Transient Overexpression of HvSERK2 Improves Barley Resistance to Powdery Mildew
Abstract
:1. Introduction
2. Results
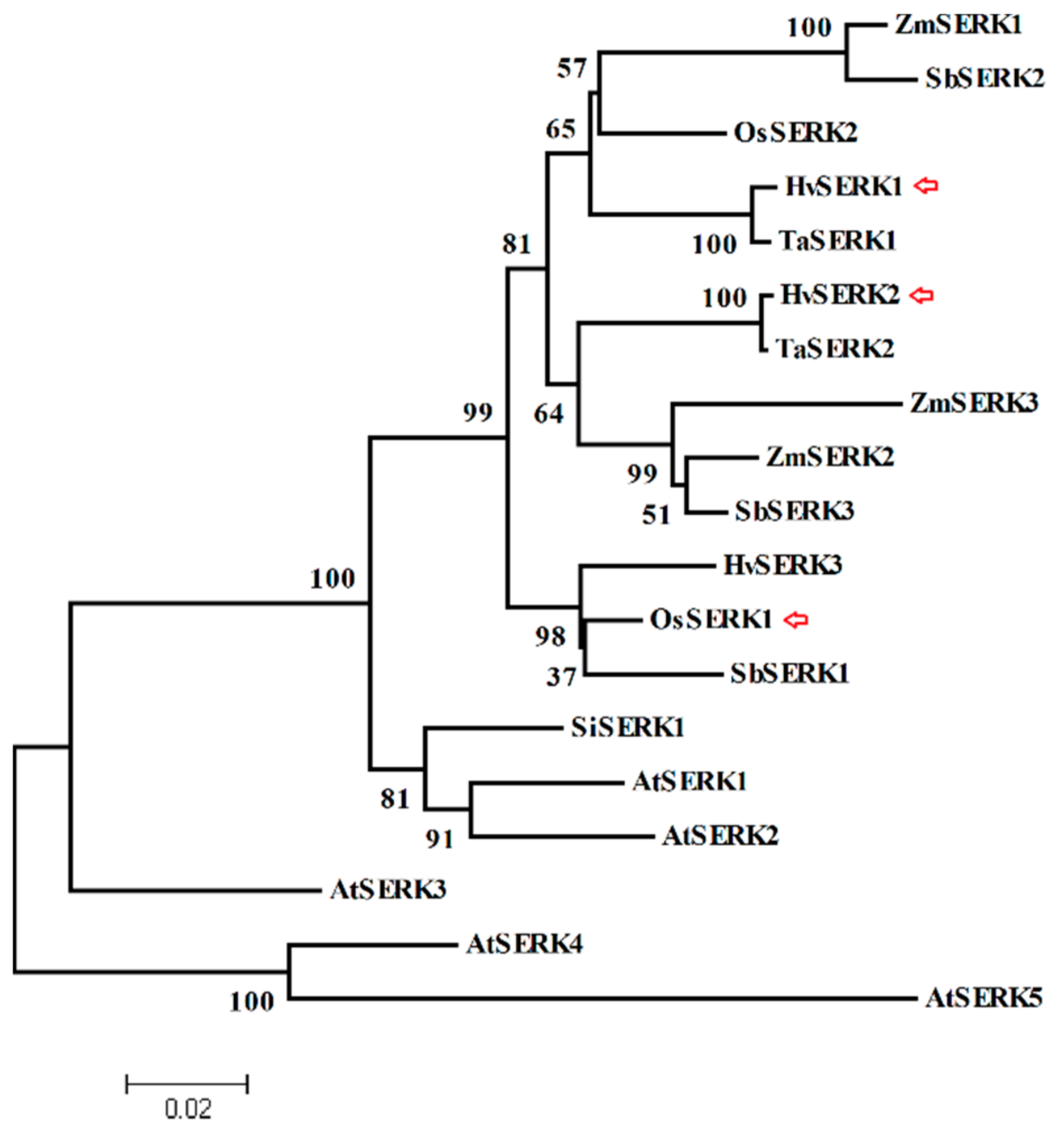
2.1. Cloning and Identification of Three SERK Genes from Barley
2.2. Characterization of the Response of Three SERK Genes to Bgh Infection
2.3. Characterization of HvSERK2 Responses to Defense Signal Molecules
2.4. Analysis of the Cis-Regulatory Motifs for Cloned HvSERK2 Promoter
2.5. Functional Analysis of HvSERK2 Promoter
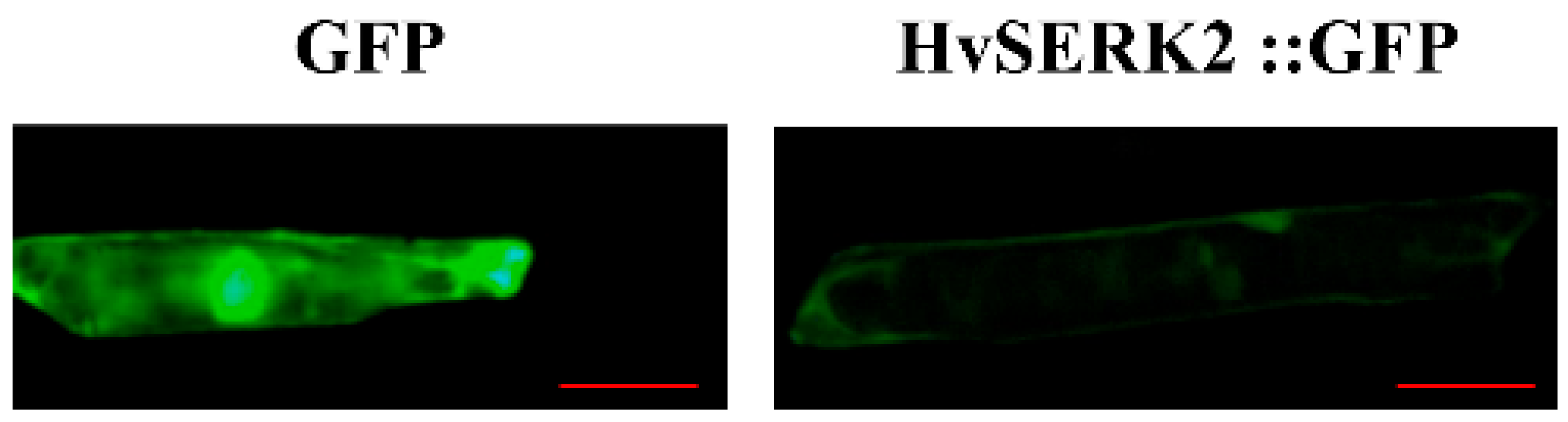
2.6. Subcellular Localization of HvSERK2
2.7. Functional Analysis of HvSERK2 in Bgh Infection by TOA
3. Discussion
4. Materials and Methods
4.1. Plant Materials, Growth Conditions and Treatment
4.2. Identification of Three SERK Genes in Barley
4.3. Cloning and In Silico Analysis of HvSERK2 Promoter
4.4. DNA, RNA Extraction and Transcription Analysis
4.5. Subcellular Localization of HvSERK2
4.6. Single-Cell Transient Overexpression Assay
4.7. Function Analysis of HvSERK2 Promoter
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| SERK | Somatic embryogenesis receptor-like kinase |
| Bgh | Blumeria graminis f. Sp. hordei |
| PAMP | Pathogen-associated molecular patterns |
| PTI | PAMP-triggered immunity |
| ETI | Effector-triggered immunity |
| RLKs | Receptor-like kinases |
| LZ | Leucine zipper |
| LRRs | Leucine rich repeats |
| SPP | Serine proline proline |
| BR | Brassinosteroid |
| Xoo | Xanthomonas oryzae pv. Oryzae |
| TOA | Transient overexpression assay |
| MeJA | Methyl jasmonate |
| SA | Salicylic acid |
| H2O2 | Hydrogen peroxide |
| ETH | Ethephon |
| ABA | Abscisic acid |
| hpt | Hours post-treatment |
| hpi | Hours post-inoculation |
References
- Zhang, Z.; Henderson, C.; Perfect, E.; Carver, T.L.W.; Thomas, B.J.; Skamnioti, P.; Gurr, S.J. Of genes and genomes, needles and haystacks: Blumeria graminis and functionality. Mol. Plant Pathol. 2005, 6, 561–575. [Google Scholar] [CrossRef] [PubMed]
- Dangl, J.L.; Dietrich, R.A.; Richberg, M.H. Death don’t have no mercy: Cell death programs in plant-microbe interactions. Plant Cell 1996, 18, 1793–1807. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, C. Early molecular events in PAMP-triggered immunity. Curr. Opin. Plant Boil. 2009, 12, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, S.T.; Coaker, G.; Day, B.; Staskawicz, B.J. Host-microbe interactions: Shaping the evolution of the plant immune response. Cell 2006, 124, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Nicaise, V.; Roux, M.; Zipfel, C. Recent advances in PAMP-triggered immunity against bacteria: Pattern recognition receptors watch over and raise the alarm. Plant Physiol. 2009, 150, 1638–1647. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, C. Plant pattern-recognition receptors. Trends Immunol. 2014, 35, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Li, J. Multi-tasking of somatic embryogenesis receptor-like protein kinases. Curr. Opin. Plant Biol. 2010, 13, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.O.; Aragão, F.J. Role of SERK genes in plant environmental response. Plant Signal. Behav. 2009, 4, 1111–1113. [Google Scholar] [CrossRef] [PubMed]
- Heese, A.; Hann, D.R.; Gimenez-Ibanez, S.; Jones, A.M.E.; He, K.; Li, J.; Schroeder, I.J.; Peck, S.C.; Rathjen, J.P. The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc. Natl. Acad. Sci. USA 2007, 104, 12217–12222. [Google Scholar] [CrossRef] [PubMed]
- Clouse, S.D. Brassinosteroid signal transduction: From receptor kinase activation to transcriptional networks regulating plant development. Plant Cell 2011, 23, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Gou, X.; Yuan, T.; Lin, H.; Asami, T.; Yoshida, S.; Russell1, S.D.; Li, J. BAK1 and BKK1 regulate brassinosteroid-dependent growth and brassinosteroid-independent cell-death pathways. Curr. Biol. 2007, 17, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Xiong, L.; Yang, Y. Rice SERK1 gene positively regulates somatic embryogenesis of cultured cell and host defense response against fungal infection. Planta 2005, 222, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zuo, S.; Schwessinger, B.; Chern, M.; Canlas, P.E.; Ruan, D.; Zhou, X.; Wang, J.; Daudi, A.; Petzold, C.J.; et al. An XA21-associated kinase (OsSERK2) regulates immunity mediated by the XA21 and XA3 immune receptors. Mol. Plant 2014, 7, 874–892. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.J.; Bushnell, W.R. Transient expression of an thocyanin genes in barley epidermal cells: Potential for use in evaluation of disease response genes. Transgenic Res. 1997, 6, 233–244. [Google Scholar] [CrossRef]
- Shirasu, K.; Nielsen, K.; Piffanelli, P.; Oliver, R.; Schulze-Lefert, P. Cell-autonomous complementation of mlo resistance using a biolistic transient expression system. Plant J. 1999, 17, 293–299. [Google Scholar] [CrossRef]
- Schweizer, P.; Pokorny, J.; Abderhalden, O.; Dudler, R. A transient assay system for the functional assessment of defense-related genes in wheat. Mol. Plant-Microbe Interact. 1999, 12, 647–654. [Google Scholar] [CrossRef]
- Rajaraman, J.; Douchkov, D.; Hensel, G.; Stefanato, F.L.; Gordon, A.; Ereful, N.; Caldararu, O.F.; Petrescu, A.J.; Kumlehn, J.; Boyd, L.A.; et al. An LRR/malectin receptor-like kinase mediates resistance to non-adapted and adapted powdery mildew fungi in barley and wheat. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhao, T.; Yu, D.; Gai, J. Isolation and functional characterization of a SERK gene from soybean (Glycine max (L.) Merr.). Plant Mol. Biol. Rep. 2011, 29, 334–344. [Google Scholar] [CrossRef]
- Walker, J.C. Structure and function of the receptor-like protein kinases of higher plants. Plant Mol. Biol. 1994, 26, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Becraft, P.W. Receptor kinase signaling in plant development. Annu. Rev. Cell Dev. Biol. 2002, 18, 163–192. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Li, G.; Song, F.; Zheng, Z. Molecular characterization and expression analysis of OsBISERK1, a gene encoding a leucine rich repeat receptor-like kinase, during disease resistance responses in rice. Mol. Biol. Rep. 2008, 35, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Chen, C.; Chen, Z. Evidence for an important role of WRKY DNA binding proteins in the regulation of NPR1 gene expression. Plant Cell 2001, 13, 1527–1540. [Google Scholar] [CrossRef] [PubMed]
- Bari, R.; Jones, J.D. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Vanacker, H.; Carver, T.L.; Foyer, C.H. Early H2O2 accumulation in mesophyll cells leads to induction of glutathione during the hypersensitive response in the barley powdery mildew interaction. Plant Physiol. 2000, 123, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Tenhaken, R.; Levine, A.; Brisson, L.F.; Dixon, R.A.; Lamb, C. Function of the oxidative burst in hypersensitive disease resistance. Proc. Natl. Acad. Sci. USA 1995, 92, 4158–4163. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.A.; Jones, J.D.G.; Dangl, J.L. Pathogen-induced, NADPH oxidase-derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nat. Genet. 2005, 37, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.E.; Pennell, R.I.; Meijer, P.J.; Ishikawa, A.; Dixon, R.A.; Lamb, C. Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 1998, 92, 773–784. [Google Scholar] [CrossRef]
- Baker, C.J.; Orlandi, E.W. Active oxygen in plant pathogenesis. Annu. Rev. Phytopathol. 1995, 33, 299–321. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, Y.; Fei, F.; Wang, Z.; Wang, W.; Cao, A.; Liu, Y.; Han, S.; Xing, L.; Wang, H.; et al. An E3 ubiquitin ligase gene CMPG1-V from Haynaldia villosa L. contributes to the powdery mildew resistance in common wheat. Plant J. 2015, 84, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Faheem, M.; Li, Y.; Arshad, M.; Cheng, J.; Zhao, J.; Wang, Z.; Xiao, J.; Wang, H.; Cao, A.; Xing, L.; et al. A disulphide isomerase gene (PDI-V) from Haynaldia villosa contributes to powdery mildew resistance in common wheat. Sci. Rep. 2016, 6, 24227. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Li, Q.; Guo, G.; He, T.; Gao, R.; Faheem, M.; Huang, J.; Lu, R.; Liu, C. Transient Overexpression of HvSERK2 Improves Barley Resistance to Powdery Mildew. Int. J. Mol. Sci. 2018, 19, 1226. https://doi.org/10.3390/ijms19041226
Li Y, Li Q, Guo G, He T, Gao R, Faheem M, Huang J, Lu R, Liu C. Transient Overexpression of HvSERK2 Improves Barley Resistance to Powdery Mildew. International Journal of Molecular Sciences. 2018; 19(4):1226. https://doi.org/10.3390/ijms19041226
Chicago/Turabian StyleLi, Yingbo, Qingwei Li, Guimei Guo, Ting He, Runhong Gao, Muhammad Faheem, Jianhua Huang, Ruiju Lu, and Chenghong Liu. 2018. "Transient Overexpression of HvSERK2 Improves Barley Resistance to Powdery Mildew" International Journal of Molecular Sciences 19, no. 4: 1226. https://doi.org/10.3390/ijms19041226
APA StyleLi, Y., Li, Q., Guo, G., He, T., Gao, R., Faheem, M., Huang, J., Lu, R., & Liu, C. (2018). Transient Overexpression of HvSERK2 Improves Barley Resistance to Powdery Mildew. International Journal of Molecular Sciences, 19(4), 1226. https://doi.org/10.3390/ijms19041226




