Abstract
Antimicrobial lipids such as fatty acids and monoglycerides are promising antibacterial agents that destabilize bacterial cell membranes, causing a wide range of direct and indirect inhibitory effects. The goal of this review is to introduce the latest experimental approaches for characterizing how antimicrobial lipids destabilize phospholipid membranes within the broader scope of introducing current knowledge about the biological activities of antimicrobial lipids, testing strategies, and applications for treating bacterial infections. To this end, a general background on antimicrobial lipids, including structural classification, is provided along with a detailed description of their targeting spectrum and currently understood antibacterial mechanisms. Building on this knowledge, different experimental approaches to characterize antimicrobial lipids are presented, including cell-based biological and model membrane-based biophysical measurement techniques. Particular emphasis is placed on drawing out how biological and biophysical approaches complement one another and can yield mechanistic insights into how the physicochemical properties of antimicrobial lipids influence molecular self-assembly and concentration-dependent interactions with model phospholipid and bacterial cell membranes. Examples of possible therapeutic applications are briefly introduced to highlight the potential significance of antimicrobial lipids for human health and medicine, and to motivate the importance of employing orthogonal measurement strategies to characterize the activity profile of antimicrobial lipids.
1. Introduction
Molecular design principles underpin the structure and function of biological assemblies such as cells, and amphiphilic molecules drive the spontaneous self-assembly of key architectural elements like phospholipid membranes [1,2,3]. Understanding the role of molecular self-assembly in directing the formation of biological macromolecular structures, including lipid bilayers, proteins, and assemblies thereof, is part of the nanoarchitectonics field [4,5], and such insights can help solve outstanding biomedical problems. Within this scope, one of the greatest public health problems in the world today is the growing rise of antibiotic-resistant bacteria and the associated challenges to treat and prevent bacterial infections [6]. Less than a century ago, the world’s first antibiotic, penicillin, was discovered, and the specificity of antibiotics to inhibit bacterial enzymes and other proteins necessary for bacterial cell function proved highly effective and a remarkable example of molecular pharmaceutics, as evidenced by marked improvements in healthcare capabilities to treat bacterial infections. As a result, many formerly fatal or debilitating diseases caused by bacterial pathogens were suddenly curable with antibiotic treatment [7].
With high potency and working against a broad spectrum of bacterial targets, antibiotics became the standard drug option to treat bacterial infections, and are also widely used as precautionary measures to treat suspected infections, even when the microbial origin is unknown and could be bacterial, fungal, or viral among other possibilities. Antibiotics are also administered prophylactically in cases where bacterial infections might arise, such as after surgical operations. In addition, antibiotics are commonly used in the agricultural sector to not only treat and prevent bacterial infections among livestock, but also serve as growth promoters to accelerate the time to reach maturity as well as increase the body mass of animals. For all these reasons, antibiotics have become ubiquitous in society and have played an outsized role in shaping modern life.
However, despite numerous benefits, the drawbacks of antibiotics being so widely prevalent are now becoming apparent as well. With increasing exposure to antibiotics and corresponding selective pressure, bacteria have evolved to become resistant to many antibiotics, and antibiotic-resistant bacteria are widespread. As a result, existing antibiotics are losing their effectiveness to treat bacterial infections, and the problem is further compounded by the dearth of new antibiotics that have been discovered in recent years. In part, the problem is economic because pharmaceutical companies have had weak interest in developing new antibiotics due to low price points, however, the more pressing scientific issue is that the chemical space available for identifying and refining antibiotics is limited. There is growing recognition that society faces an impending post-antibiotic era [8], and hence, there is an urgent need to develop new classes of antibacterial agents that work against novel molecular targets.
To address this problem, antimicrobial lipids—single-chain lipid amphiphiles that destabilize bacterial cell membranes—are attractive candidates to become next-generation antibacterial agents for treating bacterial infections. Curiously, the antibacterial properties of antimicrobial lipids have been known since early reports by Dr. Robert Koch and colleagues in the late 1880s, when it was shown that fatty acids, a prominent class of antimicrobial lipid, inhibited growth of the Bacillus anthracis pathogen that causes anthrax [9]. A few decades later, Burtenshaw and colleagues showed that antimicrobial lipids are an important component of human skin’s innate immune system [10,11], lending credence to the possibility that exogenous addition of antimicrobial lipids would be medically opportune. Despite strong promise and demonstrated results, the prospects for antimicrobial lipids faded away by the late 1940s due to the emergence of antibiotics, but have received renewed attention amidst the growing impact of antibiotic-resistant bacteria. Indeed, one attractive feature of antimicrobial lipids is that it is difficult for bacteria to mutate to become resistant to them. As such, bacterial cell cultures can be grown in the presence of antimicrobial lipids (at sub-lethal concentrations) for at least one year, without signs of drug-resistant strains emerging [12].
Particular attention is drawn to two classes of antimicrobial lipid, namely fatty acids (hydrocarbon chains with a carboxylic acid functional group [13]) and monoglycerides (esterified adducts of a fatty acid and glycerol molecule). The motivation for studying these two classes of antimicrobial lipid arose from pioneering studies by Kabara and colleagues in the 1970s, which systematically investigated the antibacterial potency of medium-chain saturated fatty acids and monoglycerides with different chain lengths [14,15,16,17]. It was discovered that lauric acid (LA), which possesses a 12 carbon-long chain, had the most potent activity to inhibit growth of Gram-positive bacteria, and its monoglyceride derivative, glycerol monolaurate (GML), exhibited even stronger inhibitory activity than LA. Importantly, both LA and GML are Generally Recognized As Safe (GRAS) by the United States Food and Drug Administration [18] and abundant in nature. These factors have led to wide exploration of LA and GML for anti-infective applications [19,20,21], including application topics such as agriculture [22] and cosmetics [13,23,24]. Other antimicrobial lipids such as capric acid, which possesses a 10 carbon-long chain, and its monoglyceride derivative, monocaprin, have also received attention. While numerous studies have been conducted to empirically investigate the inhibitory properties of antimicrobial lipids, clarifying how the physicochemical properties of antimicrobial lipids influence biological activities remains an outstanding goal in many respects.
To date, the primary means of assessing the activity profile of an antimicrobial lipid has been to evaluate how treatment affects bacterial cell growth, with the minimum inhibitory concentration (MIC) of a test compound being defined as the drug concentration at which no visible growth of bacteria occurs. While such information provides insight into the scope and potency of an antimicrobial lipid, it does not reveal mechanistic information and there is growing interest to understand how antimicrobial lipids destabilize bacterial cell membranes. Biological assays have identified that antimicrobial lipids act as bacteriostatic (growth-inhibiting) or bactericidal (killing) agents depending on the drug concentration, target bacterium and other environmental factors [13,25]. To directly observe morphological effects on bacterial cell membranes, electron microscopy techniques have been utilized to image bacterial specimens after treatment with antimicrobial lipids [26,27,28,29,30,31,32]. While this approach provides visualization of membrane damage, very high concentrations of antimicrobial lipid are typically used (5–10 mM) and the bacterial cells can only be examined after treatment and sample fixation. Similar issues exist with atomic force microscopy experiments for examining the morphology of treated bacterial cells. In general, it is difficult to resolve molecular-level interaction kinetics when working with complex biological samples such as whole bacterial cells, thereby motivating the development of model systems.
To obtain insights into real-time interaction kinetics with a more focused approach, a variety of solution-phase model membrane platforms based on small unilamellar vesicles (SUVs) and large unilamellar vesicles (LUVs) have been employed in combination with measurement techniques such as dynamic light scattering in order to detect how antimicrobial lipids cause membrane destabilization via partial solubilization as well as membrane fission [33,34,35,36,37,38,39,40]. These model systems allow detailed characterization of membrane morphological changes by using well-controlled, simplified phospholipid compositions that mimic more complex biological membranes. Time-lapsed optical microscopy imaging of giant unilamellar vesicles (GUVs) has also enabled direct visualization of membrane morphological responses, including swelling, fusion, and fission behaviors [41,42]. As another option, supported lipid bilayers (SLBs) are two-dimensional phospholipid bilayers that have emerged as a particularly useful model membrane platform because they can be studied with a wide range of surface-sensitive measurement techniques, revealing insights into the mass, viscoelastic, fluidic, and morphological properties of SLB platforms. Indeed, one particular advantage of SLB platforms is that the two-dimensional phospholipid bilayer can remodel in response to an applied membrane strain, giving rise to three-dimensional morphological responses. Until recently, there was only scant attention to employ SLB platforms for investigating antimicrobial lipids, with only one study reporting the interactions between a long-chain fatty acid and single-component, zwitterionic phospholipid SLB platform and another study investigating the interaction between a short-chain monoglyceride and SLBs derived from bacterial cell membrane extracts [43,44,45]. Extending such approaches to investigate medium-chain saturated fatty acids and monoglycerides—representing the subset of antimicrobial lipids with the highest activity—is warranted in order to develop model systems for profiling the scope and potency of these antimicrobial lipids and a subject of active investigation. Moving beyond particular experimental approaches, there is active progress towards establishing comprehensive frameworks for correlating the physicochemical properties of antimicrobial lipids with their corresponding biophysical and biological activities.
Recognizing these possibilities, the goal of this review is to introduce the latest experimental approaches for characterizing how antimicrobial lipids destabilize phospholipid membranes within the broader scope of introducing current knowledge about the biological activities of antimicrobial lipids, testing strategies, and applications for treating bacterial infections. The contents of the review are organized into three major sections. First, a general background on antimicrobial lipids is provided that describes the structural classification, spectrum of antibacterial activity, and currently understood antibacterial mechanisms linked to membrane destabilization. Then, different experimental approaches to characterize antimicrobial lipids, including cell-based biological and model membrane-based biophysical measurement techniques, are presented. The major findings obtained by each experimental approach are critically summarized, and supplemented by discussion about how biological and biophysical approaches can be integrated to better understand how the physicochemical properties of antimicrobial lipids influence molecular self-assembly and concentration-dependent interactions with model phospholipid and bacterial cell membranes. Finally, it is described how antimicrobial lipids in free form and nanostructured assemblies can be employed for therapeutic applications, including when administered via systemic and topical administration routes. Taken together, the insights presented in this review underscore the utility of employing orthogonal measurement strategies to characterize the activity profile of antimicrobial lipids.
2. Antimicrobial Lipids
2.1. Classifications
Antimicrobial lipids are defined as single-chain lipid amphiphiles that interact with bacterial cell membranes and exhibit antibacterial activity. Fatty acids are a widely studied type of antimicrobial lipid, and are composed of a single saturated or unsaturated hydrocarbon chain and a carboxylic acid group on one end. As such, fatty acids are amphipathic molecules, with the hydrocarbon chain constituting the hydrophobic part while the carboxylic acid group is hydrophilic (either polar or anionic in aqueous solutions, depending on pH conditions). For example, the carboxylic acid groups of medium-chain fatty such as capric acid and LA, have pKa values around pH 5 [46], and therefore the fatty acids are anionic (deprotonated carboxylic acid groups) around the physiological (blood) pH condition of 7.4. In addition to fatty acids, other derivatives have been reported to have antibacterial activity, with one prominent class being monoglycerides that are composed of a fatty acid connected with a glycerol molecule via an ester bond. Other synthetic versions are also possible such as related compounds with ether bonds, rendering such molecules impervious to bacterial lipases. Compared to fatty acids, monoglycerides bear the distinction of not having ionizable functional groups across relevant pH conditions, and hence, are nonionic molecules with neutral electrical charge properties and some degree of polarity.
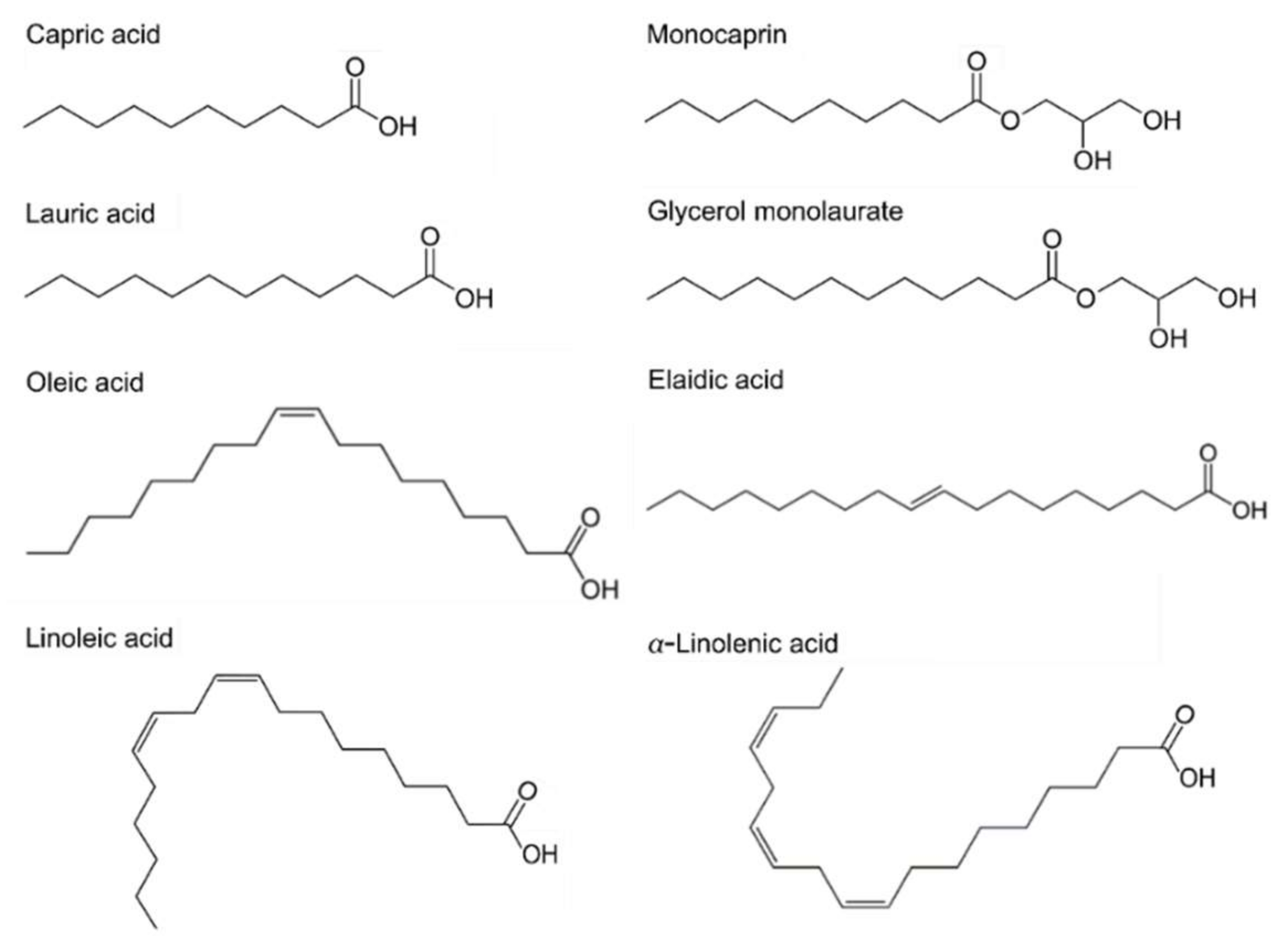
Antimicrobial lipids are classified based on their chain lengths and degrees of unsaturation. Representative compounds with different molecular structures are presented in Figure 1. In biological systems, fatty acids typically possess an even number of carbon atoms between 4 and 28, although other odd-numbered versions are possible in select systems or synthetically produced. Fatty acids that are less than 8 carbon atoms long are defined as short-chain, while those with greater than 12 carbon atoms are long-chain fatty acids and medium-chain fatty acids have between 8 and 12 carbon atoms [13,47,48]. Another important parameter is the number of degrees of unsaturation. In saturated fatty acids, all the carbon atoms are linked by single covalent bonds, while unsaturated fatty acids have one or more double bonds (degrees of unsaturation) in the carbon backbone. Specifically, unsaturated fatty acids having more than one double bond are identified as polyunsaturated fatty acids. The presence of double bonds, including the number of them and their orientation (cis- or trans-) can lead to significantly different physicochemical properties of fatty acids, even among compounds having the same hydrocarbon chain length. Thus, classifying fatty acids and their derivatives is an important part of investigating trends in antibacterial activity with respect to molecular structure and shape. To this end, extensive microbiological studies have been conducted and unsaturated fatty acids with medium and longer chains typically show greater efficacy against Gram-positive bacteria than Gram-negative bacteria [49,50]. Some studies have also been reported that focus on assessing antibacterial potency in the presence of compounds with double bonds and related properties [27,50,51,52,53].
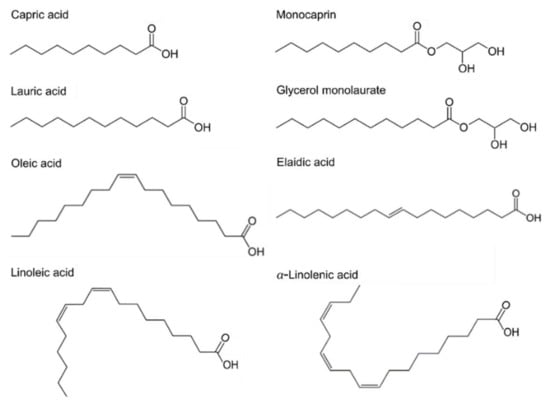
Figure 1.
Chemical structures of fatty acids and monoglycerides. Saturated fatty acids; Capric acid (C10:0), LA (C12:0). Monoglycerides; Monocaprin (MG C10:0), Glycerol monolaurate (MG C12:0). Unsaturated fatty acids; Oleic acid (C18:1), Elaidic acid (trans-C18:1). Polyunsaturated fatty acids; Linoleic acid (C18:2), Linolenic acid (C18:3). Cx:y is defined such that x is the number of carbons in the primary alkyl chain and y is the number of degrees of unsaturation.
In past works, the potency of saturated fatty acids has also been examined as a function of hydrocarbon chain length [14,30,50,53,54,55]. By systematically investigating saturated fatty acids with hydrocarbon chains ranging between 6 and 18 carbons long, it was identified that LA, which has a 12-carbon long chain, exhibits the most potent activity to inhibit growth of Gram-positive bacteria, including Staphylococcus aureus, a major causative agent of bacterial skin infections as well as systemic ones. Its 1-monoglyceride derivative, GML, showed even greater potency against S. aureus, as indicated by a lower value of the minimum inhibitory concentration (MIC) in comparison to LA [14]. In addition, capric acid and its monoglyceride derivative, monocaprin, have saturated hydrocarbon chains that are 10 carbons long, and also have high antibacterial activity, especially against Gram-negative bacteria that are commonly associated with foodborne infections such as Campylobacter jejuni [56]. The connection between antimicrobial lipid and antibacterial spectrum and potency is further discussed in the next section.
2.2. Spectrum of Antibacterial Activity
Broad-spectrum inhibitory activity of antimicrobial lipids against algae, bacteria, fungi, protozoa, and virus has been reported for several decades [13,14,15,23,57,58,59]. The antibacterial potency of fatty acids and monoglyceride derivatives has been investigated extensively against a wide range of bacteria, including pathogenic strains such as methicillin-resistant Staphylococcus aureus (MRSA), as presented in Table 1. Extensive screening of the antibacterial activities of fatty acids was conducted by Kabara and colleagues in the 1970s, and helped to establish the modern-day field of antimicrobial lipids from a chemical perspective. Fatty acids with hydrocarbon chains ranging from 6 to 18 carbons long and selected derivatives with different functionalized headgroups were evaluated, resulting in the comprehensive identification of LA (C12:0) as the most potent antimicrobial lipid to inhibit growth of Gram-positive bacteria, as mentioned above. As a general principle, the esterification of a fatty acid to its corresponding monoglyceride derivative increases antibacterial activity [14,15,17]. Another supportive study for the high antibacterial potency of LA was conducted with different types of Gram-positive bacteria, and further demonstrated that unsaturated fatty acids with 18 carbon long chains—oleic acid (C18:1), linoleic (C18:2), and linolenic acid (C18:3)—have potent antibacterial activities as well [50].

Table 1.
Antibacterial activity of fatty acids and monoglycerides against different bacteria.
Based on the findings from these pioneering studies, more selective and detailed studies focused on either specific bacterial type or particular antimicrobial lipids have been performed [27,52,60,61]. Specifically, interest on fatty acids and monoglycerides as potential therapeutic agents and/or preservatives against medically important pathogens led to the following studies of biomedical and food science relevance. Wang et al. investigated the efficacy of fatty acids and monoglycerides against Listeria monocytogenes, which is a Gram-positive bacterium that causes a series of food-borne infections. Of the tested fatty acids and monoglycerides, LA, linolenic acid, and GML had the strongest bactericidal activity at 10–20 µg/mL in brain heart infusion broth at pH 5. Interestingly, the bactericidal activity of the fatty acids depended on solution pH, showing increased activity at pH 5 as compared to pH 6, while the activity of the monoglyceride was not influenced by solution pH across this range [28]. This is consistent with the ionizable headgroup of fatty acids while monoglycerides are nonionic molecules.
Another target bacterium that is susceptible to fatty acids is Helicobacter pylori, which is a Gram-negative bacterium that can be pathogenic in the stomach or duodenum leading to diseases such as chronic gastritis, gastric ulcers, and stomach cancer. Petschow et al. tested a wide range of saturated fatty acids and corresponding monoglycerides with chain lengths from 4 to 17 carbons against H. pylori and observed the following trend in bactericidal activities. Monoglycerides with 10–14 carbon long chains showed appreciable bactericidal efficacy at 1 mM concentration and had a lower tendency of spontaneous resistance development, while LA was the only saturated fatty acid that had bactericidal potency under the tested conditions [62]. Similarly, the anti-H. pylori activity of LA and GML was demonstrated by Sun et al., in which case selected unsaturated fatty acids were evaluated along with a range of saturated fatty acids (4–16 carbon chains) and monoglycerides (12–16 carbon chains) [54]. LA completely killed H. pylori at minimum bactericidal concentration (MBC) of 1 mM, and GML showed even more potent activity than LA with a lower MBC value around 0.5 mM. Additionally, it was identified that, among unsaturated fatty acids, myristoleic (C14:1) and linolenic acid have the most potent activity against this bacterium [54]. Bergsson and colleagues also assessed inhibitory activity against Gram-negative bacteria, including H. pylori, for a wide range of fatty acids and corresponding monoglycerides [64]. Interestingly, the tested compounds that had hydrocarbon chains between 10–16 carbons long exhibited inactivation of H. pylori at 10 mM concentration, however, there was no significant activity observed against Salmonella species and Escherichia coli. Bergsson et al. conducted additional studies in order to characterize the antibacterial potency of selected antimicrobial lipids against different bacteria, and it was demonstrated that monocaprin (MG C10:0) is the most potent bactericidal agent against Chlamydia trachomatis and Neisseria gonorrhoeae [29,63]. Additional studies have been reported investigating the effects of fatty acids (with chains of 2 to 18 carbon length) on Gram-negative bacteria, including E. coli [65] and Salmonella species [66], and demonstrated that caprylic acid (C8:0) has particularly high antibacterial activity against these bacteria. Antibacterial activity of caprylic acid and its monoglyceride, monocaprylate, were also identified against fish pathogens including Edwardsiella species by Kollanoor and colleagues [69]. Of particular note, caprylic acid and monocaprylate have showed potent inhibitory activity against important foodborne pathogens, including E. coli O157:H7 [73,74,75,76,77], along with Salmonella species [78,79], L. monocytogenes [80,81], and mastitis pathogens [82].
Of relevance to skin infection, S. aureus and MRSA are a leading cause of skin and soft-tissue infections, including acute bacterial skin and skin structure infections. Within this scope, Kitahara et al. investigated a range of saturated fatty acids against conventional S. aureus, methicillin-susceptible Staphylococcus aureus (MSSA) and MRSA, and it was identified that LA is the most potent saturated fatty acid against MSSA and MRSA [19]. Potent inhibitory activity of GML has also been reported against S. aureus in the following studies. Lin et al. demonstrated that GML killed the bacteria effectively both in vitro and in vivo and reduced toxic shock syndrome [83]. Another in vivo study performed by Preuss et al. confirmed the antibacterial activity of GML against S. aureus infection [84]. In addition, Nakatsuji et al. explored the feasibility of employing LA to treat a mouse model infection caused by another Gram-positive bacterium, specifically Propionibacterium acnes [20]. Hence, multiple antimicrobial lipids have shown in vivo therapeutic potential, and there is also growing interest to investigate combinations of several antimicrobial lipids that have antibacterial potency against specific bacteria in order to achieve synergistic and broad-spectrum effects [85,86,87,88,89].
2.3. Mechanisms of Antibacterial Activity
The mechanism(s) of antibacterial activity of fatty acids and monoglycerides have been explored using diverse experimental methods to identify that they mainly target bacterial cell membranes and interrupt crucial processes involved in cellular protection and function. The membrane-lytic behavior of fatty acids and monoglycerides stem from their amphipathic properties, leading to overlapping sets of biophysical phenomena including membrane destabilization and pore formation. In particular, membrane-destabilizing activity causes increased cell permeability and cell lysis, leading to inhibition of bacterial cell growth (bacteriostatic action) or cell death (bactericidal action).
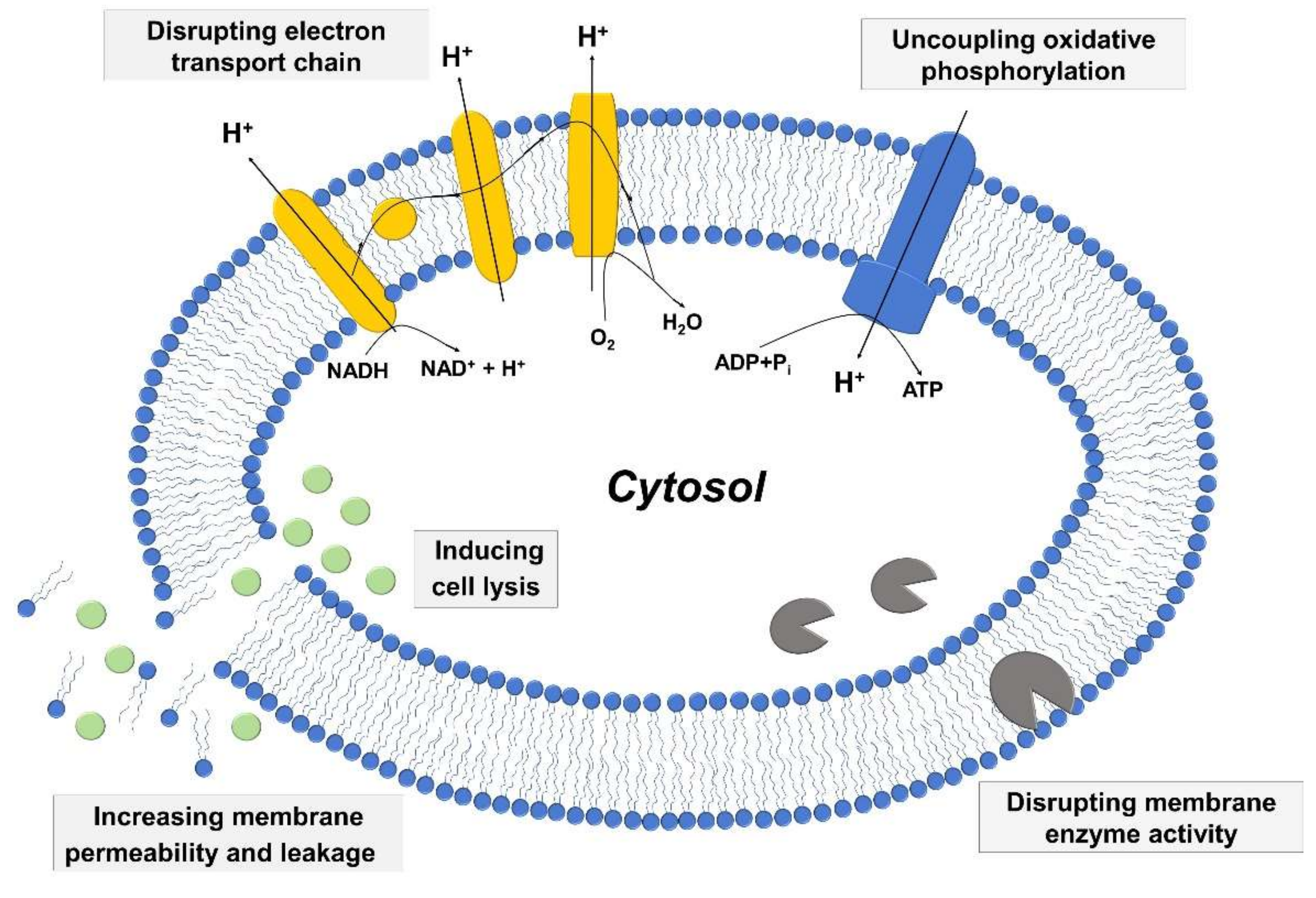
Among vital processes involving bacterial cell membranes, two of the most important ones involve the electron transport chain and oxidative phosphorylation, which are essential for energy production in bacterial cells. The two processes are interconnected, and fatty acids have the potential to disrupt the electron transport chain process by binding to electron carriers or altering membrane integrity as well as interfering with oxidative phosphorylation by decreasing the membrane potential and proton gradient. Moreover, fatty acids can directly inhibit membrane enzymes such as glucosyltransferase, presumably due to similar molecular structures of fatty acids with known small molecule inhibitors [90,91], and also target other membrane-associated proteins as well [92]. Figure 2 presents a schematic illustration describing key antibacterial activities of fatty acids and monoglycerides that relate to targeting bacterial cell membranes. The details of the mechanistic processes can be classified based on the relationship between the following three aspects: (i) increased membrane permeability and cell lysis, (ii) disruption of electron transport chain and uncoupling oxidative phosphorylation, and (iii) inhibition of membrane enzymatic activities and nutrient uptake.
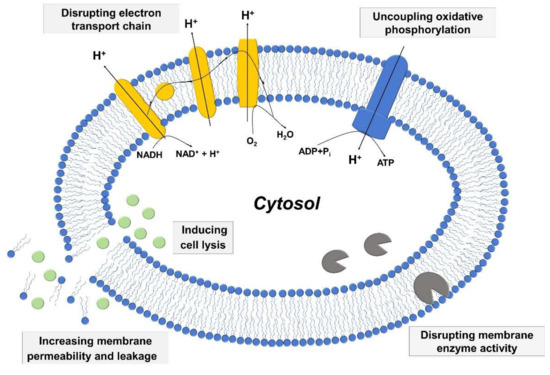
Figure 2.
Schematic representation of mechanisms behind the antibacterial activity of fatty acids and monoglycerides.
2.3.1. Increased Membrane Permeability and Cell Lysis
The interaction of antimicrobial lipids with bacterial cell membranes can destabilize the membrane and increase membrane permeability, thereby inducing leakage of cytosolic contents. In extreme cases, the increased permeability and corresponding membrane destabilization can eventually lead to cell lysis, as documented in numerous experimental studies. For example, Chamberlain et al. observed that when S. aureus cell membranes were treated with oleic acid, there was an increase in membrane permeability as determined by polarized fluorimetry. Oleic acid treatment lowered the polarization value, which indicated increased membrane fluidity and led to cell death [93]. Similarly, Greenway et al. reported that linolenic acid treatment caused the damage of S. aureus cell membranes [61]. In that case, membrane leakage after linolenic acid treatment was detected by measuring the release of biomolecules (e.g., glutamic acid) from bacteria, and absorbance spectroscopy experiments at 260 nm wavelength were conducted to measure the release kinetics, which is correlated with the extent of bacterial cell membrane leakage [94]. As such, the measurements showed that linoleic acid inhibits the growth of S. aureus by inducing a marked increase in membrane permeability—interpreted as pore formation—and this increased permeability further inhibits macromolecular synthesis and coupling of the electron transfer chain [61]. Boyaval et al. also demonstrated that the strong membrane-disruptive activity of linoleic acid causes leakage against Propionibacterium freudenreichii subsp shermanii [95]. In particular, linoleic acid interrupted bacterial cell growth by increasing membrane permeability, as measured by monitoring potassium efflux and transmembrane electrical potential. Increased permeability resulted in an increase in K+ efflux and a decrease in membrane potential. Beyond biochemical readouts, many studies have reported using electron microscopy to investigate the membrane-disruptive activity of fatty acids against various bacterial cell types [26,27,28,29,31,32]. In most of these studies, high concentrations of antimicrobial lipid in the millimolar range were used and hence, the membrane morphological damage was quite extensive and observed post-treatment. In addition, the membrane-disruptive activities of monoglycerides against different bacterial cell types have been reported [28,29]. Regarding direct observation of cell lysis, Carson et al. reported that certain unsaturated fatty acids, namely oleic acid or linoleic acid, can cause lysis of Streptococcus faecalis [96]. Thompson et al. further showed how treatment of H. pylori with linolenic acid induces cell lysis, as revealed by electron microscopy [97].
2.3.2. Disrupting Electron Transport Chain and Uncoupling Oxidative Phosphorylation
The electron transport chain is a key complex that consists of electron carriers and produces the energy source, adenosine triphosphate (ATP), and is coupled to oxidative phosphorylation through the ATP synthase, an enzyme that synthesizes ATP. The electron transport chain and ATP synthase are both located at the inner membrane of Gram-positive and Gram-negative bacteria [98]. Electrons are transported from one carrier to another until reaching the final electron accepter, which is oxygen [99]. The electron transport process is accompanied by proton (H+) transfer from the cytosol to outside of the cell, creating a proton gradient across the cytoplasmic membrane and increasing the membrane potential that provides an energy source to produce ATP via ATP synthase. When one of the steps in the electron transport chain and oxidative phosphorylation is interrupted, it is difficult for bacterial cells to have sufficient energy to function, which leads to inhibition of cell growth and eventually cell death. By measuring oxygen uptake with a Clark type oxygen electrode setup, interruption of the electron transport chain can be assessed. Following this approach, Galbraith et al. demonstrated that LA and myristic acid were the most effective saturated fatty acids to inhibit oxygen intake for Bacillus megaterium and Pseudomonas phaseolicola, while linoleic acid was the most effective unsaturated fatty acid and was active at much lower concentrations (greater potency) than saturated fatty acids [100]. In another related study, Greenway et al. investigated the disruption of the electron transport chain in S. aureus in response to linoleic acid treatment [61]. Moreover, Sheu et al. investigated the effect of fatty acid treatment on Bacillus subtilis by measuring oxygen uptake and ATP concentrations, and demonstrated that the interaction of fatty acids with the bacterial cell membrane decreased membrane integrity, resulting in decreases in oxygen uptake and ATP levels [101]. While it is difficult to directly measure the effect of fatty acid treatment on electron carrier transport in live bacteria, it is noteworthy that Peters et al. investigated electron transport in intact chloroplasts, demonstrating that palmitoleic acid (C16:1 fatty acid) mainly inhibited photosystem (PS) II by up to 90% as part of the electron transport system [102]. Although the latter study was not conduced on bacterial cell membranes, the results still provide useful insight to understand how membrane destabilization caused by antimicrobial lipids can cause severe detriments to electron transport carriers and downstream biochemical processes.
2.3.3. Inhibiting Activity of Bacterial Enzymes
Another significant mechanism by which antimicrobial lipids affect bacterial cell membranes is inhibiting the activity of membrane-associated enzymes, and there have been numerous studies exploring the effects of fatty acid treatment on specific bacterial cell enzymes. For example, Kurihara et al. observed that certain fatty acids inhibit glucan production catalyzed by glucosyltransferase (GTase) from Streptococcus sobrinus, which leads to inhibition of bacterial cell growth. GTase is an important transmembrane protein that mediates glucan production in bacterial cells and is mainly produced by Streptococcus mutans and Strep. sobrinus. Significantly, unsaturated fatty acids such as oleic acid, linoleic acid, and arachidonic acid (C20:4) exhibit even stronger inhibition of GTase activity, while there is an almost negligible effect caused by saturated fatty acids [90]. This finding is significant because it supports that antimicrobial lipids with different physicochemical properties can have unique modes of interacting with bacterial cell membranes. Another study also showed GTase inhibition by oleic acid, supporting that the antibacterial activity of unsaturated fatty acids is related, at least in part, to inhibiting membrane-associated enzymes [91].
Additionally, it has been reported that fatty acids and related derivatives can inhibit bacterial growth by inhibiting fatty acid biosynthesis. Indeed, fatty acids play important roles in bacteria because they are precursors of important cellular materials. Zheng et al. demonstrated that unsaturated fatty acids, including linoleic acid, show antibacterial activity against S. aureus by inhibiting bacterial enoyl-acyl carrier protein reductase (Fabl), which is an important enzyme involved in the fatty acid elongation process [103]. Similarly, the antibacterial activity of medium-chain saturated and unsaturated fatty acids against S. aureus was investigated in terms of inhibiting fatty acid synthesis, and the results indicated that, among the tested compounds, α-linolenic acid was particularly inhibitory [104]. To what extent the effects of antimicrobial lipids on bacterial enzymes are direct or indirect remain to be understood in the broader context of membrane destabilization processes, while it is clear that the effects of antimicrobial lipids on bacterial membranes can inhibit key enzymatic activities. Within this scope, it is particularly intriguing that unsaturated fatty acids affect bacterial enzymes, while saturated fatty acids typically have negligible effect on the same enzymes. Such findings motivate the overall motivation to establish measurement platforms for characterizing the mechanism of action and potency of antimicrobial lipids acting against phospholipid membranes, and to draw correlations with biological activities. In the following section, the main experimental techniques to characterize antimicrobial lipids are presented, including biological and biophysical methods.
3. Experimental Approaches to Characterize Antimicrobial Lipids
The inhibitory activity of antimicrobial lipids has been widely investigated by employing biological approaches based on anti-infective evaluation of bacterial specimens. Antibacterial susceptibility tests such as MIC and minimum bactericidal concentration (MBC) assays are commonly used. The MIC assay is a method of determining the minimum concentration of a test compound that inhibits bacterial growth, thereby enabling rapid screening of the antibacterial susceptibility of antimicrobial lipids against a target bacterium. A more detailed understanding about the mode of action of a test compound can be obtained by determining the MBC value, which is defined as the minimum concentration of a test compound to completely kill a target bacterium. Although both MIC and MBC assays facilitate empirical evaluation of the antibacterial potency of a drug candidate, and inform about the antibacterial spectrum of a antimicrobial lipid, the assays do not probe how antibacterial lipids destabilize bacterial cell membranes. To address such questions, electron microscopy has been widely used to visualize the antibacterial activity of antimicrobial lipids by observing morphological changes of bacterial specimens after treatment with antimicrobial lipids. While useful to look at gross morphological changes, this approach has limitations because it typically requires high lipid concentrations and the bacterial cells are analyzed after treatment and sample fixation. To facilitate real-time monitoring of the membrane interactions involving antimicrobial lipids, one of the most useful approaches has focused on developing model membrane platforms to investigate molecular-level interactions. The different experimental techniques used in biological and biophysical studies are summarized in Table 2. Following this line, details about biological and biophysical approaches are introduced in this section.

Table 2.
Summary of experimental approaches to characterize antimicrobial lipids.
3.1. Anti-Infective Evaluation of Bacterial Specimens
3.1.1. Growth Inhibition Assays
One of the most widely used experimental assays to determine the antibacterial activity of test compounds involves determining the MIC of a compound that is able to inhibit bacterial growth. Formally, the MIC is defined as the lowest concentration of test compound that inhibits observable bacterial growth, and MIC values are often used as quantitative indicators of the relative potency of new antibacterial agents [105]. The agar and broth dilution methods are the most common protocols for determining MIC values [106,107].
Kabara and colleagues conducted pioneering studies to evaluate the MIC values of fatty acids and monoglycerides against a wide range of bacteria by using the broth dilution method, and the recorded MIC values are summarized in Table 3 and Table 4 for fatty acids and monoglycerides, respectively. In addition, Galbraith and Nakatsuji also tested fatty acids against other bacteria of interest. Of note, Kitahara et al. reported an unconventional method to determine MIC values based on measuring oxygen levels using an oxygen electrode sensor named DOX-96 [19]. As mentioned above, saturated fatty acids and monoglycerides with 12-carbon long chains exhibited the most potent activity to inhibit the growth of Gram-positive bacteria, with particularly high antibacterial activity against the methicillin-resistant Staphylococcus aureus (MRSA) strain which causes serious acute skin infections in humans [108]. In general, monoglycerides have lower MIC values than fatty acid equivalents against different bacteria.

Table 3.
Selected MIC values of fatty acids against different Gram-positive bacteria.

Table 4.
Selected MIC values of monoglycerides against different Gram-positive bacteria.
While the trend in MIC results are generally reproducible in terms of evaluating releative potency, reported MIC values are sometimes quite different for the same test compound against the same bacteria, due to variations in experimental conditions. On one hand, multiple reports by Kabara and colleagues showed similar MIC values for the same compound (prepared in roughly equivalent ways in the two studies) against S. aureus, as determined by the broth dilution method [14,15]. On the other hand, the MIC values obtained for the same compound can also often be quite variable depending on the solution condition. For example, using the same measurement method, it has been reported that the MIC value of LA against S. aureus varied around 500–1000 µM and GML around 31–125 µM depending on the preparation method in PBS solution or Mueller-Hinton (MH) Broth [109]. Even greater variations are reported in the literature when considering different experimental methods. Additionally, antimicrobial lipids are particularly sensitive to temperature [110,111] and pH [21,28,31,54,60,65,66,112,113] as well, and the experimental conditions should be designed and modified appropriately depending on the type of test compound.
When it comes to antimicrobial lipids, another key issue is compound solubilization and how it affects the molecular self-assembly of compounds and resulting potency observed in the MIC experiments. Comparing the MIC values obtained for LA (C12:0) against S. aureus in studies by different groups reveals that the potency of a single compound against a single bacterium can vary by over 500-fold. In the two studies, it was noticed that different organic solvents were used for solubilizing LA molecules, and the highest test concentrations contained 1% ethanol or 5% dimethyl sulfoxide (DMSO) in the two studies, respectively. As such, the presence of organic solvents or other environmental factors likely influences the concentration-dependent molecular self-assembly of antimicrobial lipids in bulk solution, and such variations are only reflected in the MIC readout on the basis of how the compounds inhibit bacterial growth. For this reason, MIC assays provide an initial empirical assessment of antibacterial activity while additional methods are needed to further characterize the mechanism of action of a compound.
3.1.2. Infectivity Assays
While MIC readouts assess the capacity of a drug candidate to inhibit bacterial growth, the information obtained does not provide direct information about whether or not treatment with a drug can directly inactivate a bacterium by way of killing, e.g., lytic effect. To address such questions, which are particularly relevant to consider for antimicrobial lipids since they are membrane-lytic agents, other methods have been devised and involve determining the MBC, which is defined as the lowest concentration of antimicrobial agent to kill a target bacterium. Formally, the MBC value is the lowest concentration of a test compound at which ≥99.9% of the initial bacterial inoculum are killed within 24 h [114]. Normally, if the determined MBC value is no more than 4 times greater than the MIC value of a test agent, then the agent is considered to have bactericidal activity. Otherwise, the candidate is considered to have principally bacteriostatic activity.
There are many studies investigating the bactericidal activity of fatty acids and monoglycerides by determining MBC values. Sun et al. reported the MBC values of the C12:0 saturated fatty acid and monoglyceride pair, LA and GML, against H. pylori. The MBC values of LA and GML were 1 mM and 0.5 mM, respectively [54]. Wang et al. tested LA, linolenic acid, and GML and showed that all three compounds exhibit bactericidal effects against L. monocytogenes. LA, linolenic acid, and GML had MBC values of 10, 20, and 10 µg/mL, respectively, at the pH 5 condition. Interestingly, at higher pH conditions around 6, the MBC values of LA and linolenic acid increased to 20 and 100 µg/mL, respectively, while the MBC value of GML remained unchanged at 10 µg/mL and was not influenced by the change in solution pH [28]. In addition, the MBC value of LA against P. acnes was also determined to be 60 µg/mL [20]. Direct comparison of MBC values for LA and GML against S. aureus was also carried out, and it was reported that the MBC values for LA and GML are 50 mM and 0.25 mM, respectively [21]. This finding indicated that GML has about a 200-fold lower MBC value and appreciably greater bactericidal activity against S. aureus. Without directly mentioning the MBC concept, Petschow et al. counted the number of viable bacterial cells by colony-forming unit (CFU) enumeration after treatment with fatty acids or monoglycerides, and determined that LA is the only tested saturated fatty acid that showed bactericidal activity against H. pylori. In particular, treatment of H. pylori with 1 mM LA for one hour yielded a greater than 4 log10 CFU/mL reduction. Among tested monoglycerides, monocaprin and GML also showed a bactericidal effect in similar fashion [62].
Although MBC is an excellent metric to evaluate antibacterial activity and to help understand mechanistically whether or not a test compound completely kills or inhibits the growth of a bacterium, MBC values alone can also be variable depending on the technical format and experimental conditions, in analogous fashion to the challenges facing MIC determinations. Ultimately, both MIC and MBC values provide insight into the scope and potency by which antimicrobial lipids affect the infectivity of bacterial species, and can guide structure-activity relationships at the biological level. However, such assays do not provide information about how antimicrobial lipids destabilize bacterial cell membranes and hence, there is limited room to explore optimization strategies or understand the physicochemical basis underpinning the scope and potency of particular compounds. Hence, there has been more direct experimental methods to observe the interactions between antimicrobial lipids and bacterial cells.
3.1.3. Electron Microscopy
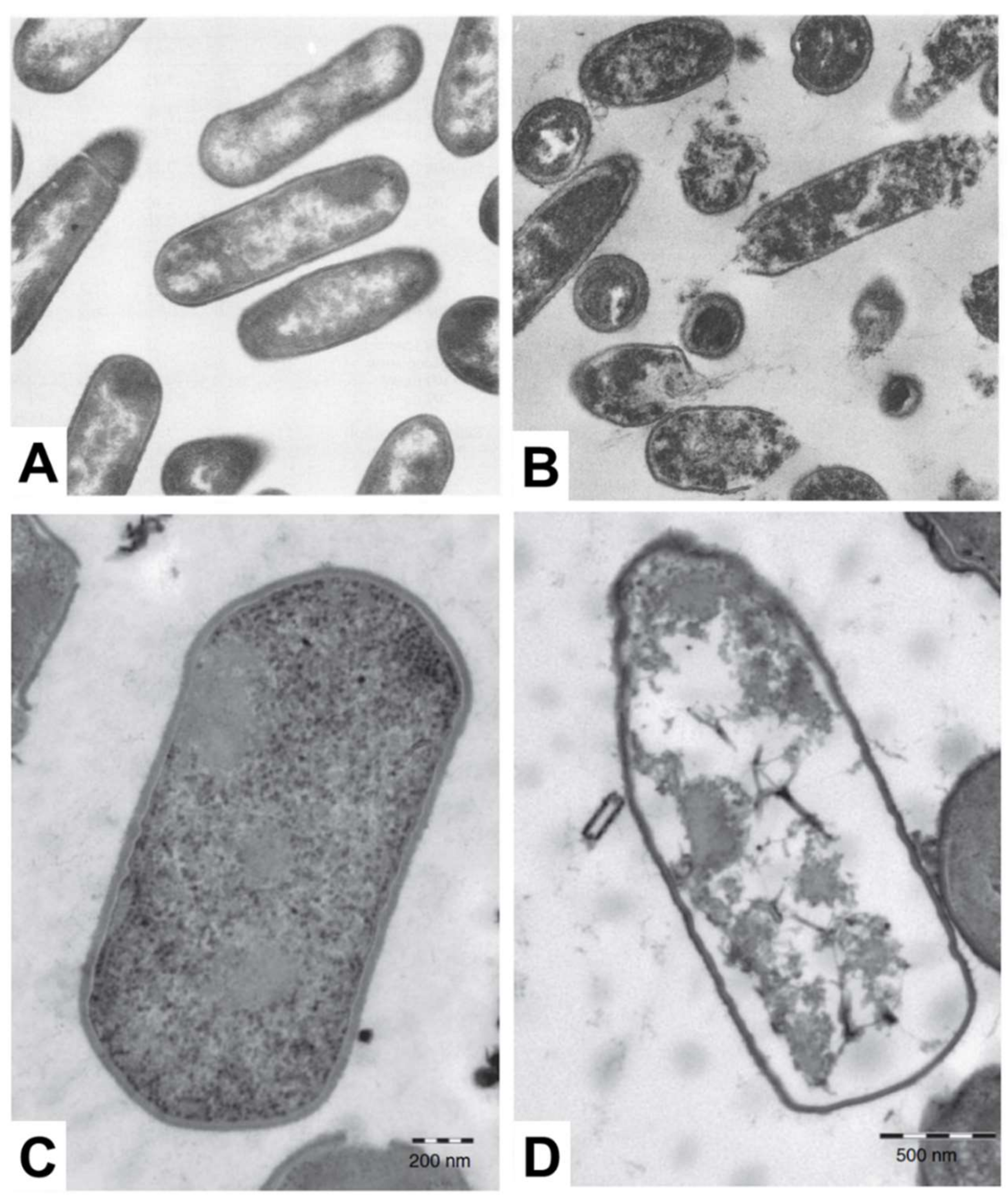
Electron microscopy is a popular measurement technique for investigating the morphological structure of bacterial cell samples and can be utilized to study the effects of antimicrobial lipid treatment [115]. Among the different techniques, scanning electron microscopy (SEM) is useful to characterize the cell surface moprhology, while transmission electron microscopy (TEM) facilitates characterization of surface morphology along with the density of inner cytoplasmic constituents [116,117]. When electron microscopy techniques were first introduced for studying antimicrobial lipids, most related studies utilized TEM for imaging the effects of treating bacteria with fatty acids. A summary of key observations made by electron microscopy analysis is reported in Table 5, including treatment conditions with antimicrobial lipid and corresponding target bacterium. In the late 1970s, Speert et al. explored the detailed mechanism of bactericidal activity underpinning how oleic acid affects Streptococcus group A. Based on TEM imaging measurements, it was observed that cytoplasmic contents become disorganized, including vacuolization in the cytosol and condensation of nucleoids [26]. Following this work, Knapp et al. observed significant membrane disruption of N. gonorrhoeae when the bacterium was treated with 10 µM arachidonic acid, as revealed in TEM experiments [27]. All of the cytoplasmic contents appeared to leak out from morphologically deformed N. gonorrhoeae cells. In contrast, however, similar treatment did not affect the surface morphology of S. aureus cells, while there was still disruption and condensation of cytoplasmic contents in the latter case. There are also some TEM studies that investigate how monoglycerides affect bacterial cells. Based on extensive screening of the antibacterial activity of fatty acids and monoglycerides, Wang et al. identified that linolenic acid and GML are the most potent fatty acids and monoglycerides, respectively, against L. monocytogenes and the antibacterial effects were further investigated by TEM. Interestingly, cell lysis occurred upon treatment with 50 µg/mL GML along with leakage of cytoplasmic contents, as presented in Figure 3A,B. On the other hand, upon treatment with 200 µg/mL linolenic acid, only irregular changes in surface morphology were detected without cell lysis [28]. The antibacterial activity of monocaprin against bacterial cells has also been studied by Bergsson and colleagues. Upon treatment with 10 mM monocaprin, the elementary body form of C. trachomatis became shrunken through morphological deformation [29]. Subsequent studies exploring the potency of monocaprin were carried out on Streptococcus group B, first by using SEM followed by TEM experiments. Significant changes in surface morphology, including size and shape, were not observed by SEM, while TEM further revealed that the bacterial cell membrane and granule structures disappeared after monocaprin treatment, although the cell wall remained intact [30]. Similarly, LA also disrupted and separated cell membranes in Clostridium perfringens leading to cytoplasmic disorganization without causing significant changes in cell wall structure, as presented in Figure 3C,D [31]. Using SEM, Shin et al. showed that after treating S. aureus and Pseudomonas aeruginosa with eicosapentaenoic acid, severe morphological disruption was observed with rough surface features becoming apparent [32].

Table 5.
Electron microscopy studies reporting how antibacterial fatty acids and monoglycerides affect bacterial cells.
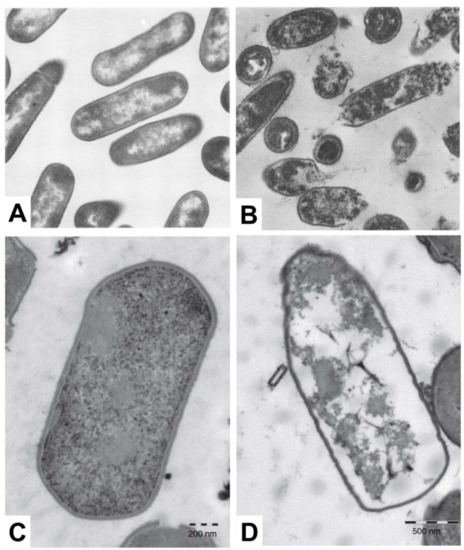
Figure 3.
TEM micrographs show the effect of treating bacterial cells with fatty acids and monoglycerides. L. monocytogenes cells that are (A) untreated or (B) treated with 50 µg/mL GML (magnification ×44,080) and C. perfringens cells that are (C) untreated or (D) treated with 1 mg/mL LA. Reproduced with permission from [28,31].
Taken together, a vast body of knowledge about how antimicrobial lipids affect bacterial cells has been elucidated by electron microscopy techniques. Among the findings, it is evident that there is a spectrum of ways in which antimicrobial lipids can perturb bacterial cells, including disruption of cell membranes and related effects such as loss of cytoplasmic contents. However, at the same time, distilling the empirical insights into general principles describing how antimicrobial lipids affect bacterial cell membranes remains difficult to achieve with electron microscopy results. Aside from the high lipid concentrations that are commonly tested, the low measurement throughput, and the divergent structural and compositional properties of different tested bacterial species, it is only possible to examine the effects of treatment after bacterial specimens have been fixed and therefore it is not possible to monitor changes in morphological properties in real-time. To address such needs, a wealth of complementary biophysical techniques has been developed based on employing model membrane systems, as discussed in the next section.
3.2. Biophysical Approaches with Model Membrane Platforms
3.2.1. Solution-Phase Liposomes
Solution-phase liposome assays have been developed to monitor the interaction kinetics between antimicrobial lipids and phospholipid membranes, by measuring changes in the size distribution of liposomes in bulk solution. One advantage of this approach is that the phospholipid compositions of the liposomes are highly simplified and therefore offer excellent control to understand how specific factors influence resulting interaction processes. The biophysical approach therefore provides a complementary approach to look at how fundamental parameters such as compound concentration affect interaction processes, while it should be noted that the simplified lipid compositions do not fully mimic the more complex membrane structures surrounding bacterial cells. Nevertheless, model membranes provide a useful tool to obtain key insights into compound-specific membrane interaction processes and compound potency. Among the measurement options, dynamic light scattering is utilized as an ensemble-average measurement technique to determine the size and polydispersity of liposomes in bulk solution, including size changes after treatment with antimicrobial lipids. In addition, electron microscopy is employed to visualize how antimicrobial lipids induce morphological changes on individual liposomes.
To date, several studies have been reported that perform complementary electron microscopy and dynamic light scattering experiments and focused on oleic acid/oleate compositions, as presented in Table 6. By using the thin film hydration method, liposomes can be prepared across a range of sizes, which are classified as small unilamellar vesicle (SUVs) up to 100 nm diameter, large unilamellar vesicles (LUV) with diameters between 100 and 400 nm [118], and giant unilamellar vesicles (GUVs) with diameters above 1 µm [119]. The studies were typically related to a “matrix effect” describing the phenomenon that, in the presence of preexisting liposomes, the rate of forming new liposomes—as induced by the fatty acids—is appreciably accelerated, and the size distribution of the newly formed liposomes depended on the size of the preexisting liposomes and/or the molar ratio of fatty acids added to the liposomes [33,120]. The first direct observation of the matrix effect was made when Blöchliger et al. showed that autocatalysis of oleate in aqueous solution was accelerated to form new liposomes in the presence of preexisting oleic acid/oleate liposomes [120]. In addition to the presence of the preexisting liposomes, the size distribution of newly formed, mixed liposomes appeared within a narrow size range and the results showed a similar size distribution to the preexisting liposomes (e.g., 50 or 100 nm diameter), while the newly formed liposomes from oleate itself without the preexisting liposomes had a broad range of sizes ranging from 50 nm to 1.5 µm diameter.

Table 6.
Investigation of fatty acid interactions with solution-phase liposomes.
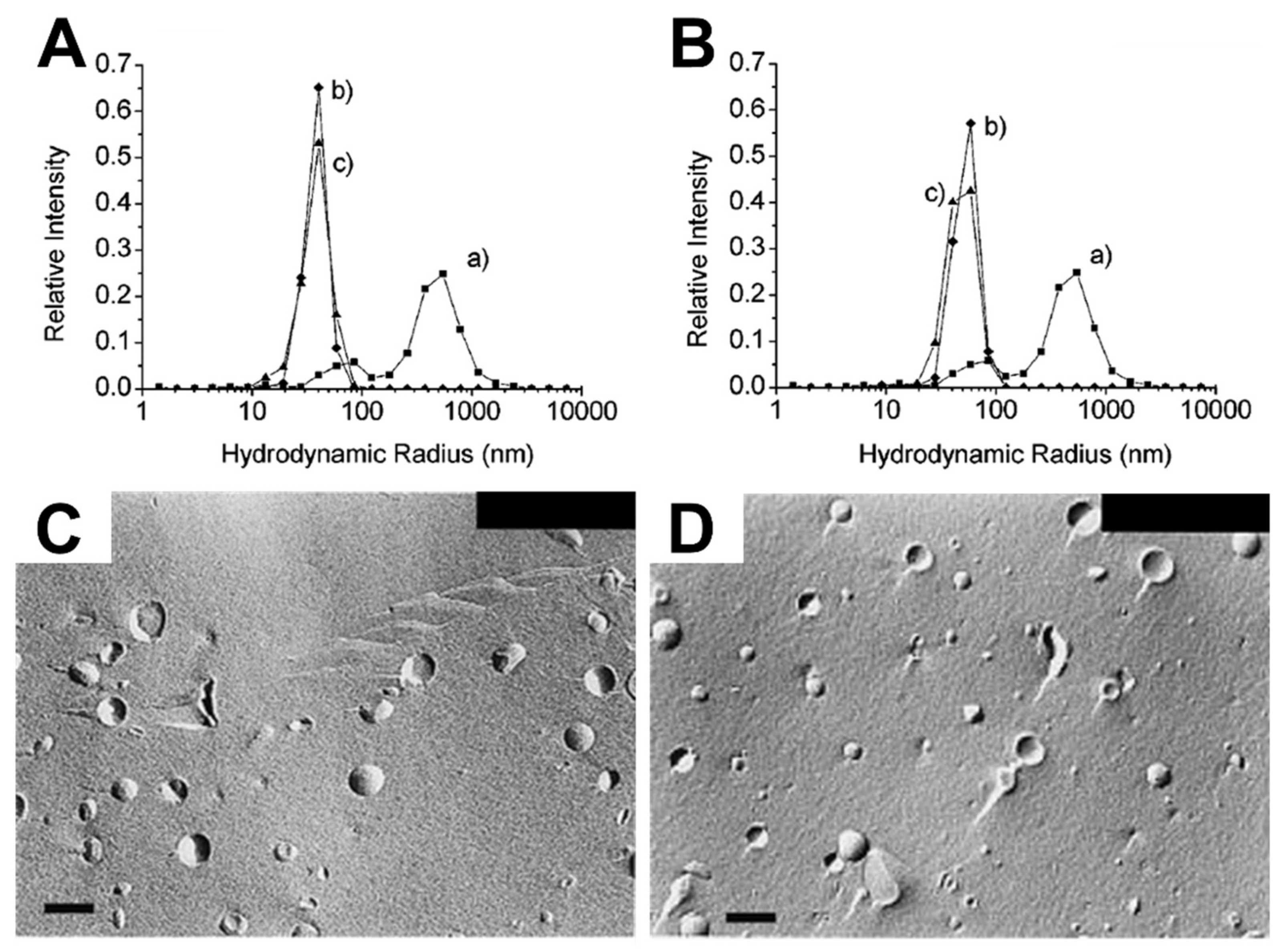
There are also numerous studies reporting the “matrix effect” when using phospholipid liposomes [33,35,36,37,38,39,40]. Lonchin et al. studied how different molar ratios of oleate added to preexisting POPC (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine) liposomes influence the size distribution of newly formed liposomes. Adding a large amount of oleate to POPC liposomes induced the formation of larger mixed POPC/oleate liposomes with increased polydispersity, while similar sizes of mixed liposomes occurred when a lower amount was added [33]. Berclaz et al. provided further evidence showing how the size distribution of newly formed liposomes varies depending on the amount of oleate added to preexisting phospholipid liposomes, as determined by cryogenic TEM (cryo-TEM) analysis [34,35]. Using DLS, Rasi et al. also investigated the formation of newly formed liposomes in response to the matrix effect caused by oleate treatment of POPC liposomes. The size distribution curves before (curve b) and after (curve c) oleate addition to preexisting 50 and 100 nm POPC SUVs were quite similar, as presented in Figure 4A,B [36]. Chungcharoenwattana and colleagues confirmed the matrix effect by employing a particular system, gel filtration chromatography combined with DLS, to measure the detailed size distribution of newly formed phospholipid/oleate liposomes across individual elution fractions [37]. Using freeze-fracture electron microscopy, it was also explored how adding different amounts of oleate to preexisting phospholipid liposomes affects the resulting liposome size distribution, whereby the addition of a small amount caused a narrower size distribution of newly formed mixed liposomes than the addition of a large amount, as shown in Figure 4C,D [38]. At a mechanistic level, it has been suggested that fatty acid interactions cause membrane fission and partial solubilization, giving rise to the matrix effect and related phenomena [38,39]. The effects of adding various fatty acids to preexisting DMPC (1,2-dimyristoyl-sn-glycero-3-phosphocholine) or POPC SUVs have also been determined by turbidity measurements based on UV/Vis spectrophotometry experiments [40]. The fatty acids exhibited rapid incorporation into the preexisting liposomes, followed by the formation of mixed phospholipids/fatty acid liposomes through size growth and subsequent fission. These changes were determined by noticing that the turbidity increased dramatically in the presence of preexisting liposomes as compared to when preexisting liposomes were absent. Hence, solution-phase liposomes provide a useful platform for investigating the types of morphological changes that occur when antimicrobial lipids are added to phospholipid membranes. Typically, the data analysis focuses on antimicrobial lipid:phospholipid ratio and the experimental readouts are largely based on gross morphological changes and changes in size distribution. Hence, the measurements provide indications that membrane interactions are occurring, however, tracking details of specific membrane interaction processes still require additonal biophysical tools.
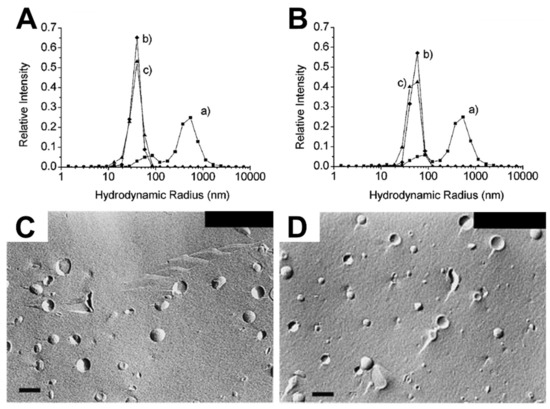
Figure 4.
DLS measurements and complementary freeze-fracture electron micrographs measuring the interaction of fatty acids with SUVs and LUVs. DLS-measured size distribution curves of POPC lipid vesicle extruded with (A) 50 nm, (B) 100 nm diameter pore filters before (curve b) and after (curve c) oleate addition, and freeze-fracture micrographs of (C) preformed 180-nm diameter Egg PC vesicles and (D) Egg PC/oleate (1:1) vesicles. The scale bars are 200 nm. Reproduced with permission from [36] (Copyright 2003, American Chemical Society) and [38].
3.2.2. Giant Unilamellar Vesicle
To directly visualize membrane morphological changes induced by antimicrobial lipids and related compounds, giant unilamellar vesicles (GUVs) in solution-based systems have been utilized to monitor interaction kinetics. Typically, the GUVs are greater than 10 µm diameter and are hence, able to be studied by optical microscopy, including fluorescence-based labeling of phase-sensitive membrane components for visualizing phenomena such as phase separation [41,42,121,122,123,124,125,126,127]. Individual GUVs are directly studied and a wide amount of information about morphological behavior, including fluctuations and membrane fission/fusion, can be monitored in real-time. Pioneering interactions studies showed that after treating GUVs with representative membrane-active, nonionic surfactants, membrane morphological responses could be tracked in real-time. Tamba et al. observed membrane disruption and leakage in response to treatment with Triton X-100 and octylglucoside [125]. Mavčič et al. assessed the influence of another nonionic surfactant, octaethyleneglycol dodecylether (C12E8), on GUVs, and dynamic morphological responses such as the shape transformation from tubular to spherical formations depending on the C12E8 concentration [126].
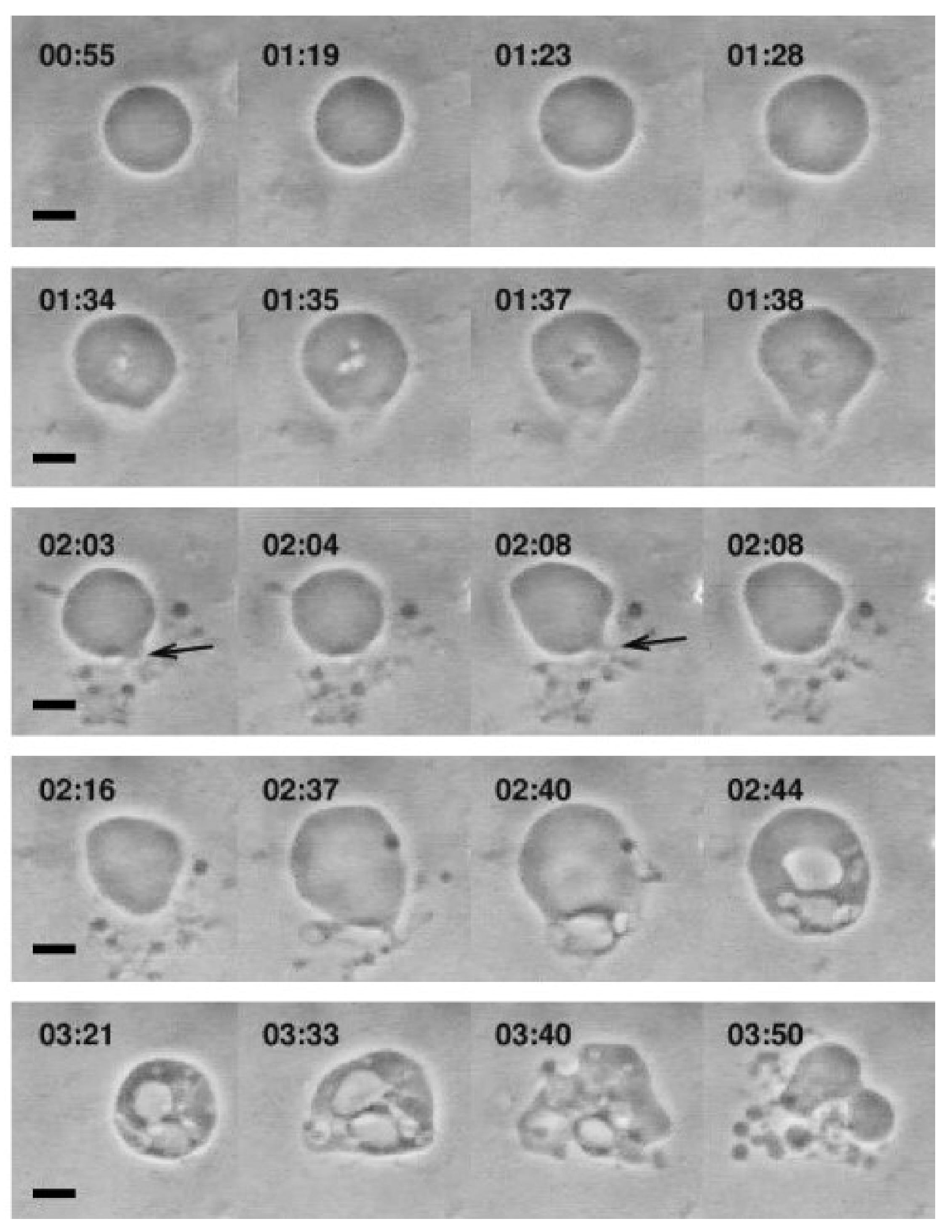
The interaction of various single-chain lipid amphiphiles with GUVs has also been investigated. Inaoka et al. investigated membrane fission in GUVs that occurred upon treatment of lysophosphatidylcholine (LPC) containing 16-carbon long alkyl chains at low concentration, e.g., 2 µM LPC, along with varying the membrane composition and amphiphile concentrations [127]. Tanaka et al. also observed similar membrane fission behavior with interesting shape changes occurring as well [125]. In that study, LPC molecules with different chain lengths ranging from 10 to 16 carbons long were tested against cholesterol-containing GUVs, and the resulting shape changes varied from prolate to asymmetrical spherical shape and membrane fission occurred above a corresponding threshold concentrations for each LPC (which decreased with increasing chain length). Furthermore, Peterlin et al. monitored morphological responses of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) GUVs upon treatment with 0.8 mM oleic acid solution, under which condition mixed oleic acid/oleate liposomes form [41]. Upon treatment, the GUVs started growing followed by various responses such as membrane invaginations, evaginations and budding, and finally creating small budding liposomes that were attached to the mother GUV, as presented in Figure 5. Recently, Mally et al. utilized phase-contrast microscopy to characterize the interactions between oleic acid and GUVs, revealing how fatty acid insertion causes an increase in GUV size followed by bursting [42]. A physical model was developed to explain the results, with particular focus on the role of membrane strain in triggering the burst after reaching a critical threshold. As such, at an observational level, GUV platforms have provided a useful tool to study the interactions between antimicrobial lipids and phospholipid membranes, and developing preliminary mechanistic models to explain the basis for membrane interactions, including concentration-dependence.
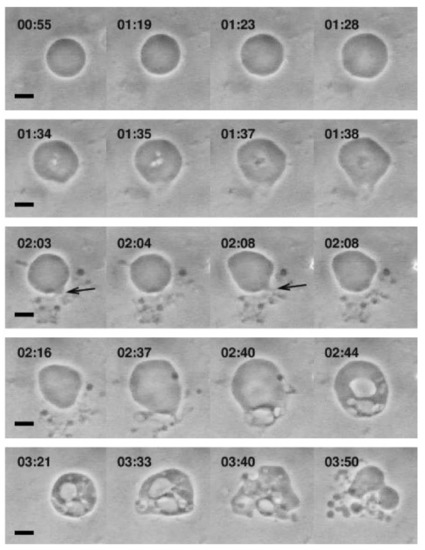
Figure 5.
Optical micrographs showing the morphological responses of POPC GUVs that occur upon treatment with 0.8 mM oleic acid solution. The scale bars are 10 µm. Reproduced with permission from [41].
3.2.3. Supported Lipid Bilayers
Expanding beyond solution-phase studies, the supported lipid bilayer (SLB) platform has emerged as a promising measurement platform to study the mechanism of action of antimicrobial lipids, including fatty acids and monoglycerides, interacting with phospholipid membranes. SLB platforms are composed of two-dimensional phospholipid bilayers that are supported on a hydrophilic support and the bilayer-substrate interaction stabilizes the model membrane [128] while preserving key functional features of membranes in general. One particular advantage of SLB platforms is that they can be studied by a wide range of surface-sensitive measurement techniques, thereby allowing detailed investigation of the interaction kinetics from multiple perspectives, including binding mass, change in viscoelastic properties, and membrane fluidity.
As mentioned above, the insertion of antimicrobial lipids and other single-chain lipid amphiphiles into phospholipid bilayers induces membrane strain, and the bilayers can undergo membrane remodeling in order to response to the applied strain. Staykova et al. observed how osmotic pressure changes can generate membrane strain in SLB platforms and presented a physical model to describe the resulting remodeling processes [129]. Specifically, the SLB platform responds by deforming to form spherical or tubular shapes protruding from the bilayer. With increasing compressive strain, the morphological response shifted from spherical to tubular protrusions, as monitored by confocal microscopy. In addition, Cambrea et al. showed that spherical protrusions can form on fluid-phase SLBs composed of phosphatidylcholine and phosphatidic acid lipids in response to changing ionic strength conditions, as determined by fluorescence microscopy [130]. The presence of phosphatidic acid (PA) molecules in the SLB caused a large negative spontaneous curvature in the bilayer, leading to deformation of the membrane to induce spherical caps when the SLB was exposed to asymmetric osmotic pressure conditions. It was identified that different geometries of the intercalating lipid molecules significantly affected membrane strain. Following this line, Seu et al. characterized how single-chain lipid amphiphiles affect SLB properties by correlating changes in membrane fluidity with corresponding lipid-phospholipid intermolecular interactions on the basis of fluorescence recovery after photobleaching (FRAP) and attenuated total reflection-fourier transform infrared spectroscopy (ATR-FTIR) experiments [131]. Specifically, it was determined that insertion of a single-chain lipid amphiphile, lysophosphatidylcholine (LPC), in the SLB platform increased membrane fluidity by reducing interactions between phospholipid molecules.
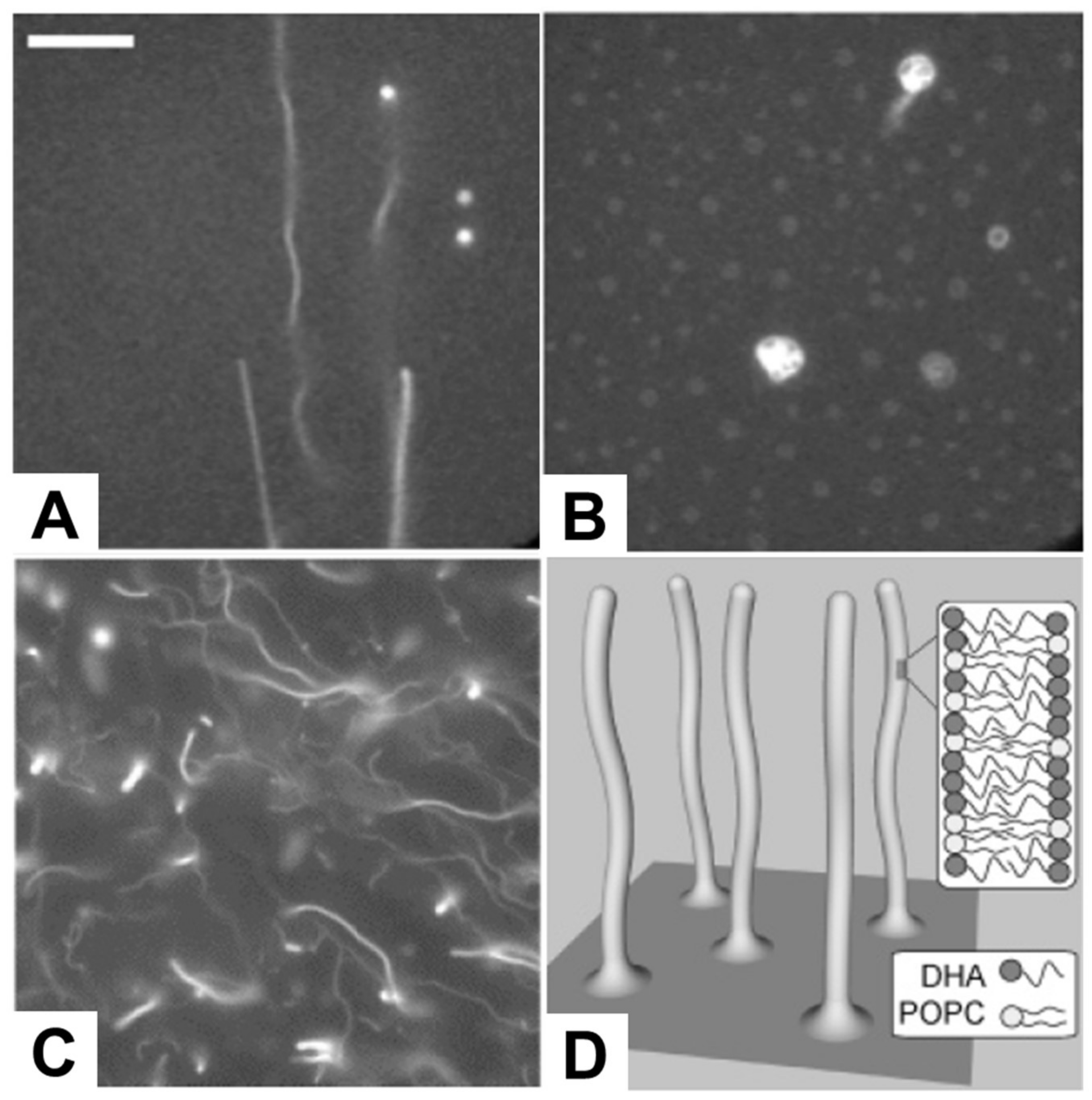
Following this line, several studies have been conducted reporting the direct observation of membrane morphological responses upon treating preformed SLB platforms with antimicrobial lipids, including fatty acids and monoglycerides, as summarized in Table 7. Giger et al. treated SLB platforms composed of dioleoylphosphatidylcholine (DOPC) and dioleoylphosphatidic acid (DOPA) phospholipids with C16 LPC, and monitored resulting membrane responses by time-lapsed fluorescence microscopy and FRAP measurements. As presented in Figure 6A,B, elongated tubule structures were observed at or above 50 µM LPC, and a subsequent decrease in the ionic strength conditions transformed the lipid structures from tubule to spherical caps with complex morphologies [128]. Within the specific context of fatty acids, Thid et al. observed similar tubule formation resulting from adding docosahexaenoic acid (DHA), a polyunsaturated long fatty acid, to POPC SLBs [43]. This study was particularly important because it demonstrated the combined use of the quartz crystal microbalance-dissipation (QCM-D) and fluorescence microscopy techniques to study membrane morphological responses in complementary fashion. This study was particularly significant because it was the first investigation reporting the interaction of a fatty acid molecule with an SLB platform and provided initial evidence suggesting that DHA causes tubule formation above its corresponding CMC value. The fluorescence microscopy results provided direct evidence of tubule formation, as evidenced in Figure 6C,D, while the QCM-D measurements provided corroborating data that DHA treatment increased the viscoelastic properties of the SLB platform. The combination of the two measurement techniques led the authors to conclude that the elongated tubules form in response to membrane strain arising from DHA incorporation. Moreover, Flynn et al. conducted a more detailed QCM-D study investigating how DHA treatment affected SLBs composed of POPC alone, POPC and phosphatidylinositol (PI), or POPC and phosphatidylserine (PS), and monitored the corresponding interaction kinetics [44]. At or above 50 µM DHA, there was a large increase in adsorbed mass and dissipation on POPC SLBs, indicating significantly strong interactions to cause perturbation. However, with the introduction of negatively charged PS or PI molecules to the SLB platform, the DHA interaction became attenuated, likely reflecting some degree of electrostatic repulsion between negatively charged SLBs and anionic DHA molecules.

Table 7.
Interactions of antimicrobial lipids and related single-chain lipid amphiphiles with SLB platforms.
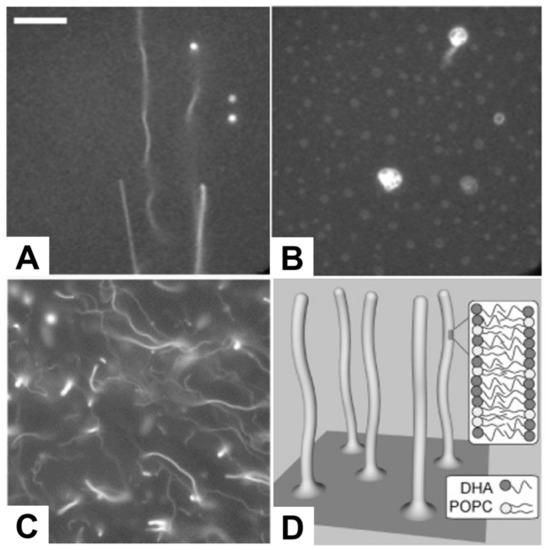
Figure 6.
Fluorescence micrographs depicting the morphological responses of SLBs. The morphological responses occurred after treatment with 50 µM lysophosphatidylcholine (LPC) in (A) 250 mM KCl and (B) 50 mM KCl, and (C) 200 µM docosahexaenoic acid (DHA) and corresponding (D) proposed mechanism of tubule formation. The scale bar in part A is 25 µm, and is valid for images in parts A and B. The image in part C is 80 µm × 80 µm. Reproduced with permission from [128] (Copyright 2008, American Chemical Society) and [43] (Copyright 2007, American Chemical Society).
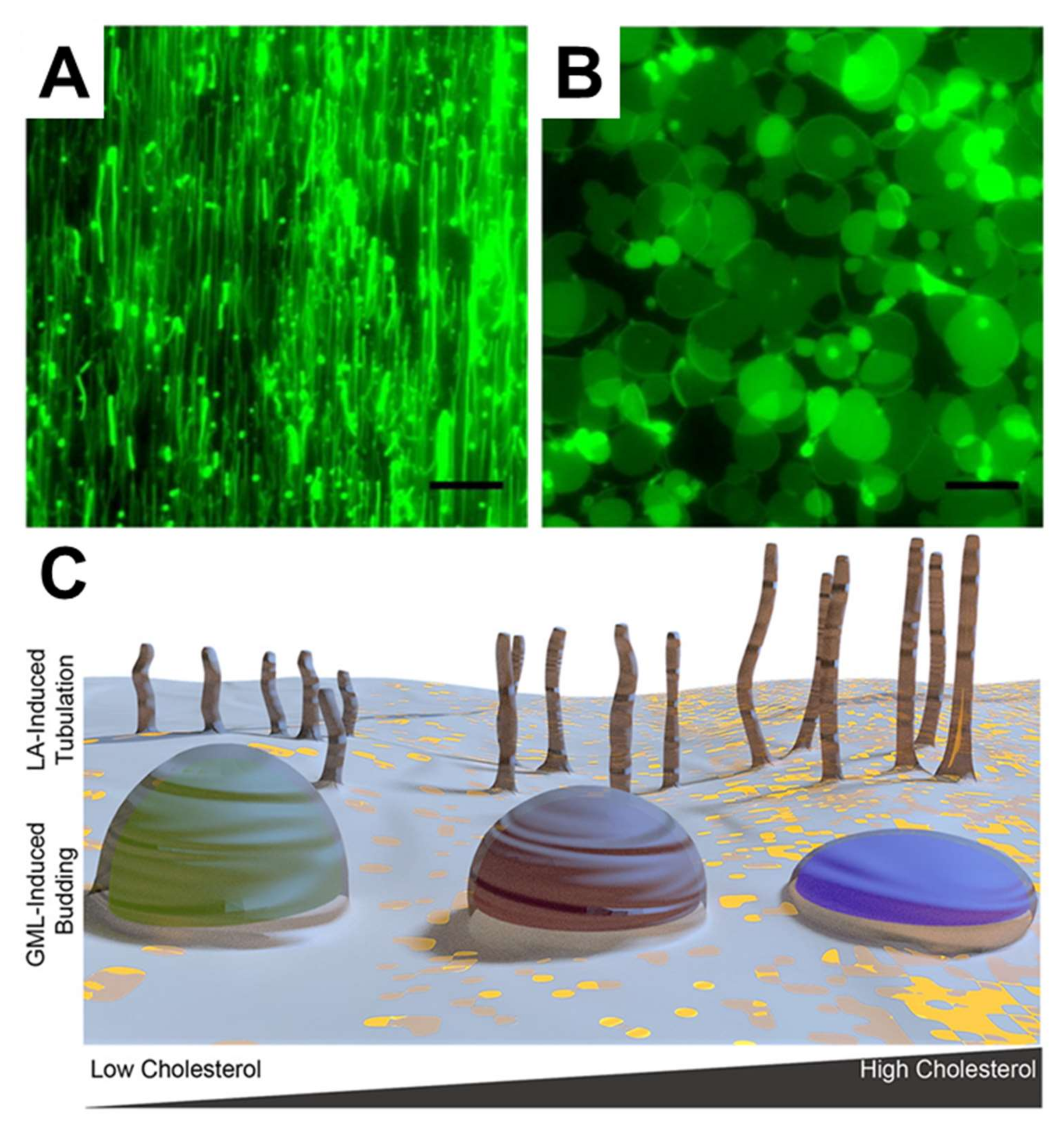
In terms of more potent and well-known antimicrobial lipids, Yoon et al. employed SLB platforms composed of zwitterionic DOPC phospholipids, to investigate how LA and GML induce membrane morphological responses in SLB platforms [109]. By employing QCM-D and fluorescence microscopy measurements, it was discovered that LA and GML both exhibit membrane-disruptive behavior against SLBs principally above their respective CMC values. Importantly, similar concentration-dependent behavior was observed in the biophysical experiments and biological experiments performed in parallel, specifically MIC determinations against S. aureus. However, the two compounds induced strikingly different types of membrane morphological responses, suggesting that antibacterial activity might result from different types of membrane interactions. In particular, LA and GML promoted the formation of elongated tubules and spherical buds formations, respectively, as presented in Figure 7A,B. These findings provide the first evidence that different classes of antimicrobial lipids can interact with phospholipid membranes in distinct ways, and a physicochemical explanation was provided based on how lipid charge (i.e., anionic fatty acids and nonionic monoglycerides) influences membrane translocation rates and corresponding effects on membrane strain. Further investigation of capric acid and monocaprin was conducted using similar experimental strategies as well [132]. Capric acid showed membrane-disruptive activity against SLBs, induced tubule formation, and increased membrane bilayer fluidity only above its CMC value. By contrast, monocaprin was active against SLBs and increase bilayer fluidity both above and below its CMC. Interestingly, monomeric and micellar monocaprin induced different types of membrane morphological response, namely elongated tubules and spherical buds, respectively. Furthermore, Kawakami et al. investigated how the presence of sterols influence membrane morphological responses triggered by antimicrobial lipids [133]. To do so, cholesterol-enriched SLBs were fabricated and it was determined that LA induced tubule formation in all cases, and the extent of membrane remodeling was greater in SLBs with higher cholesterol fractions. In marked contrast, GML addition led to bud formation and the extent of membrane remodeling was lower in SLBs with higher cholesterol fractions, as schematically depicted in Figure 7C. These distinct trends were explained in part by how cholesterol influences the elastic (stiffness) and viscous (stress relaxation) properties of SLB platforms, highlighting the importance of correlating biological activities with detailed biophysical insights.
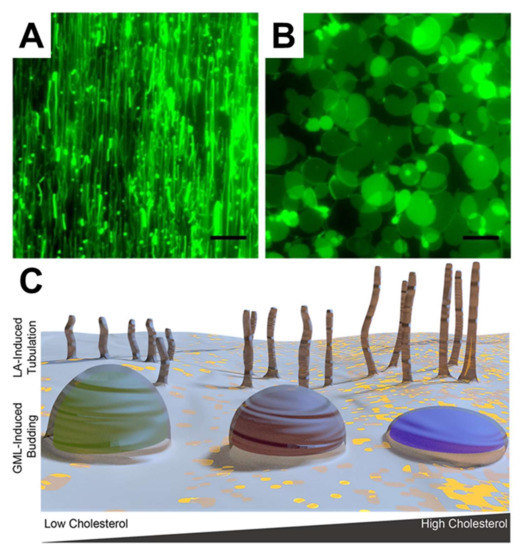
Figure 7.
Membrane morphological responses induced by LA and GML. The morphological responses occurred after treating (A) 2 mM LA and (B) 500 µM GML on DOPC SLBs. The scale bars are 20 μm. (C) schematic representation of how membrane morphological responses are induced on cholesterol-rich SLBs with treatment of LA and GML. Reproduced with permission from [109] (Copyright 2015, American Chemical Society) and [133] (Copyright 2017, American Chemical Society).
While the aforementioned studies involved SLB platforms composed of synthetic membrane compositions, Hyldgaard et al. also investigated how monocaprylate, the monoglyceride derivative of caprylic acid, causes membrane destabilization on SLBs composed of bacterial lipid extracts. QCM-D measurements showed a monotonic increase in bound mass with increasing monocaprylate concentration from 0.05 mM upwards, and appreciable shifts were detected at 5 mM test concentration, however, it was difficult to interpret the origin of the measurement responses due to the complex compositional features of the SLB platform in this case. Using AFM, it was further shown that monocaprylate preferentially interacted with liquid-disordered phase regions of the SLB platform and caused membrane defects, suggesting that the membrane interaction of monocaprylate affected membrane fluidity [45]. The studies summarized in this section support the potential of utilizing SLB platforms to study antimicrobial lipids, especially those causing cell lysis, and further translation of these characterization efforts into antimicrobial therapies is a key direction with enormous potential.
4. Examples of Therapeutic Applications
Based on the experimental capabilities being developed to study antimicrobial lipids, new insights are being obtained into how these molecules might work therapeutically to treat and prevent bacterial infections. From biological activity profiling, there is detailed information about the relative potency of free forms of antimicrobial lipids, while biophysical experiments are guiding us to better understand how structure-function relationships influence antibacterial activity and can be translated into new classes of self-assembled nanomedicines. Such capabilities are being developed for various systemic and topical applications, and the following examples highlight how antimicrobial lipids are poised to offer safe and effective medicines.
4.1. Systemic Treatment of Stomach Infection
Helicobacter pylori is a pathogenic Gram-negative bacterium that colonizes the stomach of over half of the world’s population, and is a leading cause of stomach infections, chronic gastritis, gastric ulcers, and stomach cancer [134,135]. Indeed, H. pylori infection is a serious health problem because it is estimated that ~75% of all stomach cancer cases worldwide are caused by H. pylori infection [136]. As the standard first-line treatment for H. pylori infection involves a combination of three of four compounds in total (typically a proton-pump inhibitor and/or bismuth along with two of three antibiotics), however, there are several key challenges faced, including poor patient compliance, history of past antibiotic usage, drug side effects, uncertain eradication rate, and most importantly the presence of antibiotic-resistant H. pylori strains [137,138,139]. As such, there is strong motivation to identify new antibacterial agents that work against H. pylori, particulary ones that are effective with high potency and have a low risk of drug-resistant strains emerging.
In this regard, fatty acids and monoglycerides are promising agents that have demonstrated antibacterial efficacy against H. pylori and there is a high barrier for drug-resistant strains to emerge. The antibacterial activity of fatty acids and monoglycerides was identified through extensive in vitro screening to detremine the antibacterial spectrum of various fatty acids and derivatives thereof. Petschow et al. demonstrated that LA and monoglycerides containing 10–14 carbon long chains effectively killed H. pylori at 1 mM treatment concentrations, with a low tendency for resistance development [62]. Similarly, in vitro bacterial susceptibility tests showed that saturated fatty acids and corresponding monoglycerides with 10–14 carbon long chains as well as two additional unsaturated fatty acids and monoglycerides—palmitoleic acid and monopalmitolein—showed an appreciable killing effect against H. pylori [64]. Among the findings, monocaprin and GML were the most active against H. pylori. Subsequently, Sun et al. reported in vitro inhibitory activity of fatty acids and monoglycerides against H. pylori as well [54]. According to the study, LA completely killed H. pylori at 1 mM concentration and its monoglyceride derivative, GML, showed similar bactericidal action at lower concentrations around 0.5 mM. Additionally, another unsaturated fatty acid, linolenic acid, also exhibited bactericidal activity at 0.5 mM concentration, in line with the GML potency. Polyunsaturated fatty acids such as docosahexaenoic acid (DHA) also have anti-H.pylori activity and been reported to reduce H.pylori-associated disease pathogenesis in mouse models [140,141,142,143].
To improve delivery of antimicrobial lipids in highly concentated forms (i.e., to avoid dilution effect), liposomal formulations have been developed that encapsulate the active agents within the liposomal bilayer. The development of liposomal formulations containing intercalated fatty acids are first characterized—usually entailing size, zeta potential, and loading characterization—followed by in vitro efficacy and safety studies, and then in vivo testing. Obonyo et al. developed linolenic acid-loaded liposomes (LipoLLA) and identified that LipoLLA effectively killed both spiral and coccoid forms of H. pylori by disrupting the bacterial cell membrane [144]. Compared to free linolenic acid, LipoLLA provided greater killing effect and there was a high barrier to resistance emerging. Jung et al. extended this line of investigation to better understand how LipoLLA interacts with and disrupts the membranes surrounding H. pylori cells [145]. Linolenic acid and 18-carbon long analogues were prepared in liposomal formulations, and, out of the tested species, it was confirmed that LipoLLA had the most potent antibacterial activity against H. pylori and showed complete killing of the bacterium at 200 μg/mL based on causing an increase in bacterial membrane permeability.
Motivated by these in vitro findings, Thamphiwatana et al. further investigated the therapeutic efficacy of LipoLLA to treat H. pylori infection in an in vivo mouse model [146]. After systemic administration of LipoLLA, H. pylori burden in the mouse stomach was measured in comparison to treatment with free linolenic acid or conventional triple-therapy involving antibiotics. Treatment with LipoLLA showed superior activity to reduce bacterial burden and the level of H. pylori-induced proinflammatory cytokines, indicating that LipoLLA is promising therapeutic agent to treat H. pylori infection with high biocompatibility as well. The latter feature was further confirmed recently by Zhong and colleagues by demonstrating that LipoLLA administration induced only minor changes in the gastrointestinal microbiota while conventional triple therapy with three antibiotics caused more dramatic changes in the microbiotica [147].
Following this approach, Seabra et al. reported another promising formulation that involves loading DHA into solid lipid nanoparticles [148]. The nanoparticle enhanced bactericidal activity of DHA against H. pylori by enabling delivery of poorly water-soluble DHA and the DHA-loaded nanoparticles were not cytotoxic against human adenocarcinoma cells. Taken together, the results highlight that antimicrobial lipids can be developed into therapeutically viable formulations that can be used for in vivo applications.
4.2. Topical Treatment of Skin Infection
Acne vulgaris is the most common skin disorder suffered by teenagers and young adults. The responsible bacterium is Propionibacterium acnes, which is a Gram-positive anaerobic rod that thrives inside skin pores, and is an important part of the skin microbiome. Under healthy skin conditions, the skin surface is colonized by beneficial strains of P. acnes. In marked contrast, diseased conditions are linked to a higher prevalence of pathogenic strains of P. acnes, and therapeutic reduction of P. acnes cell counts is associated with improved treatment outcomes.
Towards this goal, free fatty acids are promising antimicrobial candidates because they are naturally found on the skin surface as part of the innate immune system and exogenous addition of antimicrobial lipids can provide a therapeutic effect. While the antibacterial properties of the most potent medium-chain fatty acid, LA, have long been confirmed against a wide range of Gram-positive bacteria, only recently was it demonstrated that LA exhibits inhibitory activity against P. acnes. In particular, the antibacterial efficacy and skin cell cytotoxicity of LA was investigated in direct comparison to benzoyl peroxide (BPO), which is a first-choice medication for treating moderate acne [20]. In vitro experimental results showed that LA has a 15-times lower MIC value against P. acnes as compared to BPO, and the compound also inhibited S. aureus and Staphylococcus epidermidis (S. epidermidis), which are other types of skin bacteria. Among the tested bacteria, P. acnes was the most susceptible bacterium to LA treatment. The demonstrated in vitro efficacy was further encouraged by in vivo safety assessments in mice indicating that intradermal injection or epicutaneous application of therapeutically active concentrations of LA is safe. Ultimately, it was shown that LA treatment via both administration routes led to reduction of P. acnes cell counts on infected mouse ears, which in turn mitigated infection-related ear swelling and inflammation. In addition to LA, the inhibitory activity of capric acid against P. acnes has also been reported [149]. Capric acid was effective in vitro and in vivo, and reduced ear swelling in mice along with mitigating cytokine and chemokine levels, thereby demonstrating a combination of bactericidal and anti-inflammatory properties. However, it was noted that LA still exhibited the best treatment performance in parallel experiments, reinforcing the importance of establishing clear structure-function relationships to guide selection of antimicrobial lipids.
Regarding delivery methods, liposomal formulations have been explored for LA, in part motivated by the need to typical solubilize antimicrobial lipids in organic solvents such as DMSO that can be skin irritants. To this end, Yang et al. reported the characterization and efficacy testing of LA-loaded liposomes (termed “LipoLA”) with size diameters around 120 nm [150]. Due to their amphipathic properties, LA molecules become encapsulated in the liposomal bilayer and it was shown that complete inhibition of P. acnes could be achieved with a 51 μg/mL LA concentration in the LipoLA format. This was an improvement over the 80 μg/mL LA concentration required in free form. To understand the mechanism of antibacterial activity, the authors conducted foster resonance energy transfer (FRET) experiments to study the interaction of LipoLA with bacterial cell membranes and observed fusion and lipid exchange between the two membranes. This was further confirmed by electron microscopy experiments showing that LipoLA treatment causes bacterial cell membrane damage, and in vivo efficacy against P. acnes was demonstrated using intradermal injection of LipoLA and topical application of LipoLA gel in a mouse ear infection model [151]. Therapeutic doses of LipoLA across the two administration routes exhibited negligible toxicity, in comparison to BPO and salicylic acid—two medicines that are routinely used to treat mild to moderate acne.
In an alternative strategy, LA was loaded into nano-sized micelles to evaluate its antibacterial efficacy and loading capacity [152]. Poly(caprolactone) poly (ethylene glycol)-poly(caprolactone) (PCL-PEG-PCL) micelles were developed as one promising option. After LA was loaded into the micelles, the average particle size of the PCL-PEG-PCL micelles decreased from around 50–198 nm diameter to 27–89 nm. The antibacterial potency also increased, and the MBC value of LA against P. acnes was 80 and 40 μg/mL in the free and micellar forms, respectively. Furthermore, it was possible to tune the payload range based on varying the molecular weight of the polymer chains used in the micelles.
Solid lipid nanoparticles (SLNs) are another option for encapsulating antimicrobial lipids, and have gained attention from the cosmetic and dermatological industries. In SLNs, a solid lipid core encapsulates the active compound and release is achieved when appropriately designed SLNs “melt” upon skin contact, leaving behind the lipid mantle. SLNs have attractive features, including simple and scalable manufacturing, high payload, and biodegradability. Recently, LA has been loaded alone and in combination with retinoic acid into SLN carriers [153]. It was possible to achieve high encapsulation efficiency and inhibit growth of P. acnes, S. aureus and S. epidermidis. In the context of antimicrobial lipids, one drawback of the study was that the SLNs were active against P. acnes, both without and with encapsulated LA. Hence, the specific inhibitory effect of LA against P. acnes could not be determined, however, only SLNs loaded with LA were active against S. aureus. The inclusion of stearylamine, a cationic lipophilic amine, within the SLN composition was cited as an additional contributing factor to explain the inhibitory effect. Further testing of SLNs comprising antimicrobial lipids is warranted to develop optimized SLN versions, and it is noteworthy that SLNs in general are emerging as an industrially acceptable vehicle for skin delivery applications.
As evidenced by these selected examples, a wide range of delivery formats have been explored for treating bacterial skin infections with antimicrobial lipids. It can also be seen that most efficacy studies involve free fatty acids and the high potency of monoglycerides highlights the importance of continuing to further explore them as candidates to treat bacterial skin infections. Indeed, in addition to treating acne vulgaris, other classes of skin infections such as those caused by S. aureus might be treated with antimicrobial lipids in free form [154] or advanced formulations [155,156].
5. Conclusions
As presented in this review, there is enormous potential for employing antimicrobial lipids to combat bacterial infections for human health and medicine. Over the past few decades, significant progress has been made towards understanding the relative potency and spectrum of antibacterial activity for different classes of antimicrobial lipids, in turn identifying particularly promising drug candidates through biological investigations. In recent years, these biological investigations have been complemented by biophysical studies aimed at delineating mechanistic properties and correlating membrane interactions with physicochemical parameters such as chain length and headgroup charge. It should be noted that biophysical studies typically involve model systems that are not fully representative of more complex bacterial cell membranes, and hence, extrapolating results to yield biological insights relies on surrogate markers such as profiling molecular-level membrane interactions as an indicator of potential antibacterial activity. Such approaches are particularly useful for studying the molecular basis of interactions involving antimicrobial lipids that cause changes in membrane permeability and induce cell lysis and can predict potency and the potential of synergistic effects. Ultimately, the evolving scope of experimental tools that can be employed to characterize antimicrobial lipids is particularly powerful when integrated into orthogonal measurement strategies that combine biological and biophysical analyses. Figure 8 presents an overview of how we envision that biological and biophysical approaches can be employed synergistically to characterize amphiphilic-based antimicrobial lipids, and to translate this knowledge into biologically relevant therapeutic strategies. Indeed, while conventional drug development focuses on initially screening the biological activity of candidate compounds, we believe that molecular-level characterization can be utilized as a starting point to guide the rational design of therapeutic strategies that take into account molecular design principles.

Figure 8.
Overview of experimental strategy to characterize antimicrobial lipids based on integrating biophysical and biological approaches.
By understanding how antimicrobial lipids function and the critical role of molecular self-assembly, we are beginning to design new strategies to enhance therapeutic performance and there is accelerating progress in this direction. Recognizing the challenges of antibiotic-resistant bacteria and taking advantage of the low cost and abundant supply of antimicrobial lipids, there is excellent opportunity to further explore antimicrobial lipids as next-generation antibacterial agents for human health and medicine.
Acknowledgments
This work was supported by a National Research Foundation Proof-of-Concept grant (NRF2015NRF-POC0001-19) and an A*STAR-NTU-NHG Skin Research Grant (SRG/14028) as well as through the Center for Precision Biology at Nanyang Technological University.
Author Contributions
Bo Kyeong Yoon, Joshua A. Jackman, and Nam-Joon Cho initiated and designed the study. Bo Kyeong Yoon, Joshua A. Jackman, and Elba R. Valle-González collected the literature and prepared the manuscript. Nam-Joon Cho provided critical comments for revisions of the manuscript. All authors read and approved the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Antonietti, M.; Förster, S. Vesicles and liposomes: A self-assembly principle beyond lipids. Adv. Mater. 2003, 15, 1323–1333. [Google Scholar] [CrossRef]
- Ninham, B.W.; Larsson, K.; Nostro, P.L. Two sides of the coin. Part 2. Colloid and surface science meets real biointerfaces. Colloids Surf. B Biointerfaces 2017, 159, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Mukai, M.; Regen, S.L. Lipid raft formation driven by push and pull forces. Bull. Chem. Soc. Japan 2017, 90, 1083–1087. [Google Scholar] [CrossRef]
- Ramanathan, M.; Shrestha, L.K.; Mori, T.; Ji, Q.; Hill, J.P.; Ariga, K. Amphiphile nanoarchitectonics: From basic physical chemistry to advanced applications. Phys. Chem. Chem. Phys. 2013, 15, 10580–10611. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, M.; Yoshimoto, K.; Sisido, M.; Ariga, K. Chemistry can make strict and fuzzy controls for bio-systems: DNA nanoarchitectonics and cell-macromolecular nanoarchitectonics. Bull. Chem. Soc. Japan 2017, 90, 967–1004. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.; Wertheim, H.F.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H. Antibiotic resistance—The need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef]
- Aminov, R.I. A brief history of the antibiotic era: Lessons learned and challenges for the future. Front. Microbiol. 2010, 1, 134. [Google Scholar] [CrossRef] [PubMed]
- Alanis, A.J. Resistance to antibiotics: Are we in the post-antibiotic era? Arch. Med. Res. 2005, 36, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Thormar, H. Lipids and Essential Oils as Antimicrobial Agents; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Chen, Y.E.; Tsao, H. The skin microbiome: Current perspectives and future challenges. J. Am. Acad. Dermatol. 2013, 69, 143.e3–155.e3. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, T.; Gallo, R.L. Antimicrobial peptides: Old molecules with new ideas. J. Investig. Dermatol. 2012, 132, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef] [PubMed]
- Kabara, J.J.; Swieczkowski, D.M.; Conley, A.J.; Truant, J.P. Fatty acids and derivatives as antimicrobial agents. Antimicrob. Agents Chemother. 1972, 2, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Kabara, J.; Vrable, R.; Jie, M.L.K. Antimicrobial lipids: Natural and synthetic fatty acids and monoglycerides. Lipids 1977, 12, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Kabara, J.J. Antimicrobial agents derived from fatty acids. J. Ame. Oil Chem. Soc. 1984, 61, 397–403. [Google Scholar] [CrossRef]
- Kabara, J.J. Structure-function relationships of surfactants as antimicrobial agents. J. Soc. Cosmet. Chem. 1978, 29, 733–741. [Google Scholar]
- Kabara, J.J. GRAS antimicrobial agents for cosmetic products. J. Soc. Cosmet. Chem. 1980, 31, 1–10. [Google Scholar]
- Kitahara, T.; Koyama, N.; Matsuda, J.; Aoyama, Y.; Hirakata, Y.; Kamihira, S.; Kohno, S.; Nakashima, M.; Sasaki, H. Antimicrobial activity of saturated fatty acids and fatty amines against methicillin-resistant Staphylococcus aureus. Biol. Pharm. Bull. 2004, 27, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, T.; Kao, M.C.; Fang, J.-Y.; Zouboulis, C.C.; Zhang, L.; Gallo, R.L.; Huang, C.-M. Antimicrobial property of lauric acid against Propionibacterium acnes: Its therapeutic potential for inflammatory acne vulgaris. J. Investig. Dermatol. 2009, 129, 2480–2488. [Google Scholar] [CrossRef] [PubMed]
- Schlievert, P.M.; Peterson, M.L. Glycerol monolaurate antibacterial activity in broth and biofilm cultures. PLoS ONE 2012, 7, e40350. [Google Scholar] [CrossRef] [PubMed]
- Skřivanová, E.; Molatová, Z.; Marounek, M. Effects of caprylic acid and triacylglycerols of both caprylic and capric acid in rabbits experimentally infected with enteropathogenic Escherichia coli O103. Vet. Microbiol. 2008, 126, 372–376. [Google Scholar] [CrossRef] [PubMed]
- P Desbois, A. Potential applications of antimicrobial fatty acids in medicine, agriculture and other industries. Recent Patents Anti-Infect. Drug Discov. 2012, 7, 111–122. [Google Scholar] [CrossRef]
- Maag, H. Fatty acid derivatives: Important surfactants for household, cosmetic and industrial purposes. J. Am. Oil Chem. Soc. 1984, 61, 259–267. [Google Scholar] [CrossRef]
- Heerklotz, H. Interactions of surfactants with lipid membranes. Quart. Rev. Biophys. 2008, 41, 205–264. [Google Scholar] [CrossRef] [PubMed]
- Speert, D.P.; Wannamaker, L.W.; Gray, E.D.; Clawson, C.C. Bactericidal effect of oleic acid on group A streptococci: Mechanism of action. Infect. Immun. 1979, 26, 1202–1210. [Google Scholar] [PubMed]
- Knapp, H.R.; Melly, M.A. Bactericidal effects of polyunsaturated fatty acids. J. Infect. Dis. 1986, 154, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-L.; Johnson, E.A. Inhibition of Listeria monocytogenes by fatty acids and monoglycerides. Appl. Environ. Microbiol. 1992, 58, 624–629. [Google Scholar] [PubMed]
- Bergsson, G.; Arnfinnsson, J.; Karlsson, S.M.; Steingrímsson, Ó.; Thormar, H. In vitro inactivation of Chlamydia trachomatis by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 1998, 42, 2290–2294. [Google Scholar] [PubMed]
- Bergsson, G.; Arnfinnsson, J.; SteingrÍmsson, Ó.; Thormar, H. Killing of Gram-positive cocci by fatty acids and monoglycerides. APMIS 2001, 109, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Skřivanová, E.; Marounek, M.; Dlouha, G.; Kaňka, J. Susceptibility of Clostridium perfringens to C2–C18 fatty acids. Lett. Appl. Microbiol. 2005, 41, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Bajpai, V.; Kim, H.; Kang, S. Antibacterial activity of eicosapentaenoic acid (EPA) against foodborne and food spoilage microorganisms. LWT-Food Sci. Technol. 2007, 40, 1515–1519. [Google Scholar] [CrossRef]
- Lonchin, S.; Luisi, P.L.; Walde, P.; Robinson, B.H. A matrix effect in mixed phospholipid/fatty acid vesicle formation. J. Phys. Chem. B 1999, 103, 10910–10916. [Google Scholar] [CrossRef]
- Berclaz, N.; Müller, M.; Walde, P.; Luisi, P.L. Growth and transformation of vesicles studied by ferritin labeling and cryotransmission electron microscopy. J. Phys. Chem. B 2001, 105, 1056–1064. [Google Scholar] [CrossRef]
- Berclaz, N.; Blöchliger, E.; Müller, M.; Luisi, P.L. Matrix effect of vesicle formation as investigated by cryotransmission electron microscopy. J. Phys. Chem. B 2001, 105, 1065–1071. [Google Scholar] [CrossRef]
- Rasi, S.; Mavelli, F.; Luisi, P.L. Cooperative micelle binding and matrix effect in oleate vesicle formation. J. Phys. Chem. B 2003, 107, 14068–14076. [Google Scholar] [CrossRef]
- Chungcharoenwattana, S.; Ueno, M. Size control of mixed egg yolk phosphatidylcholine (EggPC)/oleate vesicles. Chem. Pharm. Bull. 2004, 52, 1058–1062. [Google Scholar] [CrossRef] [PubMed]
- Chungcharoenwattana, S.; Kashiwagi, H.; Ueno, M. Effect of preformed egg phosphatidylcholine vesicles on spontaneous vesiculation of oleate micelles. Colloid Polym. Sci. 2005, 283, 1180–1189. [Google Scholar] [CrossRef]
- Chungcharoenwattana, S.; Ueno, M. New vesicle formation upon oleate addition to preformed vesicles. Chem. Pharm. Bull. 2005, 53, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Rogerson, M.L.; Robinson, B.H.; Bucak, S.; Walde, P. Kinetic studies of the interaction of fatty acids with phosphatidylcholine vesicles (liposomes). Colloids Surf. B Biointerfaces 2006, 48, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Peterlin, P.; Arrigler, V.; Kogej, K.; Svetina, S.; Walde, P. Growth and shape transformations of giant phospholipid vesicles upon interaction with an aqueous oleic acid suspension. Chem. Phys. Lipids 2009, 159, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Mally, M.; Peterlin, P.; Svetina, S. Partitioning of oleic acid into phosphatidylcholine membranes is amplified by strain. J. Phys. Chem. B 2013, 117, 12086–12094. [Google Scholar] [CrossRef] [PubMed]
- Thid, D.; Benkoski, J.J.; Svedhem, S.; Kasemo, B.; Gold, J. DHA-induced changes of supported lipid membrane morphology. Langmuir 2007, 23, 5878–5881. [Google Scholar] [CrossRef] [PubMed]
- Flynn, K.R.; Martin, L.L.; Ackland, M.L.; Torriero, A.A. Real-time quartz crystal microbalance monitoring of free docosahexaenoic acid interactions with supported lipid bilayers. Langmuir 2016, 32, 11717–11727. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, M.; Sutherland, D.S.; Sundh, M.; Mygind, T.; Meyer, R.L. Antimicrobial mechanism of monocaprylate. Appl. Environ. Microbiol. 2012, 78, 2957–2965. [Google Scholar] [CrossRef] [PubMed]
- Barratt, M. Quantitative structure-activity relationships (QSARs) for skin corrosivity of organic acids, bases and phenols: Principal components and neural network analysis of extended datasets. Toxicol. In Vitro 1996, 10, 85–94. [Google Scholar] [CrossRef]
- Osborn, H.; Akoh, C. Structured lipids-novel fats with medical, nutraceutical, and food applications. Compr. Rev. Food Sci. Food Saf. 2002, 1, 110–120. [Google Scholar] [CrossRef]
- Bazinet, R.P.; Layé, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014, 15, 771. [Google Scholar] [CrossRef] [PubMed]
- Kodicek, E.; Worden, A. The effect of unsaturated fatty acids on Lactobacillus helveticus and other Gram-positive micro-organisms. Biochem. J. 1945, 39, 78. [Google Scholar] [CrossRef] [PubMed]
- Galbraith, H.; Miller, T.; Paton, A.; Thompson, J. Antibacterial activity of long chain fatty acids and the reversal with calcium, magnesium, ergocalciferol and cholesterol. J. Appl. Microbiol. 1971, 34, 803–813. [Google Scholar] [CrossRef]
- Saito, H.; Tomioka, H.; Yoneyama, T. Growth of group IV mycobacteria on medium containing various saturated and unsaturated fatty acids. Antimicrob. Agents Chemother. 1984, 26, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Feldlaufer, M.; Knox, D.; Lusby, W.; Shimanuki, H. Antimicrobial activity of fatty acids against Bacillus larvae, the causative agent of American foulbrood disease. Apidologie 1993, 24, 95–99. [Google Scholar] [CrossRef]
- Wille, J.; Kydonieus, A. Palmitoleic acid isomer (C16: 1Δ6) in human skin sebum is effective against gram-positive bacteria. Skin Pharmacol. Physiol. 2003, 16, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.Q.; O’Connor, C.J.; Roberton, A.M. Antibacterial actions of fatty acids and monoglycerides against Helicobacter pylori. FEMS Immunol. Med. Microbiol. 2003, 36, 9–17. [Google Scholar] [CrossRef]
- Heczko, P.; Lütticken, R.; Hryniewicz, W.; Neugebauer, M.; Pulverer, G. Susceptibility of Staphylococcus aureus and group A, B, C, and G streptococci to free fatty acids. J. Clin. Microbiol. 1979, 9, 333–335. [Google Scholar] [PubMed]
- Thormar, H.; Hilmarsson, H.; Bergsson, G. Stable concentrated emulsions of the 1-monoglyceride of capric acid (monocaprin) with microbicidal activities against the food-borne bacteria Campylobacter jejuni, Salmonella spp., and Escherichia coli. Appl. Environ. Microbiol. 2006, 72, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Rohrer, L.; Winterhalter, K.; Eckert, J.; Köhler, P. Killing of Giardia lamblia by human milk is mediated by unsaturated fatty acids. Antimicrob. Agents Chemother. 1986, 30, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Thormar, H.; Isaacs, C.E.; Brown, H.R.; Barshatzky, M.R.; Pessolano, T. Inactivation of enveloped viruses and killing of cells by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 1987, 31, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Hilmarsson, H.; Larusson, L.; Thormar, H. Virucidal effect of lipids on visna virus, a lentivirus related to HIV. Arch. Virol. 2006, 151, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Kanetsuna, F. Bactericidal effect of fatty acids on mycobacteria, with particular reference to the suggested mechanism of intracellular killing. Microbiol. Immunol. 1985, 29, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Greenway, D.; Dyke, K. Mechanism of the inhibitory action of linoleic acid on the growth of Staphylococcus aureus. Microbiology 1979, 115, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Petschow, B.W.; Batema, R.P.; Ford, L.L. Susceptibility of Helicobacter pylori to bactericidal properties of medium-chain monoglycerides and free fatty acids. Antimicrob. Agents Chemother. 1996, 40, 302–306. [Google Scholar] [PubMed]
- Bergsson, G.; Steingrímsson, Ó.; Thormar, H. In vitro susceptibilities of Neisseria gonorrhoeae to fatty acids and monoglycerides. Antimicrob. Agents Chemother. 1999, 43, 2790–2792. [Google Scholar] [PubMed]
- Bergsson, G.; Steingrímsson, Ó.; Thormar, H. Bactericidal effects of fatty acids and monoglycerides on Helicobacter pylori. Int. J. Antimicrob. Agents 2002, 20, 258–262. [Google Scholar] [CrossRef]
- Marounek, M.; Skřivanová, E.; Rada, V. Susceptibility of Escherichia coli to C2–C18 fatty acids. Folia Microbiologica 2003, 48, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Skřivanová, E.; Savka, O.; Marounek, M. In vitro effect of C2–C18 fatty acids on salmonellas. Folia microbiologica 2004, 49, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Benkendorff, K.; Davis, A.R.; Rogers, C.N.; Bremner, J.B. Free fatty acids and sterols in the benthic spawn of aquatic molluscs, and their associated antimicrobial properties. J. Exp. Mar. Biol. Ecol. 2005, 316, 29–44. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Kim, Y.-S.; Shin, D.-H. Antimicrobial synergistic effect of linolenic acid and monoglyceride against Bacillus cereus and Staphylococcus aureus. J. Agric. Food Chem. 2002, 50, 2193–2199. [Google Scholar] [CrossRef] [PubMed]
- Kollanoor, A.; Vasudevan, P.; Nair, M.K.M.; Hoagland, T.; Venkitanarayanan, K. Inactivation of bacterial fish pathogens by medium-chain lipid molecules (caprylic acid, monocaprylin and sodium caprylate). Aquac. Res. 2007, 38, 1293–1300. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, H.; Cui, Y.; Zhao, G.; Feng, F. Antibacterial interactions of monolaurin with commonly used antimicrobials and food components. J. Food Sci. 2009, 74. [Google Scholar] [CrossRef] [PubMed]
- Doležalová, M.; Janiš, R.; Svobodová, H.; Kašpárková, V.; Humpolíček, P.; Krejčí, J. Antimicrobial properties of 1-monoacylglycerols prepared from undecanoic (C11:0) and undecenoic (C11:1) acid. Eur. J. Lipid Sci. Technol. 2010, 112, 1106–1114. [Google Scholar] [CrossRef]
- Marounek, M.; Putthana, V.; Benada, O.; Lukešová, D. Antimicrobial activities of medium-chain fatty acids and monoacylglycerols on Cronobacter sakazakii DBM 3157T and Cronobacter malonaticus DBM 3148. Czech J. Food Sci. 2012, 30, 573–580. [Google Scholar] [CrossRef]
- Annamalai, T.; Nair, M.K.M.; Marek, P.; Vasudevan, P.; Schreiber, D.; Knight, R.; Hoagland, T.; Venkitanarayanan, K. In vitro inactivation of Escherichia coli O157: H7 in bovine rumen fluid by caprylic acid. J. Food Prot. 2004, 67, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.K.M.; Abouelezz, H.; Hoagland, T.; Venkitanarayanan, K. Antibacterial effect of monocaprylin on Escherichia coli O157: H7 in apple juice. J. Food Prot. 2005, 68, 1895–1899. [Google Scholar] [CrossRef] [PubMed]
- Amalaradjou, M.A.R.; Annamalai, T.; Marek, P.; Rezamand, P.; Schreiber, D.; Hoagland, T.; Venkitanarayanan, K. Inactivation of Escherichia coli O157: H7 in cattle drinking water by sodium caprylate. J. Food Prot. 2006, 69, 2248–2252. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Rhee, M. Marked synergistic bactericidal effects and mode of action of medium-chain fatty acids in combination with organic acids against Escherichia coli O157: H7. Appl. Environ. Microbiol. 2013, 79, 6552–6560. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Rhee, M. Synergistic antimicrobial activity of caprylic acid in combination with citric acid against both Escherichia coli O157: H7 and indigenous microflora in carrot juice. Food Microbiol. 2015, 49, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, P.; Marek, P.; Nair, M.K.M.; Annamalai, T.; Darre, M.; Khan, M.; Venkitanarayanan, K. In vitro inactivation of Salmonella enteritidis in autoclaved chicken cecal contents by caprylic acid. J. Appl. Poult. Res. 2005, 14, 122–125. [Google Scholar] [CrossRef]
- Chang, S.-s.; Redondo-Solano, M.; Thippareddi, H. Inactivation of Escherichia coli O157: H7 and Salmonella spp. on alfalfa seeds by caprylic acid and monocaprylin. Int. J. Food Microbiol. 2010, 144, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.K.M.; Vasudevan, P.; Hoagland, T.; Venkitanarayanan, K. Inactivation of Escherichia coli O157: H7 and Listeria monocytogenes in milk by caprylic acid and monocaprylin. Food Microbiol. 2004, 21, 611–616. [Google Scholar] [CrossRef]
- Garcia, M.; Amalaradjou, M.A.R.; Nair, M.K.M.; Annamalai, T.; Surendranath, S.; Lee, S.; Hoagland, T.; Dzurec, D.; Faustman, C.; Venkitanarayanan, K. Inactivation of Listeria monocytogenes on frankfurters by monocaprylin alone or in combination with acetic acid. J. Food Prot. 2007, 70, 1594–1599. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.; Joy, J.; Vasudevan, P.; Hinckley, L.; Hoagland, T.; Venkitanarayanan, K. Antibacterial effect of caprylic acid and monocaprylin on major bacterial mastitis pathogens. J. Dairy Sci. 2005, 88, 3488–3495. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Schlievert, P.M.; Anderson, M.J.; Fair, C.L.; Schaefers, M.M.; Muthyala, R.; Peterson, M.L. Glycerol monolaurate and dodecylglycerol effects on Staphylococcus aureus and toxic shock syndrome toxin-1 in vitro and in vivo. PLoS ONE 2009, 4, e7499. [Google Scholar] [CrossRef] [PubMed]
- Preuss, H.G.; Echard, B.; Dadgar, A.; Talpur, N.; Manohar, V.; Enig, M.; Bagchi, D.; Ingram, C. Effects of essential oils and monolaurin on Staphylococcus aureus: In vitro and in vivo studies. Toxicol. Mech. Methods 2005, 15, 279–285. [Google Scholar] [CrossRef] [PubMed]
- McLay, J.; Kennedy, M.; O’Rourke, A.-L.; Elliot, R.; Simmonds, R. Inhibition of bacterial foodborne pathogens by the lactoperoxidase system in combination with monolaurin. Int. J. Food Microbiol. 2002, 73, 1–9. [Google Scholar] [CrossRef]
- Choi, M.; Kim, S.; Lee, N.; Rhee, M. New decontamination method based on caprylic acid in combination with citric acid or vanillin for eliminating Cronobacter sakazakii and Salmonella enterica serovar Typhimurium in reconstituted infant formula. Int. J. Food Microbiol. 2013, 166, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Rhee, M. Highly enhanced bactericidal effects of medium chain fatty acids (caprylic, capric, and lauric acid) combined with edible plant essential oils (carvacrol, eugenol, β-resorcylic acid, trans-cinnamaldehyde, thymol, and vanillin) against Escherichia coli O157: H7. Food Control 2016, 60, 447–454. [Google Scholar]
- Hovorková, P.; Laloučková, K.; Skřivanová, E. Determination of in vitro antibacterial activity of plant oils containing medium-chain fatty acids against gram-positive pathogenic and gut commensal bacteria. Czech J. Anim. Sci. 2018, 63, 119–125. [Google Scholar] [CrossRef]
- Anacarso, I.; Quartieri, A.; De Leo, R.; Pulvirenti, A. Evaluation of the antimicrobial activity of a blend of monoglycerides against Escherichia coli and Enterococci with multiple drug resistance. Arch. Microbiol. 2018, 200, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, H.; Goto, Y.; Aida, M.; Hosokawa, M.; Takahashi, K. Antibacterial activity against cariogenic bacteria and inhibition of insoluble glucan production by free fatty acids obtained from dried Gloiopeltis furcata. Fish. Sci. 1999, 65, 129–132. [Google Scholar] [CrossRef]
- Won, S.-R.; Hong, M.-J.; Kim, Y.-M.; Li, C.Y.; Kim, J.-W.; Rhee, H.-I. Oleic acid: An efficient inhibitor of glucosyltransferase. FEBS Lett. 2007, 581, 4999–5002. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Stevens, M.J.; Neuenschwander, S.; Schwarm, A.; Kreuzer, M.; Bratus-Neuenschwander, A.; Zeitz, J.O. The transcriptome response of the ruminal methanogen Methanobrevibacter ruminantium strain M1 to the inhibitor lauric acid. BMC Res. Notes 2018, 11, 135. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, N.R.; Mehrtens, B.; Xiong, Z.; Kapral, F.; Boardman, J.; Rearick, J. Correlation of carotenoid production, decreased membrane fluidity, and resistance to oleic acid killing in Staphylococcus aureus 18Z. Infect. Immun. 1991, 59, 4332–4337. [Google Scholar] [PubMed]
- Salton, M. The adsorption of cetyltrimethylammonium bromide by bacteria, its action in releasing cellular constituents and its bactericidal effects. Microbiology 1951, 5, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Boyaval, P.; Corre, C.; Dupuis, C.; Roussel, E. Effects of free fatty acids on propionic acid bacteria. Le Lait 1995, 75, 17–29. [Google Scholar] [CrossRef]
- Carson, D.D.; Daneo-Moore, L. Effects of fatty acids on lysis of Streptococcus faecalis. J. Bacteriol. 1980, 141, 1122–1126. [Google Scholar] [PubMed]
- Thompson, L.; Cockayne, A.; Spiller, R. Inhibitory effect of polyunsaturated fatty acids on the growth of Helicobacter pylori: A possible explanation of the effect of diet on peptic ulceration. Gut 1994, 35, 1557–1561. [Google Scholar] [CrossRef] [PubMed]
- Boyer, P.D. The ATP synthase—A splendid molecular machine. Annu. Rev. Biochem. 1997, 66, 717–749. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 1961, 191, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Galbraith, H.; Miller, T. Effect of long chain fatty acids on bacterial respiration and amino acid uptake. J. Appl. Microbiol. 1973, 36, 659–675. [Google Scholar] [CrossRef]
- Sheu, C.W.; Freese, E. Effects of fatty acids on growth and envelope proteins of Bacillus subtilis. J. Bacteriol. 1972, 111, 516–524. [Google Scholar] [PubMed]
- Peters, J.S.; Chin, C.-K. Inhibition of photosynthetic electron transport by palmitoleic acid is partially correlated to loss of thylakoid membrane proteins. Plant Physiol. Biochem. 2003, 41, 117–124. [Google Scholar] [CrossRef]
- Zheng, C.J.; Yoo, J.-S.; Lee, T.-G.; Cho, H.-Y.; Kim, Y.-H.; Kim, W.-G. Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett. 2005, 579, 5157–5162. [Google Scholar] [CrossRef] [PubMed]
- Sado-Kamdem, S.L.; Vannini, L.; Guerzoni, M.E. Effect of α-linolenic, capric and lauric acid on the fatty acid biosynthesis in Staphylococcus aureus. Int. J. Food Microbiol. 2009, 129, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48 (Suppl. 1), S5–S16. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163. [Google Scholar] [CrossRef] [PubMed]
- Ericsson, H.M.; Sherris, J.C. Antibiotic sensitivity testing. Report of an international collaborative study. Acta Pathologica et Microbiologica Scandinavica 1971, 217 (Suppl. 217), 1+. [Google Scholar]
- Arias, C.A.; Murray, B.E. Antibiotic-resistant bugs in the 21st century—A clinical super-challenge. N. Engl. J. Med. 2009, 360, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.K.; Jackman, J.A.; Kim, M.C.; Cho, N.-J. Spectrum of membrane morphological responses to antibacterial fatty acids and related surfactants. Langmuir 2015, 31, 10223–10232. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, I.; Kato, N. Combined effects on antibacterial activity of fatty acids and their esters against gram-negative bacteria. Pharmacol. Effect Lipids 1978, 1978, 15–24. [Google Scholar]
- Dervichian, D. The surface properties of fatty acids and allied substances. Prog. Chem. Fats Other Lipids 1954, 2, 193–242. [Google Scholar] [CrossRef]
- Galbraith, H.; Miller, T. Physicochemical effects of long chain fatty acids on bacterial cells and their protoplasts. J. Appl. Microbiol. 1973, 36, 647–658. [Google Scholar] [CrossRef]
- Miller, R.D.; Brown, K.E.; Morse, S.A. Inhibitory action of fatty acids on the growth of Neisseria gonorrhoeae. Infect. Immun. 1977, 17, 303–312. [Google Scholar] [PubMed]
- French, G. Bactericidal agents in the treatment of MRSA infections—The potential role of daptomycin. J. Antimicrob. Chemother. 2006, 58, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Freundlich, M.M. Origin of the electron microscope. Science 1963, 142, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Ruska, E. The development of the electron microscope and of electron microscopy. Rev. Mod. Phys. 1987, 59, 627. [Google Scholar] [CrossRef]
- Williams, D.B.; Carter, C.B. The transmission electron microscope. In Transmission Electron Microscopy; Springer: Berlin, Germany, 1996; pp. 3–17. [Google Scholar]
- Gabriel, N.E.; Roberts, M.F. Spontaneous formation of stable unilamellar vesicles. Biochemistry 1984, 23, 4011–4015. [Google Scholar] [CrossRef] [PubMed]
- Angelova, M.; Soléau, S.; Méléard, P.; Faucon, F.; Bothorel, P. Preparation of giant vesicles by external AC electric fields. Kinetics and applications. In Trends in Colloid and Interface Science VI; Springer: Berlin, Germany, 1992; pp. 127–131. [Google Scholar]
- Blöchliger, E.; Blocher, M.; Walde, P.; Luisi, P.L. Matrix effect in the size distribution of fatty acid vesicles. J. Phys. Chem. B 1998, 102, 10383–10390. [Google Scholar] [CrossRef]
- Tamba, Y.; Yamazaki, M. Single giant unilamellar vesicle method reveals effect of antimicrobial peptide magainin 2 on membrane permeability. Biochemistry 2005, 44, 15823–15833. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Masum, S.M.; Tanaka, T.; Yamazaki, M. Shape changes of giant unilamellar vesicles of phosphatidylcholine induced by a de novo designed peptide interacting with their membrane interface. Langmuir 2002, 18, 9638–9641. [Google Scholar] [CrossRef]
- Tanaka, T.; Tamba, Y.; Masum, S.M.; Yamashita, Y.; Yamazaki, M. La3+ and Gd3+ induce shape change of giant unilamellar vesicles of phosphatidylcholine. Biochimica et Biophysica Acta (BBA)-Biomembr. 2002, 1564, 173–182. [Google Scholar] [CrossRef]
- Tamba, Y.; Ohba, S.; Kubota, M.; Yoshioka, H.; Yoshioka, H.; Yamazaki, M. Single GUV method reveals interaction of tea catechin (−)-epigallocatechin gallate with lipid membranes. Biophys. J. 2007, 92, 3178–3194. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Sano, R.; Yamashita, Y.; Yamazaki, M. Shape changes and vesicle fission of giant unilamellar vesicles of liquid-ordered phase membrane induced by lysophosphatidylcholine. Langmuir 2004, 20, 9526–9534. [Google Scholar] [CrossRef] [PubMed]
- Mavčič, B.; Babnik, B.; Iglič, A.; Kandušer, M.; Slivnik, T.; Kralj-Iglič, V. Shape transformation of giant phospholipid vesicles at high concentrations of C12E8. Bioelectrochemistry 2004, 63, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Inaoka, Y.; Yamazaki, M. Vesicle fission of giant unilamellar vesicles of liquid-ordered-phase membranes induced by amphiphiles with a single long hydrocarbon chain. Langmuir 2007, 23, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Giger, K.; Lamberson, E.R.; Hovis, J.S. Formation of complex three-dimensional structures in supported lipid bilayers. Langmuir 2008, 25, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Staykova, M.; Arroyo, M.; Rahimi, M.; Stone, H.A. Confined bilayers passively regulate shape and stress. Phys. Rev. Lett. 2013, 110, 028101. [Google Scholar] [CrossRef] [PubMed]
- Cambrea, L.R.; Hovis, J.S. Formation of three-dimensional structures in supported lipid bilayers. Biophys. J. 2007, 92, 3587–3594. [Google Scholar] [CrossRef] [PubMed]
- Seu, K.J.; Cambrea, L.R.; Everly, R.M.; Hovis, J.S. Influence of lipid chemistry on membrane fluidity: Tail and headgroup interactions. Biophys. J. 2006, 91, 3727–3735. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.K.; Jackman, J.A.; Kim, M.C.; Sut, T.N.; Cho, N.-J. Correlating membrane morphological responses with micellar aggregation behavior of capric acid and monocaprin. Langmuir 2017, 33, 2750–2759. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, L.M.; Yoon, B.K.; Jackman, J.A.; Knoll, W.; Weiss, P.S.; Cho, N.-J. Understanding how sterols regulate membrane remodeling in supported lipid bilayers. Langmuir 2017, 33, 14756–14765. [Google Scholar] [CrossRef] [PubMed]
- Lee, A. Prevention of Helicobacter pylori infection. Scand. J. Gastroenterol. 1996, 31 (Suppl. 215), 11–15. [Google Scholar] [CrossRef]
- Pattison, C.; Combs, M.; Marshall, B. Helicobacter pylori and peptic ulcer disease: Evolution to revolution to resolution. Am. J. Roentgenol. 1997, 168, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Amieva, M.; Peek, R.M. Pathobiology of Helicobacter pylori–induced gastric cancer. Gastroenterology 2016, 150, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Molina-Infante, J.; Lucendo, A.; Angueira, T.; Rodriguez-Tellez, M.; Perez-Aisa, A.; Balboa, A.; Barrio, J.; Martin-Noguerol, E.; Gomez-Rodriguez, B.; Botargues-Bote, J. Optimised empiric triple and concomitant therapy for Helicobacter pylori eradication in clinical practice: The OPTRICON study. Aliment. Pharmacol. Therap. 2015, 41, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Fallone, C.A.; Chiba, N.; van Zanten, S.V.; Fischbach, L.; Gisbert, J.P.; Hunt, R.H.; Jones, N.L.; Render, C.; Leontiadis, G.I.; Moayyedi, P. The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology 2016, 151, 51.e14–69.e14. [Google Scholar] [CrossRef] [PubMed]
- Chey, W.D.; Leontiadis, G.I.; Howden, C.W.; Moss, S.F. ACG clinical guideline: Treatment of Helicobacter pylori infection. Am. J. Gastroenterol. 2017, 112, 212. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.; Michel, V.; Matos, A.A.; Carvalho, P.; Oliveira, M.J.; Ferreira, R.M.; Dillies, M.-A.; Huerre, M.; Seruca, R.; Figueiredo, C. Docosahexaenoic acid inhibits Helicobacter pylori growth in vitro and mice gastric mucosa colonization. PLoS ONE 2012, 7, e35072. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.; Michel, V.; Osório, H.; El Ghachi, M.; Bonis, M.; Boneca, I.G.; De Reuse, H.; Matos, A.A.; Lenormand, P.; Seruca, R. Crosstalk between Helicobacter pylori and gastric epithelial cells is impaired by docosahexaenoic acid. PLoS ONE 2013, 8, e60657. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Lim, J.W.; Kim, J.M.; Kim, H. Anti-inflammatory mechanism of polyunsaturated fatty acids in Helicobacter pylori-infected gastric epithelial cells. Mediat. Inflamm. 2014, 2014, 128919. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.W.; Lee, S.W. The antibacterial effect of fatty acids on Helicobacter pylori infection. Korean J. Intern. Med. 2016, 31, 30. [Google Scholar] [CrossRef] [PubMed]
- Obonyo, M.; Zhang, L.; Thamphiwatana, S.; Pornpattananangkul, D.; Fu, V.; Zhang, L. Antibacterial activities of liposomal linolenic acids against antibiotic-resistant Helicobacter pylori. Mol. Pharm. 2012, 9, 2677–2685. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.W.; Thamphiwatana, S.; Zhang, L.; Obonyo, M. Mechanism of antibacterial activity of liposomal linolenic acid against Helicobacter pylori. PLoS ONE 2015, 10, e0116519. [Google Scholar] [CrossRef] [PubMed]
- Thamphiwatana, S.; Gao, W.; Obonyo, M.; Zhang, L. In vivo treatment of Helicobacter pylori infection with liposomal linolenic acid reduces colonization and ameliorates inflammation. Proc. Natl. Acad. Sci. USA 2014, 111, 17600–17605. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-X.; Shi, S.; Rong, L.; Feng, M.-Q.; Zhong, L. The impact of liposomal linolenic acid on gastrointestinal microbiota in mice. Int. J. Nanomed. 2018, 13, 1399. [Google Scholar] [CrossRef] [PubMed]
- Seabra, C.L.; Nunes, C.; Gomez-Lazaro, M.; Correia, M.; Machado, J.C.; Gonçalves, I.C.; Reis, C.A.; Reis, S.; Martins, M.C.L. Docosahexaenoic acid loaded lipid nanoparticles with bactericidal activity against Helicobacter pylori. Int. J. Pharm. 2017, 519, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Tsai, T.-H.; Chuang, L.-T.; Li, Y.-Y.; Zouboulis, C.C.; Tsai, P.-J. Anti-bacterial and anti-inflammatory properties of capric acid against Propionibacterium acnes: A comparative study with lauric acid. J. Dermatol. Sci. 2014, 73, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Pornpattananangkul, D.; Nakatsuji, T.; Chan, M.; Carson, D.; Huang, C.-M.; Zhang, L. The antimicrobial activity of liposomal lauric acids against Propionibacterium acnes. Biomaterials 2009, 30, 6035–6040. [Google Scholar] [CrossRef] [PubMed]
- Pornpattananangkul, D.; Fu, V.; Thamphiwatana, S.; Zhang, L.; Chen, M.; Vecchio, J.; Gao, W.; Huang, C.M.; Zhang, L. In vivo treatment of Propionibacterium acnes infection with liposomal lauric acids. Adv. Healthc. Mater. 2013, 2, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.-Q.-M.; Hsieh, M.-F.; Chang, K.-L.; Pho, Q.-H.; Nguyen, V.-C.; Cheng, C.-Y.; Huang, C.-M. Bactericidal effect of lauric acid-loaded PCL-PEG-PCL nano-sized micelles on skin commensal Propionibacterium acnes. Polymers 2016, 8, 321. [Google Scholar] [CrossRef]
- Silva, E.L.; de Carvalho, M.; Santos, S.; Ferreira, L. Solid lipid nanoparticles loaded with retinoic acid and lauric acid as an alternative for topical treatment of acne vulgaris. J. Nanosci. Nanotechnol. 2014, 14, 1–8. [Google Scholar] [CrossRef]
- Chen, C.-H.; Wang, Y.; Nakatsuji, T.; Liu, Y.-T.; Zouboulis, C.; Gallo, R.L.; Zhang, L.; Hsieh, M.-F.; Huang, C.-M. An innate bactericidal oleic acid effective against skin infection of methicillin-resistant Staphylococcus aureus: A therapy concordant with evolutionary medicine. J. Microbiol. Biotechnol. 2011, 21, 391–399. [Google Scholar] [PubMed]
- Zhang, H.; Cui, Y.; Zhu, S.; Feng, F.; Zheng, X. Characterization and antimicrobial activity of a pharmaceutical microemulsion. Int. J. Pharm. 2010, 395, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Jackman, J.A.; Yoon, B.K.; Li, D.; Cho, N.J. Nanotechnology formulations for antibacterial free fatty acids and monoglycerides. Molecules 2016, 21, 305. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).