Fraxinellone Attenuates Rheumatoid Inflammation in Mice
Abstract
1. Introduction
2. Results
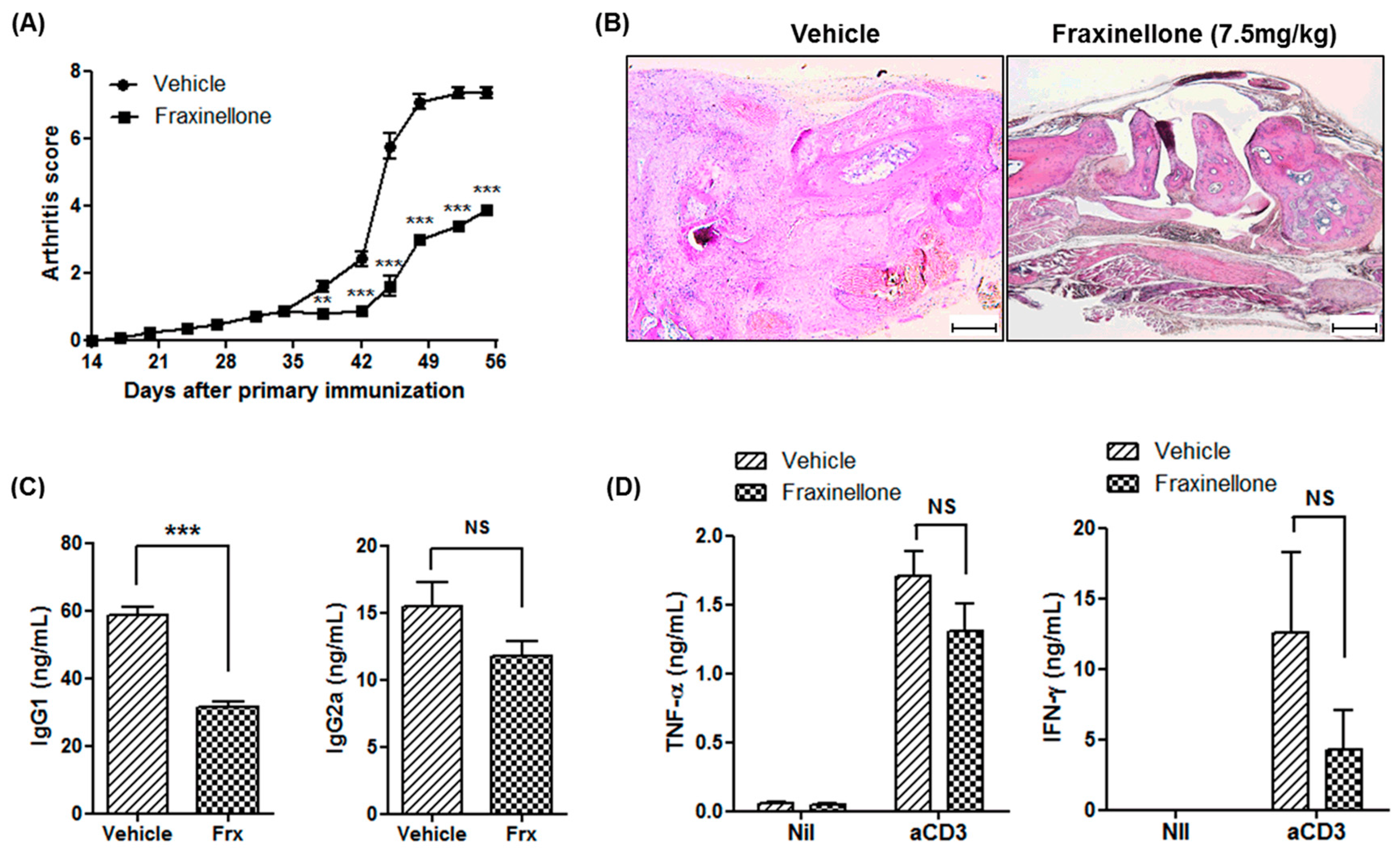
2.1. Fraxinellone Alleviates Inflammatory Arthritis in CIA Mice
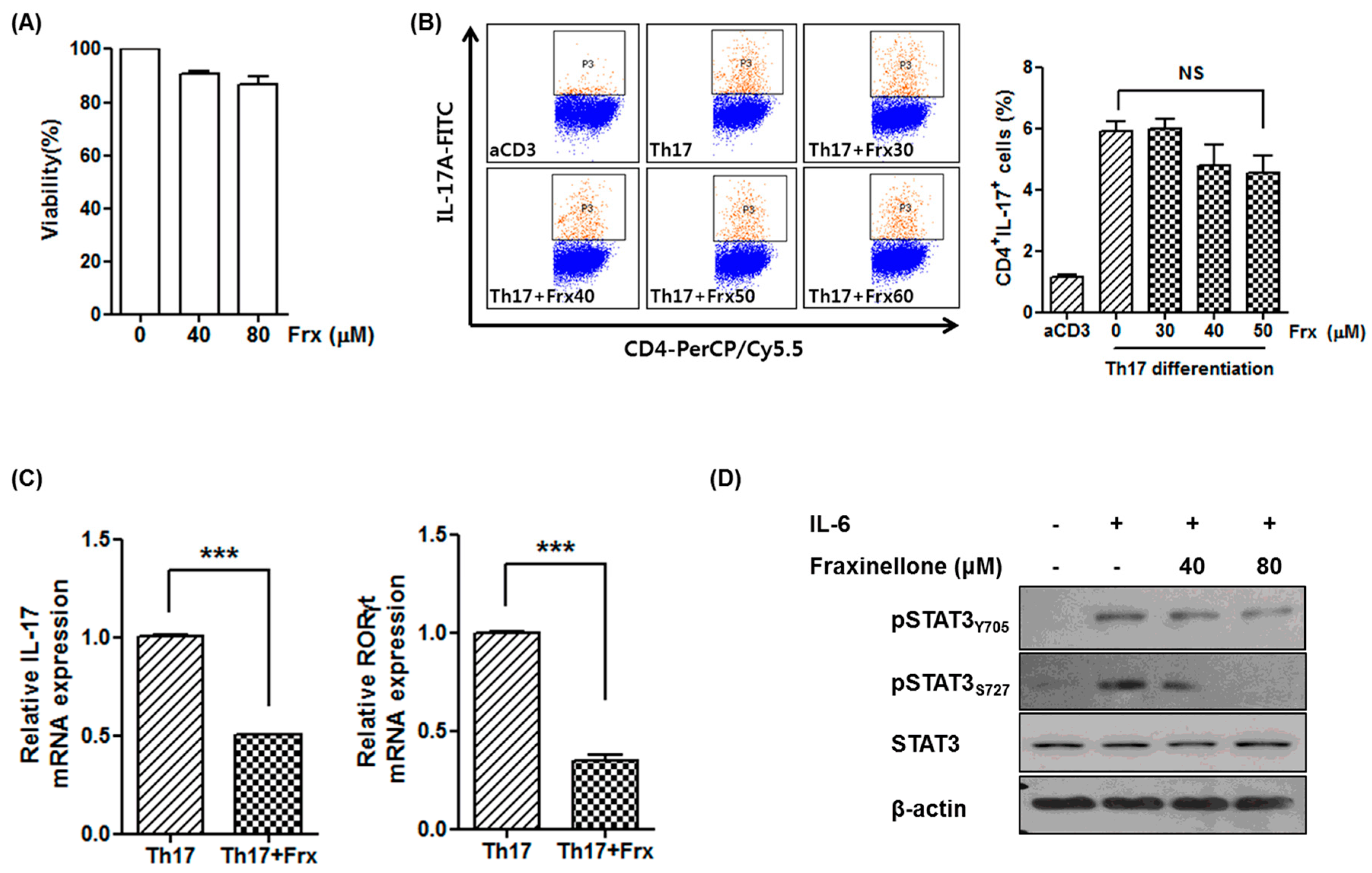
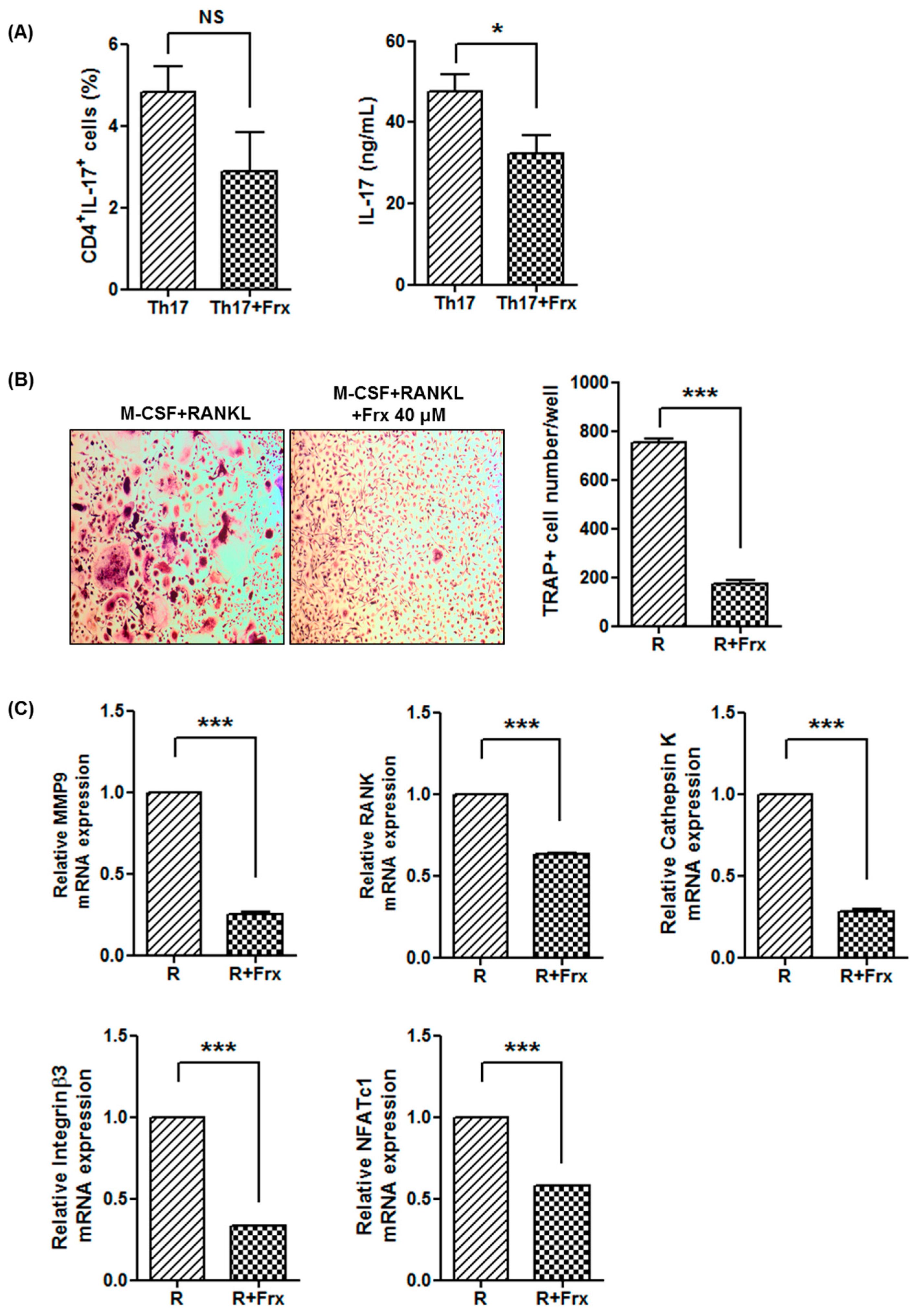
2.2. Fraxinellone Suppresses a Th17 Cell-Related Pathway
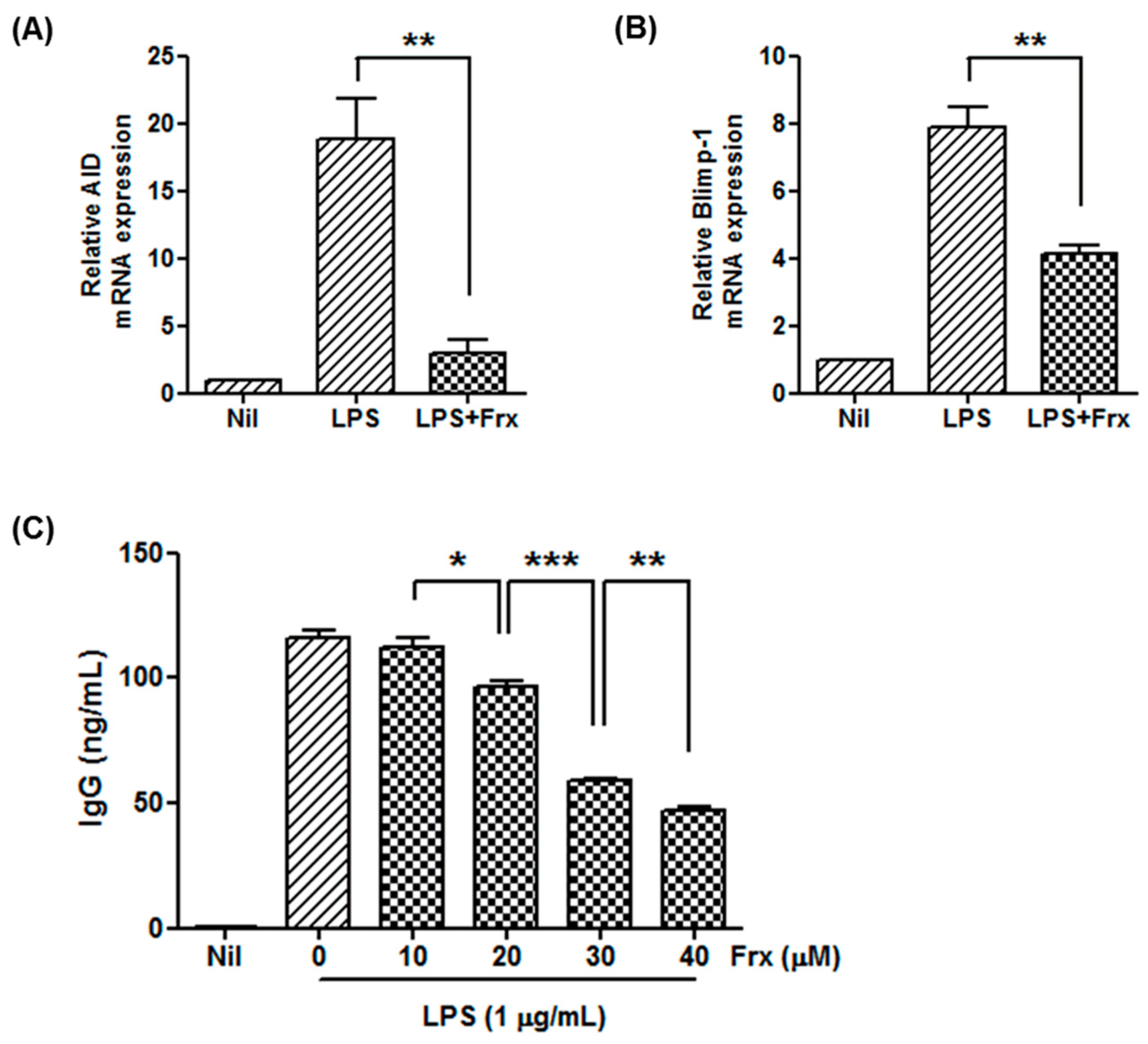
2.3. Fraxinellone Controls B Cell Function
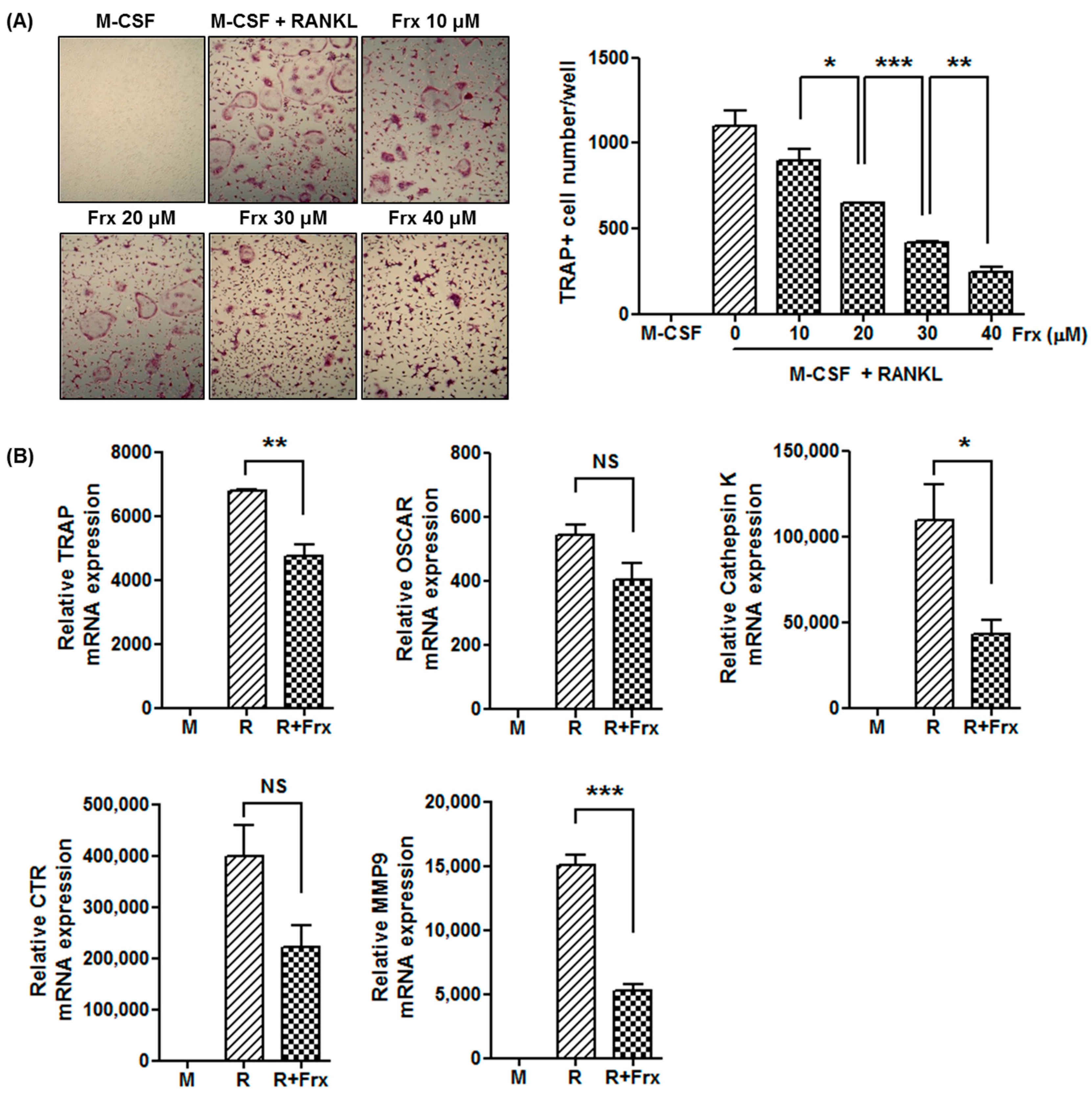
2.4. Fraxinellone Inhibits Murine Osteoclastogenesis
2.5. Fraxinellone Inhibits Th17 Differentiation and Osteoclastogenesis in Human
3. Discussion
4. Materials and Methods
4.1. Induction of CIA and Treatment with Fraxinellone
4.2. Assessment of Arthritis
4.3. Histological Evaluation
4.4. Analysis of Immunoglobulin G
4.5. Cytokine Measurement
4.6. Cytotoxicity
4.7. Culture of CD4+ T Cells and CD19+ B Cells
4.8. Flow Cytometry
4.9. Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR)
4.10. Western Blot Analysis
4.11. Osteoclastogenesis Assay
4.12. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| RA | Rhematoid arthritis |
| IL | Interleukin |
| Th17 | T helper cell producing IL-17 |
| TNF-α | Tumor necrosis factor-alpha |
| RANK | Receptor activator of nuclear factor-κB |
| RANKL | Receptor activator of nuclear factor-κB ligand |
| DMARD | Disease modifying anti-rheumatic drug |
| CIA | Collagen-induced arthritis |
| Ig | Immunoglobulin |
| IFN-γ | Interferon-gamma |
| ELISA | Enzyme-linked immunosorbent assay |
| SEM | Standard error of the mean |
| RORγt | RAR-related orphan receptor γ t |
| STAT3 | Signal transducer and activator of transcription 3 |
| pSTAT3 | Phospho-signal transducer and activator of transcription 3 |
| RT-PCR | Reverse transcriptase-polymerase chain reaction |
| Blimp-1 | B lymphocyte-induced maturation protein-1 |
| AID | Activation-induced cytidine deaminase |
| LPS | Lipopolysaccharide |
| M-CSF | Macrophage-colony stimulating factor |
| TRAP | Tartrate resistant acid phosphatase |
| MMP9 | Matrix metalloproteinase 9 |
| OSCAR | Osteoclast-associated immunoglobulin-like receptor |
| CTR | Calcitonin receptor |
| NFATc1 | Nuclear factor of activated T-cells 1. |
| NF-κB | Nuclear factor-κB |
References
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef] [PubMed]
- Miossec, P.; Korn, T.; Kuchroo, V.K. Interleukin-17 and type 17 helper T cells. N. Engl. J. Med. 2009, 361, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.B.; Emery, P.; Greenwald, M.W.; Dougados, M.; Furie, R.A.; Genovese, M.C.; Keystone, E.C.; Loveless, J.E.; Burmester, G.R.; Cravets, M.W.; et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006, 54, 2793–2806. [Google Scholar] [CrossRef] [PubMed]
- Emery, P.; Fleischmann, R.; Filipowicz-Sosnowska, A.; Schechtman, J.; Szczepanski, L.; Kavanaugh, A.; Racewicz, A.J.; van Vollenhoven, R.F.; Li, N.F.; Agarwal, S.; et al. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: Results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis Rheum. 2006, 54, 1390–1400. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.L.; Wolfe, F.; Huizinga, T.W. Rheumatoid arthritis. Lancet 2010, 376, 1094–1108. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, S.P.; Chang, H.T.; Wang, Y.T.; Tu, P.F. Pressurized liquid extraction followed by high-performance liquid chromatography for determination of seven active compounds in Cortex Dictamni. J. Chromatogr. A 2006, 1108, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, X.; Wang, P.; Li, L.; Qu, W.; Liang, J. Antimicrobial, antioxidant and cytotoxic properties of essential oil from Dictamnus angustifolius. J. Ethnopharmacol. 2015, 159, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.S.; Byun, E.; Li, B.; Lee, D.S.; Kim, Y.C.; An, R.B. Neuroprotective effects of constituents of the root bark of Dictamnus dasycarpus in mouse hippocampal cells. Arch. Pharm. Res. 2010, 33, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.S.; Yang, H.; Kim, S.H.; Sung, S.H.; Kim, Y.C. Limonoids from Dictamnus dasycarpus protect against glutamate-induced toxicity in primary cultured rat cortical cells. J. Mol. Neurosci. 2010, 42, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, Y.M.; Shin, J.S.; Park, S.J.; Choi, J.H.; Jung, H.J.; Park, H.J.; Lee, K.T. Fraxinellone inhibits lipopolysaccharide-induced inducible nitric oxide synthase and cyclooxygenase-2 expression by negatively regulating nuclear factor-κB in RAW 264.7 macrophages cells. Biol. Pharm. Bull. 2009, 32, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Won, C.; Yoo, H.; Yi, E.H.; Cho, Y.; Maeng, J.W.; Sung, S.H.; Ye, S.K.; Chung, M.H. Inhibition of double-stranded RNA-induced inducible nitric oxide synthase expression by fraxinellone and sauchinone in murine microglia. Biol. Pharm. Bull. 2009, 32, 1870–1874. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, H.; Kim, M.; Kim, H.; Lee, G.S.; An, W.G.; Cho, S.I. Anti-inflammatory activities of Dictamnus dasycarpus Turcz., root bark on allergic contact dermatitis induced by dinitrofluorobenzene in mice. J. Ethnopharmacol. 2013, 149, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.F.; Ouyang, Z.J.; Feng, L.L.; Chen, G.; Guo, W.J.; Shen, Y.; Wu, X.D.; Sun, Y.; Xu, Q. Suppression of NF-κB signaling and NLRP3 inflammasome activation in macrophages is responsible for the amelioration of experimental murine colitis by the natural compound fraxinellone. Toxicol. Appl. Pharmacol. 2014, 281, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Chen, H.; Zhou, J.; Tai, H.; Gong, H.; Wang, X.; Huang, N.; Qin, J.; Fang, T.; Wang, F.; et al. The inhibitory effect in Fraxinellone on oxidative stress-induced senescence correlates with AMP-activated protein kinase-dependent autophagy restoration. J. Cell. Physiol. 2017, 233, 3945–3954. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Qin, Y.; Gong, F.Y.; Wu, X.F.; Hua, Z.C.; Chen, T.; Xu, Q. Selective triggering of apoptosis of concanavalin A-activated T cells by fraxinellone for the treatment of T-cell-dependent hepatitis in mice. Biochem. Pharmacol. 2009, 77, 1717–1724. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Nakano, Y.; Rahman, M.A.; Yatsuzuka, R.; Kamei, C. Effects of a Dictamnus dasycarpus T. extract on allergic models in mice. Biosci. Biotechnol. Biochem. 2008, 72, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Lee, H.B.; Kim, S.; Park, Y.C.; Kim, K.; Kim, H. Decoction of Dictamnus Dasycarpus Turcz. Root Bark Ameliorates Skin Lesions and Inhibits Inflammatory Reactions in Mice with Contact Dermatitis. Pharmacogn. Mag. 2017, 13, 483–487. [Google Scholar] [PubMed]
- Kunwar, S.; Dahal, K.; Sharma, S. Anti-IL-17 therapy in treatment of rheumatoid arthritis: A systematic literature review and meta-analysis of randomized controlled trials. Rheumatol. Int. 2016, 36, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Van Baarsen, L.G.; Lebre, M.C.; van der Coelen, D.; Aarrass, S.; Tang, M.W.; Ramwadhdoebe, T.H.; Gerlag, D.M.; Tak, P.P. Heterogeneous expression pattern of interleukin 17A (IL-17A), IL-17F and their receptors in synovium of rheumatoid arthritis, psoriatic arthritis and osteoarthritis: Possible explanation for nonresponse to anti-IL-17 therapy? Arthritis Res. Ther. 2014, 16, 426. [Google Scholar] [CrossRef] [PubMed]
- Gaffen, S.L. Structure and signalling in the IL-17 receptor family. Nat. Rev. Immunol. 2009, 9, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Veldhoen, M. Interleukin 17 is a chief orchestrator of immunity. Nat. Immunol. 2017, 18, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, M.; Kinoshita, K.; Fagarasan, S.; Yamada, S.; Shinkai, Y.; Honjo, T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 2000, 102, 553–563. [Google Scholar] [CrossRef]
- Turner, C.A., Jr.; Mack, D.H.; Davis, M.M. Blimp-1, a novel zinc finger-containing protein that can drive the maturation of B lymphocytes into immunoglobulin-secreting cells. Cell 1994, 77, 297–306. [Google Scholar] [CrossRef]
- Lee, Z.H.; Kim, H.H. Signal transduction by receptor activator of nuclear factor κB in osteoclasts. Biochem. Biophys. Res. Commun. 2003, 305, 211–214. [Google Scholar] [CrossRef]
- Jimi, E.; Ghosh, S. Role of nuclear factor-κB in the immune system and bone. Immunol. Rev. 2005, 208, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Brand, D.D.; Latham, K.A.; Rosloniec, E.F. Collagen-induced arthritis. Nat. Protoc. 2007, 2, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
| Gene | Sense (5′–3′) | Anti-Sense (5′–3′) | |
|---|---|---|---|
| Mouse | IL-17 | CCTCAAAGCTCAGCGTGTCC | GAGCTCACTTTTGCGCCAAG |
| RORγt | TGTCCTGGGCTACCCTACTG | GTGCAGGAGTAGGCCACATT | |
| Blimp-1 | CTGTCAGAACGGGATGAACA | TGGGGACACTCTTTGGGTAG | |
| AID | CGTGGTGAAGAGGAGAGATAGTG | CAGTCTGAGATGTAGCGTAGGAA | |
| TRAP | TCCTGGCTCAAAAAGCAGTT | ACATAGCCCACACCGTTCTC | |
| OSCAR | CCTAGCCTCATACCCCCAG | CAAACCGCCAGGCAGATTG | |
| Cathepsin K | CAGCAGAGGTGTGTACTATG | GCGTTGTTCTTACTTCGAGC | |
| CTR | CGGACTTTGACACAGCAGAA | AGCAGCAATCGACAAGGAGT | |
| MMP9 | CTGTCCAGACCAAGGGTACAGCCT | GAGGTATAGTGGGACACATAGTGG | |
| β-Actin | GAAATCGTGCGTGACATCAAAG | TGTAGTTTCATGGATGCCACAG | |
| Human | MMP9 | TGGGGGGCAACTCGGC | GGAATGATCTAAGCCCAG |
| Cathepsin K | TGAGGCTTCTCTTGGTGTCCATAC | AAAGGGTGTCATTACTGCGGG | |
| Integrin β3 | GCAATGGGACCTTTGAGTGT | GTGGCAGACACATTGACCAC | |
| RANK | GCTCTAACAAATGTGAACCA | GCCTTGCCTGTATCACAAAC | |
| NFATc1 | GCATCACAGGGAAGACCGTGTC | GAAGTTCAATGTCGGAGTTTCTGAG | |
| β-actin | GGACTTCGAGCAAGAGATGG | TGTGTTGGGGTACAGGTCTTTG | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.M.; Lee, J.; Baek, S.Y.; Lee, J.; Jang, S.G.; Hong, S.-M.; Park, J.-S.; Cho, M.-L.; Park, S.-H.; Kwok, S.-K. Fraxinellone Attenuates Rheumatoid Inflammation in Mice. Int. J. Mol. Sci. 2018, 19, 829. https://doi.org/10.3390/ijms19030829
Jung SM, Lee J, Baek SY, Lee J, Jang SG, Hong S-M, Park J-S, Cho M-L, Park S-H, Kwok S-K. Fraxinellone Attenuates Rheumatoid Inflammation in Mice. International Journal of Molecular Sciences. 2018; 19(3):829. https://doi.org/10.3390/ijms19030829
Chicago/Turabian StyleJung, Seung Min, Jaeseon Lee, Seung Ye Baek, Juhyun Lee, Se Gwang Jang, Seung-Min Hong, Jin-Sil Park, Mi-La Cho, Sung-Hwan Park, and Seung-Ki Kwok. 2018. "Fraxinellone Attenuates Rheumatoid Inflammation in Mice" International Journal of Molecular Sciences 19, no. 3: 829. https://doi.org/10.3390/ijms19030829
APA StyleJung, S. M., Lee, J., Baek, S. Y., Lee, J., Jang, S. G., Hong, S.-M., Park, J.-S., Cho, M.-L., Park, S.-H., & Kwok, S.-K. (2018). Fraxinellone Attenuates Rheumatoid Inflammation in Mice. International Journal of Molecular Sciences, 19(3), 829. https://doi.org/10.3390/ijms19030829






