S1P4 Regulates Passive Systemic Anaphylaxis in Mice but Is Dispensable for Canonical IgE-Mediated Responses in Mast Cells
Abstract
1. Introduction
2. Results
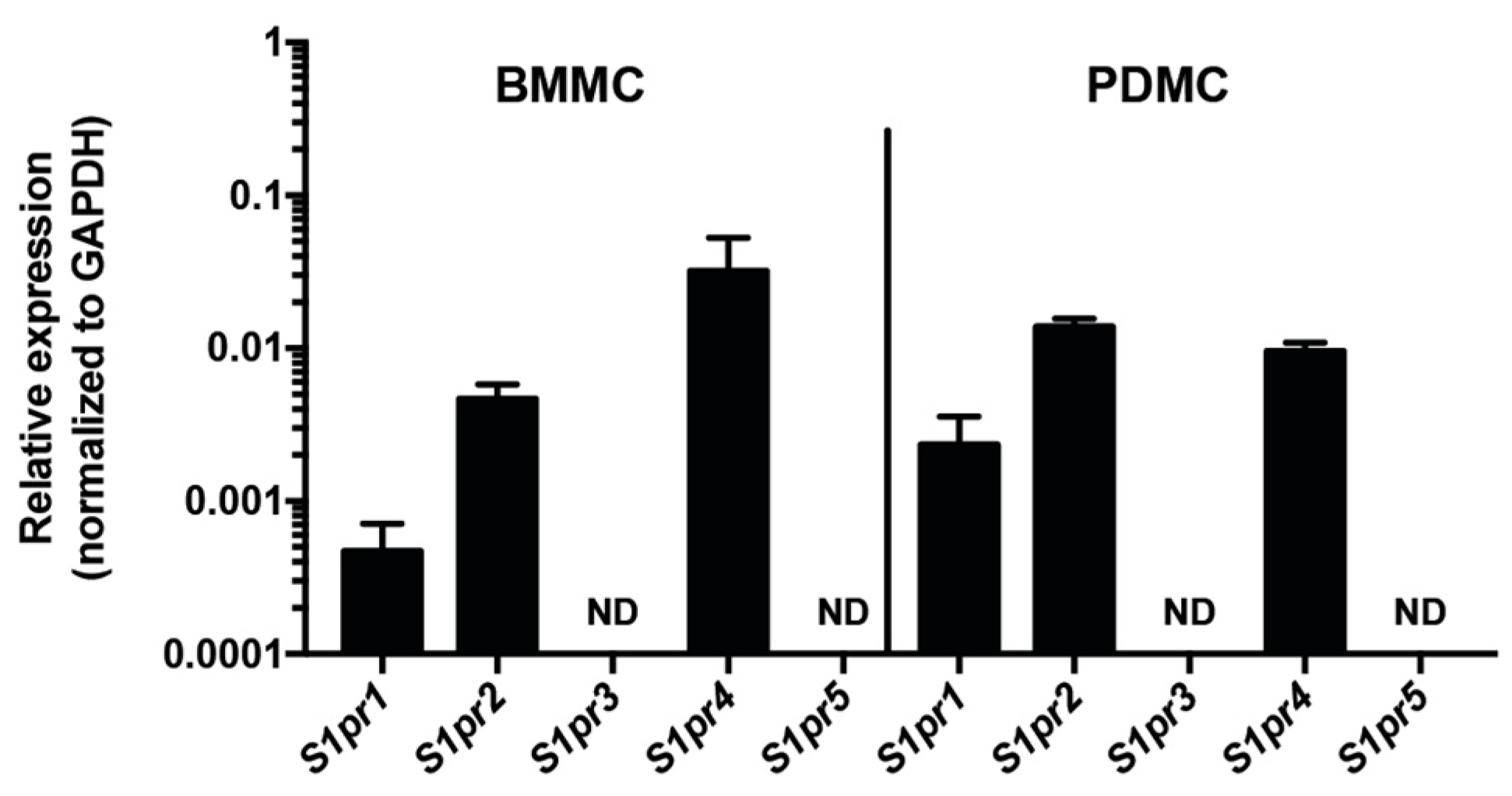
2.1. S1P4 Is Expressed in Mast Cells
2.2. Maturation and Proliferation of S1pr4-Deficient Mast Cells In Vitro
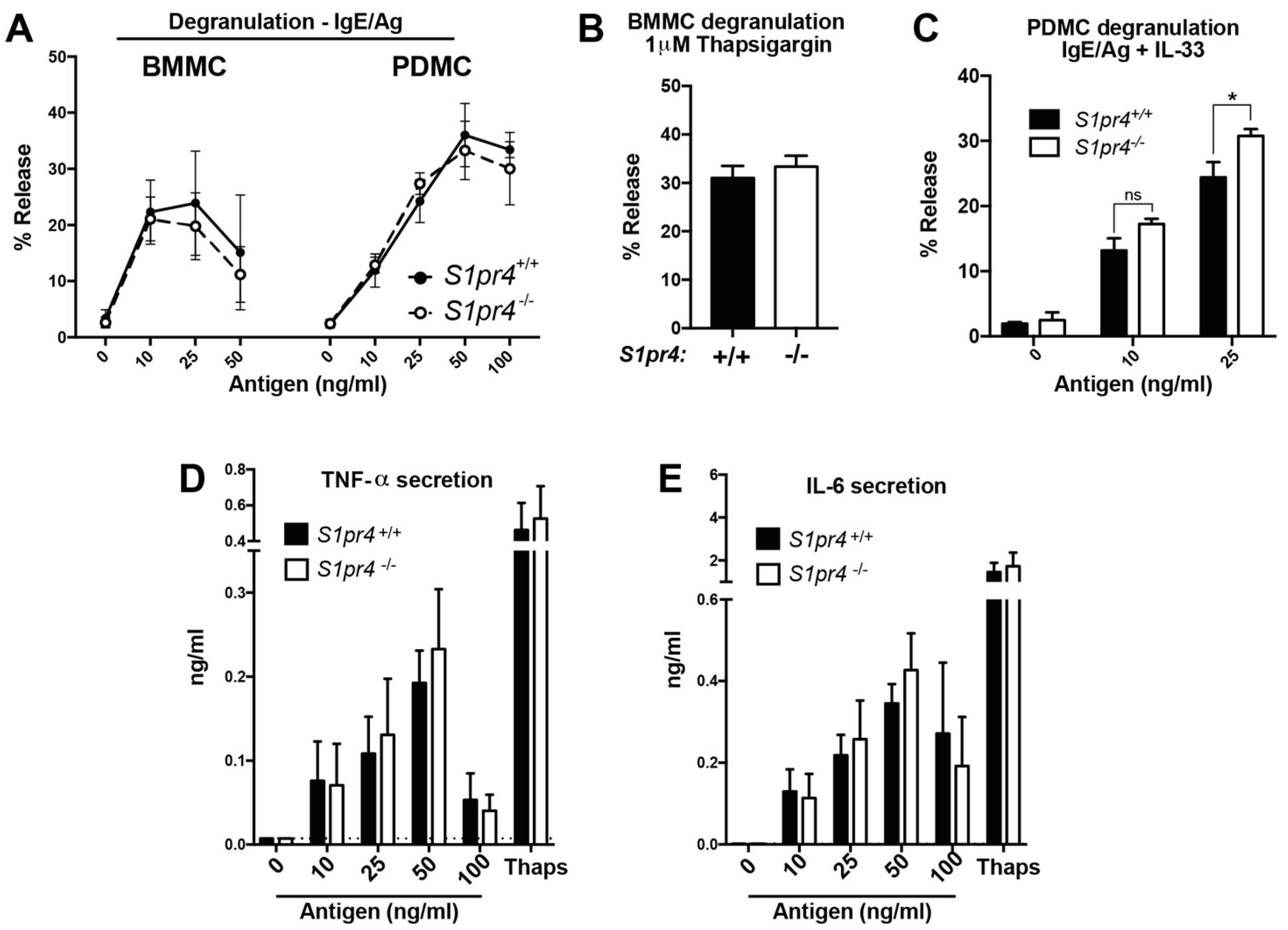
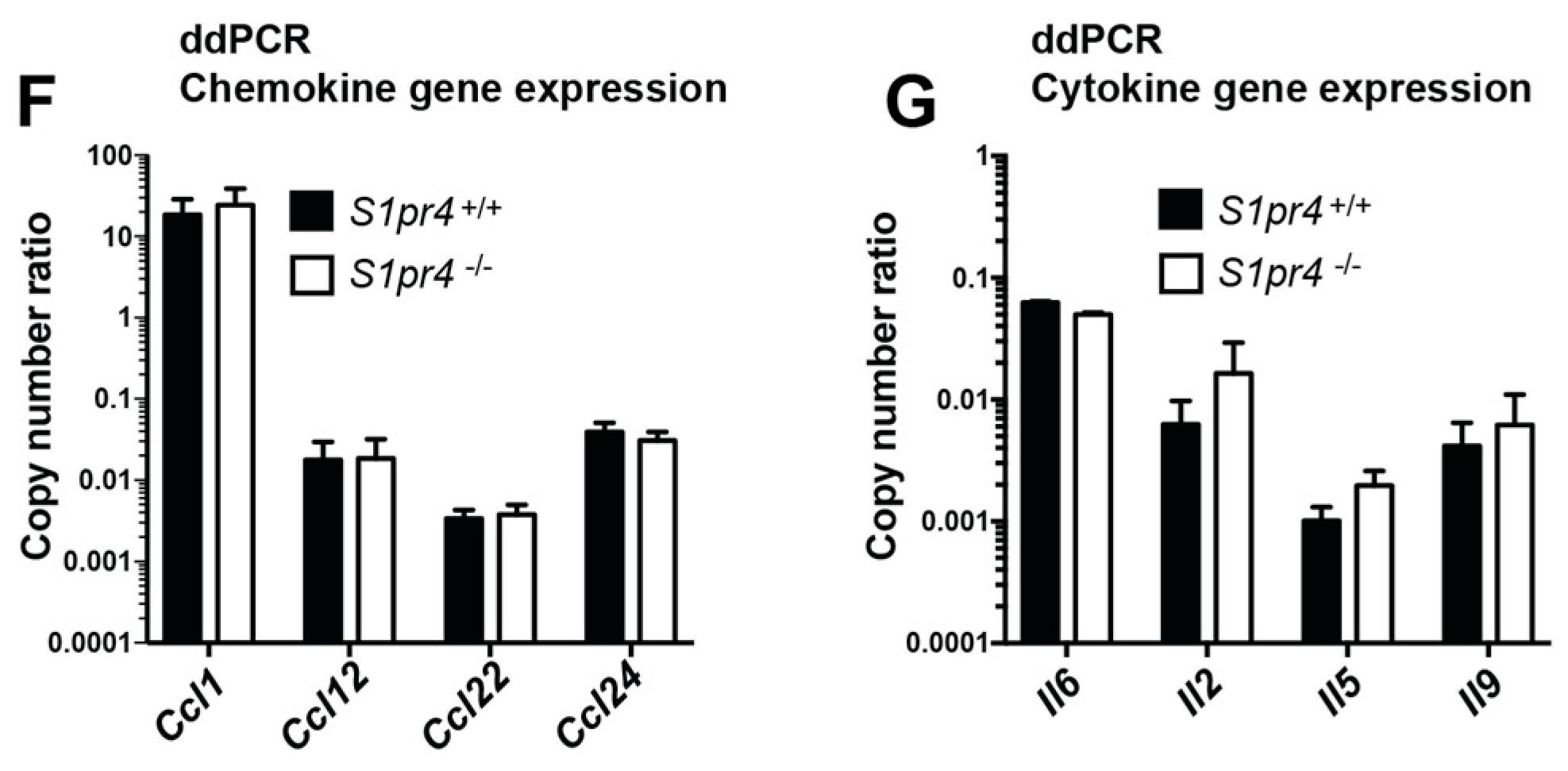
2.3. Degranulation, Cytokine and Chemokine Responses in S1pr4-Deficient Mast Cells In Vitro
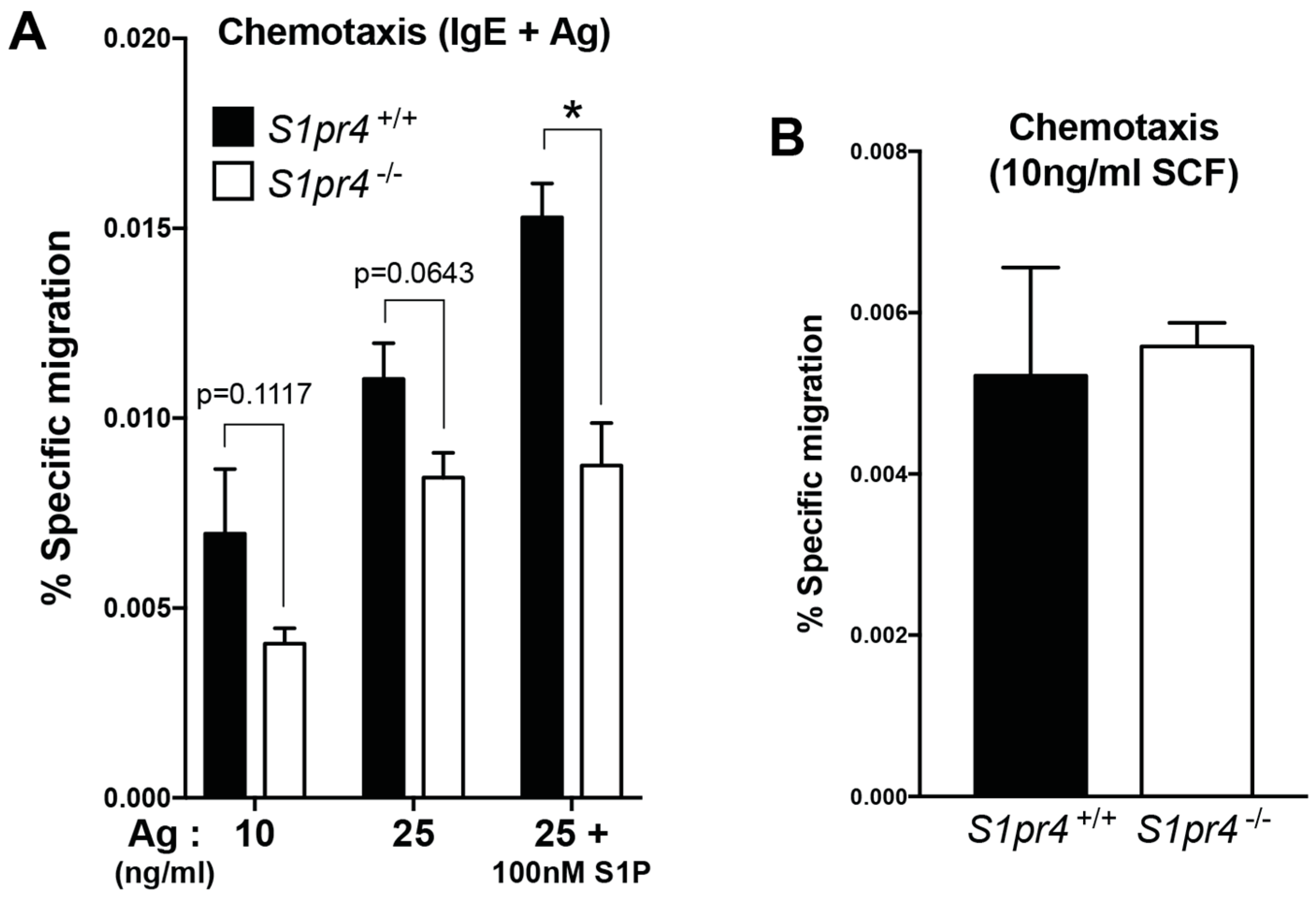
2.4. Regulation of Mast Cell Chemotaxis by S1P4
2.5. Systemic Anaphylaxis in S1pr4−/− Mice
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Mast Cell Cultures
4.3. Degranulation Assays
4.4. Flow Cytometry
4.5. Measurement of Cytokine Release
4.6. RT-PCR and Gene Expression Analysis
4.7. Chemotaxis
4.8. Passive Systemic Anaphylaxis
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| GPCR | G Protein-coupled receptor |
| S1P | Sphingosine-1-phosphate |
| S1P1-5 | Sphingosine-1-phosphate receptors 1-5 |
| S1PR | Sphingosine-1-phosphate receptor |
| FcεRI | High affinity IgE receptor, Fc Epsilon receptor I |
| Ag | Antigen |
| SCF | Stem cell factor |
| BMMC | Bone marrow-derived mast cells |
| PDMC | Peritoneum-derived mast cells |
| WT | Wild type |
| qPCR | Quantitative real-time PCR |
| FAF BSA | Fatty acid-free bovine serum albumin |
| ddPCR | Droplet digital PCR |
| PSA | Passive systemic anaphylaxis |
| mAb | Monoclonal antibody |
References
- Strub, G.M.; Maceyka, M.; Hait, N.C.; Milstien, S.; Spiegel, S. Extracellular and intracellular actions of sphingosine-1-phosphate. Adv. Exp. Med. Biol. 2010, 688, 141–155. [Google Scholar] [PubMed]
- Graler, M.H.; Bernhardt, G.; Lipp, M. EDG6, a novel G-protein-coupled receptor related to receptors for bioactive lysophospholipids, is specifically expressed in lymphoid tissue. Genomics 1998, 53, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Group, C.C.H.W. Meta-analysis of rare and common exome chip variants indentifies S1PR4 and other loci influencing blood cell traits. Nat. Genet. 2016, 8, 10. [Google Scholar]
- Allende, M.L.; Bektas, M.; Lee, B.G.; Bonifacino, E.; Kang, J.; Tuymetova, G.; Chen, W.; Saba, J.D.; Proia, R.L. Sphingosine-1-phosphate lyase deficiency produces a pro-inflammatory response while impairing neutrophil trafficking. J. Biol. Chem. 2011, 286, 7348–7358. [Google Scholar] [CrossRef] [PubMed]
- Gorlino, C.V.; Ranocchia, R.P.; Harman, M.F.; Garcia, I.A.; Crespo, M.I.; Moron, G.; Maletto, B.A.; Pistoresi-Palencia, M.C. Neutrophils exhibit differential requirements for homing molecules in their lymphatic and blood trafficking into draining lymph nodes. J. Immunol. 2014, 193, 1966–1974. [Google Scholar] [CrossRef] [PubMed]
- Schulze, T.; Golfier, S.; Tabeling, C.; Rabel, K.; Graler, M.H.; Witzenrath, M.; Lipp, M. Sphingosine-1-phospate receptor 4 (S1P4) deficiency profoundly affects dendritic cell function and TH17-cell differentiation in a murine model. FASEB J. 2011, 25, 4024–4036. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Graeler, M.H.; Goetzl, E.J. Type 4 sphingosine 1-phosphate G protein-coupled receptor (S1P4) transduces S1P effects on T cell proliferation and cytokine secretion without signaling migration. FASEB J. 2005, 19, 1731–1733. [Google Scholar] [CrossRef] [PubMed]
- Sic, H.; Kraus, H.; Madl, J.; Flittner, K.A.; von Munchow, A.L.; Pieper, K.; Rizzi, M.; Kienzler, A.K.; Ayata, K.; Rauer, S.; et al. Sphingosine-1-phosphate receptors control B-cell migration through signaling components associated with primary immunodeficiencies, chronic lymphocytic leukemia, and multiple sclerosis. J. Allergy Clin. Immunol. 2014, 134, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Kleinwort, A.; Luhrs, F.; Heidecke, C.D.; Lipp, M.; Schulze, T. S1P Signalling Differentially Affects Migration of Peritoneal B Cell Populations In Vitro and Influences the Production of Intestinal IgA In Vivo. Int. J. Mol. Sci. 2018, 19, 391. [Google Scholar] [CrossRef] [PubMed]
- Gilfillan, A.M.; Peavy, R.D.; Metcalfe, D.D. Amplification mechanisms for the enhancement of antigen-mediated mast cell activation. Immunol. Res. 2009, 43, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Blank, U.; Rivera, J. The ins and outs of IgE-dependent mast-cell exocytosis. Trends Immunol. 2004, 25, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.; Grimbaldeston, M.; Galli, S.J. Mast cells and immunoregulation/immunomodulation. Adv. Exp. Med. Biol. 2011, 716, 186–211. [Google Scholar] [PubMed]
- Galli, S.J.; Tsai, M. Mast cells: Versatile regulators of inflammation, tissue remodeling, host defense and homeostasis. J. Dermatol. Sci. 2008, 49, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Gilfillan, A.M.; Beaven, M.A. Regulation of mast cell responses in health and disease. Crit. Rev. Immunol. 2011, 31, 475–529. [Google Scholar] [CrossRef] [PubMed]
- Olivera, A.; Allende, M.L.; Proia, R.L. Shaping the landscape: Metabolic regulation of S1P gradients. Biochim. Biophys. Acta 2013, 1831, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Kulinski, J.M.; Munoz-Cano, R.; Olivera, A. Sphingosine-1-phosphate and other lipid mediators generated by mast cells as critical players in allergy and mast cell function. Eur. J. Pharmacol. 2016, 778, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Jolly, P.S.; Bektas, M.; Olivera, A.; Gonzalez-Espinosa, C.; Proia, R.L.; Rivera, J.; Milstien, S.; Spiegel, S. Transactivation of sphingosine-1-phosphate receptors by FcepsilonRI triggering is required for normal mast cell degranulation and chemotaxis. J. Exp. Med. 2004, 199, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Mitra, P.; Oskeritzian, C.A.; Payne, S.G.; Beaven, M.A.; Milstien, S.; Spiegel, S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc. Natl. Acad. Sci. USA 2006, 103, 16394–16399. [Google Scholar] [CrossRef] [PubMed]
- Olivera, A.; Urtz, N.; Mizugishi, K.; Yamashita, Y.; Gilfillan, A.M.; Furumoto, Y.; Gu, H.; Proia, R.L.; Baumruker, T.; Rivera, J. IgE-dependent activation of sphingosine kinases 1 and 2 and secretion of sphingosine 1-phosphate requires Fyn kinase and contributes to mast cell responses. J. Biol. Chem. 2006, 281, 2515–2525. [Google Scholar] [CrossRef] [PubMed]
- Olivera, A.; Beaven, M.A.; Metcalfe, D.D. Mast Cells Signal their Importance in Health and Disease. J. Allergy Clin. Immunol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, D.; Baram, D.; Mekori, Y.A. Mast cells. Physiol. Rev. 1997, 77, 1033–1079. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.M.; Swindle, E.J.; Iwaki, S.; Gilfillan, A.M. Generation, isolation, and maintenance of rodent mast cells and mast cell lines. Curr. Protoc. Immunol. 2006. [Google Scholar] [CrossRef]
- Vukman KV, M.M.; Maurer, M.; O’Neill, SM. Isolation and Culture of Peritoneal Cell-derived Mast Cells. Bio Protoc. 2014, 4, e1052. [Google Scholar] [CrossRef]
- Kashem, S.W.; Subramanian, H.; Collington, S.J.; Magotti, P.; Lambris, J.D.; Ali, H.G. protein coupled receptor specificity for C3a and compound 48/80-induced degranulation in human mast cells: Roles of Mas-related genes MrgX1 and MrgX2. Eur. J. Pharmacol. 2011, 668, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Bandara, G.; Beaven, M.A.; Olivera, A.; Gilfillan, A.M.; Metcalfe, D.D. Activated mast cells synthesize and release soluble ST2-a decoy receptor for IL-33. Eur. J. Immunol. 2015, 45, 3034–3044. [Google Scholar] [CrossRef] [PubMed]
- McNeil, B.D.; Pundir, P.; Meeker, S.; Han, L.; Undem, B.J.; Kulka, M.; Dong, X. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 2015, 519, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Mousli, M.; Hugli, T.E.; Landry, Y.; Bronner, C. Peptidergic pathway in human skin and rat peritoneal mast cell activation. Immunopharmacology 1994, 27, 1–11. [Google Scholar] [CrossRef]
- Liew, F.Y.; Girard, J.P.; Turnquist, H.R. Interleukin-33 in health and disease. Nat. Rev. Immunol. 2016, 16, 676–689. [Google Scholar] [CrossRef] [PubMed]
- Joulia, R.; L’Faqihi, F.E.; Valitutti, S.; Espinosa, E. IL-33 fine tunes mast cell degranulation and chemokine production at the single-cell level. J. Allergy Clin. Immunol. 2017, 140, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Hundley, T.R.; Gilfillan, A.M.; Tkaczyk, C.; Andrade, M.V.; Metcalfe, D.D.; Beaven, M.A. Kit and FcepsilonRI mediate unique and convergent signals for release of inflammatory mediators from human mast cells. Blood 2004, 104, 2410–2417. [Google Scholar] [CrossRef] [PubMed]
- Gilfillan, A.M.; Tkaczyk, C. Integrated signalling pathways for mast-cell activation. Nat. Rev. Immunol. 2006, 6, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.C.; Laperriere, G.; Germain, H. Droplet Digital PCR versus qPCR for gene expression analysis with low abundant targets: From variable nonsense to publication quality data. Sci. Rep. 2017, 7, 2409. [Google Scholar] [CrossRef] [PubMed]
- Rivera, J.; Proia, R.L.; Olivera, A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat. Rev. Immunol. 2008, 8, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Finkelman, F.D. Anaphylaxis: Lessons from mouse models. J. Allergy Clin. Immunol. 2007, 120, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Olivera, A.; Eisner, C.; Kitamura, Y.; Dillahunt, S.; Allende, L.; Tuymetova, G.; Watford, W.; Meylan, F.; Diesner, S.C.; Li, L.; et al. Sphingosine kinase 1 and sphingosine-1-phosphate receptor 2 are vital to recovery from anaphylactic shock in mice. J. Clin. Investig. 2010, 120, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Borkowski, T.A.; Jouvin, M.H.; Lin, S.Y.; Kinet, J.P. Minimal requirements for IgE-mediated regulation of surface Fc epsilon RI. J. Immunol. 2001, 167, 1290–1296. [Google Scholar] [CrossRef] [PubMed]
- Khodoun, M.V.; Kucuk, Z.Y.; Strait, R.T.; Krishnamurthy, D.; Janek, K.; Lewkowich, I.; Morris, S.C.; Finkelman, F.D. Rapid polyclonal desensitization with antibodies to IgE and FcepsilonRIalpha. J. Allergy Clin. Immunol. 2013, 131, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Galand, C.; Leyva-Castillo, J.M.; Yoon, J.; Han, A.; Lee, M.S.; McKenzie, A.N.J.; Stassen, M.; Oyoshi, M.K.; Finkelman, F.D.; Geha, R.S. IL-33 promotes food anaphylaxis in epicutaneously sensitized mice by targeting mast cells. J. Allergy Clin. Immunol. 2016, 138, 1356–1366. [Google Scholar] [CrossRef] [PubMed]
- Kamijo, S.; Suzuki, M.; Hara, M.; Shimura, S.; Ochi, H.; Maruyama, N.; Matsuda, A.; Saito, H.; Nakae, S.; Suto, H.; et al. Subcutaneous Allergic Sensitization to Protease Allergen Is Dependent on Mast Cells but Not IL-33: Distinct Mechanisms between Subcutaneous and Intranasal Routes. J. Immunol. 2016, 196, 3559–3569. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Tsai, M. IgE and mast cells in allergic disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Oskeritzian, C.A.; Alvarez, S.E.; Hait, N.C.; Price, M.M.; Milstien, S.; Spiegel, S. Distinct roles of sphingosine kinases 1 and 2 in human mast-cell functions. Blood 2008, 111, 4193–4200. [Google Scholar] [CrossRef] [PubMed]
- Cyster, J.G.; Schwab, S.R. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu. Rev. Immunol. 2012, 30, 69–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; van Brocklyn, J.R.; Hobson, J.P.; Movafagh, S.; Zukowska-Grojec, Z.; Milstien, S.; Spiegel, S. Sphingosine 1-phosphate stimulates cell migration through a Gi-coupled cell surface receptor. Potential involvement in angiogenesis. J. Biol. Chem. 1999, 274, 35343–35350. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Thangada, S.; Paik, J.H.; Sapkota, G.P.; Ancellin, N.; Chae, S.S.; Wu, M.; Morales-Ruiz, M.; Sessa, W.C.; Alessi, D.R.; et al. Akt-mediated phosphorylation of the G protein-coupled receptor EDG-1 is required for endothelial cell chemotaxis. Mol. Cell. 2001, 8, 693–704. [Google Scholar] [CrossRef]
- Van Brocklyn, J.R.; Graler, M.H.; Bernhardt, G.; Hobson, J.P.; Lipp, M.; Spiegel, S. Sphingosine-1-phosphate is a ligand for the G protein-coupled receptor EDG-6. Blood 2000, 95, 2624–2629. [Google Scholar] [PubMed]
- Graler, M.H.; Grosse, R.; Kusch, A.; Kremmer, E.; Gudermann, T.; Lipp, M. The sphingosine 1-phosphate receptor S1P4 regulates cell shape and motility via coupling to Gi and G12/13. J. Cell. Biochem. 2003, 89, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Dillmann, C.; Ringel, C.; Ringleb, J.; Mora, J.; Olesch, C.; Fink, A.F.; Roberts, E.; Brune, B.; Weigert, A. S1PR4 Signaling Attenuates ILT 7 Internalization To Limit IFN-α Production by Human Plasmacytoid Dendritic Cells. J. Immunol. 2016, 196, 1579–1590. [Google Scholar] [CrossRef] [PubMed]
- Cencetti, F.; Bernacchioni, C.; Tonelli, F.; Roberts, E.; Donati, C.; Bruni, P. TGFβ1 evokes myoblast apoptotic response via a novel signaling pathway involving S1P4 transactivation upstream of Rho-kinase-2 activation. FASEB J. 2013, 27, 4532–4546. [Google Scholar] [CrossRef] [PubMed]
- Matsuyuki, H.; Maeda, Y.; Yano, K.; Sugahara, K.; Chiba, K.; Kohno, T.; Igarashi, Y. Involvement of sphingosine 1-phosphate (S1P) receptor type 1 and type 4 in migratory response of mouse T cells toward S1P. Cell Mol. Immunol. 2006, 3, 429–437. [Google Scholar] [PubMed]
- Olesch, C.; Ringel, C.; Brune, B.; Weigert, A. Beyond Immune Cell Migration: The Emerging Role of the Sphingosine-1-phosphate Receptor S1PR4 as a Modulator of Innate Immune Cell Activation. Mediators Inflamm. 2017, 2017, 6059203. [Google Scholar] [CrossRef] [PubMed]
- Olivera, A.; Dillahunt, S.E.; Rivera, J. Interrogation of sphingosine-1-phosphate receptor 2 function in vivo reveals a prominent role in the recovery from IgE and IgG-mediated anaphylaxis with minimal effect on its onset. Immunol. Lett. 2013, 150, 89–96. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oskeritzian, C.A.; Price, M.M.; Hait, N.C.; Kapitonov, D.; Falanga, Y.T.; Morales, J.K.; Ryan, J.J.; Milstien, S.; Spiegel, S. Essential roles of sphingosine-1-phosphate receptor 2 in human mast cell activation, anaphylaxis, and pulmonary edema. J. Exp. Med. 2010, 207, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Gurish, M.F.; Austen, K.F. Developmental origin and functional specialization of mast cell subsets. Immunity 2012, 37, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, H.S.; Radinger, M.; Brown, J.M.; Ali, K.; Vanhaesebroeck, B.; Beaven, M.A.; Metcalfe, D.D.; Gilfillan, A.M. Btk-dependent Rac activation and actin rearrangement following FcepsilonRI aggregation promotes enhanced chemotactic responses of mast cells. J. Cell Sci. 2010, 123, 2576–2585. [Google Scholar] [CrossRef] [PubMed]
- Lennartsson, J.; Jelacic, T.; Linnekin, D.; Shivakrupa, R. Normal and oncogenic forms of the receptor tyrosine kinase kit. Stem Cells 2005, 23, 16–43. [Google Scholar] [CrossRef] [PubMed]
- Linnekin, D. Early signaling pathways activated by c-Kit in hematopoietic cells. Int. J. Biochem. Cell Biol. 1999, 31, 1053–1074. [Google Scholar] [CrossRef]
- Moon, T.C.; St Laurent, C.D.; Morris, K.E.; Marcet, C.; Yoshimura, T.; Sekar, Y.; Befus, A.D. Advances in mast cell biology: New understanding of heterogeneity and function. Mucosal. Immunol. 2010, 3, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Crivellato, E.; Ribatti, D. The mast cell: An evolutionary perspective. Biol. Rev. Camb. Philos. Soc. 2010, 85, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Lefrancais, E.; Duval, A.; Mirey, E.; Roga, S.; Espinosa, E.; Cayrol, C.; Girard, J.P. Central domain of IL-33 is cleaved by mast cell proteases for potent activation of group-2 innate lymphoid cells. Proc. Natl. Acad. Sci. USA 2014, 111, 15502–15507. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Okamoto, Y.; Yoshioka, K.; Du, W.; Takuwa, N.; Zhang, W.; Asano, M.; Shibamoto, T.; Takuwa, Y. Sphingosine-1-phosphate receptor 2 protects against anaphylactic shock through suppression of endothelial nitric oxide synthase in mice. J. Allergy Clin. Immunol. 2013, 132, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Sekar, D.; Hahn, C.; Brune, B.; Roberts, E.; Weigert, A. Apoptotic tumor cells induce IL-27 release from human DCs to activate Treg cells that express CD69 and attenuate cytotoxicity. Eur. J. Immunol. 2012, 42, 1585–1598. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, H.S.; Radinger, M.; Gilfillan, A.M. Measuring mast cell mediator release. Curr. Protoc. Immunol. 2010. [Google Scholar] [CrossRef]
- Olivera, A.; Mizugishi, K.; Tikhonova, A.; Ciaccia, L.; Odom, S.; Proia, R.L.; Rivera, J. The sphingosine kinase-sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxis. Immunity 2007, 26, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Charles, N.; Watford, W.T.; Ramos, H.L.; Hellman, L.; Oettgen, H.C.; Gomez, G.; Ryan, J.J.; O’Shea, J.J.; Rivera, J. Lyn kinase controls basophil GATA-3 transcription factor expression and induction of Th2 cell differentiation. Immunity 2009, 30, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, K.; Szallasi, Z.; Kazanietz, M.G.; Blumberg, P.M.; Mischak, H.; Mushinski, J.F.; Beaven, M.A. Ca2+-dependent and Ca2+-independent isozymes of protein kinase C mediate exocytosis in antigen-stimulated rat basophilic RBL-2H3 cells. Reconstitution of secretory responses with Ca2+ and purified isozymes in washed permeabilized cells. J. Biol. Chem. 1993, 268, 1749–1756. [Google Scholar] [PubMed]
- Liu, F.T.; Bohn, J.W.; Ferry, E.L.; Yamamoto, H.; Molinaro, C.A.; Sherman, L.A.; Klinman, N.R.; Katz, D.H. Monoclonal dinitrophenyl-specific murine IgE antibody: Preparation, isolation, and characterization. J. Immunol. 1980, 124, 2728–2737. [Google Scholar] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulinski, J.M.; Proia, R.L.; Larson, E.M.; Metcalfe, D.D.; Olivera, A. S1P4 Regulates Passive Systemic Anaphylaxis in Mice but Is Dispensable for Canonical IgE-Mediated Responses in Mast Cells. Int. J. Mol. Sci. 2018, 19, 1279. https://doi.org/10.3390/ijms19051279
Kulinski JM, Proia RL, Larson EM, Metcalfe DD, Olivera A. S1P4 Regulates Passive Systemic Anaphylaxis in Mice but Is Dispensable for Canonical IgE-Mediated Responses in Mast Cells. International Journal of Molecular Sciences. 2018; 19(5):1279. https://doi.org/10.3390/ijms19051279
Chicago/Turabian StyleKulinski, Joseph M., Richard L. Proia, Elisabeth M. Larson, Dean D. Metcalfe, and Ana Olivera. 2018. "S1P4 Regulates Passive Systemic Anaphylaxis in Mice but Is Dispensable for Canonical IgE-Mediated Responses in Mast Cells" International Journal of Molecular Sciences 19, no. 5: 1279. https://doi.org/10.3390/ijms19051279
APA StyleKulinski, J. M., Proia, R. L., Larson, E. M., Metcalfe, D. D., & Olivera, A. (2018). S1P4 Regulates Passive Systemic Anaphylaxis in Mice but Is Dispensable for Canonical IgE-Mediated Responses in Mast Cells. International Journal of Molecular Sciences, 19(5), 1279. https://doi.org/10.3390/ijms19051279




