Genome-Wide Screening and Characterization of the Dof Gene Family in Physic Nut (Jatropha curcas L.)
Abstract
1. Introduction
2. Results
2.1. Identification and Characterization of Dof Genes in Physic Nut
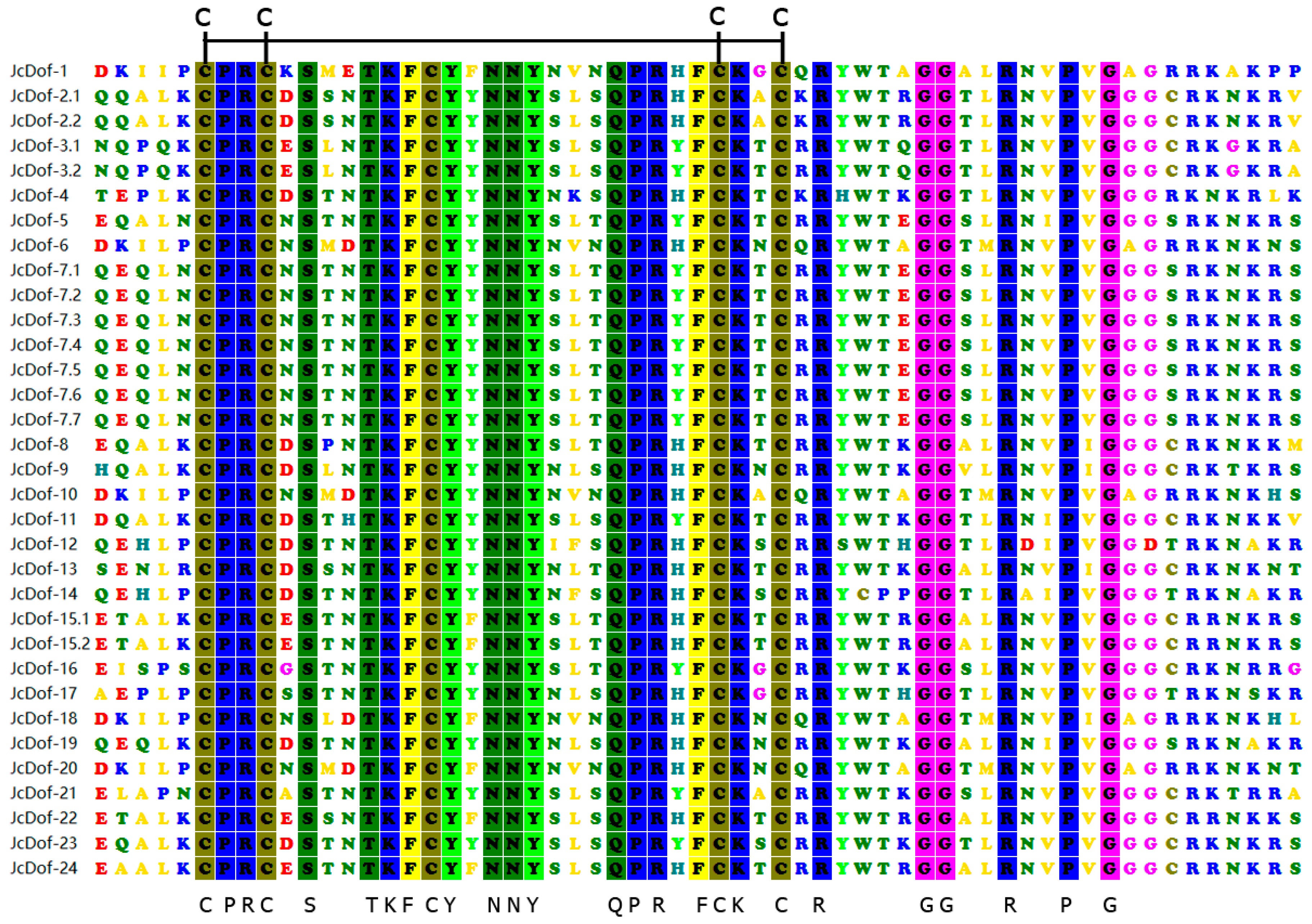
2.2. DNA-Binding Domain Conservation Analysis of JcDof Protein
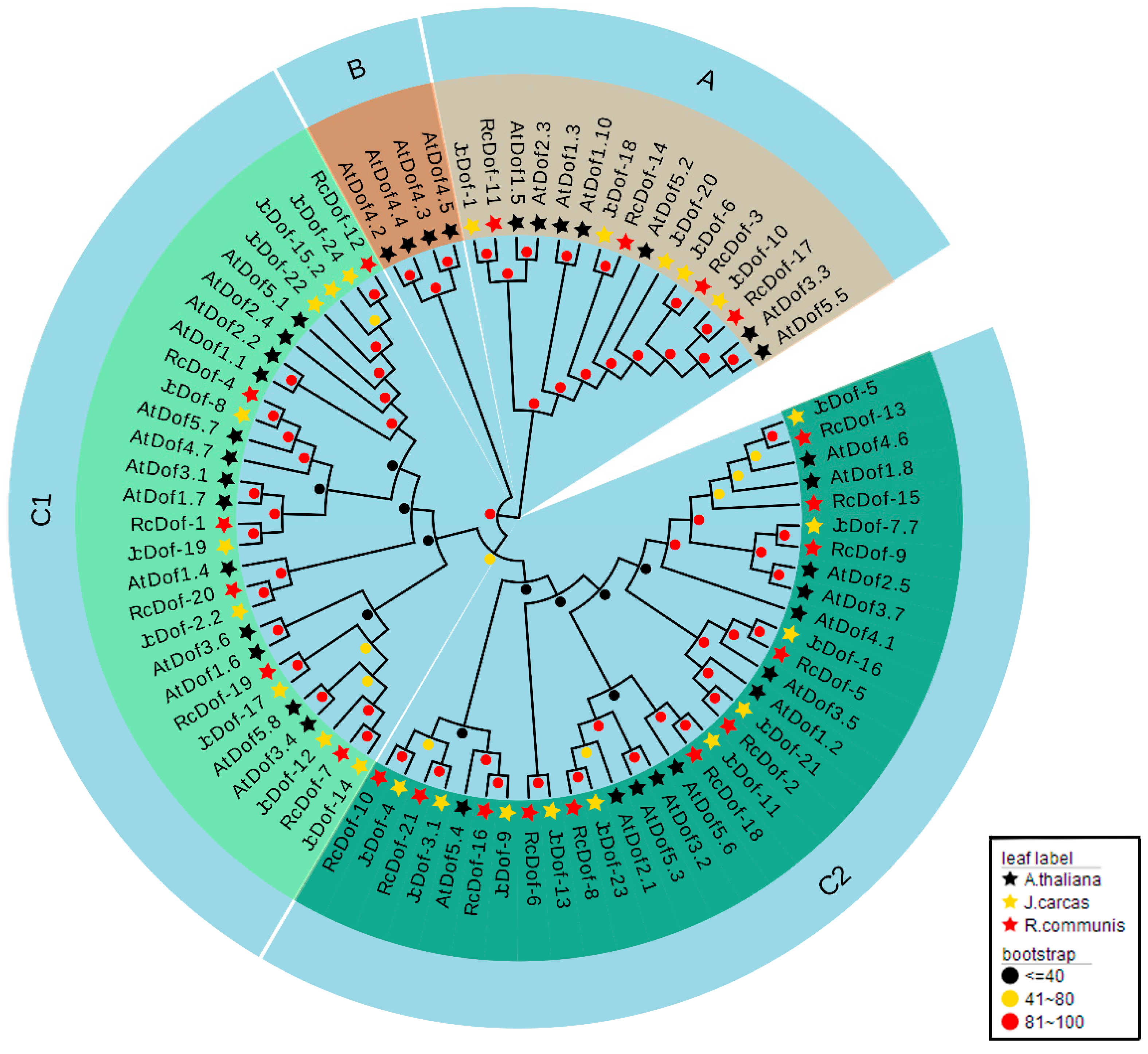
2.3. Phylogenetic Analysis and Classification of JcDof Proteins
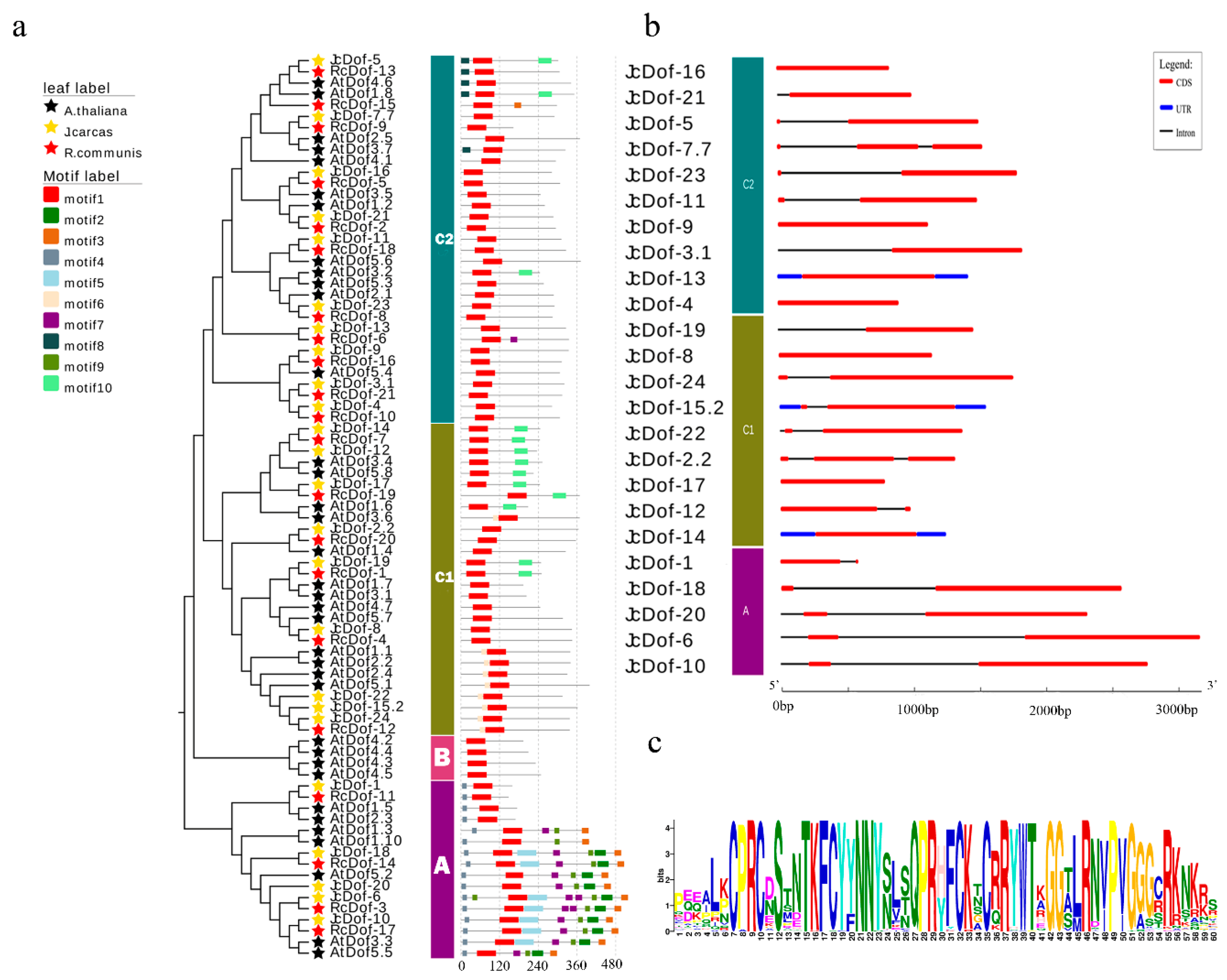
2.4. JcDof Gene Structures and Conserved Motifs in JcDof Proteins
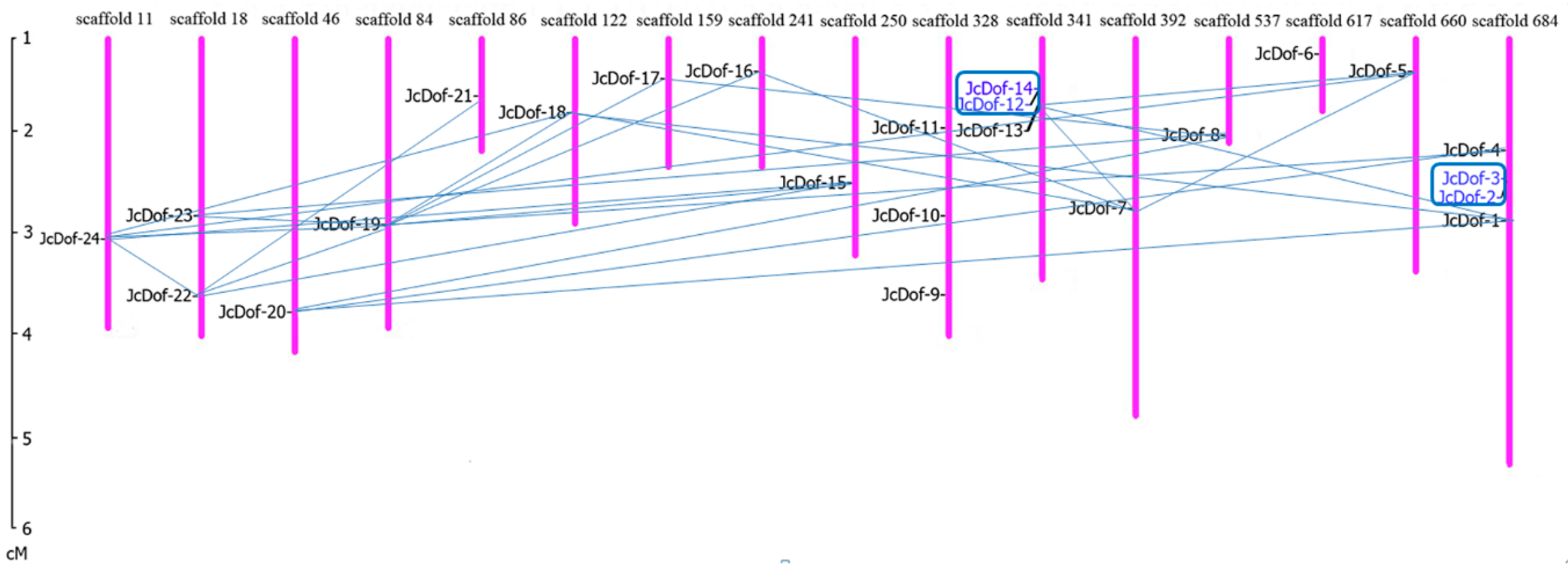
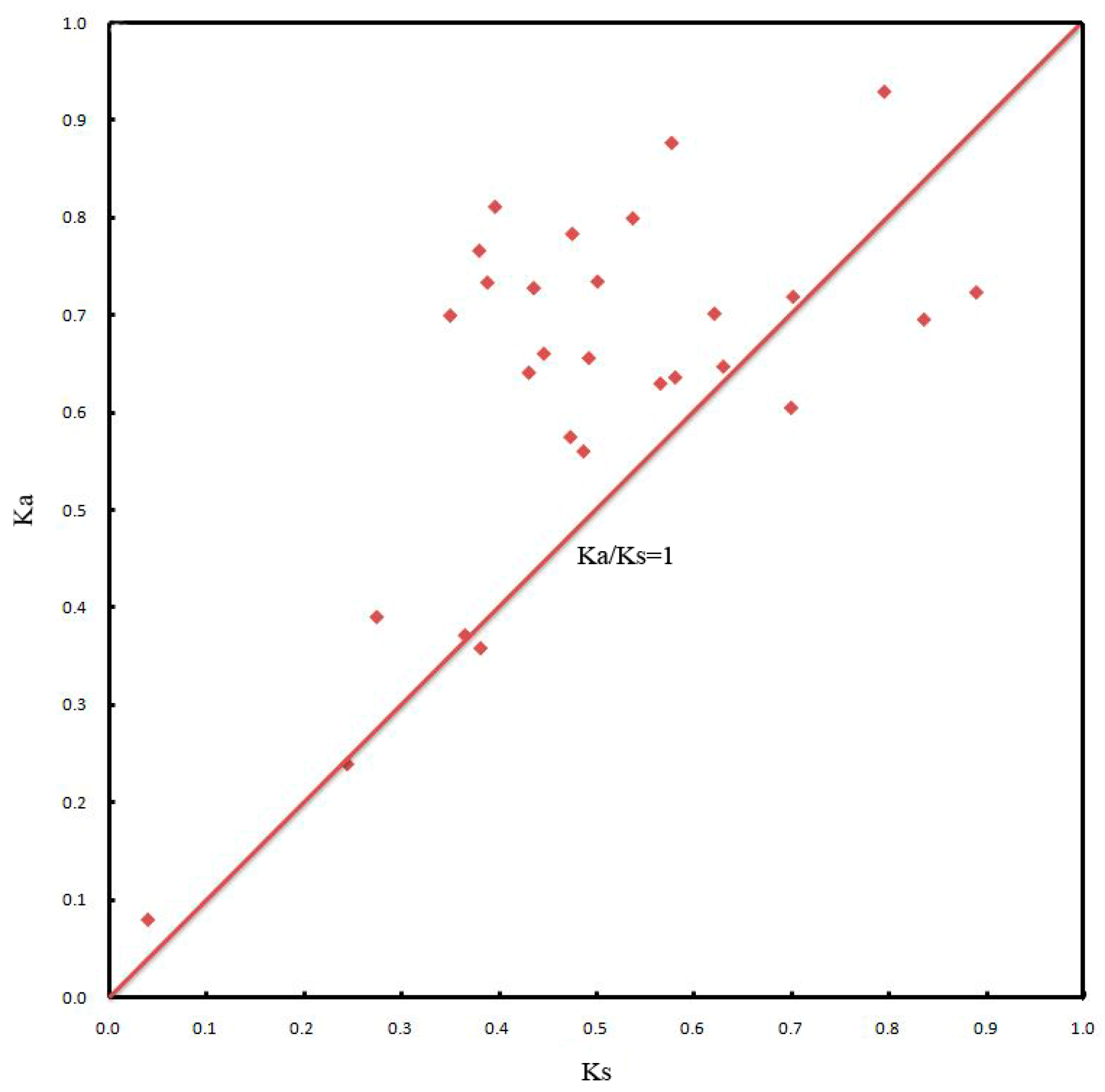
2.5. Chromosomal Locations and Gene Duplication Events of JcDof Genes
2.6. Expression Patterns of JcDof Genesunder Different Abiotic Stress and Hormone Treatments
3. Discussion
4. Materials and Methods
4.1. Data Sources
4.2. Dof Gene Identification and Characterization
4.3. DNA-Binding Domain Conservation Analysis of JcDof Protein
4.4. Phylogenetic Analysis
4.5. Gene Structure of Dof Proteins
4.6. Detection of Additional Conserved Motifs
4.7. Chromosomal Localization
4.8. Detection of Gene Duplication Events and Estimation of Synonymous (Ks) and Nonsynonymous (Ka) Substitutions per Site and Their Ratio
4.9. Expression Analysis of Physic Nut Dof Genes
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| TF | Transcription factor |
| HMM | Hidden Markov model |
| Mw | Molecular weight |
| pI | Isoelectric point |
| GA | Gibberellic acid |
| BA | 6-Benzylaminopurine |
References
- Bhasanutra, R.; Sutiponpeibun, S. Jatropha curcas oil as a substitute for diesel engine oil. Int. Energy J. 2017, 4, 56–70. [Google Scholar]
- Openshaw, K. A review of Jatropha curcas: An oil plant of unfulfilled promise. Biomass Bioenergy 2000, 19, 1–15. [Google Scholar] [CrossRef]
- Chen, M.-S.; Pan, B.-Z.; Fu, Q.; Tao, Y.-B.; Martínez-Herrera, J.; Niu, L.; Ni, J.; Dong, Y.; Zhao, M.-L.; Xu, Z.-F. Comparative transcriptome analysis between gynoecious and monoecious plants identifies regulatory networks controlling sex determination in Jatropha curcas. Front. Plant Sci. 2017, 7, 1953. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.-Z.; Xu, Z.-F. Benzyladenine treatment significantly increases the seed yield of the biofuel plant Jatropha curcas. J. Plant Growth Regul. 2011, 30, 166–174. [Google Scholar] [CrossRef]
- Makkar, H.; Becker, K.; Sporer, F.; Wink, M. Studies on nutritive potential and toxic constituents of different provenances of Jatropha curcas. J. Agric. Food Chem. 1997, 45, 3152–3157. [Google Scholar] [CrossRef]
- Dehgan, B. Phylogenetic significance of interspecific hybridization in Jatropha (Euphorbiaceae). Syst. Bot. 1984, 9, 467–478. [Google Scholar] [CrossRef]
- Carvalho, C.R.; Clarindo, W.R.; Praça, M.M.; Araújo, F.S.; Carels, N. Genome size, base composition and karyotype of Jatropha curcas L., an important biofuel plant. Plant Sci. 2008, 174, 613–617. [Google Scholar] [CrossRef]
- Rahman, A.Y.A.; Usharraj, A.O.; Misra, B.B.; Thottathil, G.P.; Jayasekaran, K.; Feng, Y.; Hou, S.; Ong, S.Y.; Ng, F.L.; Lee, L.S. Draft genome sequence of the rubber tree Hevea brasiliensis. BMC Genom. 2013, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhou, C.; Cheng, S.; Wu, Z.; Lu, W.; Han, J.; Chen, Y.; Chen, Y.; Ni, P.; Wang, Y. Integrated genome sequence and linkage map of physic nut (Jatropha curcas L.), a biodiesel plant. Plant J. 2015, 81, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, C.; Wu, P.; Chen, Y.; Li, M.; Jiang, H.; Wu, G. Global analysis of gene expression profiles in physic nut (Jatropha curcas L.) seedlings exposed to salt stress. PLoS ONE 2014, 9, e97878. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, L.; Zhang, S.; Zhu, S.; Wu, P.; Chen, Y.; Li, M.; Jiang, H.; Wu, G. Global analysis of gene expression profiles in physic nut (Jatropha curcas L.) seedlings exposed to drought stress. BMC Plant Biol. 2015, 15, 17. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.U.; Nakagawa, S. Transcription Factor Genes. In Evolution of the Human Genome I; Springer: Berlin, Germany, 2017; pp. 241–263. [Google Scholar]
- Riaño-Pachón, D.M.; Ruzicic, S.; Dreyer, I.; Mueller-Roeber, B. PlnTFDB: An integrative plant transcription factor database. BMC Bioinform. 2007, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Noguero, M.; Atif, R.M.; Ochatt, S.; Thompson, R.D. The role of the DNA-binding One Zinc Finger (DOF) transcription factor family in plants. Plant Sci. 2013, 209, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Franco-Zorrilla, J.M.; López-Vidriero, I.; Carrasco, J.L.; Godoy, M.; Vera, P.; Solano, R. DNA-binding specificities of plant transcription factors and their potential to define target genes. Proc. Natl. Acad. Sci. USA 2014, 111, 2367–2372. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Tian, F.; Yang, D.-C.; Meng, Y.-Q.; Kong, L.; Luo, J.; Gao, G. PlantTFDB 4.0: Toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2016, 45, D1040–D1045. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, S.; Schmidt, R.J. Diversity and similarity among recognition sequences of Dof transcription factors. Plant J. 1999, 17, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, S. Dof domain proteins: Plant-specific transcription factors associated with diverse phenomena unique to plants. Plant Cell Physiol. 2004, 45, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, S. The Dof family of plant transcription factors. Trends Plant Sci. 2002, 7, 555–560. [Google Scholar] [CrossRef]
- Chen, X.; Wang, D.; Liu, C.; Wang, M.; Wang, T.; Zhao, Q.; Yu, J. Maize transcription factor Zmdof1 involves in the regulation of Zm401 gene. Plant Growth Regul. 2012, 66, 271–284. [Google Scholar] [CrossRef]
- Gupta, S.; Malviya, N.; Kushwaha, H.; Nasim, J.; Bisht, N.C.; Singh, V.; Yadav, D. Insights into structural and functional diversity of Dof (DNA binding with one finger) transcription factor. Planta 2015, 241, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, J.; Park, S.W. Genome-wide analysis and expression profiling of DNA-binding with one zinc finger (Dof) transcription factor family in potato. Plant Physiol. Biochem. 2015, 94, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, S. Structure, Function, and Evolution of the Dof Transcription Factor Family. In Plant Transcription Factors; Elsevier: New York, NY, USA, 2015; pp. 183–197. [Google Scholar]
- Shu, Y.; Song, L.; Zhang, J.; Liu, Y.; Guo, C. Genome-wide identification and characterization of the Dof gene family in Medicago truncatula. Genet. Mol. Res. 2015, 14, 10645–10657. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yue, J.-J.; Wang, X.-J.; Xu, L.; Li, L.-B.; Gu, X.-P. Genome-wide identification and characterization of the Dof gene family in moso bamboo (Phyllostachys heterocycla var. pubescens). Genes Genom. 2016, 38, 733–745. [Google Scholar] [CrossRef]
- Wu, Q.; Li, D.; Li, D.; Liu, X.; Zhao, X.; Li, X.; Li, S.; Zhu, L. Overexpression of OsDof12 affects plant architecture in rice (Oryza sativa L.). Front. Plant Sci. 2015, 6, 833. [Google Scholar] [CrossRef] [PubMed]
- Lijavetzky, D.; Carbonero, P.; Vicente-Carbajosa, J. Genome-wide comparative phylogenetic analysis of the rice and Arabidopsis Dof gene families. BMC Evol. Biol. 2003, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Qiu, L.-J. Retraction: Genome-Wide Analysis of the Dof Transcription Factor Gene Family Reveals Soybean-Specific Duplicable and Functional Characteristics. PLoS ONE 2016, 11, e0167019. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.M.; McIntyre, C.L.; Gresshoff, P.M.; Xue, G.-P. Members of the Dof transcription factor family in Triticum aestivum are associated with light-mediated gene regulation. Funct. Integr. Genom. 2009, 9, 485. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Chandrasekaran, U.; Liu, A. Genome-wide analysis of the Dof transcription factors in castor bean (Ricinus communis L.). Genes Genom. 2014, 36, 527–537. [Google Scholar] [CrossRef]
- Chan, A.P.; Crabtree, J.; Zhao, Q.; Lorenzi, H.; Orvis, J.; Puiu, D.; Melake-Berhan, A.; Jones, K.M.; Redman, J.; Chen, G. Draft genome sequence of the oilseed species Ricinus communis. Nat. Biotechnol. 2010, 28, 951. [Google Scholar] [CrossRef] [PubMed]
- Marquez, Y.; Höpfler, M.; Ayatollahi, Z.; Barta, A.; Kalyna, M. Unmasking alternative splicing inside protein-coding exons defines exitrons and their role in proteome plasticity. Genome Res. 2015, 25, 995–1007. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.B.; Foley, R.C.; Oñate-Sánchez, L. Transcription factors in plant defense and stress responses. Curr. Opin. Plant Biol. 2002, 5, 430–436. [Google Scholar] [CrossRef]
- Ma, J.; Li, M.-Y.; Wang, F.; Tang, J.; Xiong, A.-S. Genome-wide analysis of Dof family transcription factors and their responses to abiotic stresses in Chinese cabbage. BMC Genom. 2015, 16, 33. [Google Scholar] [CrossRef] [PubMed]
- Lamesch, P.; Berardini, T.Z.; Li, D.; Swarbreck, D.; Wilks, C.; Sasidharan, R.; Muller, R.; Dreher, K.; Alexander, D.L.; Garcia-Hernandez, M. The Arabidopsis Information Resource (TAIR): Improved gene annotation and new tools. Nucleic Acids Res. 2011, 40, D1202–D1210. [Google Scholar] [CrossRef] [PubMed]
- Initiative, A.G. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 2000, 408, 796. [Google Scholar] [CrossRef] [PubMed]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Leister, D. Tandem and segmental gene duplication and recombination in the evolution of plant disease resistance genes. Trends Genet. 2004, 20, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Leinonen, R.; Sugawara, H.; Shumway, M.; Collaboration, I.N.S.D. The sequence read archive. Nucleic Acids Res. 2010, 39 (Suppl. 1), D19–D21. [Google Scholar] [CrossRef] [PubMed]
- Mount, D.W. Using the basic local alignment search tool (BLAST). Cold Spring Harb. Protoc. 2007, 2007. [Google Scholar] [CrossRef] [PubMed]
- Schuster-Böckler, B.; Bateman, A. An introduction to hidden Markov models. Curr. Protoc. Bioinform. 2007, 18, A.3A.1–A.3A.9. [Google Scholar]
- Letunic, I.; Doerks, T.; Bork, P. SMART 7: Recent updates to the protein domain annotation resource. Nucleic Acids Res. 2011, 40, D302–D305. [Google Scholar] [CrossRef] [PubMed]
- Marchler-Bauer, A.; Derbyshire, M.K.; Gonzales, N.R.; Lu, S.; Chitsaz, F.; Geer, L.Y.; Geer, R.C.; He, J.; Gwadz, M.; Hurwitz, D.I. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 2014, 43, D222–D226. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef] [PubMed]
- Woffelman, C. DNAMAN for Windows, Version 2.6; Lynon Biosoft, Institute of Molecular Plant Sciences, Leiden University: Leiden, The Netherlands, 1994. [Google Scholar]
- Thompson, J.D.; Gibson, T.; Higgins, D.G. Multiple sequence alignment using ClustalW and ClustalX. Curr. Protoc. Bioinform. 2002. [Google Scholar] [CrossRef] [PubMed]
- Sela, I.; Ashkenazy, H.; Katoh, K.; Pupko, T. GUIDANCE2: Accurate detection of unreliable alignment regions accounting for the uncertainty of multiple parameters. Nucleic Acids Res. 2015, 43, W7–W14. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Jin, J.; Guo, A.-Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2014, 31, 1296–1297. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37 (Suppl. 2), W202–W208. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Dong, Q.; Shao, Y.; Jiang, H.; Zhu, S.; Cheng, B.; Xiang, Y. Genome-wide survey and characterization of the WRKY gene family in Populus trichocarpa. Plant Cell Rep. 2012, 31, 1199–1217. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-H.; Tang, H.; Wang, X.; Paterson, A.H. PGDD: A database of gene and genome duplication in plants. Nucleic Acids Res. 2012, 41, D1152–D1158. [Google Scholar] [CrossRef] [PubMed]
- Kohler, A.; Rinaldi, C.; Duplessis, S.; Baucher, M.; Geelen, D.; Duchaussoy, F.; Meyers, B.C.; Boerjan, W.; Martin, F. Genome-wide identification of NBS resistance genes in Populus trichocarpa. Plant Mol. Biol. 2008, 66, 619–636. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.-H.; Jin, H.; Marler, B.; Guo, H. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Löytynoja, A.; Goldman, N. webPRANK: A phylogeny-aware multiple sequence aligner with interactive alignment browser. BMC Bioinform. 2010, 11, 579. [Google Scholar] [CrossRef] [PubMed]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Nielsen, R. Estimating synonymous and nonsynonymous substitution rates under realistic evolutionary models. Mol. Biol. Evol. 2000, 17, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Hurst, L.D. The Ka/Ks ratio: Diagnosing the form of sequence evolution. Trends Genet. 2002, 18, 486–487. [Google Scholar] [CrossRef]
- Ito, T.M.; Trevizan, C.B.; dos Santos, T.B.; de Souza, S.G.H. Genome-Wide Identification and Characterization of the Dof Transcription Factor Gene Family in Phaseolus vulgaris L. Am. J. Plant Sci. 2017, 8, 3233. [Google Scholar] [CrossRef]
- Deng, W.; Wang, Y.; Liu, Z.; Cheng, H.; Xue, Y. HemI: A toolkit for illustrating heatmaps. PLoS ONE 2014, 9, e111988. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Zhou, H.; Sheng, S.; Cao, M.; Li, Y.; Pang, X. Genome-Wide Organization and Expression Profiling of the SBP-Box Gene Family in Chinese Jujube (Ziziphus jujuba Mill.). Int. J. Mol. Sci. 2017, 18, 1734. [Google Scholar] [CrossRef] [PubMed]

| Gene ID | Protein Name | Protein Model | Position of Dof Domain | Protein Length | Mw | pI | Instability Index | Orthologous |
|---|---|---|---|---|---|---|---|---|
| 105649666 | JcDof-1 | XP_012091770.1 | 40–98 | 160 | 18,217 | 9.4 | 49.94 | AT1G29160.1 |
| 105649561 * | JcDof-2.1 | XP_012091631.1 | 67–125 | 331 | 36,503 | 8.74 | 58.25 | AT1G28310.2 |
| JcDof-2.2 | XP_012091630.1 | 67–125 | 365 | 40,036 | 8.72 | 60.68 | AT1G28310.2 | |
| 105649560 * | JcDof-3.1 | XP_012091629.1 | 39–97 | 321 | 34,862 | 9.42 | 61.1 | AT5G60850.1 |
| JcDof-3.2 | XP_012091628.1 | 39–97 | 321 | 34,862 | 9.42 | 61.1 | AT5G60850.1 | |
| 105649506 | JcDof-4 | XP_012091561.1 | 48–106 | 283 | 31,234 | 8.87 | 58.45 | AT1G28310.2 |
| 105645621 | JcDof-5 | XP_012086658.1 | 39–97 | 302 | 32,787 | 8.69 | 56.53 | AT4G24060.1 |
| 105644078 | JcDof-6 | XP_012084716.1 | 148–206 | 518 | 55,717 | 5.88 | 43.82 | AT5G62430.1 |
| 105642820 * | JcDof-7.1 | XP_012083166.1 | 38–96 | 256 | 27,889 | 9.04 | 58.51 | AT3G61850.4 |
| JcDof-7.2 | XP_012083165.1 | 41–99 | 259 | 28,222 | 9.04 | 57.94 | AT3G61850.4 | |
| JcDof-7.3 | XP_012083164.1 | 23–81 | 272 | 29,648 | 8.89 | 61.74 | AT3G61850.4 | |
| JcDof-7.4 | XP_012083162.1 | 23–81 | 272 | 29,648 | 8.89 | 61.74 | AT3G61850.4 | |
| JcDof-7.5 | XP_012083161.1 | 23–81 | 272 | 29,648 | 8.89 | 61.74 | AT3G61850.4 | |
| JcDof-7.6 | XP_012083160.1 | 38–96 | 287 | 31,275 | 8.87 | 59.86 | AT3G61850.4 | |
| JcDof-7.7 | XP_012083159.1 | 41–99 | 290 | 31,607 | 8.87 | 59.35 | AT3G61850.4 | |
| 105641716 | JcDof-8 | XP_012081705.1 | 32–90 | 344 | 36,852 | 8.97 | 52.37 | AT5G65590.1 |
| 105640671 | JcDof-9 | XP_012080436.1 | 31–89 | 334 | 36,528 | 6.86 | 57.05 | AT5G60850.1 |
| 105640546 | JcDof-10 | XP_012080278.1 | 121–179 | 471 | 51,491 | 6.61 | 54.99 | AT5G39660.1 |
| 105640379 | JcDof-11 | XP_012080063.1 | 52–110 | 312 | 34,986 | 6.75 | 39.5 | AT5G62940.1 |
| 105639962 | JcDof-12 | XP_012079559.1 | 26–84 | 236 | 24,419 | 9.17 | 48.6 | AT3G50410.1 |
| 105639655 | JcDof-13 | XP_012079164.1 | 63–121 | 326 | 34,900 | 9.08 | 48.8 | AT1G07640.3 |
| 105639642 | JcDof-14 | XP_012079148.1 | 26–84 | 246 | 25,139 | 8.72 | 41.9 | AT3G50410.1 |
| 105636282 * | JcDof-15.1 | XP_012074917.1 | 75–133 | 353 | 36,578 | 9.14 | 50.3 | AT2G37590.1 |
| JcDof-15.2 | XP_012074916.1 | 84–142 | 362 | 37,605 | 9.14 | 50.73 | AT2G37590.1 | |
| 105635894 | JcDof-16 | XP_012074418.1 | 10–68 | 282 | 30,892 | 5.14 | 48.76 | AT3G52440.1 |
| 105633699 | JcDof-17 | XP_012071724.1 | 21–79 | 245 | 25,883 | 8.52 | 39.4 | AT1G47655.1 |
| 105632564 | JcDof-18 | XP_012070363.1 | 101–159 | 497 | 53,629 | 7.78 | 43.27 | AT3G47500.1 |
| 105631489 | JcDof-19 | XP_012069011.1 | 18–76 | 249 | 26,497 | 8.26 | 47.16 | AT3G21270.1 |
| 105630455 | JcDof-20 | XP_012067660.1 | 129–187 | 465 | 51,091 | 6.8 | 47.74 | AT5G39660.1 |
| 105629142 | JcDof-21 | XP_012066060.1 | 28–86 | 287 | 32,762 | 4.65 | 51.26 | AT1G21340.1 |
| 105628246 | JcDof-22 | XP_012065018.1 | 71–129 | 315 | 33,921 | 9.23 | 51.88 | AT2G28810.1 |
| 105628152 | JcDof-23 | XP_012064896.1 | 36–94 | 290 | 32,430 | 6.65 | 41.41 | AT2G28510.1 |
| 105647749 | JcDof-24 | XP_012089351.1 | 70–128 | 338 | 35,691 | 9.19 | 50.23 | AT3G55370.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Li, J.; Gao, X.; Zhang, D.; Li, A.; Liu, C. Genome-Wide Screening and Characterization of the Dof Gene Family in Physic Nut (Jatropha curcas L.). Int. J. Mol. Sci. 2018, 19, 1598. https://doi.org/10.3390/ijms19061598
Wang P, Li J, Gao X, Zhang D, Li A, Liu C. Genome-Wide Screening and Characterization of the Dof Gene Family in Physic Nut (Jatropha curcas L.). International Journal of Molecular Sciences. 2018; 19(6):1598. https://doi.org/10.3390/ijms19061598
Chicago/Turabian StyleWang, Peipei, Jing Li, Xiaoyang Gao, Di Zhang, Anlin Li, and Changning Liu. 2018. "Genome-Wide Screening and Characterization of the Dof Gene Family in Physic Nut (Jatropha curcas L.)" International Journal of Molecular Sciences 19, no. 6: 1598. https://doi.org/10.3390/ijms19061598
APA StyleWang, P., Li, J., Gao, X., Zhang, D., Li, A., & Liu, C. (2018). Genome-Wide Screening and Characterization of the Dof Gene Family in Physic Nut (Jatropha curcas L.). International Journal of Molecular Sciences, 19(6), 1598. https://doi.org/10.3390/ijms19061598






