Nanocoatings for Chronic Wound Repair—Modulation of Microbial Colonization and Biofilm Formation
Abstract
1. Introduction
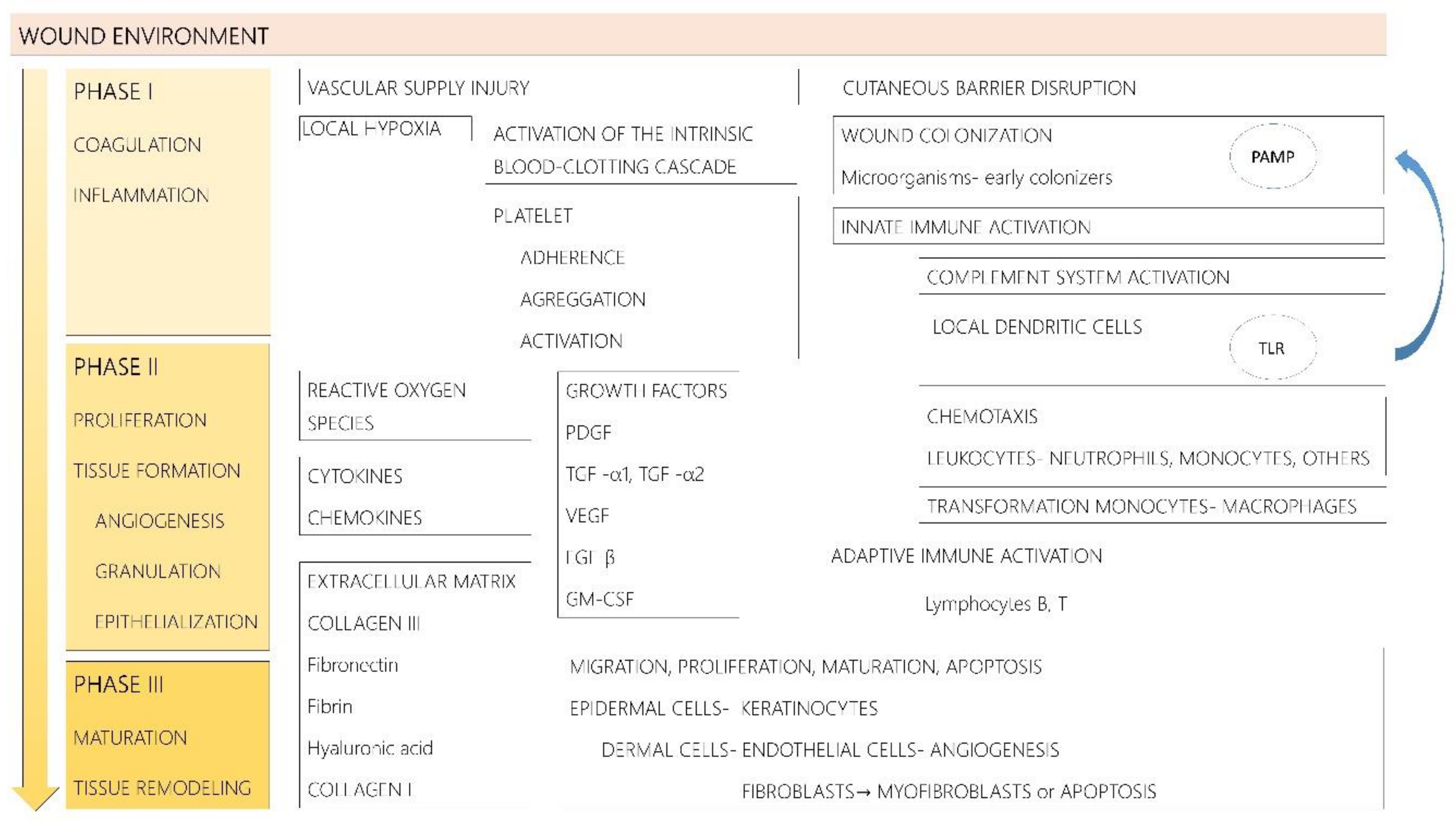
2. Pathogenesis of Chronic Wounds
2.1. Pathophysiology of Acute Wound Repair
2.2. Pathophysiology of Chronic Wound Repair
2.3. Microbial Colonization and Wound Healing
2.4. Bacterial Biofilms and Wound Healing
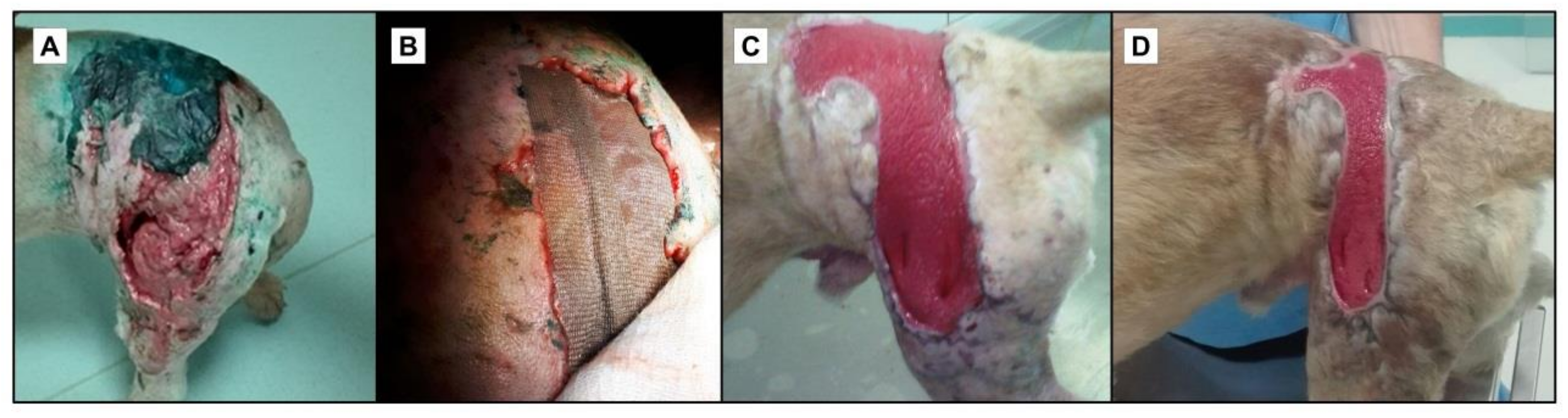
3. Limitations of Current Wound Therapy
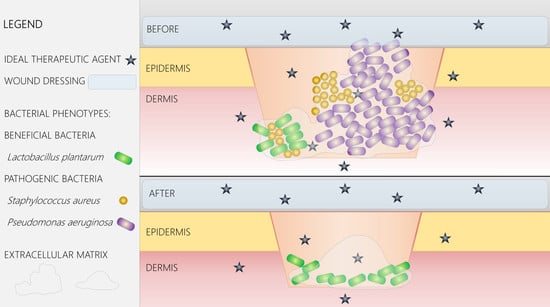
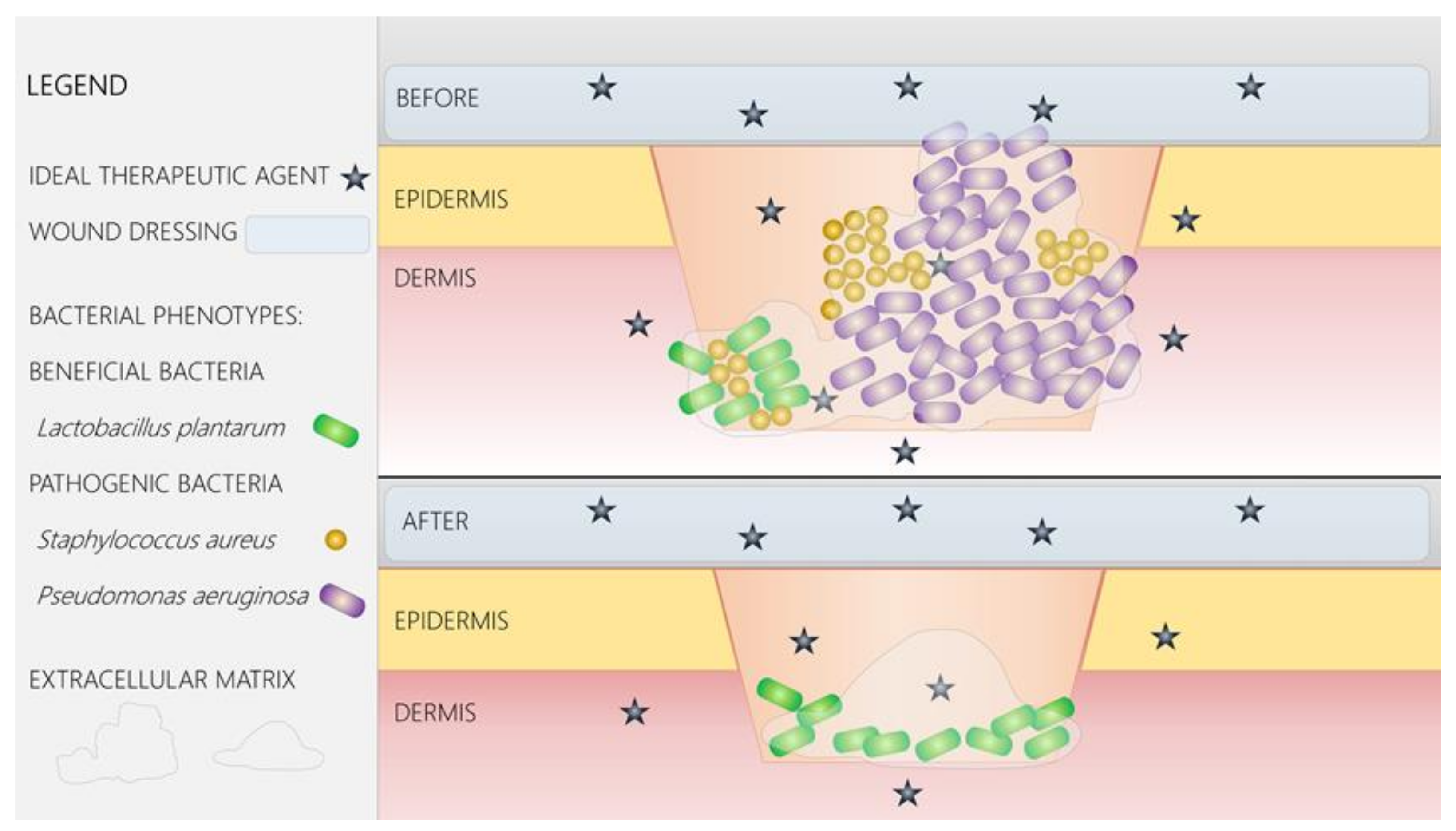
4. Advanced Wound Dressings and Coatings
5. Nano-Solutions for Wound Management
6. Antimicrobial Nanoparticles
6.1. Inorganic Nanoparticles
6.2. Organic Nanoparticles
7. Conclusions
Acknowledgments
Conflicts of Interest
References
- Yin, R.; Dai, T.; Avci, P.; Jorge, A.; de Melo, W.; Vecchio, D.; Huang, Y.; Gupta, A.; Hamblin, M. Light based anti-infectives: Ultraviolet C irradiation, photodynamic therapy, blue light, and beyond. Curr. Opin. Pharmacol. 2013, 13, 731–762. [Google Scholar] [CrossRef] [PubMed]
- Bertesteanu, S.; Triaridis, S.; Stankovic, M.; Lazar, V.; Chifiriuc, M.C.; Vlad, M.; Grigore, R. Polymicrobial wound infections: Pathophysiology and current therapeutic approaches. Int. J. Pharm. 2014, 463, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Kirker, K.R.; James, G.A. In vitro studies evaluating the effects of biofilms on wound-healing cells: A review. APMIS 2017, 125, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Rahim, K.; Saleha, S.; Zhu, X.; Huo, L.; Basit, A.; Franco, O.L. Bacterial Contribution in Chronicity of Wounds. Microb. Ecol. 2017, 73, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, part 1: Normal and chronic wounds: Biology, causes, and approaches to care. Adv. Skin Wound Care 2012, 25, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Tsiouris, C.G.; Tsiouri, M.G. Human microflora, probiotics and wound healing. Wound Med. 2017, 19, 33–38. [Google Scholar] [CrossRef]
- Jahromi, M.A.M.; Zangabad, P.S.; Basri, S.M.M.; Zangabad, K.S.; Ghamarypour, A.; Aref, A.R.; Karimi, M.; Hamblin, M.R. Nanomedicine and advanced technologies for burns: Preventing infection and facilitating wound healing. Adv. Drug Deliv. Rev. 2018, 123, 33–64. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.M.; Valgimigli, M.; Giannico, M.B.; Zaccone, V.; Perfetti, M.; D’Amario, D.; Rebuzzi, A.G.; Crea, F. From bone marrow to the arterial wall: The ongoing tale of endothelial progenitor cells. Eur. Heart J. 2009, 30, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, C.; Madalina, M.M.; Gabriela, P.L.; Calin, G.; Ioan, P.M. Considerations on the pathogenesis of chronic venous ulcers—Review. Infectio.ro 2015, 44, 19–24. [Google Scholar]
- Rai, N.K.; Tripathi, K.; Sharma, D.; Shukla, V.K. Apoptosis: A basic physiologic process in wound healing. Int. J. Low. Extrem. Wounds 2005, 4, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, part 2: Role of growth factors in normal and pathological wound healing: Therapeutic potential and methods of delivery. Adv. Skin Wound Care 2012, 25, 349–370. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L. Importance of biofilm formation in surgical infection. Br. J. Surg. 2017, 104, e85–e94. [Google Scholar] [CrossRef] [PubMed]
- Mihai, M.M.; Giurcaneanu, C.; Popa, L.G.; Nitipir, C.; Popa, M.I. Controversies and challenges of chronic wound infection diagnosis and treatment. Mod. Med. 2015, 22, 375–381. [Google Scholar]
- Robson, M.C. Wound infection. A failure of wound healing caused by an imbalance of bacteria. Surg. Clin. N. Am. 1997, 77, 637–650. [Google Scholar] [CrossRef]
- Percival, S.L.; Thomas, J.G.; Williams, D.W. Biofilms and bacterial imbalances in chronic wounds: Anti-Koch. Int. Wound J. 2010, 7, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Martindale, R.G.; Longaker, M.T.; Gurtner, G.C. From germ theory to germ therapy: Skin microbiota, chronic wounds, and probiotics. Plast. Reconstr. Surg. 2013, 132, 854e–861e. [Google Scholar] [CrossRef] [PubMed]
- Canesso, M.C.; Vieira, A.T.; Castro, T.B.; Schirmer, B.G.; Cisalpino, D.; Martins, F.S.; Rachid, M.A.; Nicoli, J.R.; Teixeira, M.M.; Barcelos, L.S. Skin wound healing is accelerated and scarless in the absence of commensal microbiota. J. Immunol. 2014, 193, 5171–5180. [Google Scholar] [CrossRef] [PubMed]
- Bessa, L.J.; Fazii, P.; di Giulio, M.; Cellini, L. Bacterial isolates from infected wounds and their antibiotic susceptibility pattern: Some remarks about wound infection. Int. Wound J. 2015, 12, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Mihai, M.M.; Holban, A.M.; Giurcaneanu, C.; Popa, L.G.; Buzea, M.; Filipov, M.; Lazar, V.; Chifiriuc, M.C.; Popa, M.I. Identification and phenotypic characterization of the most frequent bacterial etiologies in chronic skin ulcers. Rom. J. Morphol. Embryol. 2014, 55, 1401–1408. [Google Scholar] [PubMed]
- Mihai, M.M.; Popa, M.I.; Popa, L.G.; Ion, A.V.; Calugareanu, A.; Solomon, I.; Giurcaneanu, C. Antibiotics resistance phenotypes of the bacterial strains isolated from leg ulcers during 5 years in a dermatology department. Acta Dermatovenerol. 2017, 62, 7–23. [Google Scholar]
- Rhoads, D.D.; Cox, S.B.; Rees, E.J.; Sun, Y.; Wolcott, R.D. Clinical identification of bacteria in human chronic wound infections: Culturing vs. 16S ribosomal DNA sequencing. BMC Infect. Dis. 2012, 12, 321. [Google Scholar] [CrossRef] [PubMed]
- McCarty, S.M.; Cochrane, C.A.; Clegg, P.D.; Percival, S.L. The role of endogenous and exogenous enzymes in chronic wounds: A focus on the implications of aberrant levels of both host and bacterial proteases in wound healing. Wound Repair Regen. 2012, 20, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Shettigar, K.; Jain, S.; Bhat, D.V.; Acharya, R.; Ramachandra, L.; Satyamoorthy, K.; Murali, T.S. Virulence determinants in clinical Staphylococcus aureus from monomicrobial and polymicrobial infections of diabetic foot ulcers. J. Med. Microbiol. 2016, 65, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Schierle, C.F.; de la Garza, M.; Mustoe, T.A.; Galiano, R.D. Staphylococcal biofilms impair wound healing by delaying reepithelialization in a murine cutaneous wound model. Wound Repair Regen. 2009, 17, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Hochwalt, P.C.; Usui, M.L.; Underwood, R.A.; Singh, P.K.; James, G.A.; Stewart, P.S.; Fleckman, P.; Olerud, J.E. Delayed wound healing in diabetic (db/db) mice with Pseudomonas aeruginosa biofilm challenge: A model for the study of chronic wounds. Wound Repair Regen. 2010, 18, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Pastar, I.; Nusbaum, A.G.; Gil, J.; Patel, S.B.; Chen, J.; Valdes, J.; Stojadinovic, O.; Plano, L.R.; Tomic-Canic, M.; Davis, S.C. Interactions of methicillin resistant Staphylococcus aureus USA300 and Pseudomonas aeruginosa in polymicrobial wound infection. PLoS ONE 2013, 8, e56846. [Google Scholar] [CrossRef] [PubMed]
- Dalton, T.; Dowd, S.E.; Wolcott, R.D.; Sun, Y.; Watters, C.; Griswold, J.A.; Rumbaugh, K.P. An in vivo polymicrobial biofilm wound infection model to study interspecies interactions. PLoS ONE 2011, 6, e27317. [Google Scholar] [CrossRef] [PubMed]
- Hotterbeekx, A.; Kumar-Singh, S.; Goossens, H.; Malhotra-Kumar, S. In vivo and In vitro Interactions between Pseudomonas aeruginosa and Staphylococcus spp. Front. Cell. Infect. Microbiol. 2017, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; McCarty, S.M.; Lipsky, B. Biofilms and Wounds: An Overview of the Evidence. Adv. Wound Care 2015, 4, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.; Wright, J.B.; Schultz, G.; Burrell, R.; Nadworny, P. Microbial Biofilms and Chronic Wounds. Microorganisms 2017, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-H.; Tian, X. Quorum Sensing and Bacterial Social Interactions in Biofilms. Sensors 2012, 12, 2519–2538. [Google Scholar] [CrossRef] [PubMed]
- Hoiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Seth, A.K.; Geringer, M.R.; Gurjala, A.N.; Hong, S.J.; Galiano, R.D.; Leung, K.P.; Mustoe, T.A. Treatment of Pseudomonas aeruginosa biofilm-infected wounds with clinical wound care strategies: A quantitative study using an in vivo rabbit ear model. Plast. Reconstr. Surg. 2012, 129, 262e–274e. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T. The role of bacterial biofilms in chronic infections. APMIS 2013, 121, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Solano, C.; Echeverz, M.; Lasa, I. Biofilm dispersion and quorum sensing. Curr. Opin. Microbiol. 2014, 18, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Mihai, M.M.; Holban, A.M.; Giurcaneanu, C.; Popa, L.G.; Oanea, R.M.; Lazar, V.; Chifiriuc, M.C.; Popa, M.; Popa, M.I. Microbial biofilms: Impact on the pathogenesis of periodontitis, cystic fibrosis, chronic wounds and medical device-related infections. Curr. Top. Med. Chem. 2015, 15, 1552–1576. [Google Scholar] [CrossRef] [PubMed]
- Moser, C.; Pedersen, H.T.; Lerche, C.J.; Kolpen, M.; Line, L.; Thomsen, K.; Høiby, N.; Jensen, P. Biofilms and host response—Helpful or harmful. APMIS 2017, 125, 320–338. [Google Scholar] [CrossRef] [PubMed]
- Hirschfeld, J. Dynamic interactions of neutrophils and biofilms. J. Oral Microbiol. 2014, 17, 26102. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.; Fiehn, N.E. Dental biofilm infections—An update. APMIS 2017, 125, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.K.; Chae, S.W.; Go, Y.Y.; Im, G.J.; Song, J.J. In vitro Multi-Species Biofilms of Methicillin-Resistant Staphylococcus aureus and Pseudomonas aeruginosa and Their Host Interaction during in vivo Colonization of an Otitis Media Rat Model. Front. Cell. Infect. Microbiol. 2017, 7, 125. [Google Scholar] [CrossRef] [PubMed]
- Romling, U.; Balsalobre, C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J. Intern. Med. 2012, 272, 541–561. [Google Scholar] [CrossRef] [PubMed]
- James, G.A.; Swogger, E.; Wolcott, R.; Pulcini, E.; Secor, P.; Sestrich, J.; Costerton, J.W.; Stewart, P.S. Biofilms in chronic wounds. Wound Repair Regen. 2008, 16, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Centre for Diseases Control and Prevention. Available online: www.cdc.gov (accessed on 21 January 2018).
- Di Domenico, E.G.; Toma, L.; Provot, C.; Ascenzioni, F.; Sperduti, I.; Prignano, G.; Gallo, M.T.; Pimpinelli, F.; Bordignon, V.; Bernardi, T.; et al. Development of an in vitro Assay, Based on the BioFilm Ring Test(R), for Rapid Profiling of Biofilm-Growing Bacteria. Front. Microbiol. 2016, 7, 1429. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, E.G.; Farulla, I.; Prignano, G.; Gallo, M.T.; Vespaziani, M.; Cavallo, I.; Sperduti, I.; Pontone, M.; Bordignon, V.; Cilli, L.; et al. Biofilm is a major virulence determinant in bacterial colonization of chronic skin ulcers independently from the multidrug resistant phenotype. Int. J. Mol. Sci. 2017, 18, 1077. [Google Scholar] [CrossRef] [PubMed]
- Holban, A.M.; Gestal, M.C.; Grumezescu, A.M. Control of biofilm-associated infections by signaling molecules and nanoparticles. Int. J. Pharm. 2016, 510, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Hoiby, N.; Bjarnsholt, T.; Moser, C.; Bassi, G.L.; Coenye, T.; Donelli, G.; Hall-Stoodley, L.; Hola, V.; Imbert, C.; Kirketerp-Moller, K.; et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin. Microbiol. Infect. 2015, 21, S1–S25. [Google Scholar] [CrossRef] [PubMed]
- Nusbaum, A.G.; Kirsner, R.S.; Charles, C.A. Biofilms in dermatology. Skin Ther. Lett. 2012, 17, 1–5. [Google Scholar]
- Wolcott, R.D.; Rumbaugh, K.P.; James, G.; Schultz, G.; Phillips, P.; Yang, Q.; Watters, C.; Stewart, P.S.; Dowd, S.E. Biofilm maturity studies indicate sharp debridement opens a time-dependent therapeutic window. J. Wound Care 2010, 19, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Elgharably, H.; Sinha, M.; Ganesh, K.; Chaney, S.; Mann, E.; Miller, C.; Khanna, S.; Bergdall, V.K.; Powell, H.; et al. Mixed-species biofilm compromises wound healing by disrupting epidermal barrier function. J. Pathol. 2014, 233, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Daeschlein, G. Antimicrobial and antiseptic strategies in wound management. Int. Wound J. 2013, 10 (Suppl. S1), 9–14. [Google Scholar] [CrossRef] [PubMed]
- Hoiby, N.; Frederiksen, B.; Pressler, T. Eradication of early Pseudomonas aeruginosa infection. J. Cyst. Fibros. 2005, 4 (Suppl. S2), 49–54. [Google Scholar] [CrossRef] [PubMed]
- Liakos, I.; Grumezescu, A.M.; Holban, A.M. Magnetite nanostructures as novel strategies for anti-infectious therapy. Molecules 2014, 19, 12710–12726. [Google Scholar] [CrossRef] [PubMed]
- Tsiouris, C.G.; Kelesi, M.; Vasilopoulos, G.; Kalemikerakis, I.; Papageorgiou, E.G. The efficacy of probiotics as pharmacological treatment of cutaneous wounds: Meta-analysis of animal studies. Eur. J. Pharm. Sci. 2017, 104, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Valdez, J.C.; Peral, M.C.; Rachid, M.; Santana, M.; Perdigon, G. Interference of Lactobacillus plantarum with Pseudomonas aeruginosa in vitro and in infected burns: The potential use of probiotics in wound treatment. Clin. Microbiol. Infect. 2005, 11, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Peral, M.C.; Martinez, M.A.; Valdez, J.C. Bacteriotherapy with Lactobacillus plantarum in burns. Int. Wound J. 2009, 6, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound Dressings and Comparative Effectiveness Data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, S.; Coradin, T.; Desimone, M.F. Bio-inspired silica–collagen materials: Applications and perspectives in the medical field. Biomater. Sci. 2013, 1, 688–702. [Google Scholar] [CrossRef]
- Chikazua, D.; Taguchi, T.; Koyama, H.; Hikiji, H.; Fujihara, H.; Suenaga, H.; Saijo, H.; Mori, Y.; Seto, H.; Lino, M.; et al. Improvement in wound healing by a novel synthetic collagen-gel dressing in genetically diabetic mice. Asian J. Oral Maxillofac. Surg. 2010, 22, 61–67. [Google Scholar] [CrossRef]
- Holban, A.M.; Grumezescu, V.; Grumezescu, A.M.; Vasile, B.Ş.; Truşcă, R.; Cristescu, R.; Socol, G.; Iordache, F. Antimicrobial nanospheres thin coatings prepared by advanced pulsed laser technique. Beilstein J. Nanotechnol. 2014, 5, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Tocco, I.; Zavan, B.; Bassetto, F.; Vindigni, V. Nanotechnology-Based Therapies for Skin Wound Regeneration. J. Nanomater. 2012, 2012, 1–11. [Google Scholar] [CrossRef]
- Radulescu, M.; Andronescu, E.; Holban, A.; Vasile, B.; Iordache, F.; Mogoanta, L.; Mogoșanu, G.; Grumezescu, A.; Georgescu, M.; Chifiriuc, M. Antimicrobial Nanostructured Bioactive Coating Based on Fe3O4 and Patchouli Oil for Wound Dressing. Metals 2016, 6, 103. [Google Scholar] [CrossRef]
- Anghel, I.; Holban, A.M.; Grumezescu, A.M.; Andronescu, E.; Ficai, A.; Anghel, A.G.; Maganu, M.; Lazǎr, V.; Chifiriuc, M.C. Modified wound dressing with phyto-nanostructured coating to prevent staphylococcal and pseudomonal biofilm development. Nanoscale Res. Lett. 2012, 7, 690. [Google Scholar] [CrossRef] [PubMed]
- Cady, N.C.; Behnke, J.L.; Strickland, A.D. Copper-Based Nanostructured Coatings on Natural Cellulose: Nanocomposites Exhibiting Rapid and Efficient Inhibition of a Multi-Drug Resistant Wound Pathogen, A. baumannii, and Mammalian Cell Biocompatibility In Vitro. Adv. Funct. Mater. 2011, 21, 2506–2514. [Google Scholar] [CrossRef]
- Radulescu, M.; Andronescu, E.; Dolete, G.; Popescu, R.; Fufă, O.; Chifiriuc, M.; Mogoantă, L.; Bălşeanu, T.-A.; Mogoşanu, G.; Grumezescu, A. Silver Nanocoatings for Reducing the Exogenous Microbial Colonization of Wound Dressings. Materials 2016, 9, 345. [Google Scholar] [CrossRef] [PubMed]
- Meftahi, A.; Nasrolahi, D.; Babaeipour, V.; Alibakhshi, S.; Shahbazi, S. Investigation of Nano Bacterial Cellulose Coated by Sesamum Oil for Wound Dressing Application. Procedia Mater. Sci. 2015, 11, 212–216. [Google Scholar] [CrossRef]
- Anghel, I.; Grumezescu, A.M.; Holban, A.M.; Ficai, A.; Anghel, A.G.; Chifiriuc, M.C. Biohybrid nanostructured iron oxide nanoparticles and Satureja hortensis to prevent fungal biofilm development. Int. J. Mol. Sci. 2013, 14, 18110–18123. [Google Scholar] [CrossRef] [PubMed]
- Paladini, F.; di Franco, C.; Panico, A.; Scamarcio, G.; Sannino, A.; Pollini, M. In Vitro Assessment of the Antibacterial Potential of Silver Nano-Coatings on Cotton Gauzes for Prevention of Wound Infections. Materials 2016, 9, 411. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Luo, G.; Wang, Y.; Xu, R.; Wang, Y.; He, W.; Tan, J.; Xing, M.; Wu, J. Nano-silver-decorated microfibrous eggshell membrane: Processing, cytotoxicity assessment and optimization, antibacterial activity and wound healing. Sci. Rep. 2017, 7, 436. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.M.P.; Aguiar, M.I.F.D.; Rodrigues, A.B.; Miranda, M.D.C.; Araújo, M.Â.M.; Rolim, I.L.T.P.; Souza, A.M.A. Utilização de nanopartículas no tratamento de feridas: Revisão sistemática. Revista da Escola de Enfermagem da USP 2017, 51, e03272. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Beyth, N.; Houri-Haddad, Y.; Domb, A.; Khan, W.; Hazan, R. Alternative Antimicrobial Approach: Nano-Antimicrobial Materials. Evid. Based Complement. Altern. Med. 2015, 2015, 16. [Google Scholar] [CrossRef] [PubMed]
- Stan, M.S.; Constanda, S.; Grumezescu, V.; Andronescu, E.; Ene, A.M.; Holban, A.M.; Vasile, B.S.; Mogoanta, L.; Balseanu, T.A.; Mogosaamnu, G.D.; et al. Thin coatings based on ZnO@ C 18-usnic acid nanoparticles prepared by MAPLE inhibit the development of Salmonella enterica early biofilm growth. Appl. Surf. Sci. 2016, 374, 318–325. [Google Scholar] [CrossRef]
- Irie, Y.; Parsek, M.R. Quorum sensing and microbial biofilms. Curr. Top Microbiol. Immunol. 2008, 322, 67–84. [Google Scholar] [PubMed]
- Hussain, S.; Ferguson, C. Best evidence topic report. Silver sulphadiazine cream in burns. Emerg. Med. J. 2006, 23, 929–932. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.J.; Shafii, S.M.; Ko, F.; Donate, G.; Wright, T.E.; Mannari, R.J.; Payne, W.G.; Smith, D.J.; Robson, M.C. Comparative evaluation of silver-containing antimicrobial dressings and drugs. Int. Wound J. 2007, 4, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Rigo, C.; Ferroni, L.; Tocco, I.; Roman, M.; Munivrana, I.; Gardin, C.; Cairns, W.R.; Vindigni, V.; Azzena, B.; Barbante, C.; et al. Active silver nanoparticles for wound healing. Int. J. Mol. Sci. 2013, 14, 4817–4840. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; McCarty, S.M. Silver and Alginates: Role in Wound Healing and Biofilm Control. Adv. Wound Care 2015, 4, 407–414. [Google Scholar] [CrossRef] [PubMed]
- You, C.; Li, Q.; Wang, X.; Wu, P.; Ho, J.K.; Jin, R.; Zhang, L.; Shao, H.; Han, C. Silver nanoparticle loaded collagen/chitosan scaffolds promote wound healing via regulating fibroblast migration and macrophage activation. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Daghdari, S.G.; Ahmadi, M.; Saei, H.D.; Tehrani, A.A. The effect of ZnO nanoparticles on bacterial load of experimental infectious wounds contaminated with Staphylococcus aureus in mice. Nanomed. J. 2017, 4, 232–236. [Google Scholar]
- Anjum, S.; Singh, S.; Gupta, B. New Generation Chitosan/Nano ZnO/Curcuminnano composite Wound Dressings for Infection Control. Ann. Pharmacol. Pharm. 2017, 2, 1044. [Google Scholar]
- Lee, J.H.; Kim, Y.G.; Cho, M.H.; Lee, J. ZnO nanoparticles inhibit Pseudomonas aeruginosa biofilm formation and virulence factor production. Microbiol. Res. 2014, 169, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Raguvaran, R.; Manuja, B.K.; Chopra, M.; Thakur, R.; Anand, T.; Kalia, A.; Manuja, A. Sodium alginate and gum acacia hydrogels of ZnO nanoparticles show wound healing effect on fibroblast cells. Int. J. Biol. Macromol. 2017, 96, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Galateanu, B.; Bunea, M.-C.; Stanescu, P.; Vasile, E.; Casarica, A.; Iovu, H.; Hermenean, A.; Zaharia, C.; Costache, M. In Vitro Studies of Bacterial Cellulose and Magnetic Nanoparticles Smart Nanocomposites for Efficient Chronic Wounds Healing. Stem Cells Int. 2015, 2015, 195096. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Baker, A.B. Biomaterials and Nanotherapeutics for Enhancing Skin Wound Healing. Front. Bioeng. Biotechnol. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Coradin, T.; Helary, C. Silica-collagen nanocomposites for local and sustained release of Interleukin 10 (IL-10) to treat cutaneous chronic wounds. Front. Bioeng. Biotechnol. 2016. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, S.; Yi, J.; Zhang, H.; Ameer, G.A. A Cooperative Copper Metal-Organic Framework-Hydrogel System Improves Wound Healing in Diabetes. Adv. Funct. Mater. 2017, 27, 1604872. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Zhu, Y.; Huddleston, S.; Li, P.; Xiao, B.; Farha, O.K.; Ameer, G.A. Copper Metal-Organic Framework Nanoparticles Stabilized with Folic Acid Improve Wound Healing in Diabetes. ACS Nano 2018, 12, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Lellouche, J.; Kahana, E.; Elias, S.; Gedanken, A.; Banin, E. Antibiofilm activity of nanosized magnesium fluoride. Biomaterials 2009, 30, 5969–5978. [Google Scholar] [CrossRef] [PubMed]
- Kutner, A.J.; Friedman, A.J. Use of nitric oxide nanoparticulate platform for the treatment of skin and soft tissue infections. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as Antimicrobial Agent: Applications and Mode of Action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
| Therapeutic Approach | Advantages | Disadvantages | Indication | Examples |
|---|---|---|---|---|
| Wound debridement |
|
|
|
|
| Topical or systemic antibiotic therapy |
|
|
|
|
| Topical antiseptic therapy |
|
|
|
|
| Bacteriophage therapy |
|
|
|
|
| Antimicrobial peptides |
|
|
|
|
| Probiotic therapy |
|
|
|
|
| Type | Nanostructure(s) | Application | Mode of Action | Reference |
|---|---|---|---|---|
| Bioactive wound coating | Magnetite (Fe3O4) nanoparticles (NPs) and patchouli essential oil | Acute and chronic wound dressing | Inhibition of microbial colonization and biofilm formation | [65] |
| Nanophyto-modified wound dressing | Nanofluid-based Fe3O4 doped with eugenol and limonene | Fixed layer on a regular external wound cover | Anti-adherence and anti-biofilm properties against bacterial pathogens | [66] |
| Layer-by-layer (LBL) electrostatic self-assembled antimicrobial nanocoating | Chemically modified cotton substrate and copper-based NP layer | Metal-based wound care and inhibition of pathogenic bacterial infections | Inhibition of A. baumannii (multidrug resistant bacterial wound pathogen) | [67] |
| Bioactive wound coating | Silver NPs for polyester–nylon wound dressing | Reduction of exogenous microbial colonization of wound dressing | Inhibition of microbial colonization, attachment, and biofilm growth | [68] |
| Bioactive wound coating | Nano bacterial cellulose and sesame oil | Modern wound dressing | Improved healing properties and inhibition of bacterial infections | [69] |
| Nano-coated wound dressing | Fe3O4 and Satureja hortensis (SO) essential oil | Cutaneous wound dressing | Inhibition of fungal biofilm development and adherence of C. albicans | [70] |
| Nano-coated wound dressing | Silver nanocoating on cotton gauzes | Acute and chronic wound dressing | Reduction of bacterial growth and biofilm proliferation | [71] |
| Bioactive wound coating | Nano-silver-coated microfibrous eggshell membrane | Cutaneous wound dressing | Antibacterial and anti-inflammatory activity, and also acceleration of wound healing | [72] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihai, M.M.; Preda, M.; Lungu, I.; Gestal, M.C.; Popa, M.I.; Holban, A.M. Nanocoatings for Chronic Wound Repair—Modulation of Microbial Colonization and Biofilm Formation. Int. J. Mol. Sci. 2018, 19, 1179. https://doi.org/10.3390/ijms19041179
Mihai MM, Preda M, Lungu I, Gestal MC, Popa MI, Holban AM. Nanocoatings for Chronic Wound Repair—Modulation of Microbial Colonization and Biofilm Formation. International Journal of Molecular Sciences. 2018; 19(4):1179. https://doi.org/10.3390/ijms19041179
Chicago/Turabian StyleMihai, Mara Mădălina, Mădălina Preda, Iulia Lungu, Monica Cartelle Gestal, Mircea Ioan Popa, and Alina Maria Holban. 2018. "Nanocoatings for Chronic Wound Repair—Modulation of Microbial Colonization and Biofilm Formation" International Journal of Molecular Sciences 19, no. 4: 1179. https://doi.org/10.3390/ijms19041179
APA StyleMihai, M. M., Preda, M., Lungu, I., Gestal, M. C., Popa, M. I., & Holban, A. M. (2018). Nanocoatings for Chronic Wound Repair—Modulation of Microbial Colonization and Biofilm Formation. International Journal of Molecular Sciences, 19(4), 1179. https://doi.org/10.3390/ijms19041179