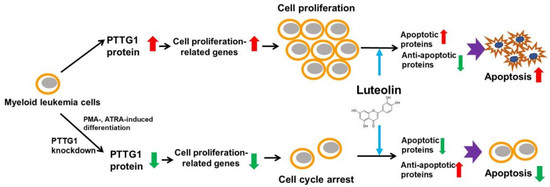
Response of Myeloid Leukemia Cells to Luteolin is Modulated by Differentially Expressed Pituitary Tumor-Transforming Gene 1 (PTTG1) Oncoprotein
Abstract
1. Introduction
2. Results
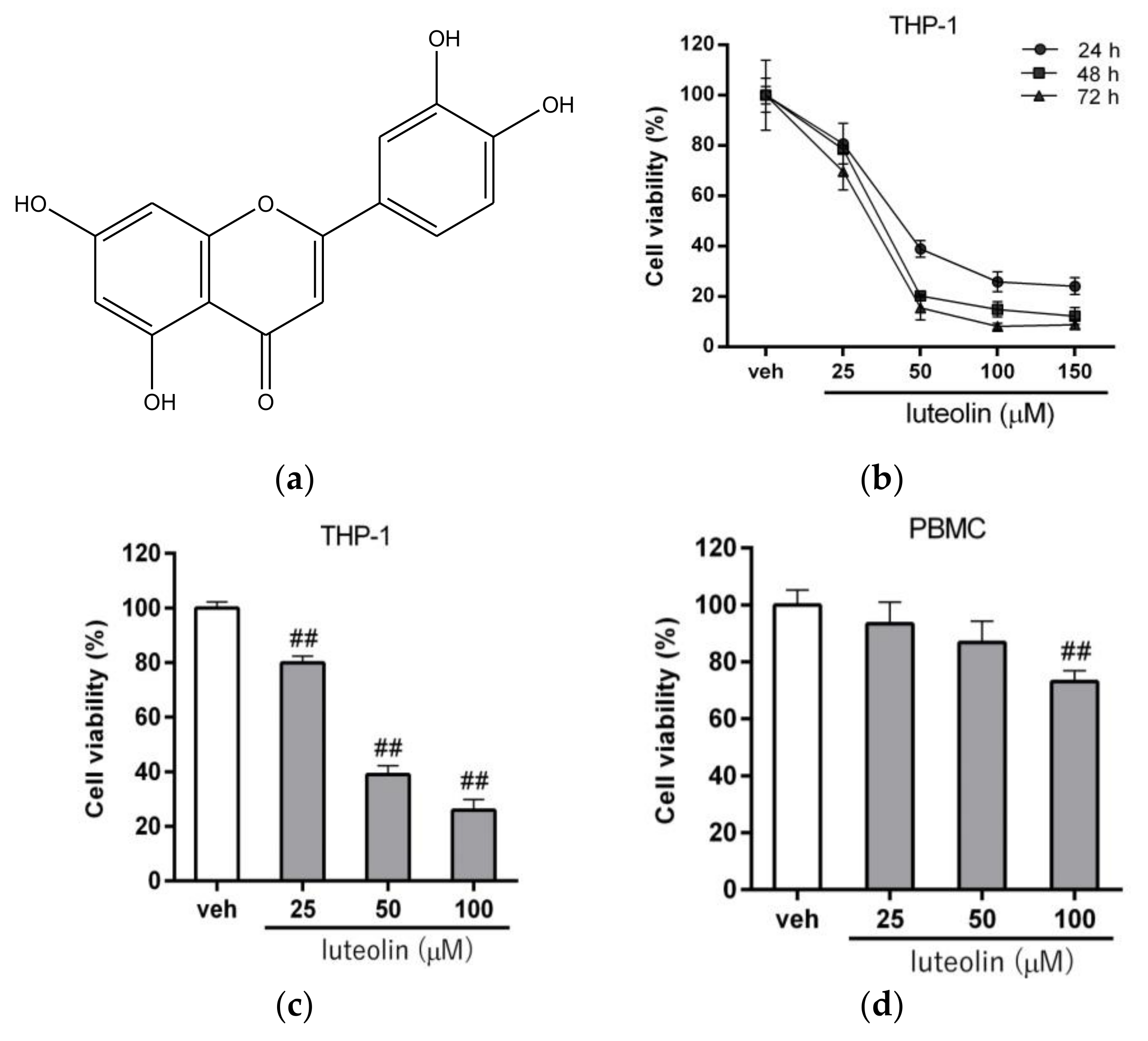
2.1. Luteolin Reduced the Viability of Human Myeloid Leukemia Cells
2.2. Effects of Luteolin on the Viability of Undifferentiated and Differentiated Leukemia Cells with Differential Pituitary Tumor-Transforming Gene 1 (PTTG1) Expression
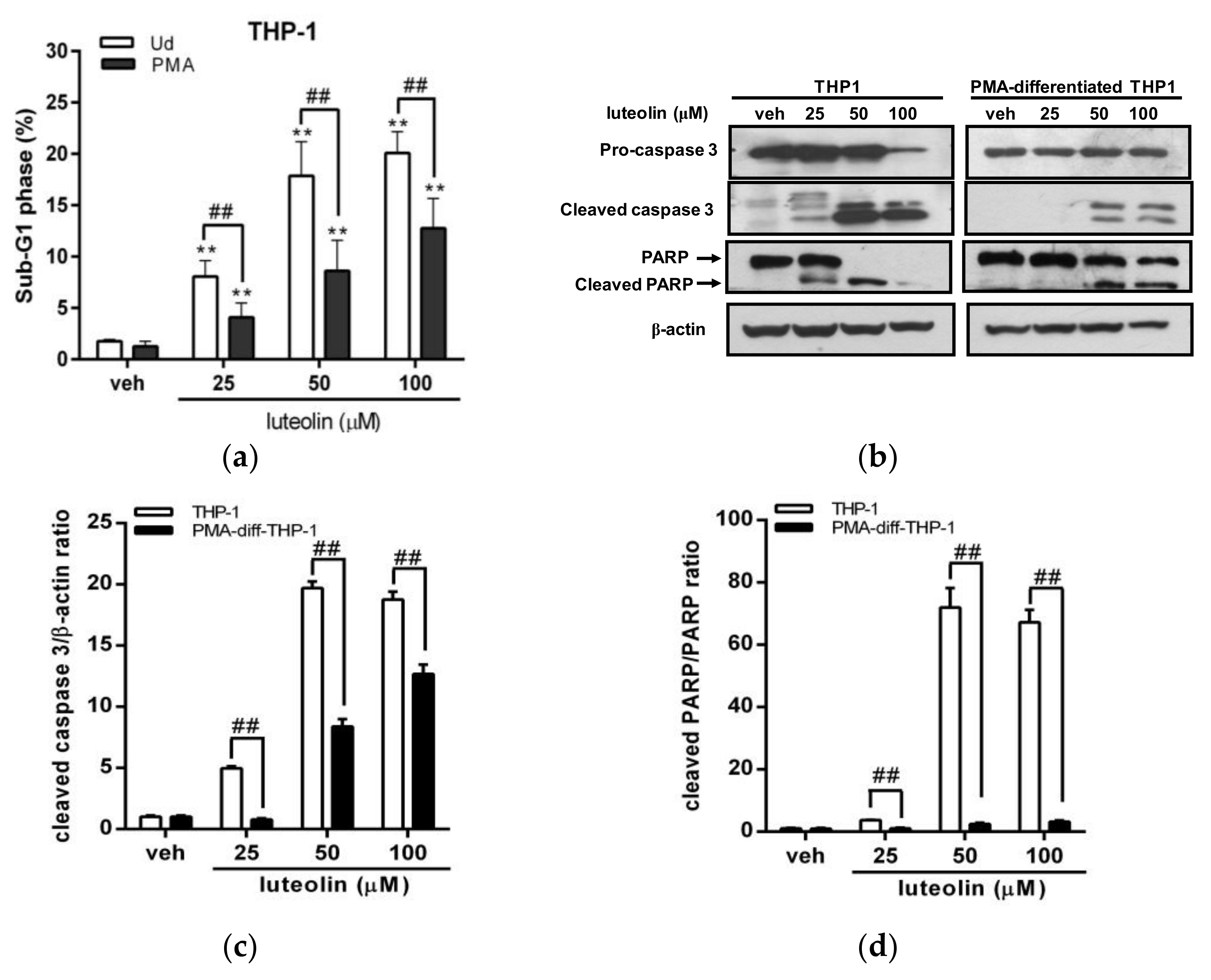
2.3. Luteolin Induced More Significant Cell Apoptosis in Undifferentiated Myeloid Leukemia Cells with Abundant PTTG1 Protein
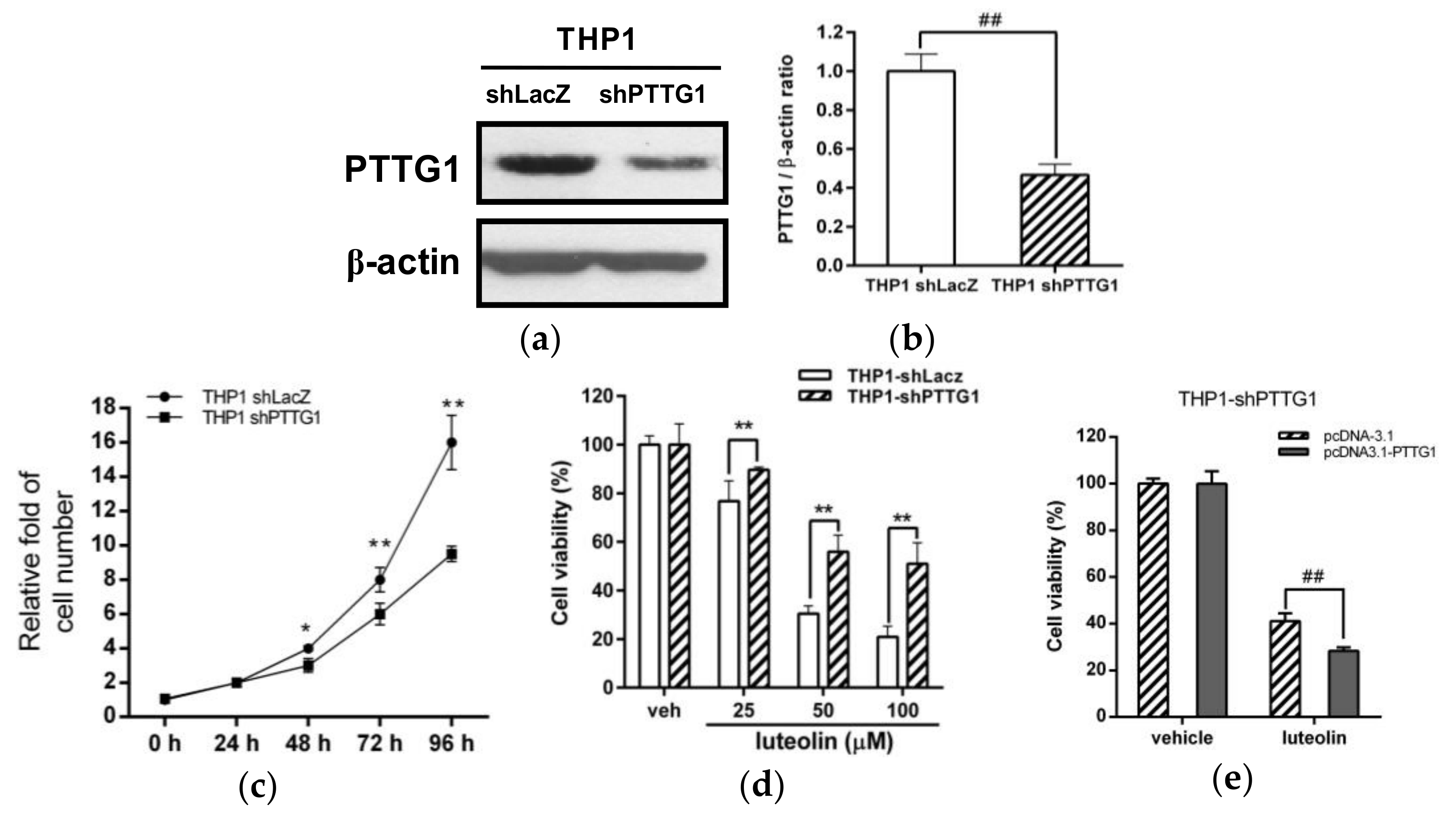
2.4. Effects of PTTG1 Knockdown on Cell Growth and the Luteolin Response in Human Myeloid Leukemia Cells
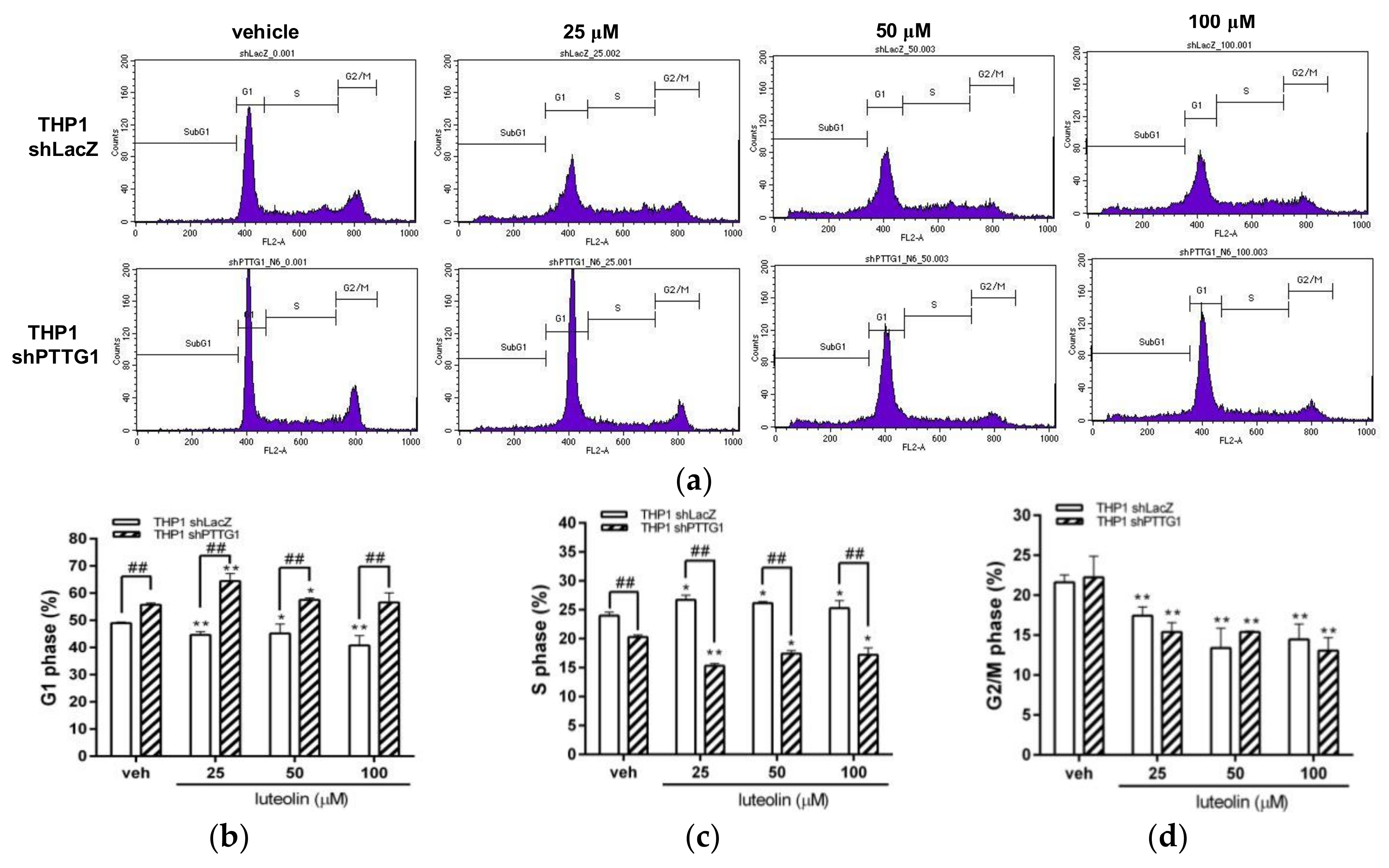
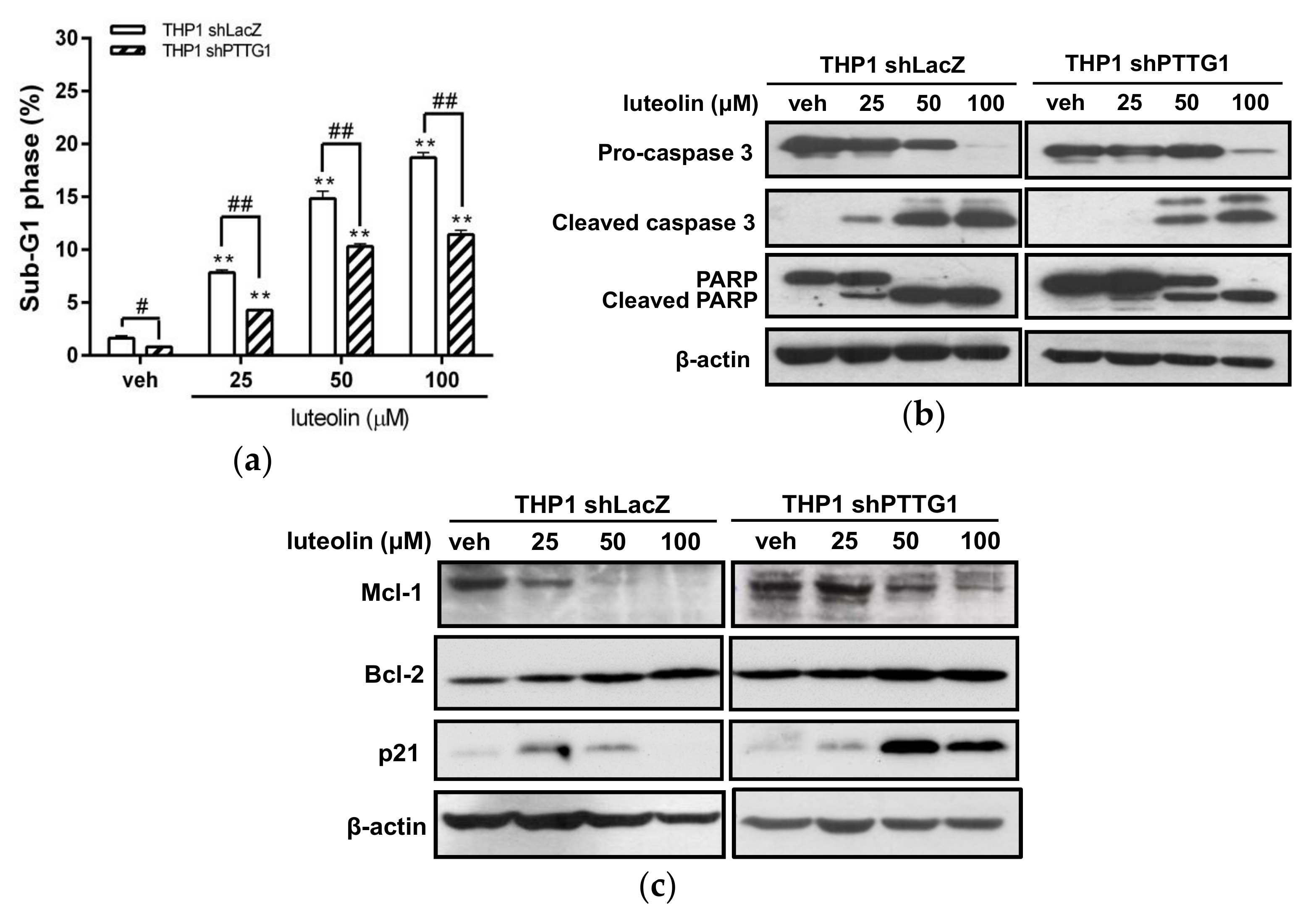
2.5. Effects of Luteolin on Cell-Cycle Distribution and Apoptosis in PTTG1-Knockdown Cells
2.6. Gene Expression Alterations in PTTG1-Knockdown Leukemia Cells
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Cell Culture
4.3. Cell Viability
4.4. Flow Cytometric Analysis
4.5. Western Blot Analysis
4.6. Generation of PTTG1-Knockdown Stable Cell Clones
4.7. Transfection of PTTG1 Expression Plasmids
4.8. RNA Preparation and Microarray Analysis
4.9. Quantitative Reverse-Transcription Polymerase Chain Reaction (Q-RT-PCR) Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rowley, J.D. Chromosomal translocations: Revisited yet again. Blood 2008, 112, 2183–2189. [Google Scholar] [CrossRef] [PubMed]
- Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 2013, 368, 2059–2074.
- Dohner, H.; Estey, E.H.; Amadori, S.; Appelbaum, F.R.; Buchner, T.; Burnett, A.K.; Dombret, H.; Fenaux, P.; Grimwade, D.; Larson, R.A.; et al. Diagnosis and management of acute myeloid leukemia in adults: Recommendations from an international expert panel, on behalf of the european leukemianet. Blood 2010, 115, 453–474. [Google Scholar] [CrossRef] [PubMed]
- Thol, F.; Schlenk, R.F.; Heuser, M.; Ganser, A. How i treat refractory and early relapsed acute myeloid leukemia. Blood 2015, 126, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Meiyanto, E.; Hermawan, A.; Anindyajati, A. Natural products for cancer-targeted therapy: Citrus flavonoids as potent chemopreventive agents. Asian Pac. J. Cancer Prev. 2012, 13, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Hartwell, L.H.; Kastan, M.B. Cell cycle control and cancer. Science 1994, 266, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Ketley, N.J.; Allen, P.D.; Kelsey, S.M.; Newland, A.C. Mechanisms of resistance to apoptosis in human aml blasts: The role of differentiation-induced perturbations of cell-cycle checkpoints. Leukemia 2000, 14, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Sordet, O.; Bettaieb, A.; Bruey, J.M.; Eymin, B.; Droin, N.; Ivarsson, M.; Garrido, C.; Solary, E. Selective inhibition of apoptosis by tpa-induced differentiation of u937 leukemic cells. Cell Death Differ. 1999, 6, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Fathi, A.T.; Karp, J.E. New agents in acute myeloid leukemia: Beyond cytarabine and anthracyclines. Curr. Oncol. Rep. 2009, 11, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Romero, F.; Multon, M.C.; Ramos-Morales, F.; Dominguez, A.; Bernal, J.A.; Pintor-Toro, J.A.; Tortolero, M. Human securin, hpttg, is associated with ku heterodimer, the regulatory subunit of the DNA-dependent protein kinase. Nucleic Acids Res. 2001, 29, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Vlotides, G.; Eigler, T.; Melmed, S. Pituitary tumor-transforming gene: Physiology and implications for tumorigenesis. Endocr. Rev. 2007, 28, 165–186. [Google Scholar] [CrossRef] [PubMed]
- Quereda, V.; Malumbres, M. Cell cycle control of pituitary development and disease. J. Mol. Endocrinol. 2009, 42, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, A.; Ramos-Morales, F.; Romero, F.; Rios, R.M.; Dreyfus, F.; Tortolero, M.; Pintor-Toro, J.A. Hpttg, a human homologue of rat pttg, is overexpressed in hematopoietic neoplasms. Evidence for a transcriptional activation function of hpttg. Oncogene 1998, 17, 2187–2193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Horwitz, G.A.; Prezant, T.R.; Valentini, A.; Nakashima, M.; Bronstein, M.D.; Melmed, S. Structure, expression, and function of human pituitary tumor-transforming gene (pttg). Mol. Endocrinol. 1999, 13, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Heaney, A.P.; Singson, R.; McCabe, C.J.; Nelson, V.; Nakashima, M.; Melmed, S. Expression of pituitary-tumour transforming gene in colorectal tumours. Lancet 2000, 355, 716–719. [Google Scholar] [CrossRef]
- Noll, J.E.; Vandyke, K.; Hewett, D.R.; Mrozik, K.M.; Bala, R.J.; Williams, S.A.; Kok, C.H.; Zannettino, A.C. Pttg1 expression is associated with hyperproliferative disease and poor prognosis in multiple myeloma. J. Hematol. Oncol. 2015, 8, 106. [Google Scholar] [CrossRef] [PubMed]
- Hamid, T.; Malik, M.T.; Kakar, S.S. Ectopic expression of pttg1/securin promotes tumorigenesis in human embryonic kidney cells. Mol. Cancer 2005, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Pei, L. Identification of c-myc as a down-stream target for pituitary tumor-transforming gene. J. Biol. Chem. 2001, 276, 8484–8491. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, S.; Ross, K.N.; Lander, E.S.; Golub, T.R. A molecular signature of metastasis in primary solid tumors. Nat. Genet. 2003, 33, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.C.; Ruan, J.W.; Lua, I.; Li, M.H.; Chen, W.L.; Wang, J.R.; Kao, R.H.; Chen, J.H. Overexpressed hpttg1 promotes breast cancer cell invasion and metastasis by regulating gef-h1/rhoa signalling. Oncogene 2012, 31, 3086–3097. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.E.; Franklyn, J.A.; McCabe, C.J. Pituitary tumor-transforming gene and its binding factor in endocrine cancer. Expert Rev. Mol. Med. 2010, 12, e38. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Eigler, T. Transcriptional targets for pituitary tumor-transforming gene-1. J. Mol. Endocrinol. 2009, 43, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.Y.; Yen, J.H.; Kao, R.H.; Chen, J.H. Down-regulation of the oncogene pttg1 via the klf6 tumor suppressor during induction of myeloid differentiation. PLoS ONE 2013, 8, e71282. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.J.; Hsu, T.S.; Chao, J.I. Opposing securin and p53 protein expression in the oxaliplatin-induced cytotoxicity of human colorectal cancer cells. Toxicol. Lett. 2006, 167, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lazaro, M. Distribution and biological activities of the flavonoid luteolin. Mini Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.Y.; Wu, M.J.; Chang, H.Y.; Tai, M.H.; Ho, C.T.; Yen, J.H. Up-regulation of mir-34a expression in response to the luteolin-induced neurite outgrowth of pc12 cells. J. Agric. Food Chem. 2015, 63, 4148–4159. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.F.; Chiu, S.P.; Wu, M.J.; Chen, P.Y.; Yen, J.H. Luteolin induces microrna-132 expression and modulates neurite outgrowth in pc12 cells. PLoS ONE 2012, 7, e43304. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Wu, M.J.; Liu, I.Y.; Su, J.D.; Yen, J.H. Neurotrophic and cytoprotective action of luteolin in pc12 cells through erk-dependent induction of nrf2-driven ho-1 expression. J. Agric. Food Chem. 2010, 58, 4477–4486. [Google Scholar] [CrossRef] [PubMed]
- Ju, W.; Wang, X.; Shi, H.; Chen, W.; Belinsky, S.A.; Lin, Y. A critical role of luteolin-induced reactive oxygen species in blockage of tumor necrosis factor-activated nuclear factor-kappab pathway and sensitization of apoptosis in lung cancer cells. Mol. Pharmacol. 2007, 71, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.Y.; Jeong, Y.; Tyner, A.L.; Park, J.H. Induction of cell cycle arrest and apoptosis in ht-29 human colon cancer cells by the dietary compound luteolin. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G66–G75. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zhou, Q.; Shi, X.L.; Jiang, B.H. Luteolin inhibits insulin-like growth factor 1 receptor signaling in prostate cancer cells. Carcinogenesis 2007, 28, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Attoub, S.; Hassan, A.H.; Vanhoecke, B.; Iratni, R.; Takahashi, T.; Gaben, A.M.; Bracke, M.; Awad, S.; John, A.; Kamalboor, H.A.; et al. Inhibition of cell survival, invasion, tumor growth and histone deacetylase activity by the dietary flavonoid luteolin in human epithelioid cancer cells. Eur. J. Pharmacol. 2011, 651, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.S.; Zhou, J.; Ong, C.N.; Shen, H.M. Luteolin induces g1 arrest in human nasopharyngeal carcinoma cells via the akt-gsk-3beta-cyclin d1 pathway. Cancer Lett. 2010, 298, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.T.; Park, O.J.; Lee, Y.K.; Sung, M.J.; Hur, H.J.; Kim, M.S.; Ha, J.H.; Kwon, D.Y. Anti-tumor effect of luteolin is accompanied by amp-activated protein kinase and nuclear factor-kappab modulation in hepg2 hepatocarcinoma cells. Int. J. Mol. Med. 2011, 28, 25–31. [Google Scholar] [PubMed]
- Ko, W.G.; Kang, T.H.; Lee, S.J.; Kim, Y.C.; Lee, B.H. Effects of luteolin on the inhibition of proliferation and induction of apoptosis in human myeloid leukaemia cells. Phytother. Res. 2002, 16, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.C.; Huang, T.C.; Lai, C.S.; Pan, M.H. Induction of apoptosis by luteolin through cleavage of bcl-2 family in human leukemia hl-60 cells. Eur. J. Pharmacol. 2005, 509, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.W.; Kong, J.Y.; Han, S.Y. Flavonoids as receptor tyrosine kinase flt3 inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 1768–1770. [Google Scholar] [CrossRef] [PubMed]
- Sak, K.; Kasemaa, K.; Everaus, H. Potentiation of luteolin cytotoxicity by flavonols fisetin and quercetin in human chronic lymphocytic leukemia cell lines. Food Funct. 2016, 7, 3815–3824. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Jiang, L.; Lin, X.; Tseng, K.F.; Lu, Z.; Wang, X. Luteolin, a novel p90 ribosomal s6 kinase inhibitor, suppresses proliferation and migration in leukemia cells. Oncol. Lett. 2017, 13, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Seelinger, G.; Merfort, I.; Wolfle, U.; Schempp, C.M. Anti-carcinogenic effects of the flavonoid luteolin. Molecules 2008, 13, 2628–2651. [Google Scholar] [CrossRef] [PubMed]
- Tuorkey, M.J. Molecular targets of luteolin in cancer. Eur. J. Cancer Prev. 2016, 25, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Pandurangan, A.K.; Esa, N.M. Luteolin, a bioflavonoid inhibits colorectal cancer through modulation of multiple signaling pathways: A review. Asian Pac. J. Cancer Prev. 2014, 15, 5501–5508. [Google Scholar] [CrossRef] [PubMed]
- Horinaka, M.; Yoshida, T.; Shiraishi, T.; Nakata, S.; Wakada, M.; Nakanishi, R.; Nishino, H.; Matsui, H.; Sakai, T. Luteolin induces apoptosis via death receptor 5 upregulation in human malignant tumor cells. Oncogene 2005, 24, 7180–7189. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.M. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Okada, N.; Tanabe, H.; Tazoe, H.; Ishigami, Y.; Fukutomi, R.; Yasui, K.; Isemura, M. Differentiation-associated alteration in sensitivity to apoptosis induced by (−)-epigallocatechin-3-o-gallate in hl-60 cells. Biomed. Res. 2009, 30, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Heaney, A.P.; Lu, W.; Chen, J.; Melmed, S. Pituitary tumor transforming gene causes aneuploidy and p53-dependent and p53-independent apoptosis. J. Biol. Chem. 2000, 275, 36502–36505. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Ren, S.G.; Horwitz, G.A.; Wang, Z.; Melmed, S. Pituitary tumor transforming gene (pttg) regulates placental jeg-3 cell division and survival: Evidence from live cell imaging. Mol. Endocrinol. 2000, 14, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Saez, C.; Pereda, T.; Borrero, J.J.; Espina, A.; Romero, F.; Tortolero, M.; Pintor-Toro, J.A.; Segura, D.I.; Japon, M.A. Expression of hpttg proto-oncogene in lymphoid neoplasias. Oncogene 2002, 21, 8173–8177. [Google Scholar] [CrossRef] [PubMed]
- Caporali, S.; Alvino, E.; Levati, L.; Esposito, A.I.; Ciomei, M.; Brasca, M.G.; Del Bufalo, D.; Desideri, M.; Bonmassar, E.; Pfeffer, U.; et al. Down-regulation of the pttg1 proto-oncogene contributes to the melanoma suppressive effects of the cyclin-dependent kinase inhibitor pha-848125. Biochem. Pharmacol. 2012, 84, 598–611. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.H.; Chuah, Q.Y.; Yang, P.M.; Chen, C.T.; Chao, J.C.; Lin, M.D.; Chiu, S.J. Securin enhances the anti-cancer effects of 6-methoxy-3-(3′,4′,5′-trimethoxy-benzoyl)-1h-indole (bpr0l075) in human colorectal cancer cells. PLoS ONE 2012, 7, e36006. [Google Scholar] [CrossRef] [PubMed]
- Castilla, C.; Flores, M.L.; Medina, R.; Perez-Valderrama, B.; Romero, F.; Tortolero, M.; Japon, M.A.; Saez, C. Prostate cancer cell response to paclitaxel is affected by abnormally expressed securin pttg1. Mol. Cancer Ther. 2014, 13, 2372–2383. [Google Scholar] [CrossRef] [PubMed]
- Cassier, P.A.; Castets, M.; Belhabri, A.; Vey, N. Targeting apoptosis in acute myeloid leukaemia. Br. J. Cancer 2017, 117, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Chesnokova, V.; Zonis, S.; Rubinek, T.; Yu, R.; Ben-Shlomo, A.; Kovacs, K.; Wawrowsky, K.; Melmed, S. Senescence mediates pituitary hypoplasia and restrains pituitary tumor growth. Cancer Res. 2007, 67, 10564–10572. [Google Scholar] [CrossRef] [PubMed]
- Belmar, J.; Fesik, S.W. Small molecule mcl-1 inhibitors for the treatment of cancer. Pharmacol. Ther. 2015, 145, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Gartel, A.L.; Radhakrishnan, S.K. Lost in transcription: P21 repression, mechanisms, and consequences. Cancer Res. 2005, 65, 3980–3985. [Google Scholar] [CrossRef] [PubMed]
- Mahyar-Roemer, M.; Roemer, K. P21 waf1/cip1 can protect human colon carcinoma cells against p53-dependent and p53-independent apoptosis induced by natural chemopreventive and therapeutic agents. Oncogene 2001, 20, 3387–3398. [Google Scholar] [CrossRef] [PubMed]
- Gorospe, M.; Cirielli, C.; Wang, X.; Seth, P.; Capogrossi, M.C.; Holbrook, N.J. P21(waf1/cip1) protects against p53-mediated apoptosis of human melanoma cells. Oncogene 1997, 14, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wu, D.; Shao, K.; Ye, B.; Huang, J.; Gao, Y. Mir-15a-5p negatively regulates cell survival and metastasis by targeting cxcl10 in chronic myeloid leukemia. Am. J. Transl. Res. 2017, 9, 4308–4316. [Google Scholar] [PubMed]
- Song, G.; Li, Y.; Jiang, G. Role of vegf/vegfr in the pathogenesis of leukemias and as treatment targets (review). Oncol. Rep. 2012, 28, 1935–1944. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, Z.; Zhou, J. Tumor necrosis factor alpha in the onset and progression of leukemia. Exp. Hematol. 2017, 45, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Alexandrova, E.M.; Moll, U.M. Role of p53 family members p73 and p63 in human hematological malignancies. Leuk. Lymphoma 2012, 53, 2116–2129. [Google Scholar] [CrossRef] [PubMed]
- Tomkova, K.; Tomka, M.; Zajac, V. Contribution of p53, p63, and p73 to the developmental diseases and cancer. Neoplasma 2008, 55, 177–181. [Google Scholar] [PubMed]
- Li, Y.; Zhou, Z.; Chen, C. Ww domain-containing e3 ubiquitin protein ligase 1 targets p63 transcription factor for ubiquitin-mediated proteasomal degradation and regulates apoptosis. Cell Death Differ. 2008, 15, 1941–1951. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Aqeilan, R.I.; Neale, M.; Candi, E.; Salomoni, P.; Knight, R.A.; Croce, C.M.; Melino, G. The e3 ubiquitin ligase itch controls the protein stability of p63. Proc. Natl. Acad. Sci. USA 2006, 103, 12753–12758. [Google Scholar] [CrossRef] [PubMed]
- Graziano, V.; De Laurenzi, V. Role of p63 in cancer development. Biochim. Biophys. Acta 2011, 1816, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, M.; Lim, J.; Kim, Y.; Han, K.; Cho, B.S.; Kim, H.J. Acute myeloid leukemia associated with fgfr1 abnormalities. Int. J. Hematol. 2013, 97, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, M.; Chou, F.S.; Link, K.A.; Mizukawa, B.; Perry, R.L.; Carroll, M.; Mulloy, J.C. Aml xenograft efficiency is significantly improved in nod/scid-il2rg mice constitutively expressing human scf, gm-csf and il-3. Leukemia 2010, 24, 1785–1788. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, F.E.; Cashman, J.D.; Hogge, D.E.; Humphries, R.K.; Eaves, C.J. Nod/scid mice engineered to express human il-3, gm-csf and steel factor constitutively mobilize engrafted human progenitors and compromise human stem cell regeneration. Leukemia 2004, 18, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Luo, R.T.; Shrestha, M.; Thirman, M.J.; Mulloy, J.C. The full transforming capacity of mll-af4 is interlinked with lymphoid lineage commitment. Blood 2017, 130, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Yoshimi, A.; Balasis, M.E.; Vedder, A.; Feldman, K.; Ma, Y.; Zhang, H.; Lee, S.C.; Letson, C.; Niyongere, S.; Lu, S.X.; et al. Robust patient-derived xenografts of mds/mpn overlap syndromes capture the unique characteristics of cmml and jmml. Blood 2017, 130, 397–407. [Google Scholar] [CrossRef] [PubMed]


| Gene Symbol | Description | Log2 (shPTTG1/shLacZ) |
|---|---|---|
| CXCL10 | chemokine (C-X-C motif) ligand 10 | −3.39 |
| VEGFA | vascular endothelial growth factor A | −2.74 |
| TNF | tumor necrosis factor | −2.33 |
| TP63 | tumor protein p63 | −2.05 |
| FGFR1 | fibroblast growth factor receptor 1 | −1.78 |
| CCL2 | chemokine (C-C motif) ligand 2 | −1.56 |
| F3 | coagulation factor III | −1.47 |
| STAT1 | signal transducer and activator of transcription 1 91 kDa | −1.40 |
| IL12A | interleukin 12A | −1.38 |
| CDK6 | cyclin-dependent kinase 6 | −1.35 |
| KIT | v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog | −1.27 |
| PTPRC | protein tyrosine phosphatase receptor type C | −1.27 |
| JUN | jun proto-oncogene | −1.24 |
| CD40 | CD40 molecule TNF receptor superfamily member 5 | −1.24 |
| VEGFC | vascular endothelial growth factor C | −1.23 |
| PRAME | preferentially expressed antigen in melanoma | −1.21 |
| IL12RB2 | interleukin 12 receptor beta 2 | −1.16 |
| VASH2 | vasohibin 2 | −1.13 |
| TGFBR2 | transforming growth factor beta receptor II (70/80 kDa) | −1.09 |
| KRAS | v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog | −1.02 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.-Y.; Tien, H.-J.; Chen, S.-F.; Horng, C.-T.; Tang, H.-L.; Jung, H.-L.; Wu, M.-J.; Yen, J.-H. Response of Myeloid Leukemia Cells to Luteolin is Modulated by Differentially Expressed Pituitary Tumor-Transforming Gene 1 (PTTG1) Oncoprotein. Int. J. Mol. Sci. 2018, 19, 1173. https://doi.org/10.3390/ijms19041173
Chen P-Y, Tien H-J, Chen S-F, Horng C-T, Tang H-L, Jung H-L, Wu M-J, Yen J-H. Response of Myeloid Leukemia Cells to Luteolin is Modulated by Differentially Expressed Pituitary Tumor-Transforming Gene 1 (PTTG1) Oncoprotein. International Journal of Molecular Sciences. 2018; 19(4):1173. https://doi.org/10.3390/ijms19041173
Chicago/Turabian StyleChen, Pei-Yi, Hsin-Jung Tien, Shih-Fen Chen, Chi-Ting Horng, Huei-Lin Tang, Hui-Ling Jung, Ming-Jiuan Wu, and Jui-Hung Yen. 2018. "Response of Myeloid Leukemia Cells to Luteolin is Modulated by Differentially Expressed Pituitary Tumor-Transforming Gene 1 (PTTG1) Oncoprotein" International Journal of Molecular Sciences 19, no. 4: 1173. https://doi.org/10.3390/ijms19041173
APA StyleChen, P.-Y., Tien, H.-J., Chen, S.-F., Horng, C.-T., Tang, H.-L., Jung, H.-L., Wu, M.-J., & Yen, J.-H. (2018). Response of Myeloid Leukemia Cells to Luteolin is Modulated by Differentially Expressed Pituitary Tumor-Transforming Gene 1 (PTTG1) Oncoprotein. International Journal of Molecular Sciences, 19(4), 1173. https://doi.org/10.3390/ijms19041173