Within the Brain: The Renin Angiotensin System
Abstract
1. Introduction
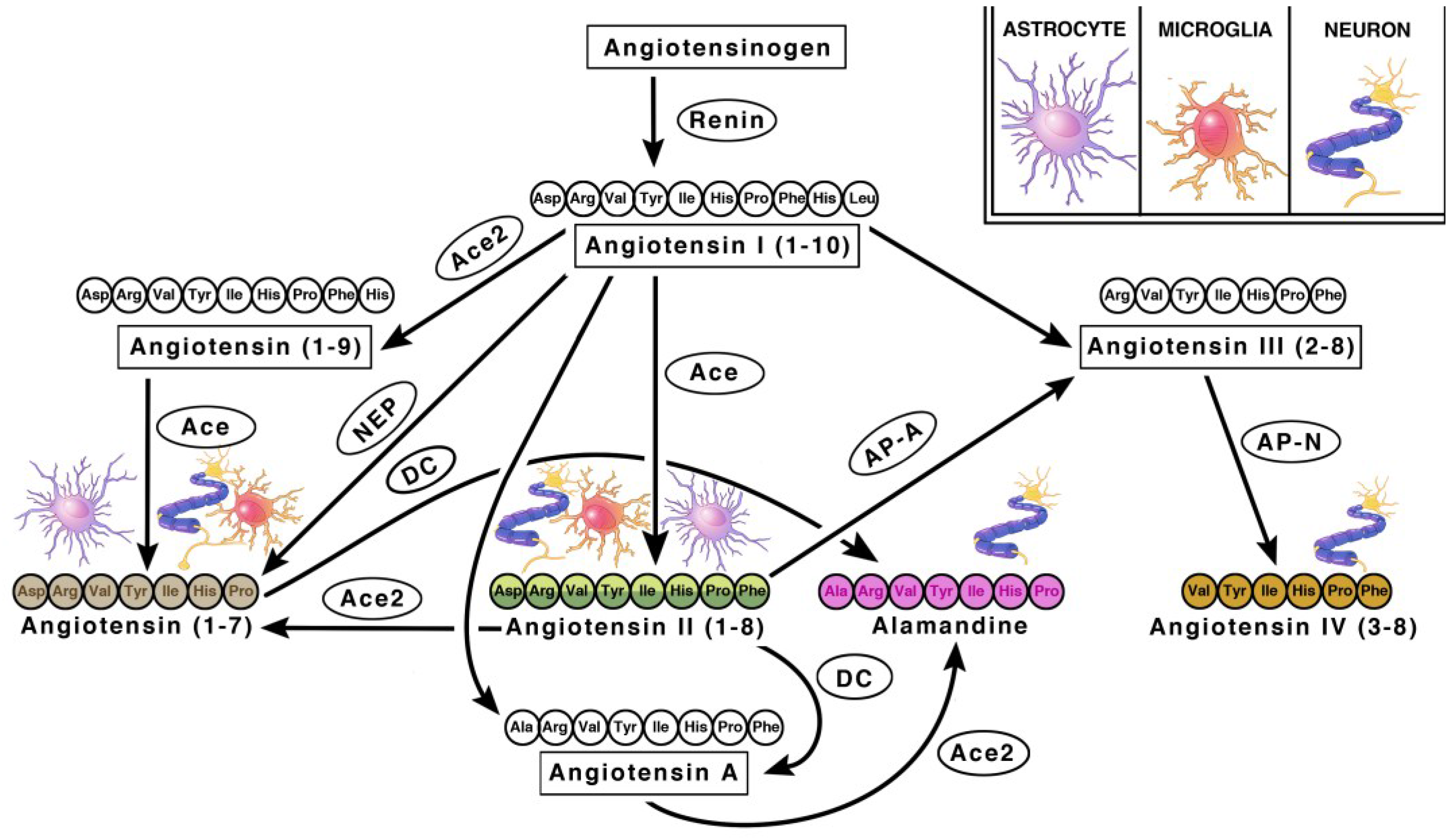
2. Central Renin Angiotensin System
2.1. Angiotensin Ligands and Peptidases
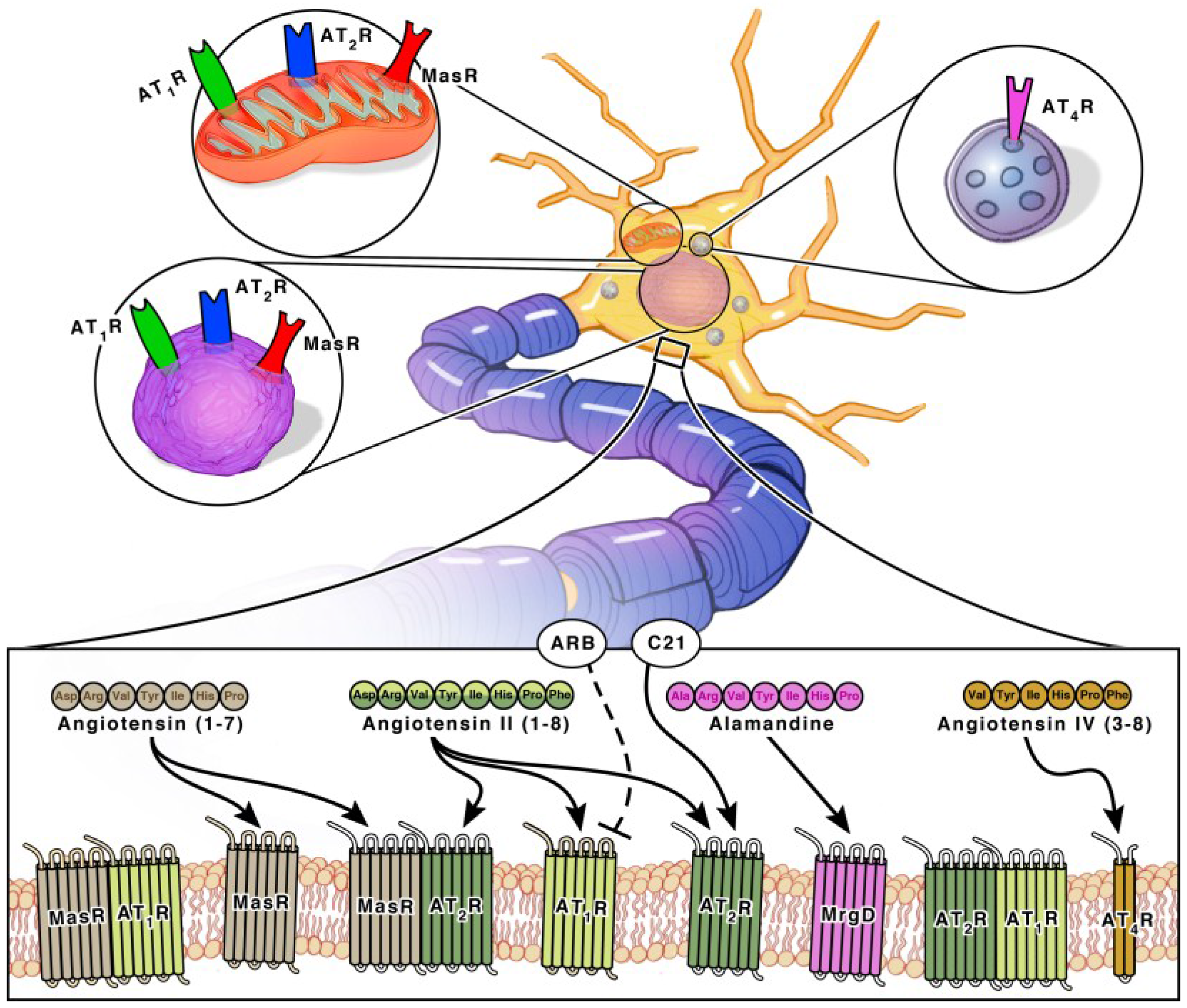
2.2. Angiotensin Receptors and Peptidases
2.3. Renin in the Brain
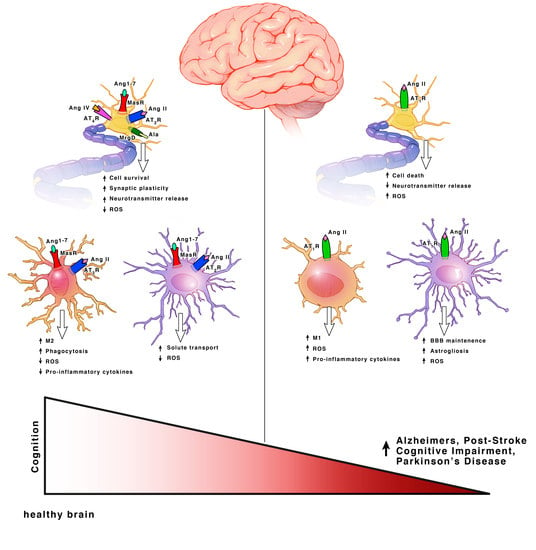
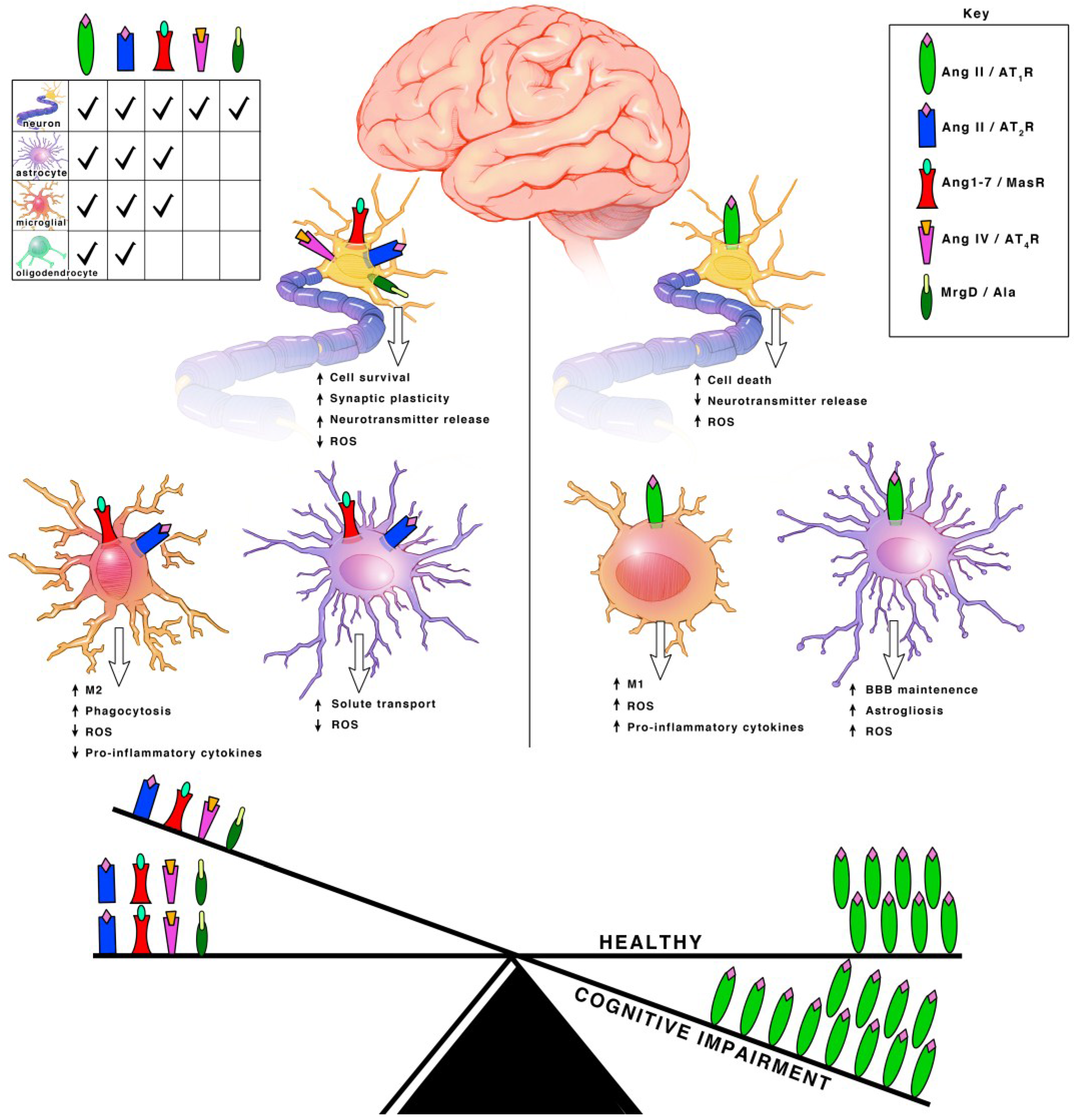
3. Angiotensin Receptor Functions within Each Cell Type
3.1. Neurons
3.1.1. Angiotensin II Type I Receptor (AT1R) and Angiotensin Converting Enzyme (ACE)
3.1.2. Angiotensin II Type II Receptor (AT2R)
3.1.3. Mas Receptor (MasR) and ACE
3.1.4. Angiotensin IV Receptor (AT4R)
3.2. Microglia
3.2.1. AT1R and ACE
3.2.2. AT2R and MasR
3.3. Astrocytes and Oligodendrocytes
3.3.1. AT1R and ACE
3.3.2. AT2R, MasR and ACE2
3.3.3. AT4R
4. Cognitive Benefits of RAS Modulators in PreClinical Animal Models
4.1. AT1R Blockers (ARBs)
4.1.1. Telmisartan
4.1.2. Valsartan
4.1.3. Losartan
4.1.4. Candesartan
4.2. Enzyme Inhibitors
4.2.1. Renin Inhibitors
4.2.2. ACE Inhibitors
4.3. AT2R Agonists
Compound 21 (C21)
5. Mechanisms of Neuroprotection
Brain-Derived Neurotrophic Factor (BDNF)
6. Translational and Clinical Implications
7. Concluding Remarks
Acknowledgments
Conflicts of Interest
References
- Tigerstedt, R.; Bergman, P.G. Niere und Kreislauf. Arch. Physiol. 1898, 8, 223–227. [Google Scholar]
- Igic, R.; Skrbic, R. The renin-angiotensin system and its blockers. Srp. Arh. Celok. Lek. 2014, 142, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Mentz, R.J.; Bakris, G.L.; Waeber, B.; McMurray, J.J.; Gheorghiade, M.; Ruilope, L.M.; Maggioni, A.P.; Swedberg, K.; Pina, I.L.; Fiuzat, M.; et al. The past, present and future of renin-angiotensin aldosterone system inhibition. Int. J. Cardiol. 2013, 167, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Artham, S.; Fouda, A.Y.; El-Remessy, A.B.; Fagan, S.C. Vascular protective effects of angiotensin receptor blockers: Beyond blood pressure. Recept. Clin. Investig. 2015, 2, e774. [Google Scholar] [CrossRef][Green Version]
- Fouda, A.Y.; Artham, S.; El-Remessy, A.B.; Fagan, S.C. Renin-angiotensin system as a potential therapeutic target in stroke and retinopathy: Experimental and clinical evidence. Clin. Sci. 2016, 130, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Hamel, E.; Royea, J.; Ongali, B.; Tong, X.K. Neurovascular and cognitive failure in Alzheimer’s disease: Benefits of cardiovascular therapy. Cell. Mol. Neurobiol. 2016, 36, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Poyan Mehr, A.; Kreutz, R. Physiology of local renin-angiotensin systems. Physiol. Rev. 2006, 86, 747–803. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Ruiz-Ortega, M.; Lorenzo, O.; Ruperez, M.; Esteban, V.; Egido, J. Inflammation and angiotensin II. Int. J. Biochem. Cell Biol. 2003, 35, 881–900. [Google Scholar] [CrossRef]
- Wright, J.W.; Harding, J.W. The brain renin-angiotensin system: A diversity of functions and implications for CNS diseases. Pflugers Arch. 2013, 465, 133–151. [Google Scholar] [CrossRef] [PubMed]
- Lenkei, Z.; Palkovits, M.; Corvol, P.; Cortes, C.L. Expression of angiotensin type-1 (AT1) and type-2 (AT2) receptor mRNAs in the adult rat brain: A functional neuroanatomical review. Front. Neuroendocrinol. 1997, 18, 383–439. [Google Scholar] [CrossRef] [PubMed]
- Bodiga, V.L.; Bodiga, S. Renin angiotensin system in cognitive function and dementia. Asian J. Neurosci. 2013, 2013, 102602. [Google Scholar] [CrossRef]
- Grobe, J.L.; Xu, D.; Sigmund, C.D. An intracellular renin-angiotensin system in neurons: Fact, hypothesis, or fantasy. Physiology (Bethesda) 2008, 23, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.W.; Harding, J.W. Brain renin-angiotensin—A new look at an old system. Prog. Neurobiol. 2011, 95, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, K.E.; Koronyo, Y.; Salumbides, B.C.; Sheyn, J.; Pelissier, L.; Lopes, D.H.; Shah, K.H.; Bernstein, E.A.; Fuchs, D.T.; Yu, J.J.; et al. Angiotensin-converting enzyme overexpression in myelomonocytes prevents Alzheimer's-like cognitive decline. J. Clin. Investig. 2014, 124, 1000–1012. [Google Scholar] [CrossRef] [PubMed]
- Elased, K.M.; Cunha, T.S.; Marcondes, F.K.; Morris, M. Brain angiotensin-converting enzymes: Role of angiotensin-converting enzyme 2 in processing angiotensin II in mice. Exp. Physiol. 2008, 93, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Gao, L.; Lu, J.; Zhang, Y. ACE2-Ang-(1–7)-Mas axis in brain: A potential target for prevention and treatment of ischemic stroke. Curr. Neuropharmacol. 2013, 11, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Tetzner, A.; Gebolys, K.; Meinert, C.; Klein, S.; Uhlich, A.; Trebicka, J.; Villacanas, O.; Walther, T. G-protein-coupled receptor MrgD is a receptor for angiotensin-(1–7) involving adenylyl cyclase, cAMP, and phosphokinase A. Hypertension 2016, 68, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Labandeira-Garcia, J.L.; Rodriguez-Perez, A.I.; Garrido-Gil, P.; Rodriguez-Pallares, J.; Lanciego, J.L.; Guerra, M.J. Brain renin-angiotensin system and microglial polarization: Implications for aging and neurodegeneration. Front. Aging Neurosci. 2017, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.A.; Ishrat, T.; Pillai, B.; Bunting, K.M.; Patel, A.; Vazdarjanova, A.; Waller, J.L.; Arbab, A.S.; Ergul, A.; Fagan, S.C. Role of angiotensin system modulation on progression of cognitive impairment and brain MRI changes in aged hypertensive animals—A randomized double-blind pre-clinical study. Behav. Brain Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Lazartigues, E. Angiotensin-converting enzyme 2 in the brain: Properties and future directions. J. Neurochem. 2008, 107, 1482–1494. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.L.; Fu, J.L.; Geng, Z.; Yang, J.J.; Sun, X.J. The neuroprotective effect of losartan through inhibiting AT1/ASK1/MKK4/JNK3 pathway following cerebral I/R in rat hippocampal CA1 region. CNS Neurosci. Ther. 2012, 18, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Lenkei, Z.; Palkovits, M.; Corvol, P.; Llorens-Cortes, C. Distribution of angiotensin II type 2 receptor (AT2) mRNA expression in the adult rat brain. J. Compara. Neurol. 1996, 373, 322–339. [Google Scholar] [CrossRef]
- Crozier, R.A.; Ajit, S.K.; Kaftan, E.J.; Pausch, M.H. MrgD activation inhibits KCNQ/M-currents and contributes to enhanced neuronal excitability. J. Neurosci. 2007, 27, 4492–4496. [Google Scholar] [CrossRef] [PubMed]
- Kangussu, L.M.; Almeida-Santos, A.F.; Moreira, F.A.; Fontes, M.A.P.; Santos, R.A.S.; Aguiar, D.C.; Campagnole-Santos, M.J. Reduced anxiety-like behavior in transgenic rats with chronically overproduction of angiotensin-(1–7): Role of the Mas receptor. Behav. Brain Res. 2017, 331, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Mateos, L.; Perez-Alvarez, M.J.; Wandosell, F. Angiotensin II type-2 receptor stimulation induces neuronal VEGF synthesis after cerebral ischemia. Biochim. Biophys. Acta 2016, 1862, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Maul, B.; von Bohlen und Halbach, O.; Becker, A.; Sterner-Kock, A.; Voigt, J.P.; Siems, W.E.; Grecksch, G.; Walther, T. Impaired spatial memory and altered dendritic spine morphology in angiotensin II type 2 receptor-deficient mice. J. Mol. Med. (Berl.) 2008, 86, 563–571. [Google Scholar] [CrossRef] [PubMed]
- AbdAlla, S.; Lother, H.; Abdel-tawab, A.M.; Quitterer, U. The angiotensin II AT2 receptor is an AT1 receptor antagonist. J. Biol. Chem. 2001, 276, 39721–39726. [Google Scholar] [CrossRef] [PubMed]
- Costa-Besada, M.A.; Valenzuela, R.; Garrido-Gil, P.; Villar-Cheda, B.; Parga, J.A.; Lanciego, J.L.; Labandeira-Garcia, J.L. Paracrine and intracrine angiotensin 1–7/Mas receptor axis in the substantia nigra of rodents, monkeys, and humans. Mol. Neurobiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, J.; Villela, D.C.; Teichmann, A.; Munter, L.M.; Mayer, M.C.; Mardahl, M.; Kirsch, S.; Namsolleck, P.; Lucht, K.; Benz, V.; et al. Evidence for heterodimerization and functional interaction of the angiotensin type 2 receptor and the receptor MAS. Hypertension 2017, 69, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Haack, K.K.; Zucker, I.H. Angiotensin II regulates ACE and ACE2 in neurons through p38 mitogen-activated protein kinase and extracellular signal-regulated kinase 1/2 signaling. Am. J. Physiol. Cell Physiol. 2013, 304, C1073–C1079. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Sriramula, S.; Lazartigues, E. ACE2/ANG-(1–7)/Mas pathway in the brain: The axis of good. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R804–R817. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, P.E.; Chappell, M.C.; Ferrario, C.M.; Tallant, E.A. Distinct roles for ANG II and ANG-(1–7) in the regulation of angiotensin-converting enzyme 2 in rat astrocytes. Am. J. Physiol. Cell Physiol. 2006, 290, C420–C426. [Google Scholar] [CrossRef] [PubMed]
- Chai, S.Y.; Bastias, M.A.; Clune, E.F.; Matsacos, T.J.; Mustafa, T.; Lee, J.H.; McDowall, S.G.; Mendelsohn, F.; Albiston, A.L.; Paxinos, G. Distribution of angiotensin IV binding sites (AT4 receptor) in the human forebrain, midbrain and pons as visualised by in vitro receptor autoradiography. J. Chem. Neuroanat. 2000, 20, 339–348. [Google Scholar] [CrossRef]
- Wright, J.W.; Kawas, L.H.; Harding, J.W. The development of small molecule angiotensin IV analogs to treat Alzheimer's and Parkinson's diseases. Prog. Neurobiol. 2015, 125, 26–46. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.W.; Harding, J.W. The brain angiotensin system and extracellular matrix molecules in neural plasticity, learning, and memory. Prog. Neurobiol. 2004, 72, 263–293. [Google Scholar] [CrossRef] [PubMed]
- Royea, J.; Zhang, L.; Tong, X.K.; Hamel, E. Angiotensin IV receptors mediate the cognitive and cerebrovascular benefits of losartan in a mouse model of Alzheimer’s disease. J. Neurosci. 2017, 37, 5562–5573. [Google Scholar] [CrossRef] [PubMed]
- Zawada, W.M.; Mrak, R.E.; Biedermann, J.; Palmer, Q.D.; Gentleman, S.M.; Aboud, O.; Griffin, W.S. Loss of angiotensin II receptor expression in dopamine neurons in Parkinson’s disease correlates with pathological progression and is accompanied by increases in Nox4- and 8-OH guanosine-related nucleic acid oxidation and caspase-3 activation. Acta Neuropathol. Commun. 2015, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, R.; Costa-Besada, M.A.; Iglesias-Gonzalez, J.; Perez-Costas, E.; Villar-Cheda, B.; Garrido-Gil, P.; Melendez-Ferro, M.; Soto-Otero, R.; Lanciego, J.L.; Henrion, D.; et al. Mitochondrial angiotensin receptors in dopaminergic neurons. Role in cell protection and aging-related vulnerability to neurodegeneration. Cell Death Dis. 2016, 7, e2427. [Google Scholar] [CrossRef] [PubMed]
- Villar-Cheda, B.; Costa-Besada, M.A.; Valenzuela, R.; Perez-Costas, E.; Melendez-Ferro, M.; Labandeira-Garcia, J.L. The intracellular angiotensin system buffers deleterious effects of the extracellular paracrine system. Cell Death Dis. 2017, 8, e3044. [Google Scholar] [CrossRef] [PubMed]
- Fernando, R.N.; Luff, S.E.; Albiston, A.L.; Chai, S.Y. Sub-cellular localization of insulin-regulated membrane aminopeptidase, IRAP to vesicles in neurons. J. Neurochem. 2007, 102, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.M.; Barnes, N.M.; Costal, B.; Coughlan, J.; Kelly, M.E.; Naylor, R.J.; Tomkins, D.M.; Williams, T.J. Angiotensin-converting enxyme inhibition, angiotensin and cognition. J. Cardiovasc. Pharmacol. 1992, 19, 63–71. [Google Scholar] [CrossRef]
- Tota, S.; Kamat, P.K.; Saxena, G.; Hanif, K.; Najmi, A.K.; Nath, C. Central angiotensin converting enzyme facilitates memory impairment in intracerebroventricular streptozotocin treated rats. Behav. Brain Res. 2012, 226, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, D.; Udayabanu, M.; Kumar, M.; Aneja, R.; Katyal, A. Involvement of angiotensin converting enzyme in cerebral hypoperfusion induced anterograde memory impairment and cholinergic dysfunction in rats. Neuroscience 2008, 155, 626–639. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Gil, P.; Rodriguez-Pallares, J.; Dominguez-Meijide, A.; Guerra, M.J.; Labandeira-Garcia, J.L. Brain angiotensin regulates iron homeostasis in dopaminergic neurons and microglial cells. Exp. Neurol. 2013, 250, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Ou, Z.; Jiang, T.; Gao, Q.; Tian, Y.Y.; Zhou, J.S.; Wu, L.; Shi, J.Q.; Zhang, Y.D. Mitochondrial-dependent mechanisms are involved in angiotensin II-induced apoptosis in dopaminergic neurons. J. Renin Angiotensin Aldosterone Syst. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Guimond, M.O.; Gallo-Payet, N. How does angiotensin AT2 receptor activation help neuronal differentiation and improve neuronal pathological situations? Front. Endocrinol. (Lausanne) 2012, 3, 164. [Google Scholar] [CrossRef] [PubMed]
- Bedecs, K.; Elbaz, N.; Sutren, M.; Masson, M.; Susini, C.; Strosberg, A.D.; Nahmias, C. Angiotensin II type 2 receptors mediate inhibition of mitogen-activated protein kinase cascade and functional activation of SHP-1 tyrosine phosphatase. Biochem. J. 1997, 325, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Smeyne, R.J.; Vendrell, M.; Hayward, M.; Baker, S.J.; Miao, G.G.; Schilling, K.; Robertson, L.M.; Curran, T.; Morgan, J.I. Continuous c-fos expression precedes programmed cell death in vivo. Nature 1993, 363, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Gwathmey, T.M.; Shaltout, H.A.; Pendergrass, K.D.; Pirro, N.T.; Figueroa, J.P.; Rose, J.C.; Diz, D.I.; Chappell, M.C. Nuclear angiotensin II type 2 (AT2) receptors are functionally linked to nitric oxide production. Am. J. Physiol. Renal Physiol. 2009, 296, F1484–F1493. [Google Scholar] [CrossRef] [PubMed]
- Villar-Cheda, B.; Rodriguez-Pallares, J.; Valenzuela, R.; Munoz, A.; Guerra, M.J.; Baltatu, O.C.; Labandeira-Garcia, J.L. Nigral and striatal regulation of angiotensin receptor expression by dopamine and angiotensin in rodents: Implications for progression of Parkinson’s disease. Eur. J. Neurosci. 2010, 32, 1695–706. [Google Scholar] [CrossRef] [PubMed]
- Bennion, D.M.; Haltigan, E.; Regenhardt, R.W.; Steckelings, U.M.; Sumners, C. Neuroprotective mechanisms of the ACE2-angiotensin-(1–7)-Mas axis in stroke. Curr. Hypertens. Rep. 2015, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Arroja, M.M.; Reid, E.; McCabe, C. Therapeutic potential of the renin angiotensin system in ischaemic stroke. Exp. Transl. Stroke Med. 2016, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Gard, P.R. Cognitive-enhancing effects of angiotensin IV. BMC Neurosci 2008, 9 (Suppl. 2), S15. [Google Scholar] [CrossRef] [PubMed]
- Stragier, B.; Demaegdt, H.; de Bundel, D.; Smolders, I.; Sarre, S.; Vauquelin, G.; Ebinger, G.; Michotte, Y.; Vanderheyden, P. Involvement of insulin-regulated aminopeptidase and/or aminopeptidase N in the angiotensin IV-induced effect on dopamine release in the striatum of the rat. Brain Res. 2007, 1131, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Kramar, E.A.; Armstrong, D.L.; Ikeda, S.; Wayner, M.J.; Harding, J.W.; Wright, J.W. The effects of angiotensin IV analogs on long-term potentiation within the CA1 region of the hippocampus in vitro. Brain Res. 2001, 897, 114–121. [Google Scholar] [CrossRef]
- Lee, J.; Chai, S.Y.; Mendelsohn, F.; Morris, M. Potentiation of cholinergic transmission in the rat hippocampus by angiotensin IV and LVV-hemorphin-7. Neuropharmacology 2001, 40, 618–623. [Google Scholar] [CrossRef]
- Davis, C.J.; Kramar, E.A.; De, A.; Meighan, P.C.; Simasko, S.M.; Wright, J.W.; Harding, J.W. AT4 receptor activation increases intracellular calcium influx and induces a non-N-methyl-d-aspartate dependent form of long-term potentiation. Neuroscience 2006, 137, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- McCoy, A.T.; Benoist, C.C.; Wright, J.W.; Kawas, L.H.; Bule-Ghogare, J.M.; Zhu, M.; Appleyard, S.M.; Wayman, G.A.; Harding, J.W. Evaluation of metabolically stabilized angiotensin IV analogs as procognitive/antidementia agents. J. Pharmacol. Exp. Ther. 2013, 344, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Gil, P.; Valenzuela, R.; Villar-Cheda, B.; Lanciego, J.L.; Labandeira-Garcia, J.L. Expression of angiotensinogen and receptors for angiotensin and prorenin in the monkey and human substantia nigra: An intracellular renin-angiotensin system in the nigra. Brain Struct. Funct. 2013, 218, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Regenhardt, R.W.; Desland, F.; Mecca, A.P.; Pioquinto, D.J.; Afzal, A.; Mocco, J.; Sumners, C. Anti-inflammatory effects of angiotensin-(1–7) in ischemic stroke. Neuropharmacology 2013, 71, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Pallares, J.; Rey, P.; Parga, J.A.; Munoz, A.; Guerra, M.J.; Labandeira-Garcia, J.L. Brain angiotensin enhances dopaminergic cell death via microglial activation and NADPH-derived ROS. Neurobiol. Dis. 2008, 31, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Biancardi, V.C.; Stranahan, A.M.; Krause, E.G.; de Kloet, A.D.; Stern, J.E. Cross talk between AT1 receptors and Toll-like receptor 4 in microglia contributes to angiotensin II-derived ROS production in the hypothalamic paraventricular nucleus. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H404–H415. [Google Scholar] [CrossRef] [PubMed]
- Fouda, A.Y.; Pillai, B.; Dhandapani, K.M.; Ergul, A.; Fagan, S.C. Role of interleukin-10 in the neuroprotective effect of the angiotensin type 2 Receptor agonist, compound 21, after ischemia/reperfusion injury. Eur. J. Pharmacol. 2017, 799, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Shi, P.; Sumners, C. Direct anti-inflammatory effects of angiotensin-(1–7) on microglia. J. Neurochem. 2016, 136, 163–171. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C.A.; Vinh, A.; Miller, A.A.; Hallberg, A.; Alterman, M.; Callaway, J.K.; Widdop, R.E. Direct angiotensin AT2 receptor stimulation using a novel AT2 receptor agonist, compound 21, evokes neuroprotection in conscious hypertensive rats. PLoS ONE 2014, 9, e95762. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, D.; Matute, C. Angiotensin receptor-like immunoreactivity in adult brain white matter astrocytes and oligodendrocytes. Glia 2001, 35, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Fuchtbauer, L.; Toft-Hansen, H.; Khorooshi, R.; Owens, T. Expression of astrocytic type 2 angiotensin receptor in central nervous system inflammation correlates with blood-brain barrier breakdown. J. Mol. Neurosci. 2010, 42, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.I.; Katayama, T.; Prat, A. Glial influence on the blood brain barrier. Glia 2013, 61, 1939–1958. [Google Scholar] [CrossRef] [PubMed]
- Wosik, K.; Cayrol, R.; Dodelet-Devillers, A.; Berthelet, F.; Bernard, M.; Moumdjian, R.; Bouthillier, A.; Reudelhuber, T.L.; Prat, A. Angiotensin II controls occludin function and is required for blood brain barrier maintenance: Relevance to multiple sclerosis. J. Neurosci. 2007, 27, 9032–9042. [Google Scholar] [CrossRef] [PubMed]
- Labandeira-Garcia, J.L.; Garrido-Gil, P.; Rodriguez-Pallares, J.; Valenzuela, R.; Borrajo, A.; Rodriguez-Perez, A.I. Brain renin-angiotensin system and dopaminergic cell vulnerability. Front. Neuroanat. 2014, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Hosomi, N.; Hitomi, H.; Pelisch, N.; Fu, H.; Masugata, H.; Murao, K.; Ueno, M.; Matsumoto, M.; Nishiyama, A. Angiotensin II induces human astrocyte senescence through reactive oxygen species production. Hypertens. Res. 2011, 34, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Villapol, S.; Balarezo, M.G.; Affram, K.; Saavedra, J.M.; Symes, A.J. Neurorestoration after traumatic brain injury through angiotensin II receptor blockage. Brain 2015, 138, 3299–3315. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Zhu, D.; Ji, L.; Tian, M.; Xu, C.; Shi, J. Angiotensin-(1–7) improves cognitive function in rats with chronic cerebral hypoperfusion. Brain Res. 2014, 1573, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Zhang, D.L.; Sun, Y.; Zhao, Y.X.; Zhao, K.X.; Pu, D.; Xiao, Q. Angiotensin-(1–7) administration attenuates Alzheimer’s disease-like neuropathology in rats with streptozotocin-induced diabetes via Mas receptor activation. Neuroscience 2017, 346, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Valero-Esquitino, V.; Lucht, K.; Namsolleck, P.; Monnet-Tschudi, F.; Stubbe, T.; Lucht, F.; Liu, M.; Ebner, F.; Brandt, C.; Danyel, L.A.; et al. Direct angiotensin type 2 receptor (AT2R) stimulation attenuates T-cell and microglia activation and prevents demyelination in experimental autoimmune encephalomyelitis in mice. Clin. Sci. (Lond.) 2015, 128, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Holownia, A.; Braszko, J.J. The effect of angiotensin II and IV on ERK1/2 and CREB signalling in cultured rat astroglial cells. Naunyn Schmiedebergs Arch. Pharmacol. 2007, 376, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Albiston, A.L.; McDowall, S.G.; Matsacos, D.; Sim, P.; Clune, E.; Mustafa, T.; Lee, J.; Mendelsohn, F.A.; Simpson, R.J.; Connolly, L.M.; et al. Evidence that the angiotensin IV (AT4) receptor is the enzyme insulin-regulated aminopeptidase. J. Biol. Chem. 2001, 276, 48623–48626. [Google Scholar] [CrossRef] [PubMed]
- Yeatman, H.; Albiston, A.; Chai, S. Insulin-regulated aminopeptidase in astrocytes: Role in Alzheimer’s disease? Alzheimer's Dement. 2011, 7, S668. [Google Scholar] [CrossRef]
- Kane, R.L.; Butler, M.; Fink, H.A.; Brasure, M.; Davila, H.; Desai, P.; Jutkowitz, E.; McCreedy, E.; Nelson, V.A.; McCarten, J.R.; et al. Interventions to Prevent Age-Related Cognitive Decline, Mild Cognitive Impairment, and Clinical Alzheimer's-Type Dementia; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2017. [Google Scholar]
- Zhuang, S.; Wang, H.F.; Wang, X.; Li, J.; Xing, C.M. The association of renin-angiotensin system blockade use with the risks of cognitive impairment of aging and Alzheimer’s disease: A meta-analysis. J. Clin. Neurosci. 2016, 33, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, T.; Iwasaki, K.; Takasaki, K.; Uchida, K.; Naito, T.; Nogami, A.; Kubota, K.; Shindo, T.; Uchida, N.; Katsurabayashi, S.; et al. Telmisartan, a partial agonist of peroxisome proliferator-activated receptor γ, improves impairment of spatial memory and hippocampal apoptosis in rats treated with repeated cerebral ischemia. Brain Res. 2010, 1353, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Washida, K.; Ihara, M.; Nishio, K.; Fujita, Y.; Maki, T.; Yamada, M.; Takahashi, J.; Wu, X.; Kihara, T.; Ito, H.; et al. Nonhypotensive dose of telmisartan attenuates cognitive impairment partially due to peroxisome proliferator-activated receptor-γ activation in mice with chronic cerebral hypoperfusion. Stroke 2010, 41, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Wincewicz, D.; Braszko, J.J. Angiotensin II AT1 receptor blockade by telmisartan reduces impairment of spatial maze performance induced by both acute and chronic stress. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Prathab Balaji, S.; Vijay Chand, C.; Justin, A.; Ramanathan, M. Telmisartan mediates anti-inflammatory and not cognitive function through PPAR-γ agonism via SARM and MyD88 signaling. Pharmacol. Biochem. Behav. 2015, 137, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Haruyama, N.; Fujisaki, K.; Yamato, M.; Eriguchi, M.; Noguchi, H.; Torisu, K.; Tsuruya, K.; Kitazono, T. Improvement in spatial memory dysfunction by telmisartan through reduction of brain angiotensin II and oxidative stress in experimental uremic mice. Life Sci. 2014, 113, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Kishi, T.; Hirooka, Y.; Sunagawa, K. Telmisartan protects against cognitive decline via up-regulation of brain-derived neurotrophic factor/tropomyosin-related kinase B in hippocampus of hypertensive rats. J. Cardiol. 2012, 60, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Min, L.J.; Mogi, M.; Shudou, M.; Jing, F.; Tsukuda, K.; Ohshima, K.; Iwanami, J.; Horiuchi, M. Peroxisome proliferator-activated receptor-γ activation with angiotensin II type 1 receptor blockade is pivotal for the prevention of blood-brain barrier impairment and cognitive decline in type 2 diabetic mice. Hypertension 2012, 59, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.K.; Kumar, J.A. Effect of mentat, a herbal formulation on experimental models of Alzheimer’s disease and central cholinergic markers in rats. Fitoterapia 1995, 66, 216. [Google Scholar]
- Danion, J.M.; Weingartner, H.; Singer, L. Is cognitive psychopathology plausible? Illustrations from memory research. Can. J. Psychiatry 1996, 41, S5–S13. [Google Scholar] [CrossRef] [PubMed]
- Jagetia, G.C.; Rao, S.K.; Baliga, M.S.; S Babu, K. The evaluation of nitric oxide scavenging activity of certain herbal formulations in vitro: A preliminary study. Phytother. Res. 2004, 18, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.M.; Routtenberg, A. Angiotensin injected into the neostriatum after learning disrupts retention performance. Science 1977, 196, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Ma, Y.L.; Wayner, M.J.; Armstrong, D.L. Impaired retention by angiotensin II mediated by the AT1 receptor. Peptides 1995, 16, 1069–1071. [Google Scholar] [CrossRef]
- Raghavendra, V.; Chopra, K.; Kulkarni, S.K. Brain renin angiotensin system (RAS) in stress-induced analgesia and impaired retention. Peptides 1999, 20, 335–342. [Google Scholar] [CrossRef]
- Barnes, J.M.; Barnes, N.M.; Costall, B.; Horovitz, Z.P.; Ironside, J.W.; Naylor, R.J.; Williams, T.J. Angiotensin II inhibits cortical cholinergic function: Implications for cognition. J. Cardiovasc. Pharmacol. 1990, 16, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Anil Kumar, K.V.; Nagwar, S.; Thyloor, R.; Satyanarayana, S. Anti-stress and nootropic activity of drugs affecting the renin-angiotensin system in rats based on indirect biochemical evidence. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Douma, B.R.; Korte, S.M.; Buwalda, B.; la Fleur, S.E.; Bohus, B.; Luiten, P.G. Repeated blockade of mineralocorticoid receptors, but not of glucocorticoid receptors impairs food rewarded spatial learning. Psychoneuroendocrinology 1998, 23, 33–44. [Google Scholar] [CrossRef]
- Ongali, B.; Nicolakakis, N.; Tong, X.K.; Aboulkassim, T.; Papadopoulos, P.; Rosa-Neto, P.; Lecrux, C.; Imboden, H.; Hamel, E. Angiotensin II type 1 receptor blocker losartan prevents and rescues cerebrovascular, neuropathological and cognitive deficits in an Alzheimer’s disease model. Neurobiol. Dis. 2014, 68, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Braszko, J.J.; Walesiuk, A.; Wielgat, P. Cognitive effects attributed to angiotensin II may result from its conversion to angiotensin IV. J. Renin Angiotensin Aldosterone Syst. 2006, 7, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Jana, M.; Kundu, M.; Corbett, G.T.; Rangaswamy, S.B.; Mishra, R.K.; Luan, C.H.; Gonzalez, F.J.; Pahan, K. HMG-CoA reductase inhibitors bind to PPARalpha to upregulate neurotrophin expression in the brain and improve memory in mice. Cell Metab. 2015, 22, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Avanzini, G.; Depaulis, A.; Tassinari, A.; de Curtis, M. Do seizures and epileptic activity worsen epilepsy and deteriorate cognitive function? Epilepsia 2013, 54 (Suppl. 8), 14–21. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Xin, Y.; HaiQin, W.; GuiLian, Z.; Ru, Z.; ShuQin, Z.; HuQing, W.; Li, Y.; Yun, D. The PPARγ agonist rosiglitazone prevents cognitive impairment by inhibiting astrocyte activation and oxidative stress following pilocarpine-induced status epilepticus. Neurol. Sci. 2012, 33, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wu, H.; Yu, X.; Zhang, G.; Zhang, R.; Zhan, S.; Wang, H.; Bu, N.; Ma, X.; Li, Y. Angiotensin II and its receptor in activated microglia enhanced neuronal loss and cognitive impairment following pilocarpine-induced status epilepticus. Mol. Cell. Neurosci. 2015, 65, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Marchese, N.A.; Artur de laVillarmois, E.; Basmadjian, O.M.; Perez, M.F.; Baiardi, G.; Bregonzio, C. Brain angiotensin II AT1 receptors are involved in the acute and long-term amphetamine-induced neurocognitive alterations. Psychopharmacology (Berl.) 2016, 233, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Braszko, J.J.; Wincewicz, D.; Jakubow, P. Candesartan prevents impairment of recall caused by repeated stress in rats. Psychopharmacology (Berl.) 2013, 225, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Ishrat, T.; Pillai, B.; Soliman, S.; Fouda, A.Y.; Kozak, A.; Johnson, M.H.; Ergul, A.; Fagan, S.C. Low-dose candesartan enhances molecular mediators of neuroplasticity and subsequent functional recovery after ischemic stroke in rats. Mol. Neurobiol. 2015, 51, 1542–1553. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.; Ishrat, T.; Pillai, A.; Somanath, P.R.; Ergul, A.; El-Remessy, A.B.; Fagan, S.C. Candesartan induces a prolonged proangiogenic effect and augments endothelium-mediated neuroprotection after oxygen and glucose deprivation: Role of vascular endothelial growth factors A and B. J. Pharmacol. Exp. Ther. 2014, 349, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Fouda, A.Y.; Alhusban, A.; Ishrat, T.; Pillai, B.; Eldahshan, W.; Waller, J.L.; Ergul, A.; Fagan, S.C. Brain-derived neurotrophic factor knockdown blocks the angiogenic and protective effects of angiotensin modulation after experimental stroke. Mol. Neurobiol. 2017, 54, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Alhusban, A.; Kozak, A.; Ergul, A.; Fagan, S.C. AT1 receptor antagonism is proangiogenic in the brain: BDNF a novel mediator. J. Pharmacol. Exp. Ther. 2013, 344, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Gadelha, A.; Vendramini, A.M.; Yonamine, C.M.; Nering, M.; Berberian, A.; Suiama, M.A.; Oliveira, V.; Lima-Landman, M.T.; Breen, G.; Bressan, R.A.; et al. Convergent evidences from human and animal studies implicate angiotensin I-converting enzyme activity in cognitive performance in schizophrenia. Transl. Psychiatry 2015, 5, e691. [Google Scholar] [CrossRef] [PubMed]
- Schwengel, K.; Namsolleck, P.; Lucht, K.; Clausen, B.H.; Lambertsen, K.L.; Valero-Esquitino, V.; Thone-Reineke, C.; Muller, S.; Widdop, R.E.; Denton, K.M.; et al. Angiotensin AT2-receptor stimulation improves survival and neurological outcome after experimental stroke in mice. J. Mol. Med. (Berl.) 2016, 94, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Wincewicz, D.; Juchniewicz, A.; Waszkiewicz, N.; Braszko, J.J. Angiotensin II type 1 receptor blockade by telmisartan prevents stress-induced impairment of memory via HPA axis deactivation and up-regulation of brain-derived neurotrophic factor gene expression. Pharmacol. Biochem. Behav. 2016, 148, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.C.; Greene-Schloesser, D.; Payne, V.; Diz, D.I.; Hsu, F.C.; Kooshki, M.; Mustafa, R.; Riddle, D.R.; Zhao, W.; Chan, M.D.; et al. Chronic administration of the angiotensin-converting enzyme inhibitor, ramipril, prevents fractionated whole-brain irradiation-induced perirhinal cortex-dependent cognitive impairment. Radiat. Res. 2012, 178, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Jing, F.; Mogi, M.; Sakata, A.; Iwanami, J.; Tsukuda, K.; Ohshima, K.; Min, L.J.; Steckelings, U.M.; Unger, T.; Dahlof, B.; et al. Direct stimulation of angiotensin II type 2 receptor enhances spatial memory. J. Cereb. Blood Flow Metab. 2012, 32, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Sakata, A.; Mogi, M.; Iwanami, J.; Tsukuda, K.; Min, L.J.; Fujita, T.; Iwai, M.; Ito, M.; Horiuchi, M. Sex-different effect of angiotensin II type 2 receptor on ischemic brain injury and cognitive function. Brain Res. 2009, 1300, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Min, L.J.; Mogi, M.; Tsukuda, K.; Jing, F.; Ohshima, K.; Nakaoka, H.; Kan-No, H.; Wang, X.L.; Chisaka, T.; Bai, H.Y.; et al. Direct stimulation of angiotensin II type 2 receptor initiated after stroke ameliorates ischemic brain damage. Am. J. Hypertens. 2014, 27, 1036–1044. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wan, Y.; Wallinder, C.; Plouffe, B.; Beaudry, H.; Mahalingam, A.K.; Wu, X.; Johansson, B.; Holm, M.; Botoros, M.; Karlen, A.; et al. Design, synthesis, and biological evaluation of the first selective nonpeptide AT2 receptor agonist. J. Med. Chem. 2004, 47, 5995–6008. [Google Scholar] [CrossRef] [PubMed]
- Alhusban, A.; Fouda, A.Y.; Bindu, P.; Ishrat, T.; Soliman, S.; Fagan, S.C. Compound 21 is pro-angiogenic in the brain and results in sustained recovery after ischemic stroke. J. Hypertens. 2015, 33, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Iwanami, J.; Mogi, M.; Tsukuda, K.; Jing, F.; Ohshima, K.; Wang, X.L.; Nakaoka, H.; Kan-no, H.; Chisaka, T.; Bai, H.Y.; et al. Possible synergistic effect of direct angiotensin II type 2 receptor stimulation by compound 21 with memantine on prevention of cognitive decline in type 2 diabetic mice. Eur. J. Pharmacol. 2014, 724, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.N.; Ali, Q.; Samuel, P.; Steckelings, U.M.; Hussain, T. Angiotensin II type 2 receptor and receptor Mas are colocalized and functionally interdependent in obese zucker rat kidney. Hypertension 2017, 70, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Numakawa, T.; Suzuki, S.; Kumamaru, E.; Adachi, N.; Richards, M.; Kunugi, H. BDNF function and intracellular signaling in neurons. Histol. Histopathol. 2010, 25, 237–258. [Google Scholar] [PubMed]
- Becker, B.K.; Wang, H.J.; Tian, C.; Zucker, I.H. BDNF contributes to angiotensin II-mediated reductions in peak voltage-gated K+ current in cultured CATH.a cells. Physiol. Rep. 2015, 3, e12598. [Google Scholar] [CrossRef] [PubMed]
- Parkhurst, C.N.; Yang, G.; Ninan, I.; Savas, J.N.; Yates, J.R., 3rd; Lafaille, J.J.; Hempstead, B.L.; Littman, D.R.; Gan, W.B. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 2013, 155, 1596–1609. [Google Scholar] [CrossRef] [PubMed]
- Umschweif, G.; Liraz-Zaltsman, S.; Shabashov, D.; Alexandrovich, A.; Trembovler, V.; Horowitz, M.; Shohami, E. Angiotensin receptor type 2 activation induces neuroprotection and neurogenesis after traumatic brain injury. Neurotherapeutics 2014, 11, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Savaskan, E.; Hock, C.; Olivieri, G.; Bruttel, S.; Rosenberg, C.; Hulette, C.; Muller-Spahn, F. Cortical alterations of angiotensin converting enzyme, angiotensin II and AT1 receptor in Alzheimer’s dementia. Neurobiol. Aging 2001, 22, 541–546. [Google Scholar] [CrossRef]
- Ho, J.K.; Nation, D.A. Memory is preserved in older adults taking AT1 receptor blockers. Alzheimers Res. Ther. 2017, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Debette, S.; Markus, H.S. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: Systematic review and meta-analysis. BMJ 2010, 341, c3666. [Google Scholar] [CrossRef] [PubMed]
- Levi Marpillat, N.; Macquin-Mavier, I.; Tropeano, A.I.; Bachoud-Levi, A.C.; Maison, P. Antihypertensive classes, cognitive decline and incidence of dementia: A network meta-analysis. J. Hypertens. 2013, 31, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Wharton, W.; Goldstein, F.C.; Zhao, L.; Steenland, K.; Levey, A.I.; Hajjar, I. Modulation of renin-angiotensin system may slow conversion from mild cognitive impairment to Alzheimer’s disease. J. Am. Geriatr. Soc. 2015, 63, 1749–1756. [Google Scholar] [CrossRef] [PubMed]
- Yasar, S.; Xia, J.; Yao, W.; Furberg, C.D.; Xue, Q.L.; Mercado, C.I.; Fitzpatrick, A.L.; Fried, L.P.; Kawas, C.H.; Sink, K.M.; et al. Antihypertensive drugs decrease risk of Alzheimer disease: Ginkgo evaluation of memory study. Neurology 2013, 81, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.; Booth, A.; Peters, R. Potential for specific dihydropyridine calcium channel blockers to have a positive impact on cognitive function in humans: A systematic review. Ther. Adv. Chronic Dis. 2015, 6, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.F.; Kataoka, K.; Tokutomi, Y.; Nako, H.; Nakamura, T.; Toyama, K.; Sueta, D.; Koibuchi, N.; Yamamoto, E.; Ogawa, H.; et al. Perindopril, a centrally active angiotensin-converting enzyme inhibitor, prevents cognitive impairment in mouse models of Alzheimer’s disease. FASEB J. 2011, 25, 2911–2920. [Google Scholar] [CrossRef] [PubMed]
- Panahpour, H.; Dehghani, G.A.; Bohlooli, S. Enalapril attenuates ischaemic brain oedema and protects the blood-brain barrier in rats via an anti-oxidant action. Clin. Exp. Pharmacol. Physiol. 2014, 41, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.R.; Abo-Youssef, A.M.; Messiha, B.A.; Khattab, M.M. Tempol and perindopril protect against lipopolysaccharide-induced cognition impairment and amyloidogenesis by modulating brain-derived neurotropic factor, neuroinflammation and oxido-nitrosative stress. Naunyn Schmiedebergs Arch. Pharmacol. 2016, 389, 637–656. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, T.G.; Smith, V.; Raghu, G. Development of novel agents for idiopathic pulmonary fibrosis: Progress in target selection and clinical trial design. Chest 2015, 148, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Hugo, J.; Ganguli, M. Dementia and cognitive impairment: Epidemiology, diagnosis, and treatment. Clin. Geriatr. Med. 2014, 30, 421–442. [Google Scholar] [CrossRef] [PubMed]
- Labandeira-Garcia, J.L.; Rodriguez-Pallares, J.; Villar-Cheda, B.; Rodriguez-perez, A.I.; garrido-Gil, P.; Guerra, M.J. Aging, angiotensin system and dopaminergic degeneration in the substantia nigra. Agining Dis. 2011, 2, 257–274. [Google Scholar]
- De Kloet, A.D.; Krause, E.G.; Woods, S.C. The renin angiotensin system and the metabolic syndrome. Physiol. Behav. 2010, 100, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Kisley, L.R.; Sakai, R.R.; Fluharty, S.J. Estrogen decreases hypothalamic angiotensin II AT1 receptor binding and mRNA in the female rat. Brain Res. 1999, 844, 34–42. [Google Scholar] [CrossRef]
- Dean, S.A.; Tan, J.; O’Brien, E.R.; Leenen, F.H. 17β-estradiol downregulates tissue angiotensin-converting enzyme and ANG II type 1 receptor in female rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R759–R766. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Perez, A.I.; Valenzuela, R.; Villar-Cheda, B.; Guerra, M.J.; Labandeira-Garcia, J.L. Dopaminergic neuroprotection of hormonal replacement therapy in young and aged menopausal rats: Role of the brain angiotensin system. Brain 2012, 135 Pt 1, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Johnren, O.; Sanvitto, G.L.; Egidy, G.; Saavedra, J.M. Angiotensin II AT1A receptor mRNA expression is induced by estrogen–progesterone in dopaminergic neurons of the female rat arcuate nucleus. J. Neurosci. 1997, 17, 8283–8292. [Google Scholar]
- Dai, S.Y.; Zhang, Y.P.; Peng, W.; Shen, Y.; He, J.J. Central infusion of angiotensin II type 2 receptor agonist compound 21 attenuates DOCA/NaCl-induced hypertension in female rats. Oxid. Med. Cell. Longev. 2016, 2016, 3981790. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Zhang, Z.; Beltz, T.G.; Guo, F.; Hay, M.; Johnson, A.K. Estrogen regulation of the brain renin-angiotensin system in protection against angiotensin II-induced sensitization of hypertension. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H191–H198. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, A.; Oryan, S.; Nematbakhsh, M. Role of Mas receptor antagonist (A779) on pressure diuresis and natriuresis and renal blood flow in the absence of angiotensin II receptors type 1 and 2 in female and male rats. J. Physiol. Pharmacol. 2014, 65, 633–639. [Google Scholar] [PubMed]
- De Morais, S.D.B.; Shanks, J.; Zucker, I.H. Integrative physiological aspects of brain ras in hypertension. Curr. Hypertens. Rep. 2018, 20, 10. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, L.; Menikdiwela, K.; LeMieux, M.; Dufour, J.M.; Kaur, G.; Kalupahana, N.; Moustaid-Moussa, N. The renin angiotensin system, oxidative stress and mitochondrial function in obesity and insulin resistance. Biochim. Biophys. Acta 2017, 1863, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jackson, L.; Eldahshan, W.; Fagan, S.C.; Ergul, A. Within the Brain: The Renin Angiotensin System. Int. J. Mol. Sci. 2018, 19, 876. https://doi.org/10.3390/ijms19030876
Jackson L, Eldahshan W, Fagan SC, Ergul A. Within the Brain: The Renin Angiotensin System. International Journal of Molecular Sciences. 2018; 19(3):876. https://doi.org/10.3390/ijms19030876
Chicago/Turabian StyleJackson, LaDonya, Wael Eldahshan, Susan C. Fagan, and Adviye Ergul. 2018. "Within the Brain: The Renin Angiotensin System" International Journal of Molecular Sciences 19, no. 3: 876. https://doi.org/10.3390/ijms19030876
APA StyleJackson, L., Eldahshan, W., Fagan, S. C., & Ergul, A. (2018). Within the Brain: The Renin Angiotensin System. International Journal of Molecular Sciences, 19(3), 876. https://doi.org/10.3390/ijms19030876