Protective Effect of Artemisia argyi and Its Flavonoid Constituents against Contrast-Induced Cytotoxicity by Iodixanol in LLC-PK1 Cells
Abstract
:1. Introduction
2. Results and Discussion
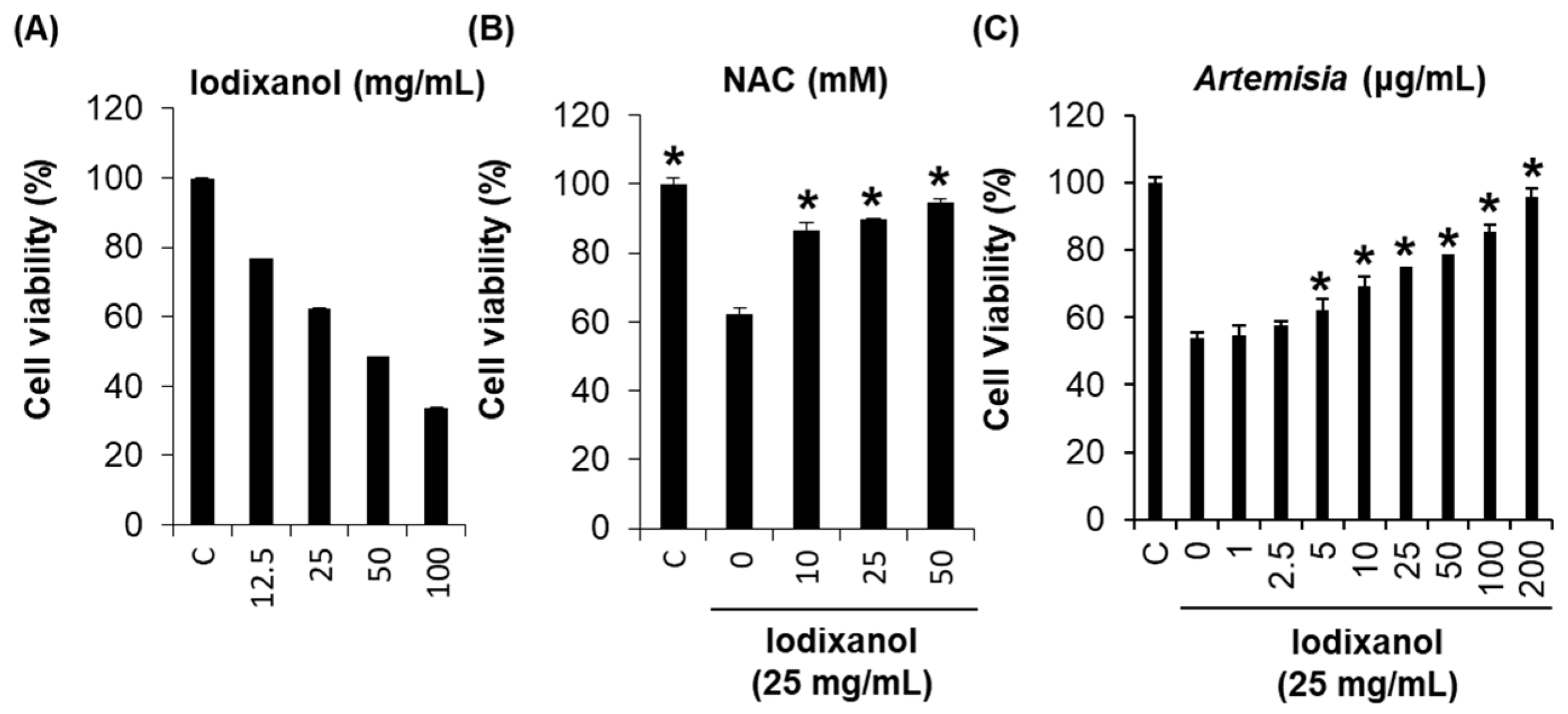
2.1. Effect of Artemisia argyi Extract on Iodixanol-Induced Nephrotoxicity in LLC-PK1 Cells
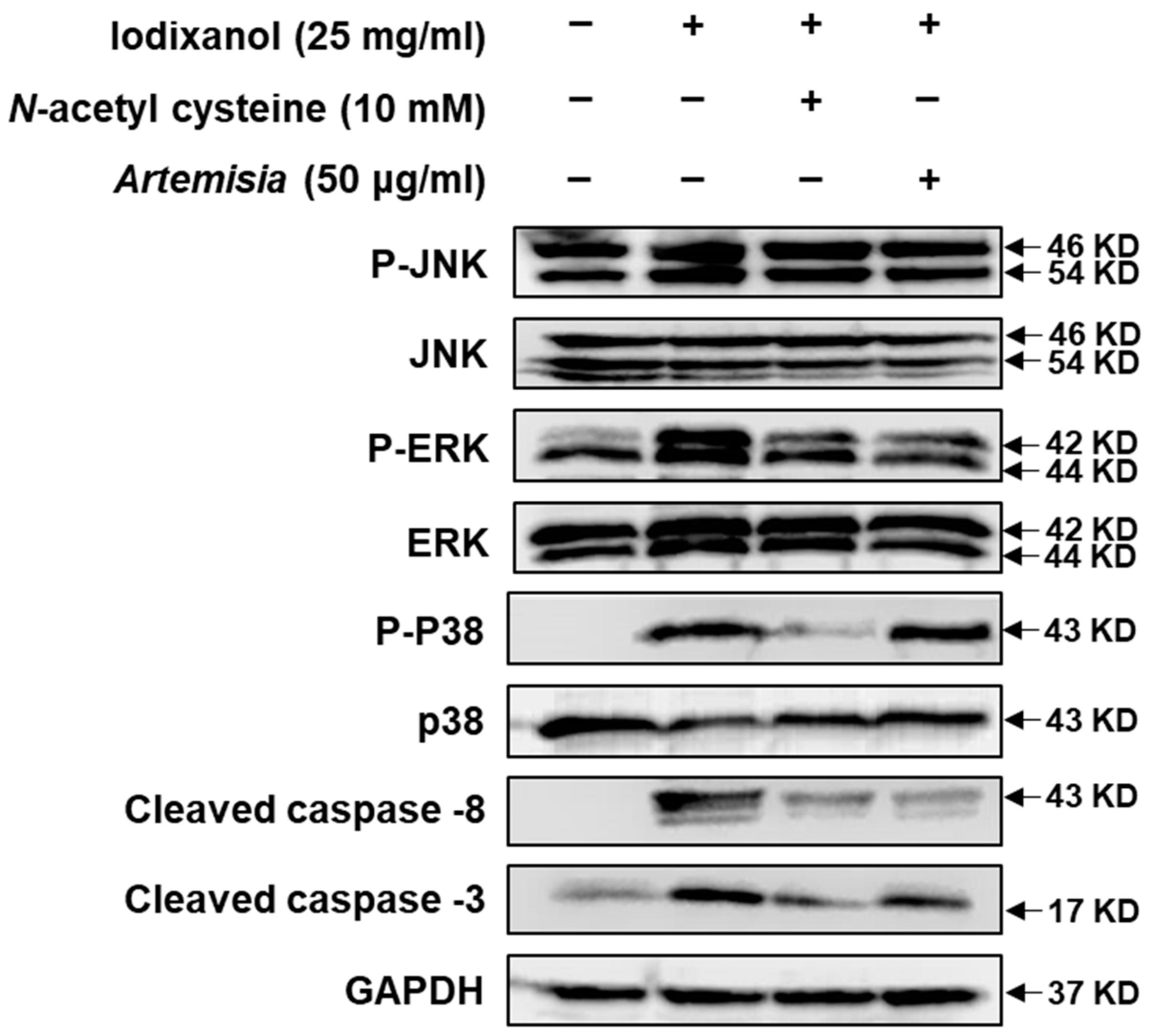
2.2. Effect of Artemisia argyi Extract on the Expression of Apoptosis-Related Proteins in LLC-PK1 Cells Exposed to Iodixanol
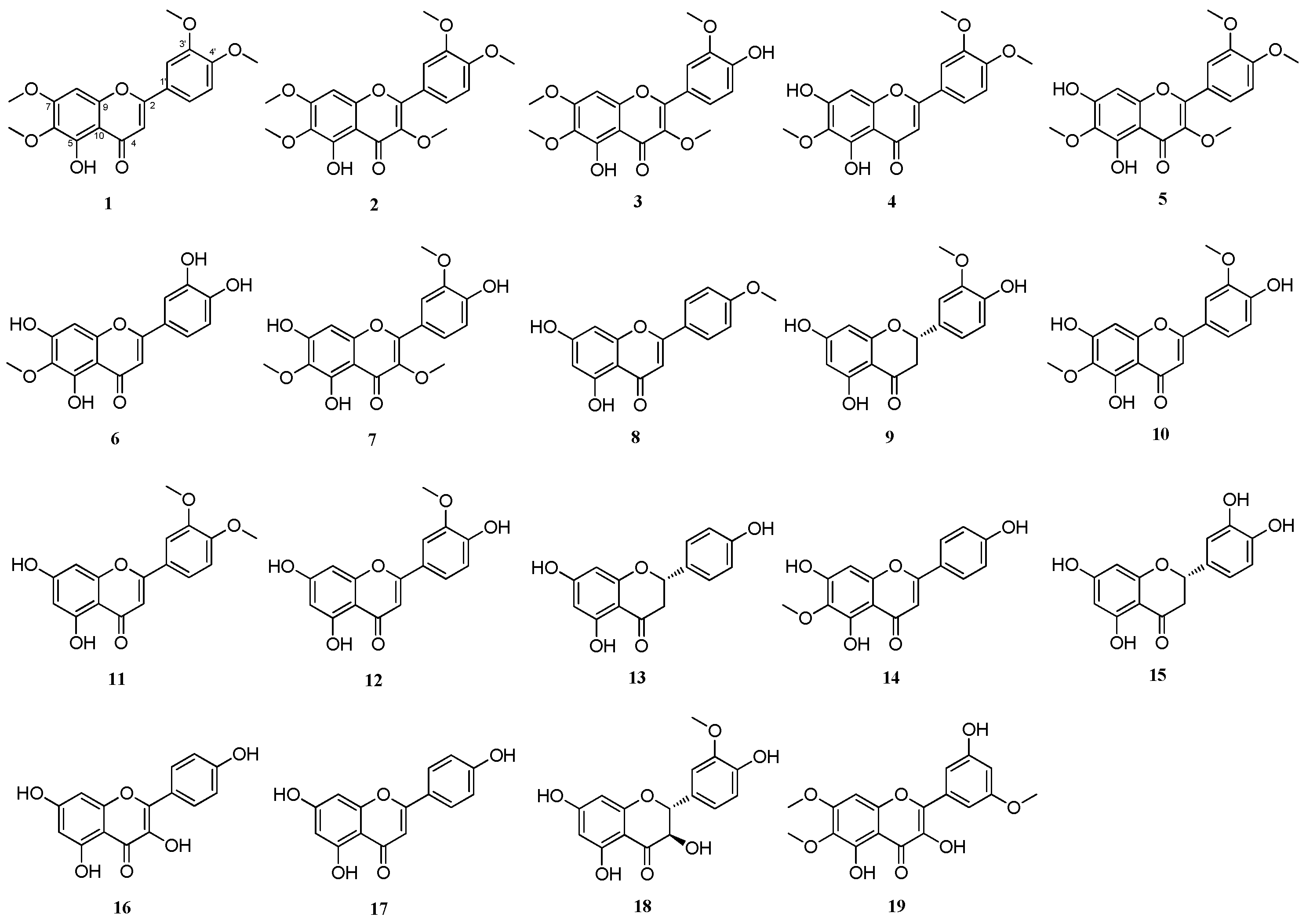
2.3. DPPH-Radical-Scavenging Effects of Flavonoid Compounds Isolated from Artemisia argyi Extracts
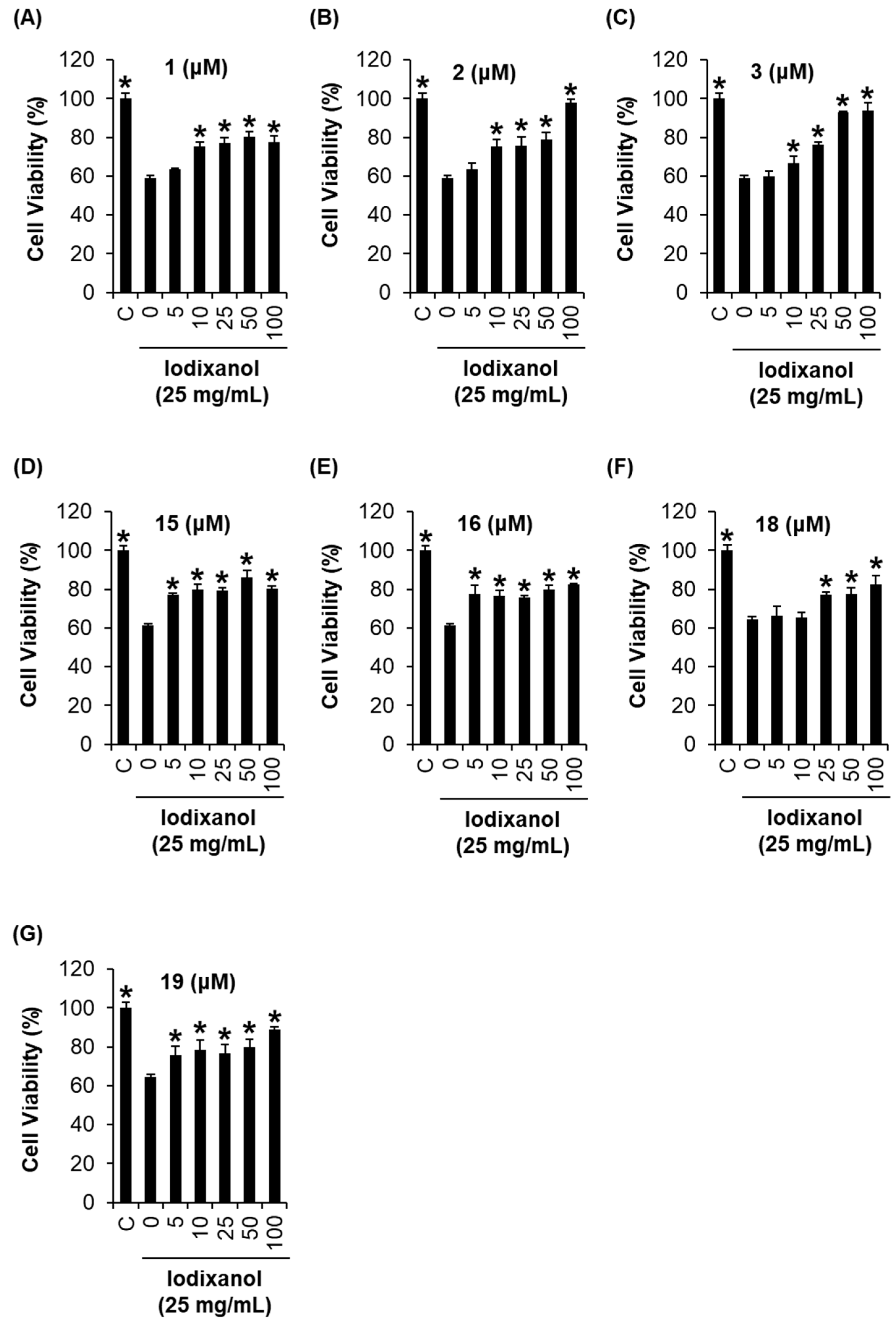
2.4. Comparison of the Protective Effects of the Flavonoid Compounds Isolated from Artemisia argyi Extracts against Iodixanol-Induced Nephrotoxicity in LLC-PK1 Cells
2.5. DL and OB Evaluation of Flavonoid Compounds with Profound Protective Effects against Iodixanol-Induced Nephrotoxicity in LLC-PK1 Cells
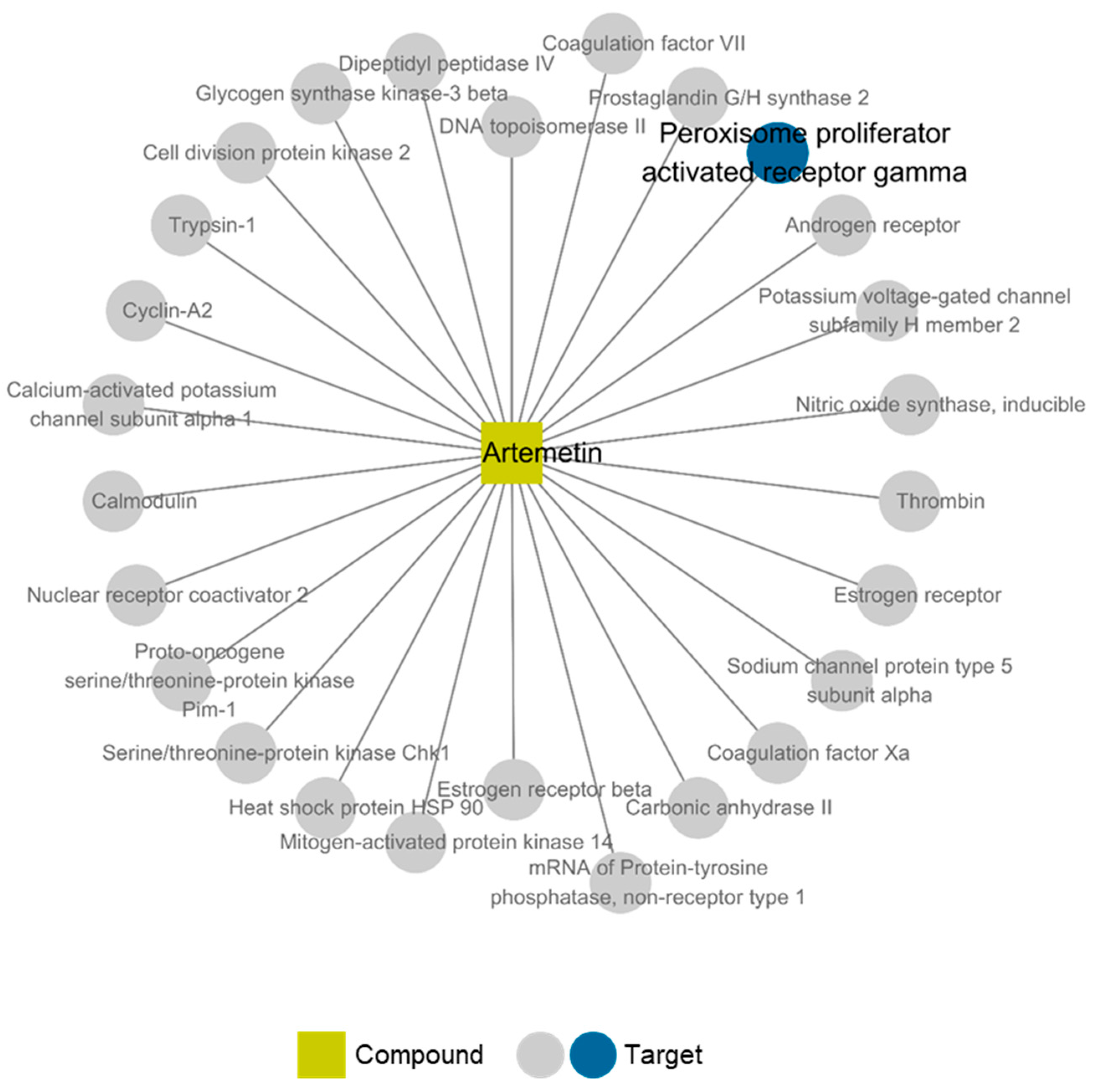
2.6. Compound-Target Network of Compound 2
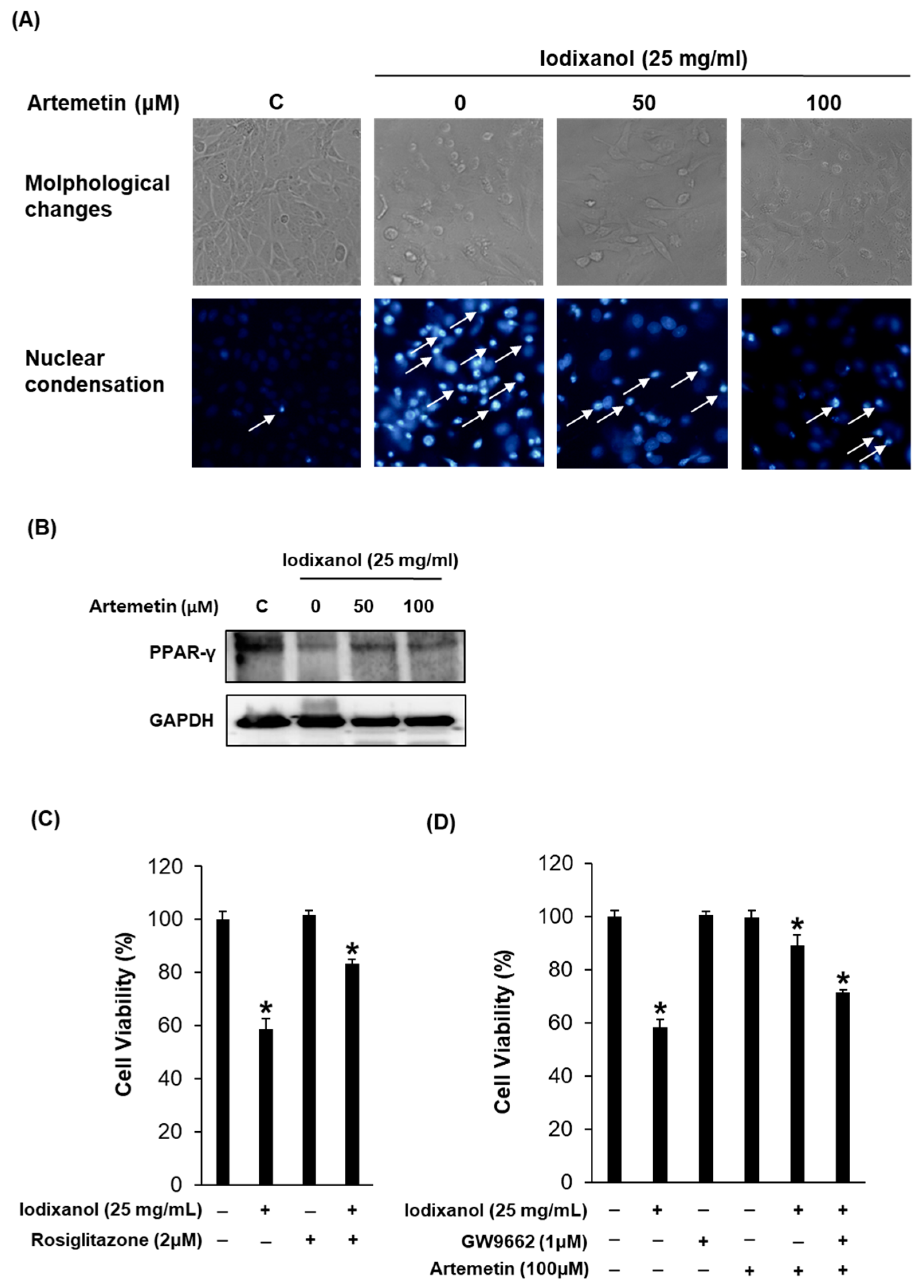
2.7. Effects of Artemetin on the Morphological Changes, Degree of Nuclear Condensation and Protein Expression of PPAR-γ in the Iodixanol-Induced Nephrotoxicity of LLC-PK1 Cells
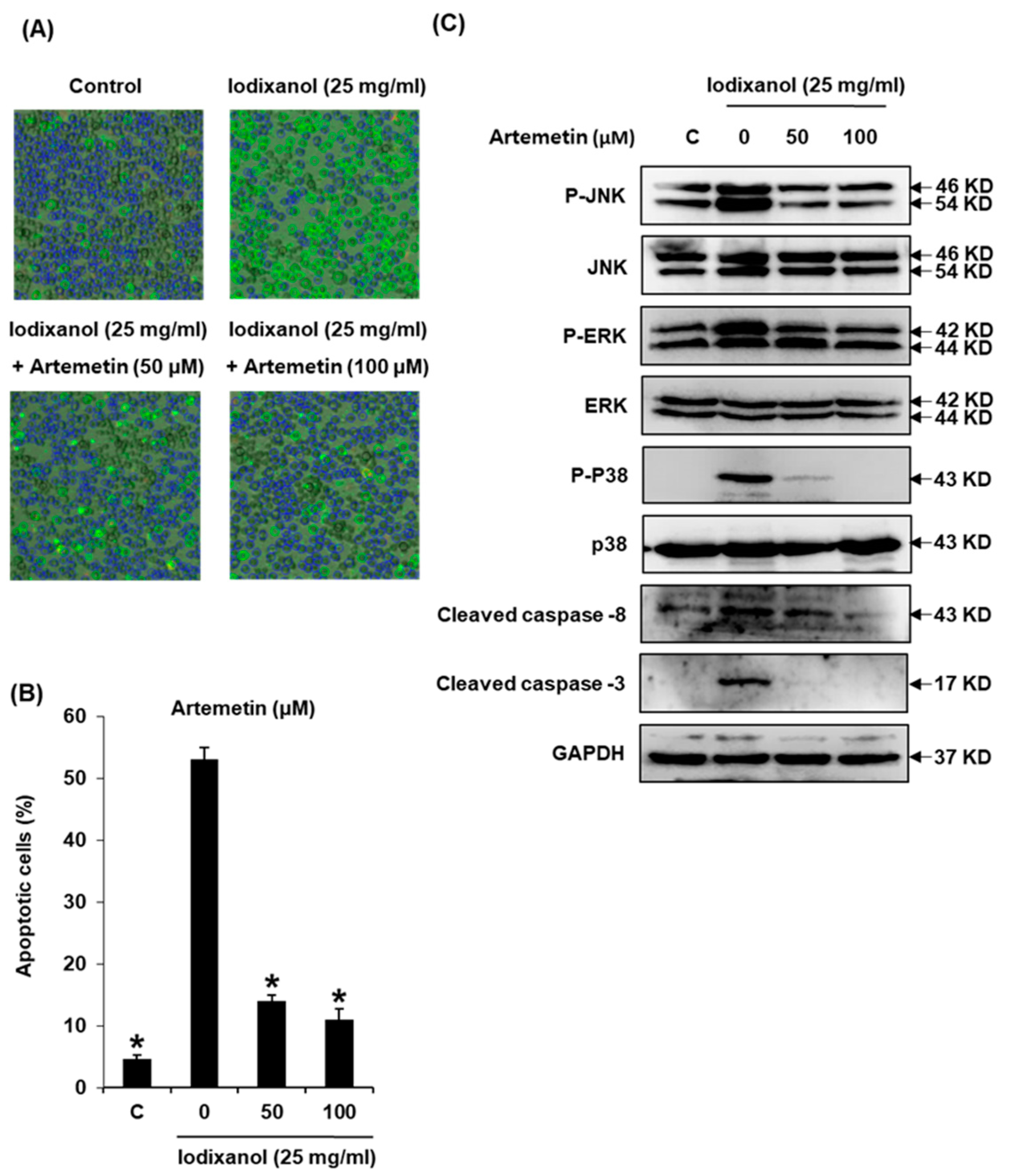
2.8. Effects of Artemetin on Iodixanol-Induced Apoptosis in LLC-PK1 Cells
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Materials
3.3. Extraction and Isolation of Flavonoids from A. argyi
3.4. General Experimental Procedures
3.5. DPPH Radical-Scavenging Assay
3.6. Cell Culture
3.7. Renoprotective Effect against Iodixanol-Induced Damage in Kidney Cells
3.8. Effect of PPAR-γ Ligand (Rosiglitazone) and PPAR-γ Antagonist (GW9662) against Iodixanol-Induced Damage in Kidney Cells
3.9. Nuclear Staining with Hoechst 33342
3.10. Image-Based Cytometric Assay
3.11. Western Blotting Analysis
3.12. Oral-Bioavailability (OB) and Drug-Likeness (DL) Evaluation
3.13. Network Analysis
3.14. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Hajdu, Z.; Hohmann, J.; Forgo, P.; Mathe, I.; Molnar, J.; Zupko, I. Antiproliferative activity of artemisia asiatica extract and its constituents on human tumor cell lines. Planta Med. 2014, 80, 1692–1697. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Yi, Y.S.; Sung, G.H.; Yang, W.S.; Park, J.G.; Yoon, K.; Yoon, D.H.; Song, C.; Lee, Y.; Rhee, M.H.; et al. Anti-inflammatory activities and mechanisms of artemisia asiatica ethanol extract. J. Ethnopharmacol. 2014, 152, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Molnar, J.; Szebeni, G.J.; Csupor-Loffler, B.; Hajdu, Z.; Szekeres, T.; Saiko, P.; Ocsovszki, I.; Puskas, L.G.; Hohmann, J.; Zupko, I. Investigation of the antiproliferative properties of natural sesquiterpenes from artemisia asiatica and onopordum acanthium on HL-60 cells in vitro. Int. J. Mol. Sci. 2016, 17, 83. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Han, Y.M.; Lee, J.S.; Ko, K.H.; Hong, S.P.; Kim, E.H.; Hahm, K.B. Nrf2-mediated mucoprotective and anti-inflammatory actions of artemisia extracts led to attenuate stress related mucosal damages. J. Clin. Biochem. Nutr. 2015, 56, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Oh, T.Y.; Kim, Y.S.; Sim, H.; Park, S.J.; Jang, E.J.; Park, J.S.; Baik, H.W.; Hahm, K.B. Artemisia asiatica extracts protect against ethanol-induced injury in gastric mucosa of rats. J. Gastroenterol. Hepatol. 2008, 23, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.Y.; Ahn, G.J.; Choi, S.M.; Ahn, B.O.; Kim, W.B. Increased susceptibility of ethanol-treated gastric mucosa to naproxen and its inhibition by DA-9601, an Artemisia asiatica extract. World J. Gastroenterol. 2005, 11, 7450–7456. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.O.; Chung, H.G.; Lee, W.H.; Lee, H.W.; Suk, K. Inhibition of microglial neurotoxicity by ethanol extract of Artemisia asiatica Nakai. Phytother. Res. 2008, 22, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.C.; Choi, E.J.; Oh, H.M.; Lee, S.; Lee, J.K.; Lee, M.S.; Shin, Y.I.; Choi, S.J.; Chae, J.R.; Lee, K.M.; et al. DA-9601, a standardized extract of Artemisia asiatica, blocks TNF-alpha-induced IL-8 and CCL20 production by inhibiting p38 kinase and NF-kappa B pathways in human gastric epithelial cells. World J. Gastroenterol. 2006, 12, 4850–4858. [Google Scholar] [PubMed]
- Hahm, K.B.; Kim, J.H.; You, B.M.; Kim, Y.S.; Cho, S.W.; Yim, H.; Ahn, B.O.; Kim, W.B. Induction of apoptosis with an extract of Artemisia asiatica attenuates the severity of cerulein-induced pancreatitis in rats. Pancreas 1998, 17, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.W.; Hwang, H.S.; Kim, Y.S.; Kim, H.J.; Shin, Y.S.; Jittreetat, T.; Kim, C.H. Protective effect of Artemisia asiatica (Pamp.) Nakai ex Kitam ethanol extract against cisplatin-induced apoptosis of human HaCaT keratinocytes: Involvement of NF-kappa B- and Bcl-2-controlled mitochondrial signaling. Phytomedicine 2015, 22, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Wu, H.; Dong, Y.G.; Lin, B.O.; Xu, G.; Ma, Y.B. Application of eupatilin in the treatment of osteosarcoma. Oncol. Lett. 2015, 10, 2505–2510. [Google Scholar] [CrossRef] [PubMed]
- Nijveldt, R.J.; van Nood, E.; van Hoorn, D.E.; Boelens, P.G.; van Norren, K.; van Leeuwen, P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Ahlenstiel, T.; Burkhardt, G.; Kohler, H.; Kuhlmann, M.K. Bioflavonoids attenuate renal proximal tubular cell injury during cold preservation in Euro-Collins and University of Wisconsin solutions. Kidney Int. 2003, 63, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, C.; Rossi, M.; McLaughlin, J.K.; Negri, E.; Talamini, R.; Lagiou, P.; Montella, M.; Ramazzotti, V.; Franceschi, S.; LaVecchia, C. Flavonoids and the risk of renal cell carcinoma. Cancer Epidemiol. Biomark. Prev. 2007, 16, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Sadat, U. Radiographic contrast-media-induced acute kidney injury: Pathophysiology and prophylactic strategies. ISRN Radiol 2013, 2013, 496438. [Google Scholar] [CrossRef] [PubMed]
- Andreucci, M.; Solomon, R.; Tasanarong, A. Side effects of radiographic contrast media: Pathogenesis, risk factors, and prevention. Biomed. Res. Int. 2014, 2014, 741018. [Google Scholar] [CrossRef] [PubMed]
- Quintavalle, C.; Brenca, M.; De Micco, F.; Fiore, D.; Romano, S.; Romano, M.F.; Apone, F.; Bianco, A.; Zabatta, M.A.; Troncone, G.; et al. In vivo and in vitro assessment of pathways involved in contrast media-induced renal cells apoptosis. Cell Death Dis. 2011, 2, e155. [Google Scholar] [CrossRef] [PubMed]
- Yokomaku, Y.; Sugimoto, T.; Kume, S.; Araki, S.; Isshiki, K.; Chin-Kanasaki, M.; Sakaguchi, M.; Nitta, N.; Haneda, M.; Koya, D.; et al. Asialoerythropoietin prevents contrast-induced nephropathy. J. Am. Soc. Nephrol. 2008, 19, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Sadat, U.; Usman, A.; Boyle, J.R.; Hayes, P.D.; Solomon, R.J. Contrast Medium-Induced Acute Kidney Injury. Cardiorenal Med. 2015, 5, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Briguori, C.; Donnarumma, E.; Quintavalle, C.; Fiore, D.; Condorelli, G. Contrast-induced acute kidney injury: Potential new strategies. Curr. Opin. Nephrol. Hypertens. 2015, 24, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Sheu, S.H.; Liu, I.H.; Lee, C.C.; Hsieh, C.C.; Yen, H.W.; Lai, W.T.; Chang, J.G. Impact of short-duration administration of N-acetylcysteine, probucol and ascorbic acid on contrast-induced cytotoxicity. J. Nephrol. 2012, 25, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Dodd, S.; Dean, O.; Copolov, D.L.; Malhi, G.S.; Berk, M. N-acetylcysteine for antioxidant therapy: Pharmacology and clinical utility. Expert Opin. Biol. Ther. 2008, 8, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Pu, L.Y.; Lu, L.; Wang, X.H.; Zhang, F.; Rao, J.H. N-acetylcysteine attenuates reactive-oxygen-species-mediated endoplasmic reticulum stress during liver ischemia-reperfusion injury. World J. Gastroenterol. 2014, 20, 15289–15298. [Google Scholar] [CrossRef] [PubMed]
- Ratliff, B.B.; Abdulmahdi, W.; Pawar, R.; Wolin, M.S. Oxidant mechanisms in renal injury and disease. Antioxid. Redox Signal 2016, 25, 119–146. [Google Scholar] [CrossRef] [PubMed]
- Romano, G.; Briguori, C.; Quintavalle, C.; Zanca, C.; Rivera, N.V.; Colombo, A.; Condorelli, G. Contrast agents and renal cell apoptosis. Eur. Heart J. 2008, 29, 2569–2576. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Lee, D.; Jang, H.J.; Jang, D.S.; Kwon, H.C.; Kim, K.H.; Kim, S.N.; Hwang, G.S.; Kang, K.S.; Eom, D.W. Protective effect of artemisia asiatica extract and its active compound eupatilin against cisplatin-induced renal damage. Evid. Based Complement. Alternat. Med. 2015, 2015, 483980. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.K.; Jang, H.J.; Kim, S.S.; Oh, M.Y.; Lee, D.H.; Eom, D.W.; Kang, K.S.; Kwan, H.C.; Ham, J.Y.; Park, C.S.; et al. Protective effect of eupatilin against renal ischemia-reperfusion injury in mice. Transpl. Proc. 2015, 47, 757–762. [Google Scholar] [CrossRef] [PubMed]
- He, X.Y.; Li, L.W.; Tan, H.; Chen, J.Y.; Zhou, Y.L. Atorvastatin attenuates contrast-induced nephropathy by modulating inflammatory responses through the regulation of JNK/p38/Hsp27 expression. J. Pharmacol. Sci. 2016, 131, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Wang, L.; Jiang, N.; Mou, S.; Zhang, M.; Gu, L.; Shao, X.; Wang, Q.; Qi, C.; Li, S.; et al. NLRP3 inflammasome mediates contrast media-induced acute kidney injury by regulating cell apoptosis. Sci. Rep. 2016, 6, 34682. [Google Scholar] [CrossRef] [PubMed]
- Seeliger, E.; Sendeski, M.; Rihal, C.S.; Persson, P.B. Contrast-induced kidney injury: Mechanisms, risk factors, and prevention. Eur. Heart J. 2012, 33, 2007–2015. [Google Scholar] [CrossRef] [PubMed]
- Hizoh, I.; Haller, C. Radiocontrast-induced renal tubular cell apoptosis: Hypertonic versus oxidative stress. Investig. Radiol. 2002, 37, 428–434. [Google Scholar] [CrossRef]
- Hizoh, I.; Strater, J.; Schick, C.S.; Kubler, W.; Haller, C. Radiocontrast-induced DNA fragmentation of renal tubular cells in vitro: Role of hypertonicity. Nephrol. Dial Transpl. 1998, 13, 911–918. [Google Scholar] [CrossRef]
- Guo, S.X.; Fang, Q.; You, C.G.; Jin, Y.Y.; Wang, X.G.; Hu, X.L.; Han, C.M. Effects of hydrogen-rich saline on early acute kidney injury in severely burned rats by suppressing oxidative stress induced apoptosis and inflammation. J. Transl. Med. 2015, 13, 183. [Google Scholar] [CrossRef] [PubMed]
- Dabaghi-Barbosa, P.; Mariante Rocha, A.; Franco da Cruz Lima, A.; Heleno de Oliveira, B.; Benigna Martinelli de Oliveira, M.; Gunilla Skare Carnieri, E.; Cadena, S.M.; Eliane Merlin Rocha, M. Hispidulin: Antioxidant properties and effect on mitochondrial energy metabolism. Free Radic. Res. 2005, 39, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.R.; Ho, Y.L.; Huang, S.C.; Huang, T.H.; Lai, S.C.; Tsai, J.C.; Wang, C.Y.; Huang, G.J.; Chang, Y.S. Antioxidant, anti-inflammatory and antiproliferative activities of Kalanchoe gracilis (L.) DC stem. Am. J. Chin. Med. 2011, 39, 1275–1290. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Lee, S.; Woo, Y.; Lim, Y. Relationships between Structure and Anti-oxidative Effects of Hydroxyflavones. Bull. Korean Chem. Soc. 2009, 30, 1397–1400. [Google Scholar]
- Small, D.M.; Morais, C.; Coombes, J.S.; Bennett, N.C.; Johnson, D.W.; Gobe, G.C. Oxidative stress-induced alterations in PPAR-gamma and associated mitochondrial destabilization contribute to kidney cell apoptosis. Am. J. Physiol.-Renal Physiol. 2014, 307, F814–F822. [Google Scholar] [CrossRef] [PubMed]
- Kiss-Toth, E.; Roszer, T. PPAR gamma in kidney physiology and pathophysiology. PPAR Res. 2008, 2008, 183108. [Google Scholar] [CrossRef] [PubMed]
- Panchapakesan, U.; Pollock, C.A.; Chen, X.M. The effect of high glucose and PPAR-gamma agonists on PPAR-gamma expression and function in HK-2 cells. Am. J. Physiol.-Renal Physiol. 2004, 287, F528–F534. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.L.; Lin, C.Y.; Chou, H.C.; Chang, C.C.; Lo, H.Y.; Juan, S.H. Perfluorooctanesulfonate Mediates Renal Tubular Cell Apoptosis through PPARgamma Inactivation. PLoS ONE 2016, 11, e0155190. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Hu, S.; Zou, J.; Xiao, J.; Zhang, X.; Fu, C.; Feng, X.; Ye, Z. Peroxisome proliferator-activated receptor gamma prevents the production of NOD-like receptor family, pyrin domain containing 3 inflammasome and interleukin 1beta in HK-2 renal tubular epithelial cells stimulated by monosodium urate crystals. Mol. Med. Rep. 2015, 12, 6221–6226. [Google Scholar] [CrossRef] [PubMed]
- Lepenies, J.; Hewison, M.; Stewart, P.M.; Quinkler, M. Renal PPAR gamma mRNA expression increases with impairment of renal function in patients with chronic kidney disease. Nephrology 2010, 15, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Tarawneh, A.; Leon, F.; Pettaway, S.; Elokely, K.M.; Klein, M.L.; Lambert, J.; Mansoor, A.; Cutler, S.J. Flavonoids from Perovskia atriplicifolia and their in vitro displacement of the respective radioligands for human opioid and cannabinoid receptors. J. Nat. Prod. 2015, 78, 1461–1465. [Google Scholar] [CrossRef] [PubMed]
- Nakasugi, T.; Nakashima, M.; Komai, K. Antimutagens in gaiyou (Artemisia argyi levl. et vant.). J. Agric. Food Chem. 2000, 48, 3256–3266. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Aisa, H.A.; Shakhidoyatov, K.M. Flavones from Artemisia rupestris. Chem. Nat. Compd. 2012, 48, 685–686. [Google Scholar] [CrossRef]
- Yu, H.; Chen, J.; Xu, X.; Li, Y.; Zhao, H.; Fang, Y.; Li, X.; Zhou, W.; Wang, W.; Wang, Y. A systematic prediction of multiple drug-target interactions from chemical, genomic, and pharmacological data. PLoS ONE 2012, 7, e37608. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mabry, T.J. Flavonoids from Artemisia-Frigida. Phytochemistry 1981, 20, 1389–1395. [Google Scholar] [CrossRef]
- Elansari, M.A.; Barron, D.; Abdalla, M.F.; Saleh, N.A.M.; Lequere, J.L. Flavonoid constituents of stachys-aegyptiaca. Phytochemistry 1991, 30, 1169–1173. [Google Scholar] [CrossRef]
- Martinez, V.; Barbera, O.; Sanchezparareda, J.; Marco, J.A. Phenolic and acetylenic metabolites from Artemisia-Assoana. Phytochemistry 1987, 26, 2619–2624. [Google Scholar] [CrossRef]
- Hanamura, S.; Hanaya, K.; Shoji, M.; Sugai, T. Synthesis of acacetin and resveratrol 3,5-di-O-beta-glucopyranoside using lipase-catalyzed regioselective deacetylation of polyphenol glycoside peracetates as the key step. J. Mol. Catal. B Enzym. 2016, 128, 19–26. [Google Scholar] [CrossRef]
- Ibrahim, A.R.; Galal, A.M.; Ahmed, M.S.; Mossa, G.S. O-demethylation and sulfation of 7-methoxylated flavanones by Cunninghamella elegans. Chem. Pharm. Bull. (Tokyo) 2003, 51, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.; Freitas, M.; Tome, S.M.; Silva, A.M.; Porto, G.; Fernandes, E. Modulation of human neutrophils’ oxidative burst by flavonoids. Eur. J. Med. Chem. 2013, 67, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kim, H.M.; Lee, S.; Kim, H.Y.; Um, B.H.; Ahn, Y.H. Apicin, a new flavonoid from Artemisia apiacea. Bull. Korean Chem. Soc. 2006, 27, 1225–1226. [Google Scholar] [CrossRef]
- Shu, R.G.; Hu, H.W.; Zhang, P.Z.; Ge, F. Triterpenes and flavonoids from Mosla chinensis. Chem. Nat. Compd. 2012, 48, 706–707. [Google Scholar] [CrossRef]
- Turkkan, B.; Ozyurek, M.; Bener, M.; Guclu, K.; Apak, R. Synthesis, characterization and antioxidant capacity of naringenin-oxime. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 85, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.H.; Li, N.G.; Wang, Z.J.; Tang, Y.P.; Dong, Z.X.; Zhang, W.; Zhang, P.X.; Gu, T.; Wu, W.Y.; Yang, J.P.; et al. Synthesis and biological evaluation of methylated scutellarein analogs based on metabolic mechanism of scutellarin in vivo. Eur. J. Med. Chem. 2015, 106, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Amrutha, K.; Nanjan, P.; Shaji, S.K.; Sunilkumar, D.; Subhalakshmi, K.; Rajakrishna, L.; Banerji, A. Discovery of lesser known flavones as inhibitors of NF-kappa B signaling in MDA-MB-231 breast cancer cells-A SAR study. Bioorg. Med. Chem. Lett. 2014, 24, 4735–4742. [Google Scholar] [CrossRef] [PubMed]
- Nagao, T.; Abe, F.; Kinjo, J.; Okabe, H. Antiproliferative constituents in plants 10. Flavones from the leaves of Lantana montevidensis BRIQ. and consideration of structure-activity relationship. Biol. Pharm. Bull. 2002, 25, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Teles, Y.C.F.; Horta, C.C.R.; Agra, M.D.; Siheri, W.; Boyd, M.; Igoli, J.O.; Gray, A.I.; de Souza, M.D.V. New sulphated flavonoids from Wissadula periplocifolia (L.) C. Presl (Malvaceae). Molecules 2015, 20, 20161–20172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyriakou, E.; Primikyri, A.; Charisiadis, P.; Katsoura, M.; Gerothanassis, I.P.; Stamatis, H.; Tzakos, A.G. Unexpected enzyme-catalyzed regioselective acylation of flavonoid aglycones and rapid product screening. Org. Biomol. Chem. 2012, 10, 1739–1742. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Z.; Zhang, Y.Q.; Liu, Q.; Sun, C.L.; Li, J.; Yang, P.; Wang, X. Preparative separation of phenolic compounds from chimonanthus praecox flowers by high-speed counter-current chromatography using a stepwise elution mode. Molecules 2016, 21, 1016. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Choi, Y.O.; Kim, K.H.; Chin, Y.-W.; Namgung, H.; Yamabe, N.; Jung, K. Protective effect of α-mangostin against iodixanol-induced apoptotic damage in LLC-PK1 cells. Bioorg. Med. Chem. Lett. 2016, 26, 3806–3809. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.L.; Kang, K.S. Protective effect of ginsenoside Rh3 against anticancer drug-induced apoptosis in LLC-PK1 kidney cells. J. Ginseng. Res. 2017, 41, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.H.; Kim, D.K.; Shin, Y.; Kim, H.Y.; Song, B.; Lee, E.Y.; Kim, J.K.; You, H.J.; Cheong, H.; Shin, D.H. Migration and invasion of drug-resistant lung adenocarcinoma cells are dependent on mitochondrial activity. Exp. Mol. Med. 2016, 48, e277. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, W.; Huang, C.; Li, Y.; Yu, H.; Wang, Y.; Duan, J.; Ling, Y. A novel chemometric method for the prediction of human oral bioavailability. Int. J. Mol. Sci. 2012, 13, 6964–6982. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Yamanishi, Y.; Kotera, M.; Kanehisa, M.; Goto, S. Drug-target interaction prediction from chemical, genomic and pharmacological data in an integrated framework. Bioinformatics 2010, 26, i246–i254. [Google Scholar] [CrossRef] [PubMed]
| Compound | IC50 (μM) |
|---|---|
| 6 | 5.04 ± 2.11 |
| 15 | 8.21 ± 1.74 |
| 16 | 37.06 ± 1.41 |
| 18 | 39.58 ± 1.13 |
| 19 | 19.05 ± 2.39 |
| Ascorbic acid | 2.06 ± 3.12 |
| Compound | OB (%) | DL | MW | AlogP | Hdon | Hacc |
|---|---|---|---|---|---|---|
| 2 | 49.55 | 0.48 | 388.4 | 2.31 | 1 | 8 |
| 3 | 27.36 | 0.44 | 374.37 | 2.05 | 2 | 8 |
| 18 | 36.16 | 0.25 | 286.25 | 2.07 | 4 | 6 |
| 19 | 41.88 | 0.24 | 286.25 | 1.77 | 4 | 6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Kim, C.-E.; Park, S.-Y.; Kim, K.O.; Hiep, N.T.; Lee, D.; Jang, H.-J.; Lee, J.W.; Kang, K.S. Protective Effect of Artemisia argyi and Its Flavonoid Constituents against Contrast-Induced Cytotoxicity by Iodixanol in LLC-PK1 Cells. Int. J. Mol. Sci. 2018, 19, 1387. https://doi.org/10.3390/ijms19051387
Lee D, Kim C-E, Park S-Y, Kim KO, Hiep NT, Lee D, Jang H-J, Lee JW, Kang KS. Protective Effect of Artemisia argyi and Its Flavonoid Constituents against Contrast-Induced Cytotoxicity by Iodixanol in LLC-PK1 Cells. International Journal of Molecular Sciences. 2018; 19(5):1387. https://doi.org/10.3390/ijms19051387
Chicago/Turabian StyleLee, Dahae, Chang-Eop Kim, Sa-Yoon Park, Kem Ok Kim, Nguyen Tuan Hiep, Dongho Lee, Hyuk-Jai Jang, Jae Wook Lee, and Ki Sung Kang. 2018. "Protective Effect of Artemisia argyi and Its Flavonoid Constituents against Contrast-Induced Cytotoxicity by Iodixanol in LLC-PK1 Cells" International Journal of Molecular Sciences 19, no. 5: 1387. https://doi.org/10.3390/ijms19051387
APA StyleLee, D., Kim, C.-E., Park, S.-Y., Kim, K. O., Hiep, N. T., Lee, D., Jang, H.-J., Lee, J. W., & Kang, K. S. (2018). Protective Effect of Artemisia argyi and Its Flavonoid Constituents against Contrast-Induced Cytotoxicity by Iodixanol in LLC-PK1 Cells. International Journal of Molecular Sciences, 19(5), 1387. https://doi.org/10.3390/ijms19051387








