SOX7 Target Genes and Their Contribution to Its Tumor Suppressive Function
Abstract
1. Introduction
2. Results
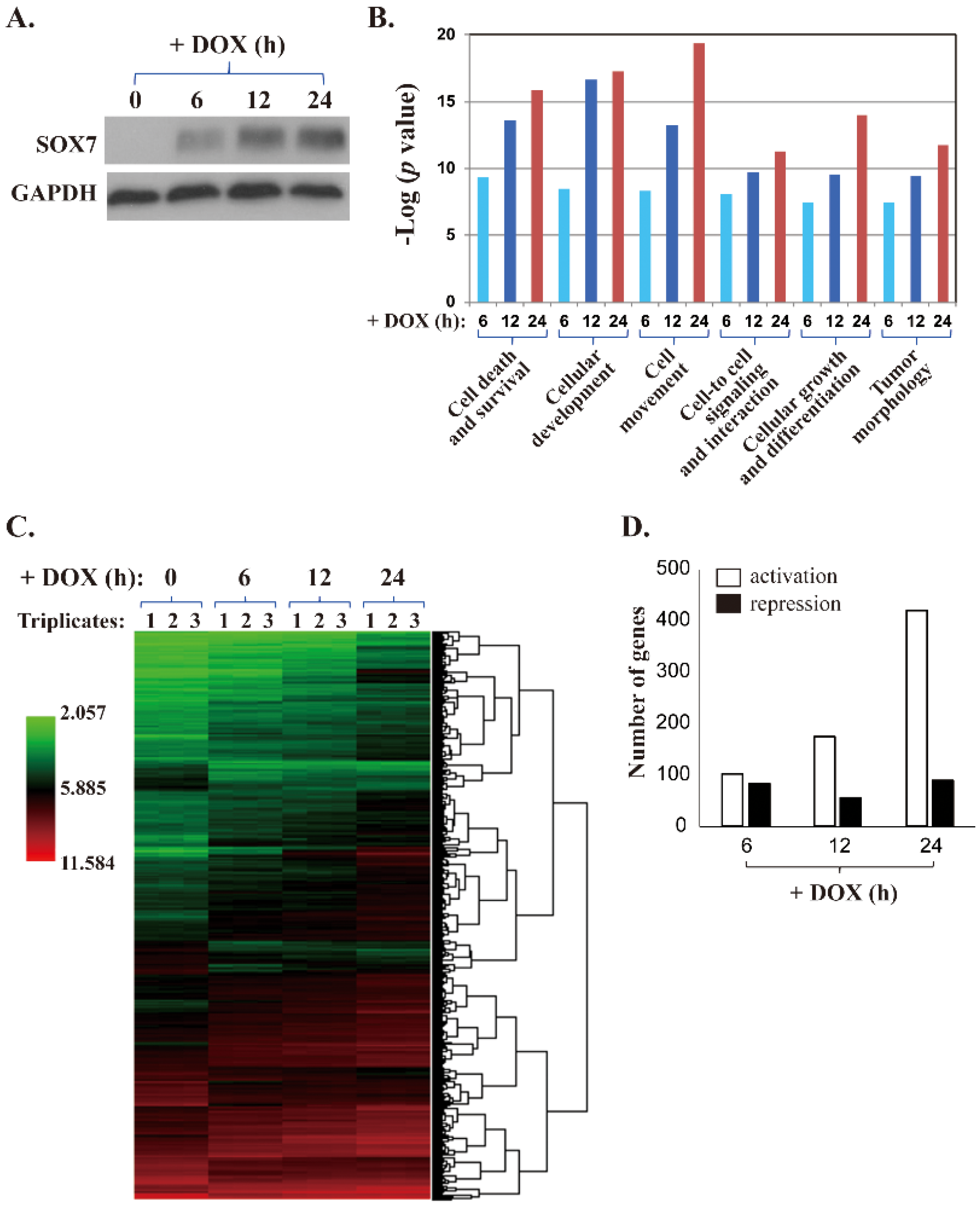
2.1. Microarray Analyses of SOX7 Target Genes
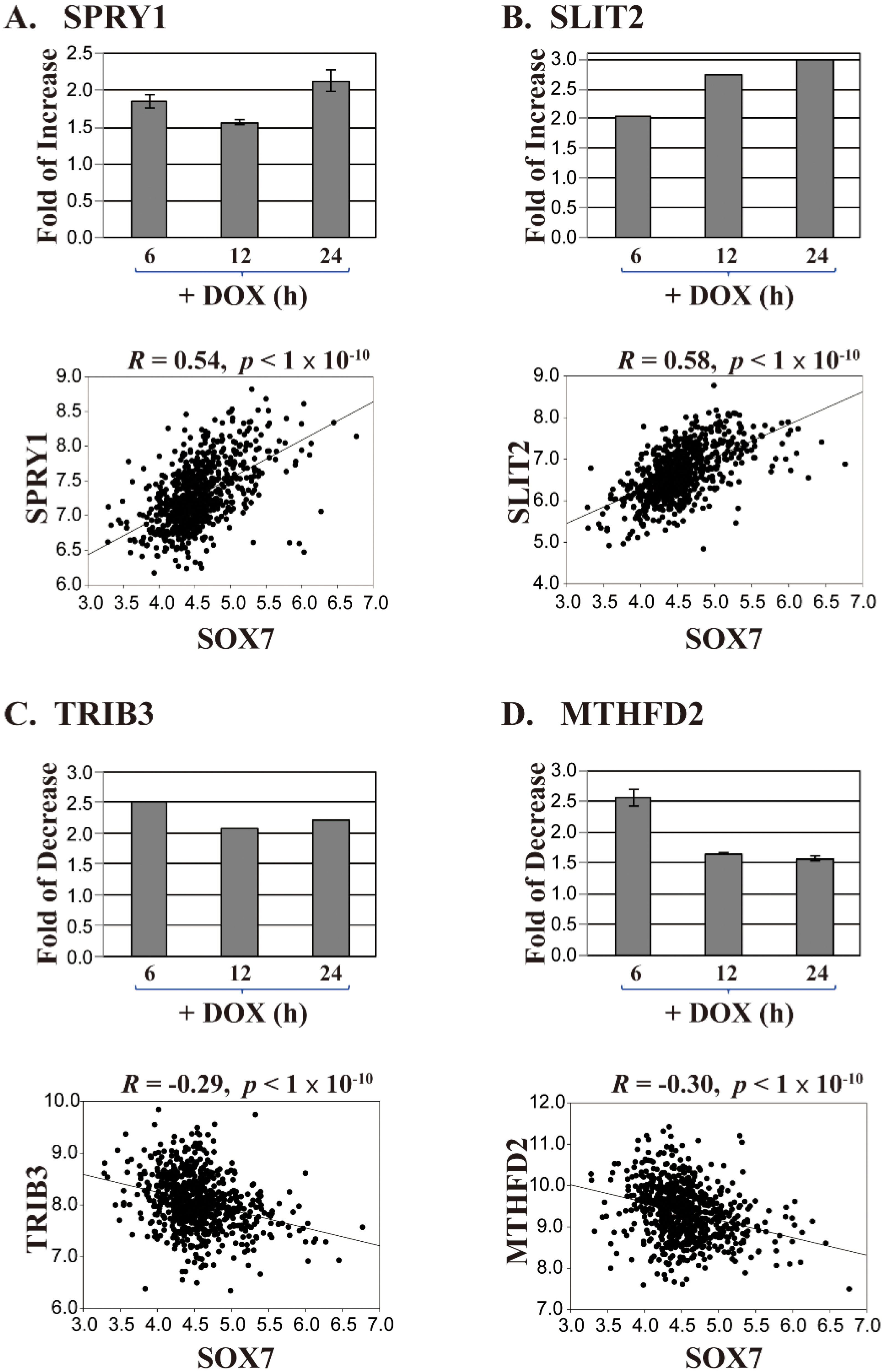
2.2. Identifying Essential SOX7 Target Genes in Breast Cancer Development
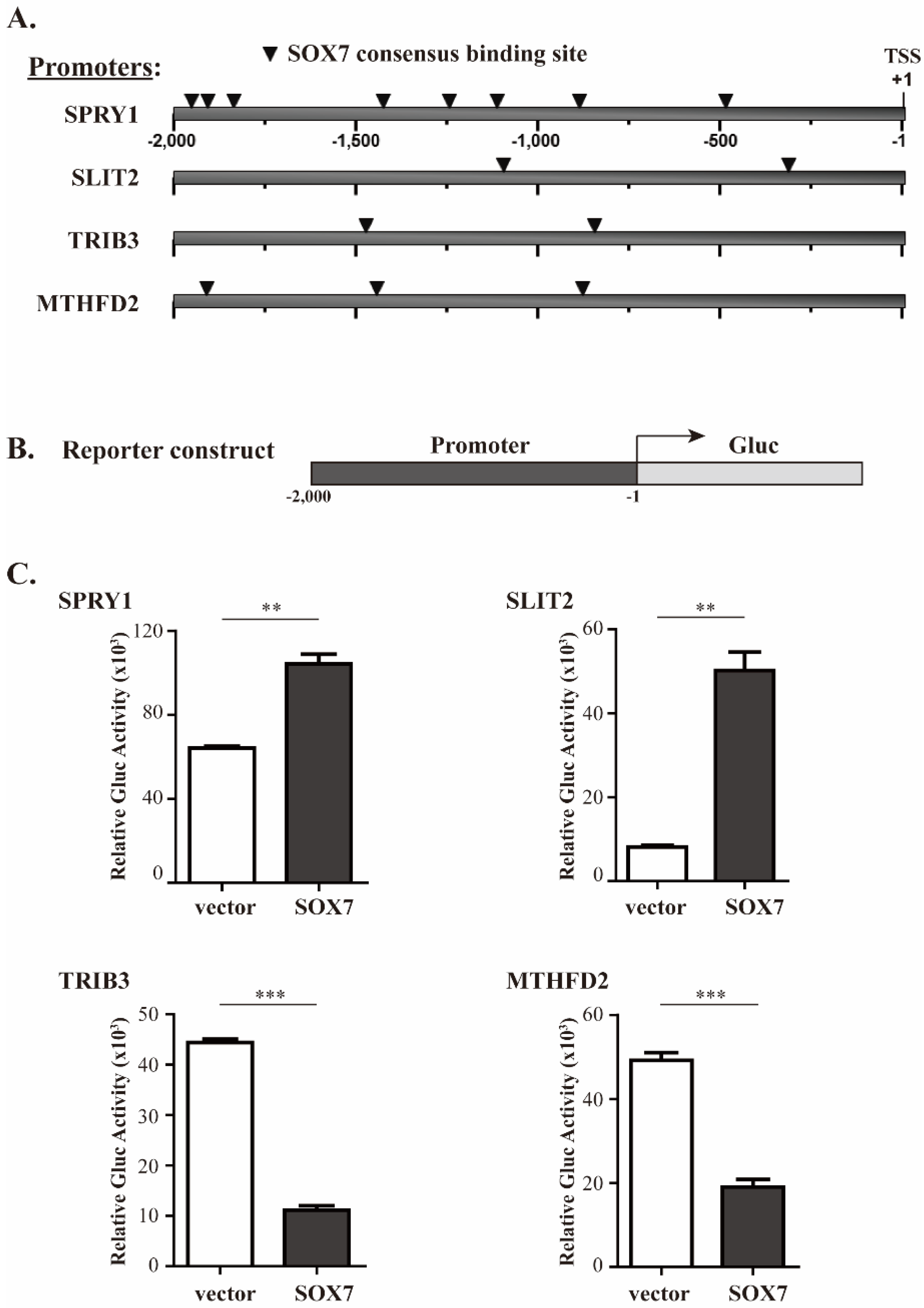
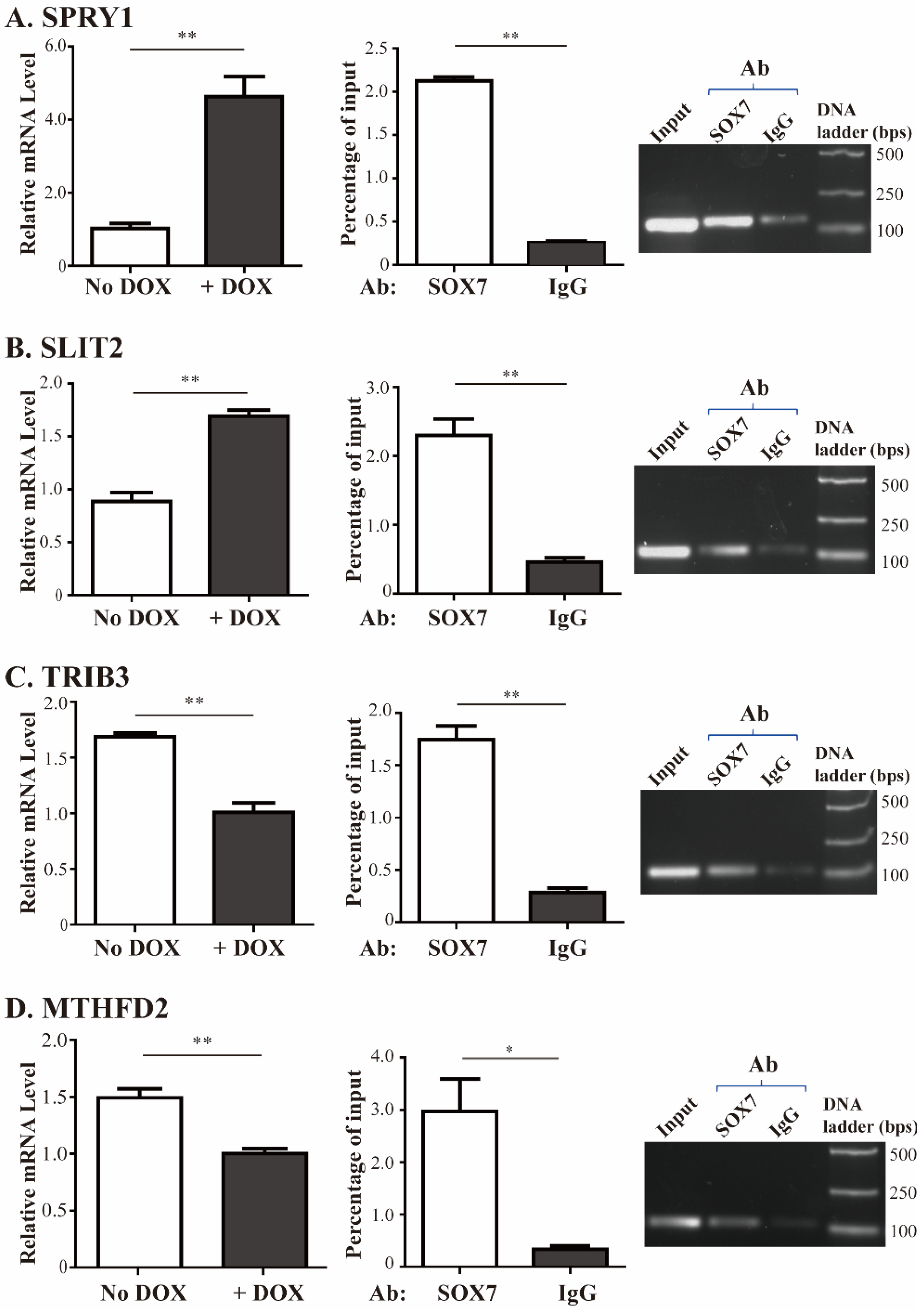
2.3. Validation of SOX7 Regulation of Its Four Target Genes
2.4. Investigating Contributions of SOX7 Target Genes to Its Tumor Suppressive Role
3. Discussion
4. Materials and Methods
4.1. Oligonucleotides, DNA Vectors, and Antibodies
4.2. Cell Culture, Lentiviral Production and Infection
4.3. Microarray Analysis
4.4. Chromatin Immunoprecipitation (ChIP) Assay
4.5. Proliferation Assays
4.6. Reverse Transcription and Quantitative PCR Analyses
4.7. Reporter Assay
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| SOX | SRY-related high mobility group (HMG) box |
| SPRY1 | sprouty homolog 1 |
| SLIT2 | slit guidance ligand 2 |
| TRIB3 | Tribbles homolog 3 |
| MTHFD2 | methylenetetrahydrofolate dehydrogenase (NADP+ dependent) |
| shRNA | short hairpin RNA |
| DOX | Doxycycline |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| Gluc | Gaussia luciferase |
| RT-PCR | reverse-transcription polymerase chain reaction |
| SEAP | secreted alkaline phosphatase |
| ChIP | Chromatin Immunoprecipitation |
References
- Sarkar, A.; Hochedlinger, K. The sox family of transcription factors: Versatile regulators of stem and progenitor cell fate. Cell Stem Cell 2013, 12, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M. Expression of human SOX7 in normal tissues and tumors. Int. J. Mol. Med. 2002, 9, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Wat, M.J.; Beck, T.F.; Hernandez-Garcia, A.; Yu, Z.; Veenma, D.; Garcia, M.; Holder, A.M.; Wat, J.J.; Chen, Y.; Mohila, C.A.; et al. Mouse model reveals the role of SOX7 in the development of congenital diaphragmatic hernia associated with recurrent deletions of 8p23.1. Hum. Mol. Genet. 2012, 21, 4115–4125. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Shen, H.; Ishida, S.; Dickson, C. SOX7 and GATA-4 are competitive activators of Fgf-3 transcription. J. Biol. Chem. 2004, 279, 28564–28573. [Google Scholar] [CrossRef] [PubMed]
- Futaki, S.; Hayashi, Y.; Emoto, T.; Weber, C.N.; Sekiguchi, K. Sox7 plays crucial roles in parietal endoderm differentiation in F9 embryonal carcinoma cells through regulating Gata-4 and Gata-6 expression. Mol. Cell. Biol. 2004, 24, 10492–10503. [Google Scholar] [CrossRef] [PubMed]
- Niimi, T.; Hayashi, Y.; Futaki, S.; Sekiguchi, K. SOX7 and SOX17 regulate the parietal endoderm-specific enhancer activity of mouse laminin alpha1 gene. J. Biol. Chem. 2004, 279, 38055–38061. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; Mazan, A.; Gandillet, A.; Pearson, S.; Lacaud, G.; Kouskoff, V. SOX7 regulates the expression of VE-cadherin in the haemogenic endothelium at the onset of haematopoietic development. Development 2012, 139, 1587–1598. [Google Scholar] [CrossRef] [PubMed]
- Chiang, I.K.; Fritzsche, M.; Pichol-Thievend, C.; Neal, A.; Holmes, K.; Lagendijk, A.; Overman, J.; D’Angelo, D.; Omini, A.; Hermkens, D.; et al. SoxF factors induce Notch1 expression via direct transcriptional regulation during early arterial development. Development 2017, 144, 2629–2639. [Google Scholar] [CrossRef] [PubMed]
- Lilly, A.J.; Costa, G.; Largeot, A.; Fadlullah, M.Z.; Lie, A.L.M.; Lacaud, G.; Kouskoff, V. Interplay between SOX7 and RUNX1 regulates hemogenic endothelial fate in the yolk sac. Development 2016, 143, 4341–4351. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhong, D.; Lau, S.; Liu, X.; Dong, X.Y.; Sun, X.; Yang, V.W.; Vertino, P.M.; Moreno, C.S.; Varma, V.; et al. Sox7 Is an independent checkpoint for beta-catenin function in prostate and colon epithelial cells. Mol. Cancer Res. 2008, 6, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; He, H.; Yao, W.; Zhu, Y.; Zhou, X.; Gui, M.; Lu, J.; Xi, H.; Deng, Z.; Fan, M. SOX7 Suppresses Wnt Signaling by Disrupting beta-Catenin/BCL9 Interaction. DNA Cell Biol. 2017, 37, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ge, Z.; Song, S.; Zhang, S.; Yan, H.; Huang, B.; Zhang, Y. Decreased expression of SOX7 is correlated with poor prognosis in lung adenocarcinoma patients. Pathol. Oncol. Res. 2012, 18, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.D.; Qin, G.Q.; Dai, Q.S.; Han, Z.D.; Chen, S.M.; Ling, X.H.; Fu, X.; Cai, C.; Chen, J.H.; Chen, X.B.; et al. SOXs in human prostate cancer: Implication as progression and prognosis factors. BMC Cancer 2012, 12, 248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, S.; Dong, W.; Li, L.; Feng, Y.; Pan, L.; Han, Z.; Wang, X.; Ren, G.; Su, D.; et al. SOX7, down-regulated in colorectal cancer, induces apoptosis and inhibits proliferation of colorectal cancer cells. Cancer Lett. 2009, 277, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Zhang, L.Y.; Wang, H.; Yang, B.; Han, T.; Zhao, X.L.; Wang, W.; Wang, X.Q.; Lin, G.W. Methylation of the CpG island near SOX7 gene promoter is correlated with the poor prognosis of patients with myelodysplastic syndrome. Tohoku J. Exp. Med. 2012, 227, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Hayano, T.; Garg, M.; Yin, D.; Sudo, M.; Kawamata, N.; Shi, S.; Chien, W.; Ding, L.W.; Leong, G.; Mori, S.; et al. SOX7 is down-regulated in lung cancer. J. Exp. Clin. Cancer Res. 2013, 32, 17. [Google Scholar] [CrossRef] [PubMed]
- Stovall, D.B.; Wan, M.; Miller, L.D.; Cao, P.; Maglic, D.; Zhang, Q.; Stampfer, M.R.; Liu, W.; Xu, J.; Sui, G. The regulation of SOX7 and its tumor suppressive role in breast cancer. Am. J. Pathol. 2013, 183, 1645–1653. [Google Scholar] [CrossRef] [PubMed]
- Pesole, G.; Liuni, S.; Grillo, G.; Saccone, C. Structural and compositional features of untranslated regions of eukaryotic mRNAs. Gene 1997, 205, 95–102. [Google Scholar] [CrossRef]
- Bai, Q.L.; Hu, C.W.; Wang, X.R.; Shang, J.X.; Yin, G.F. MiR-616 promotes proliferation and inhibits apoptosis in glioma cells by suppressing expression of SOX7 via the Wnt signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5630–5637. [Google Scholar] [PubMed]
- Yan, L.; Ma, J.; Zhu, Y.; Zan, J.; Wang, Z.; Ling, L.; Li, Q.; Lv, J.; Qi, S.; Cao, Y.; et al. miR-24-3p promotes cell migration and proliferation in lung cancer by targeting SOX7. J. Cell. Biochem. 2017, 119, 3989–3998. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wang, W.; Ding, W.; Zhang, L. MiR-9 is involved in TGF-beta1-induced lung cancer cell invasion and adhesion by targeting SOX7. J. Cell. Mol. Med. 2017, 21, 2000–2008. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, J.; Yu, Z.; Li, J.; Sun, R.; Kan, Q. miR-935 Promotes Liver Cancer Cell Proliferation and Migration by Targeting SOX7. Oncol. Res. 2017, 25, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Zhang, S.; Sun, S.; Zhu, J.; Xiao, Y. MiR-595 targeting regulation of SOX7 expression promoted cell proliferation of human glioblastoma. Biomed. Pharmacother. 2016, 80, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Chen, B.; Wu, Q.; Hu, K.; Xi, X.; Zhu, W.; Zhong, X.; Chen, J. Overexpression of miR-664 is associated with enhanced osteosarcoma cell migration and invasion ability via targeting SOX7. Clin. Exp. Med. 2017, 17, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, G.; Zeng, W.; Wang, J.; Li, Z.; Wang, B.; Tian, B.; Lu, D.; Zhang, X.; Gao, G.; et al. Long noncoding RNA AB073614 promotes the malignance of glioma by activating Wnt/beta-catenin signaling through downregulating SOX7. Oncotarget 2017, 8, 65577–65587. [Google Scholar] [PubMed]
- Lefebvre, V.; Dumitriu, B.; Penzo-Mendez, A.; Han, Y.; Pallavi, B. Control of cell fate and differentiation by Sry-related high-mobility-group box (Sox) transcription factors. Int. J. Biochem. Cell Biol. 2007, 39, 2195–2214. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.D.; Coffman, L.G.; Chou, J.W.; Black, M.A.; Bergh, J.; D’Agostino, R., Jr.; Torti, S.V.; Torti, F.M. An iron regulatory gene signature predicts outcome in breast cancer. Cancer Res. 2011, 71, 6728–6737. [Google Scholar] [CrossRef] [PubMed]
- Ohler, U.; Niemann, H. Identification and analysis of eukaryotic promoters: Recent computational approaches. Trends Genet. 2001, 17, 56–60. [Google Scholar] [CrossRef]
- Ghosh, D. Object-oriented transcription factors database (ooTFD). Nucleic Acids Res. 2000, 28, 308–310. [Google Scholar] [CrossRef] [PubMed]
- Harley, V.R.; Lovell-Badge, R.; Goodfellow, P.N. Definition of a consensus DNA binding site for SRY. Nucleic Acids Res. 1994, 22, 1500–1501. [Google Scholar] [CrossRef] [PubMed]
- Wegner, M. From head to toes: The multiple facets of Sox proteins. Nucleic Acids Res. 1999, 27, 1409–1420. [Google Scholar] [CrossRef] [PubMed]
- Hua, F.; Mu, R.; Liu, J.; Xue, J.; Wang, Z.; Lin, H.; Yang, H.; Chen, X.; Hu, Z. TRB3 interacts with SMAD3 promoting tumor cell migration and invasion. J. Cell Sci. 2011, 124, 3235–3246. [Google Scholar] [CrossRef] [PubMed]
- Izrailit, J.; Berman, H.K.; Datti, A.; Wrana, J.L.; Reedijk, M. High throughput kinase inhibitor screens reveal TRB3 and MAPK-ERK/TGFbeta pathways as fundamental Notch regulators in breast cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 1714–1719. [Google Scholar] [CrossRef] [PubMed]
- Kormish, J.D.; Sinner, D.; Zorn, A.M. Interactions between SOX factors and Wnt/beta-catenin signaling in development and disease. Dev. Dyn. 2010, 239, 56–68. [Google Scholar] [PubMed]
- Hanafusa, H.; Torii, S.; Yasunaga, T.; Matsumoto, K.; Nishida, E. Shp2, an SH2-containing protein-tyrosine phosphatase, positively regulates receptor tyrosine kinase signaling by dephosphorylating and inactivating the inhibitor Sprouty. J. Biol. Chem. 2004, 279, 22992–22995. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Bui Nguyen, T.M.; Kovalenko, D.; Adhikari, N.; Grindle, S.; Polster, S.P.; Friesel, R.; Ramakrishnan, S.; Hall, J.L. Sprouty1 inhibits angiogenesis in association with up-regulation of p21 and p27. Mol. Cell. Biochem. 2010, 338, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Lo, T.L.; Yusoff, P.; Fong, C.W.; Guo, K.; McCaw, B.J.; Phillips, W.A.; Yang, H.; Wong, E.S.; Leong, H.F.; Zeng, Q.; et al. The ras/mitogen-activated protein kinase pathway inhibitor and likely tumor suppressor proteins, sprouty 1 and sprouty 2 are deregulated in breast cancer. Cancer Res. 2004, 64, 6127–6136. [Google Scholar] [CrossRef] [PubMed]
- Dallol, A.; Morton, D.; Maher, E.R.; Latif, F. SLIT2 axon guidance molecule is frequently inactivated in colorectal cancer and suppresses growth of colorectal carcinoma cells. Cancer Res. 2003, 63, 1054–1058. [Google Scholar] [PubMed]
- Li, K.; Wang, F.; Cao, W.B.; Lv, X.X.; Hua, F.; Cui, B.; Yu, J.J.; Zhang, X.W.; Shang, S.; Liu, S.S.; et al. TRIB3 Promotes APL Progression through Stabilization of the Oncoprotein PML-RARalpha and Inhibition of p53-Mediated Senescence. Cancer Cell 2017, 31, 697–710e697. [Google Scholar] [CrossRef] [PubMed]
- Wennemers, M.; Bussink, J.; Scheijen, B.; Nagtegaal, I.D.; van Laarhoven, H.W.; Raleigh, J.A.; Varia, M.A.; Heuvel, J.J.; Rouschop, K.M.; Sweep, F.C.; et al. Tribbles homolog 3 denotes a poor prognosis in breast cancer and is involved in hypoxia response. Breast Cancer Res. 2011, 13, R82. [Google Scholar] [CrossRef] [PubMed]
- Lehtinen, L.; Ketola, K.; Makela, R.; Mpindi, J.P.; Viitala, M.; Kallioniemi, O.; Iljin, K. High-throughput RNAi screening for novel modulators of vimentin expression identifies MTHFD2 as a regulator of breast cancer cell migration and invasion. Oncotarget 2013, 4, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Selcuklu, S.D.; Donoghue, M.T.; Rehmet, K.; de Souza Gomes, M.; Fort, A.; Kovvuru, P.; Muniyappa, M.K.; Kerin, M.J.; Enright, A.J.; Spillane, C. MicroRNA-9 inhibition of cell proliferation and identification of novel miR-9 targets by transcriptome profiling in breast cancer cells. J. Biol. Chem. 2012, 287, 29516–29528. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Liu, Y.; He, C.; Tao, L.; He, X.; Song, H.; Zhang, G. Increased MTHFD2 expression is associated with poor prognosis in breast cancer. Tumour Biol. 2014, 35, 8685–8690. [Google Scholar] [CrossRef] [PubMed]
- Lizama, C.O.; Hawkins, J.S.; Schmitt, C.E.; Bos, F.L.; Zape, J.P.; Cautivo, K.M.; Borges Pinto, H.; Rhyner, A.M.; Yu, H.; Donohoe, M.E.; et al. Repression of arterial genes in hemogenic endothelium is sufficient for haematopoietic fate acquisition. Nat. Commun. 2015, 6, 7739. [Google Scholar] [CrossRef] [PubMed]
- Xu, E.E.; Sasaki, S.; Speckmann, T.; Nian, C.; Lynn, F.C. SOX4 Allows Facultative beta-Cell Proliferation Through Repression of Cdkn1a. Diabetes 2017, 66, 2213–2219. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Hernandez, J.M.; Delgado-Olguin, P.; Aguillon-Huerta, V.; Furlan-Magaril, M.; Recillas-Targa, F.; Coral-Vazquez, R.M. Sox9 represses alpha-sarcoglycan gene expression in early myogenic differentiation. J. Mol. Biol. 2009, 394, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sui, G.; Shi, Y. Gene silencing by a DNA vector-based RNAi technology. Methods Mol. Biol. 2005, 309, 205–218. [Google Scholar] [PubMed]
- Stovall, D.B.; Wan, M.; Zhang, Q.; Dubey, P.; Sui, G. DNA vector-based RNA interference to study gene function in cancer. J. Vis. Exp. 2012, e4129. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Huang, W.; Kute, T.E.; Miller, L.D.; Zhang, Q.; Hatcher, H.; Wang, J.; Stovall, D.B.; Russell, G.B.; Cao, P.D.; et al. Yin Yang 1 plays an essential role in breast cancer and negatively regulates p27. Am. J. Pathol. 2012, 180, 2120–2133. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Kulkarni, A.; Wang, Y.H. ATR preferentially interacts with common fragile site FRA3B and the binding requires its kinase activity in response to aphidicolin treatment. Mutat. Res. 2010, 686, 39–46. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Stovall, D.B.; Wan, M.; Zhang, Q.; Chou, J.W.; Li, D.; Sui, G. SOX7 Target Genes and Their Contribution to Its Tumor Suppressive Function. Int. J. Mol. Sci. 2018, 19, 1451. https://doi.org/10.3390/ijms19051451
Zhang Y, Stovall DB, Wan M, Zhang Q, Chou JW, Li D, Sui G. SOX7 Target Genes and Their Contribution to Its Tumor Suppressive Function. International Journal of Molecular Sciences. 2018; 19(5):1451. https://doi.org/10.3390/ijms19051451
Chicago/Turabian StyleZhang, Yumeng, Daniel B. Stovall, Meimei Wan, Qiang Zhang, Jeff W. Chou, Dangdang Li, and Guangchao Sui. 2018. "SOX7 Target Genes and Their Contribution to Its Tumor Suppressive Function" International Journal of Molecular Sciences 19, no. 5: 1451. https://doi.org/10.3390/ijms19051451
APA StyleZhang, Y., Stovall, D. B., Wan, M., Zhang, Q., Chou, J. W., Li, D., & Sui, G. (2018). SOX7 Target Genes and Their Contribution to Its Tumor Suppressive Function. International Journal of Molecular Sciences, 19(5), 1451. https://doi.org/10.3390/ijms19051451



