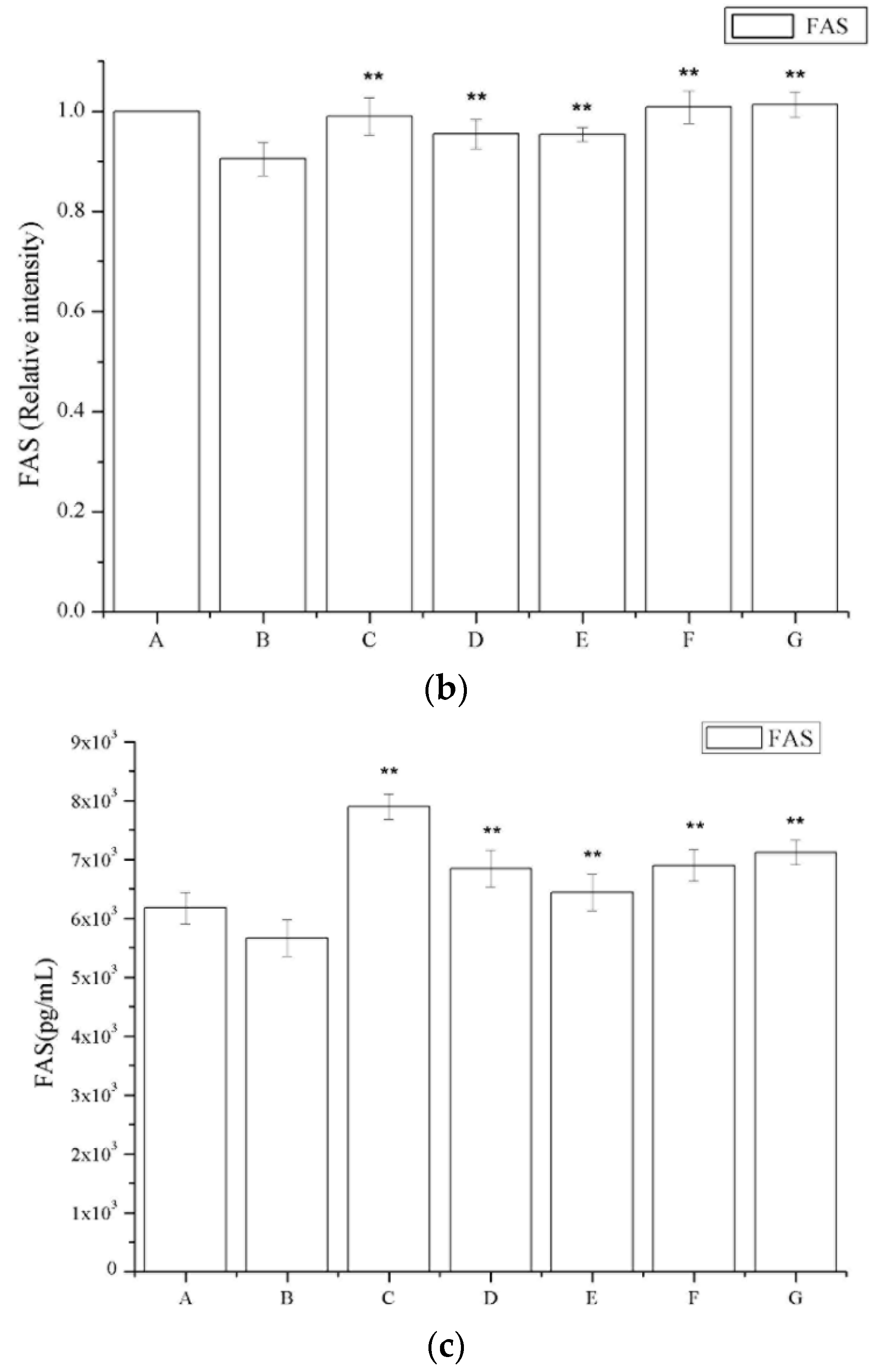
2.6. The Effect of Periostracum Cicadae on TGF-β1- and Fas-Positive Cells
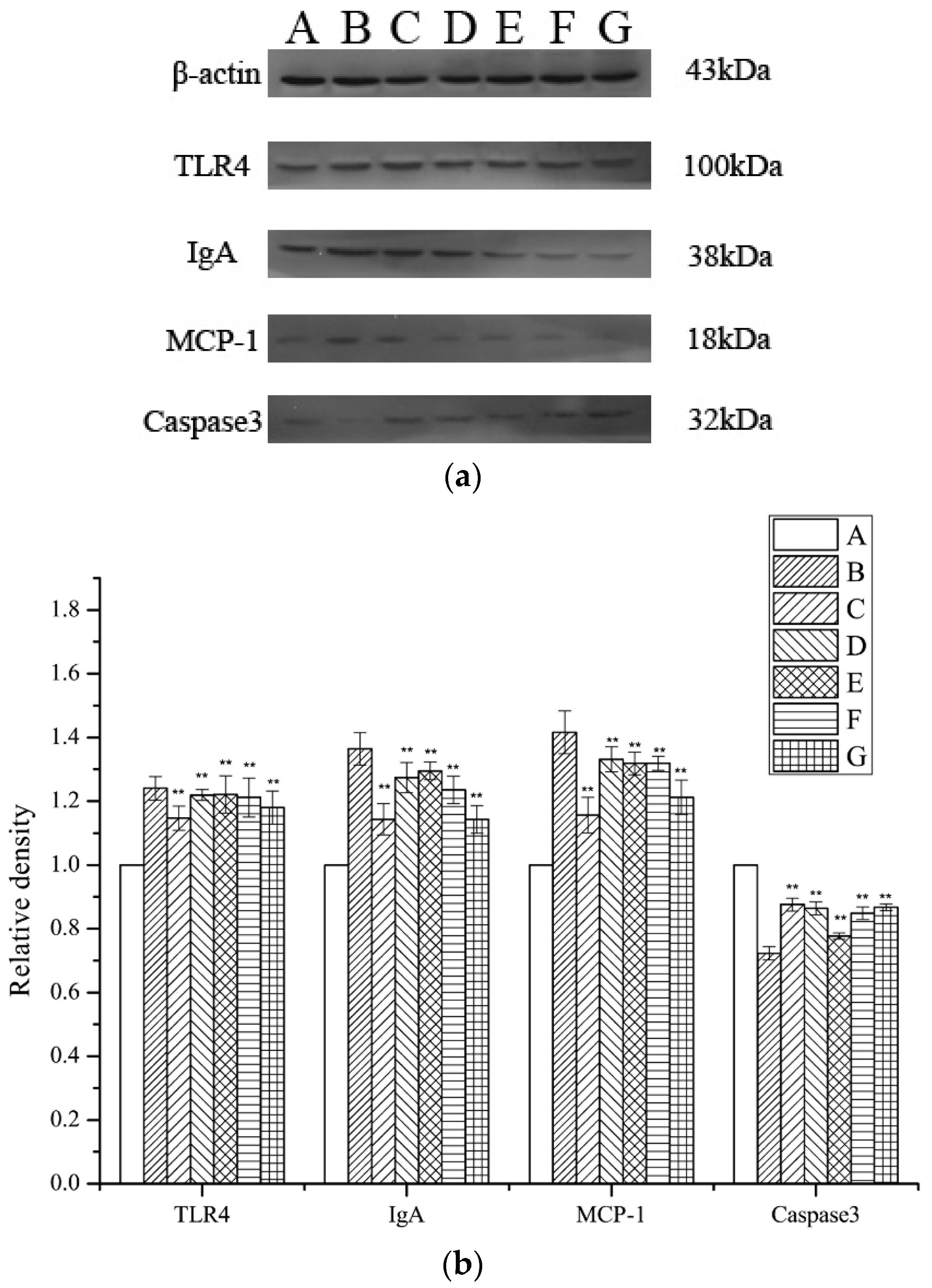
2.7. Assessment of TLR4, MCP-1, IgA, and Caspase 3 Expression Levels
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Preparation of Prednisone Acetate Extract
4.3. Animals
4.4. Establishment of a IgAN Model
4.5. Sample Collection and Preparation
4.6. Biochemical Analysis of Serum Samples
4.7. Histopathological Examination
4.8. Immunofluorescence
4.9. TUNEL Assay
4.10. Immunohistochemical Staining
4.11. Western Blot Analysis
4.12. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Hogg, R.J.; Wyatt, R.J. A randomized controlled trial of mycophenolate mofetil in patients with IgA nephropathy. BMC Nephrol. 2004, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.Y.; Chen, X.M. Immunoglobulin A nephropathy in China: Progress and challenges. Am. J. Nephrol. 2009, 30, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Kiryluk, K.; Julian, B.A.; Wyatt, R.J.; Scolari, F.; Zhang, H.; Novak, J.; Gharavi, A.G. Genetic studies of IgA nephropathy: Past, present, and future. Pediatr. Nephrol. 2010, 25, 2257–2268. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, G. Natural history of idiopathic IgA nephropathy and factors predictive of disease outcome. Semin. Nephrol. 2004, 24, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Boyd, J.K.; Cheung, C.K.; Molyneux, K.; Feehally, J.; Barratt, J. An update on the pathogenesis and treatment of IgA nephropathy. Kidney Int. 2012, 81, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Horie, A.; Hiki, Y.; Odani, H.; Yasuda, Y.; Takahashi, M.; Kato, M. IgA1 molecules produced by tonsillar lymphocytes are under-O-glycosylated in IgA nephropathy. Am. J. Kidney Dis. 2003, 42, 486–496. [Google Scholar] [CrossRef]
- Ibels, L.S.; Gyory, A.Z.; Caterson, R.J.; Pollock, C.A.; Mahony, J.F.; Waugh, D.A. Primary iga nephropathy: Natural history and factors of importance in the progression of renal impairment. Kidney Int. Suppl. 1997, 61, 67–70. [Google Scholar]
- Ohno, I.; Hosoya, T.; Gomi, H.; Ichida, K.; Okabe, H.; Hikita, M. Serum uric acid and renal prognosis in patients with IgA nephropathy. Nephron 2001, 87, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Barratt, J.; Feehally, J. IgA Nephropathy. J. Am. Soc. Nephrol. 2005, 16, 2088–2097. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Lim, B.J.; Bae, Y.S.; Kwon, Y.E.; Kim, Y.L.; Nam, K.H.; Park, K.S.; An, S.Y.; Koo, H.M.; Doh, F.M.; et al. Using the Oxford classification of IgA nephropathy to predict long-term outcomes of Henoch-Schonlein purpura nephritis in adults. Mod. Pathol. 2014, 27, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Coppo, R.; Andrulli, S.; Amore, A.; Gianoglio, B.; Conti, G.; Peruzzi, L.; Locatelli, F.; Cagnoli, L. Predictors of outcome in Henoch-Schonlein nephritis in children and adults. Am. J. Kidney Dis. 2006, 47, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Bogdanovic, R. Henoch-Schonlein purpura nephritis in children: Risk factors, prevention and treatment. Acta Paediatr. 2009, 98, 1882–1889. [Google Scholar] [CrossRef] [PubMed]
- Roberts, I.S.; Cook, H.T.; Troyanov, S.; Alpers, C.E.; Amore, A.; Barratt, J.; Berthoux, F.; Bonsib, S.; Bruijn, J.A.; Cattran, D.C.; et al. The Oxford classification of IgA nephropathy: Pathology definitions, correlations, and reproducibility. Kidney Int. 2009, 76, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Coppo, R.; Troyanov, S.; Camilla, R.; Hogg, R.J.; Cattran, D.C.; Cook, H.T.; Feehally, J.; Roberts, I.S.; Amore, A.; Alpers, C.E.; et al. The Oxford IgA nephropathy clinicopathological classification is valid for children as well as adults. Kidney Int. 2010, 77, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Alamartine, E.; Sauron, C.; Laurent, B.; Sury, A.; Seffert, A.; Mariat, C. The use of the Oxford classification of IgA nephropathy to predict renal survival. Clin. J. Am. Soc. Nephrol. 2011, 6, 2384–2388. [Google Scholar] [CrossRef] [PubMed]
- Herzenberg, A.M.; Fogo, A.B.; Reich, H.N.; Troyanov, S.; Bavbek, N.; Massat, A.E.; Hunley, T.E.; Hladunewich, M.A.; Julian, B.A.; Fervenza, F.C.; et al. Validation of the Oxford classification of IgA nephropathy. Kidney Int. 2011, 80, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Katafuchi, R.; Ninomiya, T.; Nagata, M.; Mitsuiki, K.; Hirakata, H. Validation study of oxford classification of IgA nephropathy: The significance of extracapillary proliferation. Clin. J. Am. Soc. Nephrol. 2011, 6, 2806–2813. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.F.; Wang, S.X.; Jiang, L.; Lv, J.C.; Liu, L.J.; Chen, Y.Q.; Zhu, S.N.; Liu, G.; Zou, W.Z.; Zhang, H.; et al. Pathologic predictors of renal outcome and therapeutic efficacy in IgA nephropathy: Validation of the oxford classification. Clin. J. Am. Soc. Nephrol. 2011, 6, 2175–2184. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.T.; Peng, W.H.; Yeh, F.T.; Tsai, H.Y.; Chang, Y.S. Studies on the anticonvulsive, sedative and hypothermic effects of Periostracum Cicadae extracts. J. Ethnopharmacol. 1991, 35, 83–90. [Google Scholar] [CrossRef]
- Xu, M.Z.; Lee, W.S.; Han, J.M.; Oh, H.W.; Park, D.S.; Tian, G.R.; Jeong, T.S.; Park, H.Y. Antioxidant and anti-inflammatory activities of N-acetyldopamine dimers from Periostracum Cicadae. Bioorg. Med. Chem. 2006, 14, 7826–7834. [Google Scholar] [CrossRef] [PubMed]
- Noda, N.; Kubota, S.; Miyata, Y.; Miyahara, K. Optically active N-acetyldopamine dimer of the crude drug “Zentai”, the cast-off shell of the Cicada, Cryptotympana sp. Chem. Pharm. Bull. 2000, 48, 1749–1752. [Google Scholar] [CrossRef] [PubMed]
- Shin, T.Y.; Park, J.H.; Kim, H.M. Effect of Cryptotympana atrata extract on compound 48/80-induced anaphylactic reactions. J. Ethnopharmacol. 1999, 66, 319–325. [Google Scholar] [CrossRef]
- Zaidi, M.; Singh, N.; Kamran, M.; Ansari, N.; Nasr, S.H.; Acharya, A. Acute onset of hematuria and proteinuria associated with multiorgan involvement of the heart, liver, pancreas, kidneys, and skin in a patient with Henoch-Schönlein purpura. Kidney Int. 2008, 73, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Sumingan, N.; Tan, J.; Alhous, H.; McWilliam, L.; Ballardie, F. Henoch Schonlein purpura with nephritis in adults: Adverse prognostic indicators in a UK population. QJM 2006, 99, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Szeto, C.C.; Choi, P.C.; To, K.F.; Li, P.K.; Hui, J.; Chow, K.M.; Leung, C.B.; Lui, S.F.; Mac-Moune Lai, F. Grading of acute and chronic renal lesions in Henoch-Schonlein purpura. Mod. Pathol. 2001, 14, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.H.; Wu, S.C.; Huang, T.P.; Yu, C.L.; Tsai, C.Y. Increased excretion of tumor necrosis factor alpha and interleukin 1 β in urine from patients with IgA nephropathy and Schonlein-Henoch purpura. Nephron 1996, 74, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, K.; Kobayashi, M.; Muro, K.; Yoh, K.; Yamagata, K.; Koyama, A. Specific T-cell receptor usage with cytokinemia in Henoch-Schonlein purpura nephritis associated with Staphylococcus aureus infection. J. Intern. Med. 2001, 249, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Kawahara, S.; Sakagami, Y.; Takagawa, K.; Akazawa, H.; Kamitsuji, H.; Yoshioka, A. Immunogold labelling of cytokines in glomeruli in children with various renal diseases. Nephron 1999, 83, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, Y.; Hosoya, M.; Suzuki, H. Possible pathologenic role of interleukin-5 and eosino cationic protein in Henoch-Schonlein purpura nephritis. Pediatr. Int. 2005, 47, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Michalska, M.; Gluba, A.; Mikhailidis, D.P.; Nowak, P.; Bielecka-Dabrowa, A.; Rysz, J.; Banach, M. The role of polyphenols in cardiovascular disease. Med. Sci. Monit. 2010, 16, Ra110–Ra119. [Google Scholar] [PubMed]
- El-Achkar, T.M.; Huang, X.; Plotkin, Z.; Sandoval, R.M.; Rhodes, G.J.; Dagher, P.C. Sepsis induces changes in the expression and distribution of Toll-like receptor 4 in the rat kidney. Am. J. Physiol. Renal Physiol. 2006, 290, F1034–F1043. [Google Scholar] [CrossRef] [PubMed]
- Zager, R.A.; Johnson, A.C.; Lund, S.; Randolph-Habecker, J. Toll-like receptor (TLR4) shedding and depletion: Acute proximal tubular cell responses to hypoxic and toxic injury. Am. J. Physiol. Renal Physiol. 2007, 292, F304–F312. [Google Scholar] [CrossRef] [PubMed]
- Patole, P.S.; Pawar, R.D.; Lech, M.; Zecher, D.; Schmidt, H.; Segerer, S.; Ellwart, A.; Henger, A.; Kretzler, M.; Anders, H.J. Expression and regulation of Toll-like receptors in lupus-like immune complex glomerulonephritis of MRL-Fas(lpr) mice. Nephrol. Dial. Transplant. 2006, 21, 3062–3073. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, N.; Yoshikai, Y.; Matsuo, S.; Kikuchi, T.; Iwami, K.; Nagai, Y.; Takeuchi, O.; Akira, S.; Matsuguchi, T. Roles of toll-like receptors in C-C chemokine production by renal tubular epithelial cells. J. Immunol. 2002, 169, 2026–2033. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, P.; Hang, L.; Wullt, B.; Irjala, H.; Svanborg, C. Toll-like receptor 4 expression and cytokine responses in the human urinary tract mucosa. Infect. Immun. 2004, 72, 3179–3186. [Google Scholar] [CrossRef] [PubMed]
- Wolfs, T.G.; Buurman, W.A.; van Schadewijk, A.; de Vries, B.; Daemen, M.A.; Hiemstra, P.S.; van’t Veer, C. In vivo expression of Toll-like receptor 2 and 4 by renal epithelial cells: IFN-γ and TNF-α mediated up-regulation during inflammation. J. Immunol. 2002, 168, 1286–1293. [Google Scholar] [CrossRef] [PubMed]
- El-Achkar, T.M.; Wu, X.R.; Rauchman, M.; McCracken, R.; Kiefer, S.; Dagher, P.C. Tamm-Horsfall protein protects the kidney from ischemic injury by decreasing inflammation and altering TLR4 expression. Am. J. Physiol. Renal Physiol. 2008, 295, F534–F544. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.M.; Zhang, H.D.; Jin, Y.M.; Zhu, B.B.; Chen, N. PPAR-γ agonists inhibit TGF-β1-induced chemokine expression in human tubular epithelial cells. Acta Pharmacol. Sin. 2009, 30, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ruan, X.Z.; Powis, S.H.; Fernando, R.; Mon, W.Y.; Wheeler, D.C.; Moorhead, J.F.; Varghese, Z. EPA and DHA reduce LPS-induced inflammation responses in HK-2 cells: Evidence for a PPAR-γ-dependent mechanism. Kidney Int. 2005, 67, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Hosohata, K. Role of Oxidative Stress in Drug-Induced Kidney Injury. Int. J. Mol. Sci. 2016, 17, 1826. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.; Kim, H.J.; Noh, H.J.; Chang, Y.C.; Chae, Y.M.; Kim, K.H.; Jeon, J.P.; Lee, T.S.; Oh, H.K.; Lee, Y.S.; et al. TGF-β1 siRNA suppresses the tubulointerstitial fibrosis in the kidney of ureteral obstruction. Exp. Mol. Pathol. 2006, 81, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.; Docherty, N.G.; Griffin, B.; Howlin, J.; McArdle, E.; McMahon, R.; Schmid, H.; Kretzler, M.; Droguett, A.; Mezzano, S.; et al. IHG-1 amplifies TGF-β1 signaling and is increased in renal fibrosis. J. Am. Soc. Nephrol. 2008, 19, 1672–1680. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Border, W.A.; Anderson, I.; McCourt, M.; Huang, Y.; Noble, N.A. Combining TGF-β inhibition and angiotensin II blockade results in enhanced antifibrotic effect. Kidney Int. 2004, 66, 1774–1784. [Google Scholar] [CrossRef] [PubMed]
- Martini, S.; Kramer, S.; Loof, T.; Wang-Rosenke, Y.; Daig, U.; Budde, K.; Neumayer, H.H.; Peters, H. S1P modulator FTY720 limits matrix expansion in acute anti-thy1 mesangioproliferative glomerulonephritis. Am. J. Physiol. Renal Physiol. 2007, 292, F1761–F1770. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.W.; Hsueh, S.; Wu, M.S.; Lai, P.C.; Huang, J.Y.; Wu, C.H.; Hu, S.A.; Chen, J.F.; Huang, C.C. Glomerular transforming growth factor-β1 mRNA as a marker of glomerulosclerosis-application in renal biopsies. Nephron 1997, 77, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, H.; Kashihara, N.; Makino, H.; Yamasaki, Y.; Ota, A. Apoptosis in glomerular sclerosis. Kidney Int. 1996, 49, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Neumann, L.; Pforr, C.; Beaudouin, J.; Pappa, A.; Fricker, N.; Krammer, P.H.; Lavrik, I.N.; Eils, R. Dynamics within the CD95 death-inducing signaling complex decide life and death of cells. Mol. Syst. Biol. 2010, 6, 352. [Google Scholar] [CrossRef] [PubMed]
- Medema, J.P.; Scaffidi, C.; Kischkel, F.C.; Shevchenko, A.; Mann, M.; Krammer, P.H.; Peter, M.E. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC). EMBO J. 1997, 16, 2794–2804. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.C.; Sun, J.M.; Wei, Z.L.; Yang, X.F.; Zhang, Y.C.; Xin, Y. Expression of Fas ligand and caspase-3 contributes to formation of immune escape in gastric cancer. World J. Gastroenterol. 2003, 9, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Nho, R.S.; Peterson, M.; Hergert, P.; Henke, C.A. FoxO3a (Forkhead Box O3a) deficiency protects Idiopathic Pulmonary Fibrosis (IPF) fibroblasts from type I polymerized collagen matrix-induced apoptosis via caveolin-1 (cav-1) and Fas. PLoS ONE 2013, 8, e61017. [Google Scholar] [CrossRef] [PubMed]
- Ran, X.; Diao, J.X. Huangzhi Oral Liquid Prevents Arrhythmias by Upregulating Caspase-3 and Apoptosis Network Proteins in Myocardial Ischemia-Reperfusion Injury in Rats. Evid.-Based Complement. Altern. Med. 2015, 2015, 518926. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Lou, T.Q.; Cheng, C.L. The modification of laboratory IgA nephropathy model. J. Zhongshan Univ. (Med.) 2006, 27, 184–187. [Google Scholar]
- Peng, W.; Liu, Z.R. Comparison of two rat models of IgA nephropathy. J. South Med. Univ. 2008, 28, 1842–1845. [Google Scholar]
- Lu, H.; Chen, L.L.; Jiang, X.Y.; Sun, L.Z. Temporal and spatial expression of podocyte-associated molecules are accompanied by proteinuria in IgA nephropathy rat model. Physiol. Res. 2013, 62, 35–45. [Google Scholar] [PubMed]

| Group | Controls | IgAN Model Group | Prednisone Acetate Group | Tripterygium Glycoside Tablet Group | Periostracum Cicadae Group | ||
|---|---|---|---|---|---|---|---|
| 0.5 g/kg | 1 g/kg | 2 g/kg | |||||
| IgA deposits | − | ++++ | + | ++ | +++ | ++ | + |
| Control | BSA Was Replaced by Distilled Water; PBS Was Used as Injection Control | |
|---|---|---|
| IgAN | BSA, 4 mL/kg | Intragastrically every 2 days for 8 weeks |
| CCL4/castor oil (25%, v/v) | subcutaneous injection (S.C.), 0.4 mL, once a week for 9 weeks | |
| LPS, 0.05 mg | Caudal iv, once every two weeks for six weeks at the 6th week | |
| 25% ginger ale, 10 mL/kg | Once every two days for four consecutive weeks on the 9th week | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Wang, Y.; Nuerbiye, A.; Cheng, P.; Wang, J.-H.; Kasimu, R.; Li, H. Effects of Periostracum Cicadae on Cytokines and Apoptosis Regulatory Proteins in an IgA Nephropathy Rat Model. Int. J. Mol. Sci. 2018, 19, 1599. https://doi.org/10.3390/ijms19061599
Yang L, Wang Y, Nuerbiye A, Cheng P, Wang J-H, Kasimu R, Li H. Effects of Periostracum Cicadae on Cytokines and Apoptosis Regulatory Proteins in an IgA Nephropathy Rat Model. International Journal of Molecular Sciences. 2018; 19(6):1599. https://doi.org/10.3390/ijms19061599
Chicago/Turabian StyleYang, Lu, Yan Wang, Aobulikasimu Nuerbiye, Ping Cheng, Jin-Hui Wang, Rena Kasimu, and Hong Li. 2018. "Effects of Periostracum Cicadae on Cytokines and Apoptosis Regulatory Proteins in an IgA Nephropathy Rat Model" International Journal of Molecular Sciences 19, no. 6: 1599. https://doi.org/10.3390/ijms19061599
APA StyleYang, L., Wang, Y., Nuerbiye, A., Cheng, P., Wang, J.-H., Kasimu, R., & Li, H. (2018). Effects of Periostracum Cicadae on Cytokines and Apoptosis Regulatory Proteins in an IgA Nephropathy Rat Model. International Journal of Molecular Sciences, 19(6), 1599. https://doi.org/10.3390/ijms19061599





