Cell Propagation of Cholera Toxin CTA ADP-Ribosylating Factor by Exosome Mediated Transfer
Abstract
:1. Introduction
2. Results
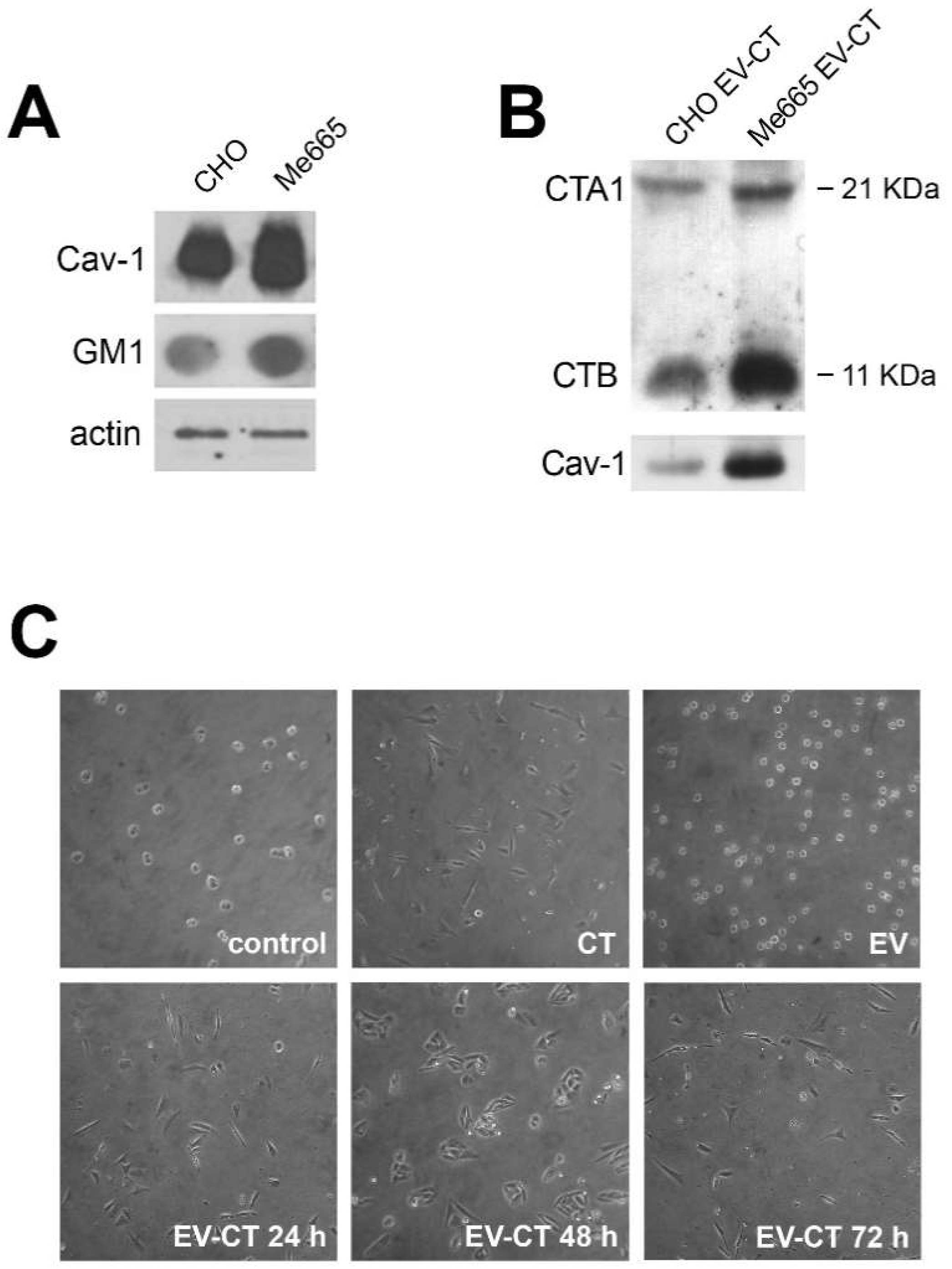
2.1. Extracellular Vesicles Isolated from CHO and Me665 Cells Upon CT Incubation Contain Cholera Toxin
2.2. Quantification and Characterization of Fluorescent Exosomes Containing CT Demonstrate the CTA Subunit Inclusion
2.3. Intracellular Distribution of CT in Me665 Cells
2.4. CTA Active Subunit Is Retrieved in Association HSP90/PDI with Respect to F-exo Biogenesis
2.5. Functional Activation of Adenylate Cyclase Following F-exo Direct Transfer to Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Isolation of EVs from CHO and Me665 Cell Culture Supernatants
4.3. Morphological Analysis of CHO Cells after CT, EV and EV-CT Treatment
4.4. Dot Blot and Western Blot Analysis
4.5. Generation and Quantification of F-exo
4.6. Iodixanol/OptiPrepTM Gradient Separation
4.7. Chymotrypsin Treatment of exo
4.8. cAMP Assay
4.9. Confocal Microscopy
4.10. F-exo and F-exo CT Transfer
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chatterjee, D.; Chaudhuri, K. Association of cholera toxin with Vibrio choleraeouter membrane vesicles which are internalized by human intestinal epithelial cells. FEBS Lett. 2011, 585, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Gill, D.M.; Rappaport, R.S. Origin of the Enzymatically Active AI Fragment of Cholera Toxin. J. Infect. Dis. 1979, 139, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Sandvig, K.; Deurs, B.V. Transport of protein toxins into cells: Pathways used by ricin, cholera toxin and Shiga toxin. FEBS Lett. 2002, 529, 49–53. [Google Scholar] [CrossRef]
- Sánchez, J.; Holmgren, J. Cholera toxin structure, gene regulation and pathophysiological and immunological aspects. Cell. Mol. Life Sci. 2008, 65, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Parton, R.G. Ultrastructural localization of gangliosides; GM1 is concentrated in caveolae. J. Histochem. Cytochem. 1994, 42, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.; Banerjee, T.; Navarro-Garcia, F.; Huerta, J.; Massey, S.; Burlingame, M.; Pande, A.H.; Tatulian, S.A.; Teter, K. A therapeutic chemical chaperone inhibits cholera intoxication and unfolding/translocation of the cholera toxin A1 subunit. PLoS ONE 2011, 6, e18825. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Le, P.U.; Nabi, I.R. Ganglioside GM1 levels are a determinant of the extent of caveolae/raft-dependent endocytosis of cholera toxin to the Golgi apparatus. J. Cell Sci. 2004, 117, 1421. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Lo Cicero, A.; Stahl, P.D.; Raposo, G. Extracellular vesicles shuffling intercellular messages: For good or for bad. Curr. Opin. Cell Biol. 2015, 35, 69–77. [Google Scholar] [CrossRef] [PubMed]
- El Andaloussi, S.; Lakhal, S.; Mäger, I.; Wood, M.J.A. Exosomes for targeted siRNA delivery across biological barriers. Adv. Drug Deliv. Rev. 2013, 65, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteom. 2010, 73, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Raposo, G. Exosomes—Vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 2009, 21, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Abrami, L.; Brandi, L.; Moayeri, M.; Brown, M.J.; Krantz, B.A.; Leppla, S.H.; van der Goot, F.G. Hijacking multivesicular bodies enables long-term and exosome-mediated long-distance action of anthrax toxin. Cell Rep. 2013, 5, 986–996. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Sun, S.; Feng, D.; Zhao, W.L.; Sui, S.F. A novel strategy for the invasive toxin: Hijacking exosome-mediated intercellular trafficking. Traffic 2009, 10, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Coscia, C.; Parolini, I.; Sanchez, M.; Biffoni, M.; Boussadia, Z.; Zanetti, C.; Fiani, M.L.; Sargiacomo, M. Generation, Quantification, and Tracing of Metabolically Labeled Fluorescent Exosomes. In Lentiviral Vectors and Exosomes as Gene and Protein Delivery Tools; Federico, M., Ed.; Springer: New York, NY, USA, 2016; pp. 217–235. [Google Scholar]
- Felicetti, F.; Parolini, I.; Bottero, L.; Fecchi, K.; Errico, M.C.; Raggi, C.; Biffoni, M.; Spadaro, F.; Lisanti, M.P.; Sargiacomo, M.; et al. Caveolin-1 tumor-promoting role in human melanoma. Int. J. Cancer J. Int. Cancer 2009, 125, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Morinaga, N.; Kaihou, Y.; Vitale, N.; Moss, J.; Noda, M. Involvement of ADP-ribosylation Factor 1 in Cholera Toxin-induced Morphological Changes of Chinese Hamster Ovary Cells. J. Biol. Chem. 2001, 276, 22838–22843. [Google Scholar] [CrossRef] [PubMed]
- Record, M.; Carayon, K.; Poirot, M.; Silvente-Poirot, S. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim. Biophys. Acta 2014, 1841, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.S.; Yin, Y.; Lee, T.; Lai, R.C.; Yeo, R.W.; Zhang, B.; Choo, A.; Lim, S.K. Therapeutic MSC exosomes are derived from lipid raft microdomains in the plasma membrane. J. Extracell. Vesicles 2013, 2, 22614. [Google Scholar] [CrossRef] [PubMed]
- Lotvall, J.; Hill, A.F.; Hochberg, F.; Buzas, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef] [PubMed]
- Ernst, K.; Schnell, L.; Barth, H. Host Cell Chaperones Hsp70/Hsp90 and Peptidyl-Prolyl Cis/Trans Isomerases Are Required for the Membrane Translocation of Bacterial ADP-Ribosylating Toxins. Curr. Top. Microbiol. Immunol. 2017, 406, 163–198. [Google Scholar] [PubMed]
- Guerrant, R.L.; Brunton, L.L.; Schnaitman, T.C.; Rebhun, L.I.; Gilman, A.G. Cyclic adenosine monophosphate and alteration of Chinese hamster ovary cell morphology: A rapid, sensitive in vitro assay for the enterotoxins of Vibrio cholerae and Escherichia coli. Infect. Immun. 1974, 10, 320–327. [Google Scholar] [PubMed]
- Wernick, N.L.B.; Chinnapen, D.J.F.; Cho, J.A.; Lencer, W.I. Cholera Toxin: An Intracellular Journey into the Cytosol by Way of the Endoplasmic Reticulum. Toxins 2010, 2, 310–325. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.; Burress, H.; Banerjee, T.; Ray, S.; Curtis, D.; Tatulian, S.A.; Teter, K. Substrate-induced unfolding of protein disulfide isomerase displaces the cholera toxin A1 subunit from its holotoxin. PLoS Pathog. 2014, 10, e1003925. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.; Navarro-Garcia, F.; Huerta, J.; Burress, H.; Massey, S.; Ireton, K.; Teter, K. Hsp90 Is Required for Transfer of the Cholera Toxin A1 Subunit from the Endoplasmic Reticulum to the Cytosol. J. Biol. Chem. 2010, 285, 31261–31267. [Google Scholar] [CrossRef] [PubMed]
- Pande, A.H.; Scaglione, P.; Taylor, M.; Nemec, K.N.; Tuthill, S.; Moe, D.; Holmes, R.K.; Tatulian, S.A.; Teter, K. Conformational instability of the cholera toxin A1 polypeptide. J. Mol. Biol. 2007, 374, 1114–1128. [Google Scholar] [CrossRef] [PubMed]
- Ampapathi, R.S.; Creath, A.L.; Lou, D.I.; Craft, J.W., Jr.; Blanke, S.R.; Legge, G.B. Order-disorder-order transitions mediate the activation of cholera toxin. J. Mol. Biol. 2008, 377, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Kaper, J.B.; Morris, J.G.; Levine, M.M. Cholera. Clin. Microbiol. Rev. 1995, 8, 48–86. [Google Scholar] [PubMed]
- Lencer, W.I.; Tsai, B. The intracellular voyage of cholera toxin: Going retro. Trends Biochem. Sci. 2003, 28, 639–945. [Google Scholar] [CrossRef] [PubMed]
- Saslowsky, D.E.; Lencer, W.I. Conversion of apical plasma membrane sphingomyelin to ceramide attenuates the intoxication of host cells by cholera toxin. Cell. Microbiol. 2008, 10, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.A.; Fujinaga, Y.; Lencer, W.I. Uncoupling of the cholera toxin-G(M1) ganglioside receptor complex from endocytosis, retrograde Golgi trafficking, and downstream signal transduction by depletion of membrane cholesterol. J. Biol. Chem. 2002, 277, 16249–16256. [Google Scholar] [CrossRef] [PubMed]
- Massol, R.H.; Larsen, J.E.; Fujinaga, Y.; Lencer, W.I.; Kirchhausen, T. Cholera toxin toxicity does not require functional Arf6- and dynamin-dependent endocytic pathways. Mol. Biol. Cell 2004, 15, 3631–3641. [Google Scholar] [CrossRef] [PubMed]
- Chinnapen, D.J.; Hsieh, W.T.; te Welscher, Y.M.; Saslowsky, D.E.; Kaoutzani, L.; Brandsma, E.; D’Auria, L.; Park, H.; Wagner, J.S.; Drake, K.R.; et al. Lipid sorting by ceramide structure from plasma membrane to ER for the cholera toxin receptor ganglioside GM1. Dev. Cell 2012, 23, 573–586. [Google Scholar] [CrossRef] [PubMed]
- de Gassart, A.; Géminard, C.; Février, B.; Raposo, G.; Vidal, M. Lipid raft-associated protein sorting in exosomes. Blood 2003, 102, 4336. [Google Scholar] [CrossRef] [PubMed]
- Calzolari, A.; Raggi, C.; Deaglio, S.; Sposi, N.M.; Stafsnes, M.; Fecchi, K.; Parolini, I.; Malavasi, F.; Peschle, C.; Sargiacomo, M.; et al. TfR2 localizes in lipid raft domains and is released in exosomes to activate signal transduction along the MAPK pathway. J. Cell Sci. 2006, 119 Pt 21, 4486–4498. [Google Scholar] [CrossRef] [PubMed]
- Parolini, I.; Federici, C.; Raggi, C.; Lugini, L.; Palleschi, S.; De Milito, A.; Coscia, C.; Iessi, E.; Logozzi, M.; Molinari, A.; et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 2009, 284, 34211–34222. [Google Scholar] [CrossRef] [PubMed]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef] [PubMed]
- Almagro-Moreno, S.; Pruss, K.; Taylor, R.K. Intestinal Colonization Dynamics of Vibrio cholerae. PLoS Pathog. 2015, 11, e1004787. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, M.; Battistini, A.; Araco, A.; Roda, L.G.; D’Agnolo, G. The Role of the Reactive Disulfide Bond in the Interaction of Cholera-Toxin Functional Regions. Eur. J. Biochem. 1979, 93, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. In Current Protocols in Cell Biology/Editorial Board; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; pp. 3.22.1–3.22.29. [Google Scholar]
- Kormelink, T.G.; Arkesteijn, G.J.; Nauwelaers, F.A.; van den Engh, G.; Nolte-’t Hoen, E.N.; Wauben, M.H. Prerequisites for the analysis and sorting of extracellular vesicle subpopulations by high-resolution flow cytometry. Cytom. Part A J. Int. Soc. Anal. Cytol. 2015, 89, 135–147. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanetti, C.; Gallina, A.; Fabbri, A.; Parisi, S.; Palermo, A.; Fecchi, K.; Boussadia, Z.; Carollo, M.; Falchi, M.; Pasquini, L.; et al. Cell Propagation of Cholera Toxin CTA ADP-Ribosylating Factor by Exosome Mediated Transfer. Int. J. Mol. Sci. 2018, 19, 1521. https://doi.org/10.3390/ijms19051521
Zanetti C, Gallina A, Fabbri A, Parisi S, Palermo A, Fecchi K, Boussadia Z, Carollo M, Falchi M, Pasquini L, et al. Cell Propagation of Cholera Toxin CTA ADP-Ribosylating Factor by Exosome Mediated Transfer. International Journal of Molecular Sciences. 2018; 19(5):1521. https://doi.org/10.3390/ijms19051521
Chicago/Turabian StyleZanetti, Cristiana, Angelo Gallina, Alessia Fabbri, Sofia Parisi, Angela Palermo, Katia Fecchi, Zaira Boussadia, Maria Carollo, Mario Falchi, Luca Pasquini, and et al. 2018. "Cell Propagation of Cholera Toxin CTA ADP-Ribosylating Factor by Exosome Mediated Transfer" International Journal of Molecular Sciences 19, no. 5: 1521. https://doi.org/10.3390/ijms19051521
APA StyleZanetti, C., Gallina, A., Fabbri, A., Parisi, S., Palermo, A., Fecchi, K., Boussadia, Z., Carollo, M., Falchi, M., Pasquini, L., Fiani, M. L., & Sargiacomo, M. (2018). Cell Propagation of Cholera Toxin CTA ADP-Ribosylating Factor by Exosome Mediated Transfer. International Journal of Molecular Sciences, 19(5), 1521. https://doi.org/10.3390/ijms19051521





