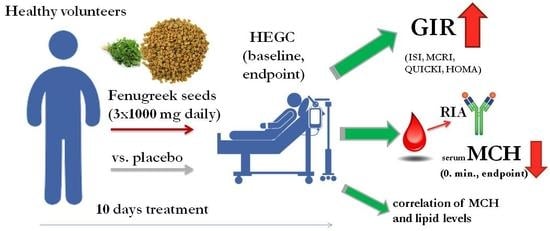
Insulin-Sensitizer Effects of Fenugreek Seeds in Parallel with Changes in Plasma MCH Levels in Healthy Volunteers
Abstract
1. Introduction
1.1. Major Pathologic Features of T2DM
1.2. Mechanisms Contributing to T2DM Onset And Progression
1.3. Countermeasures to T2DM Progression
1.4. MCH Properties
1.5. MCHR Distribution and Major Activities
1.6. Differential Effects of MCH and Relevance to Diabetes Research
1.7. Objectives of Present Investigation
2. Results
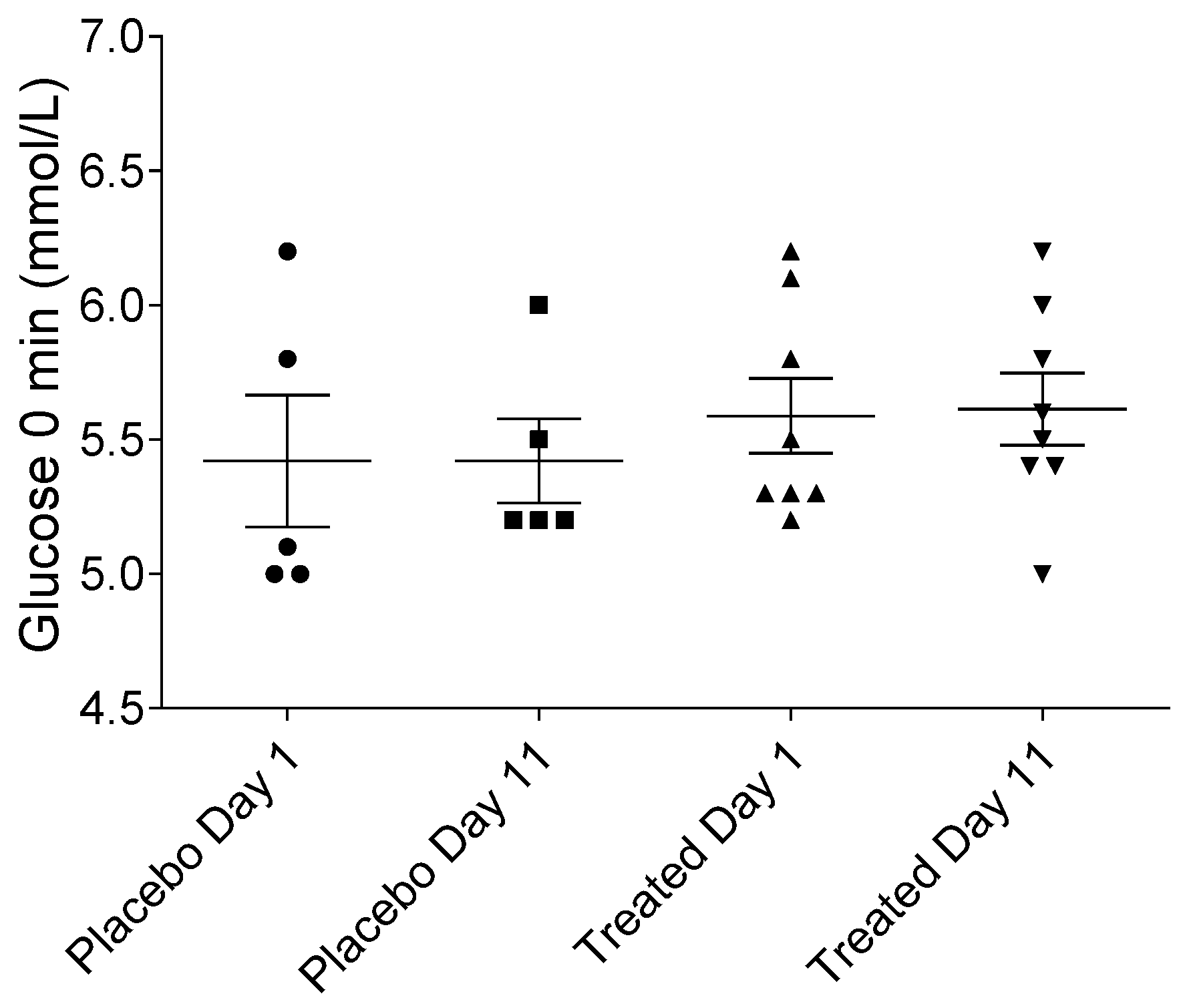
2.1. Fasting Serum Glucose Levels in Placebo- and Fenugreek-Treated Subjects
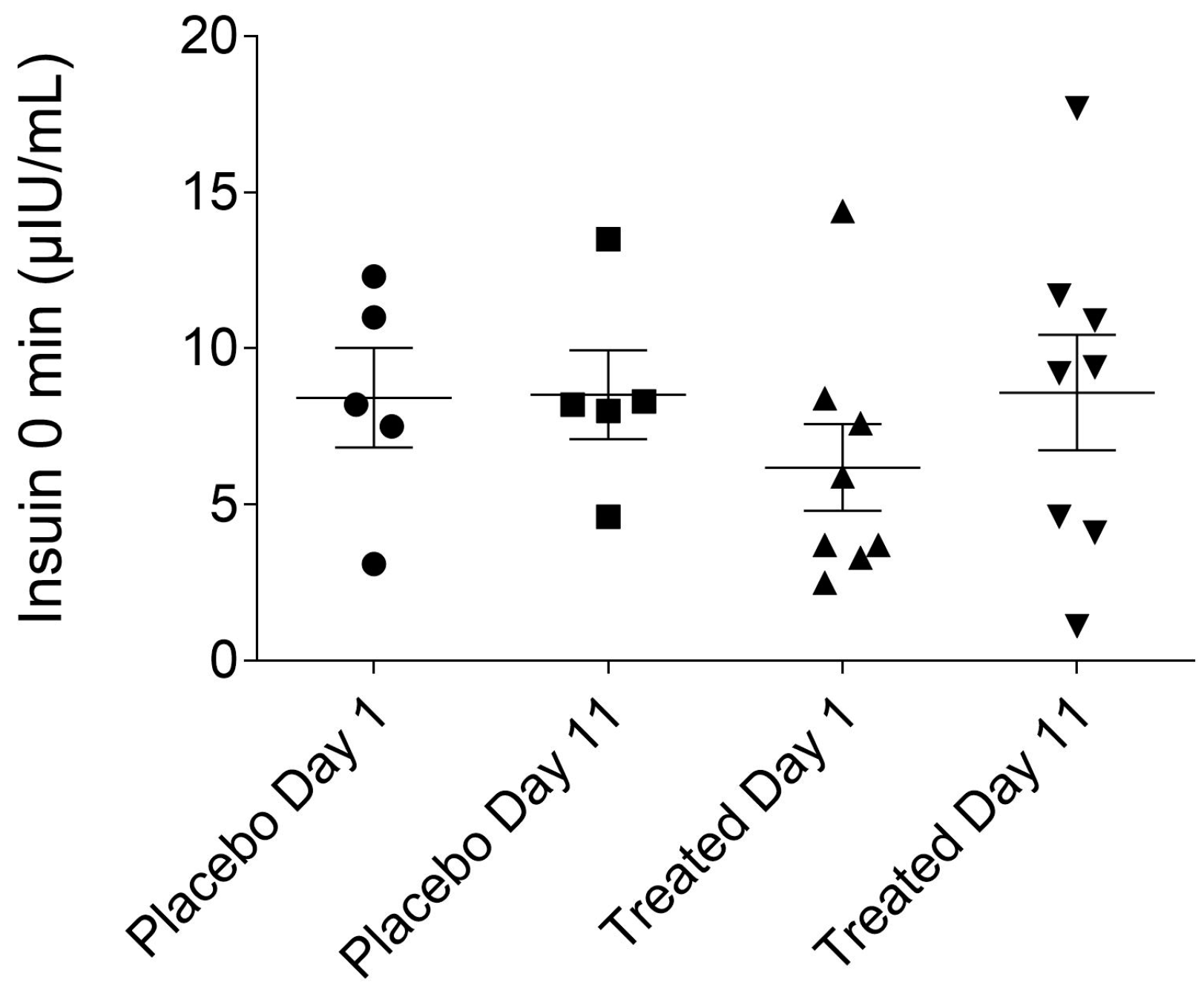
2.2. Fasting Plasma Insulin Levels of the Placebo- and Fenugreek-Treated Subjects
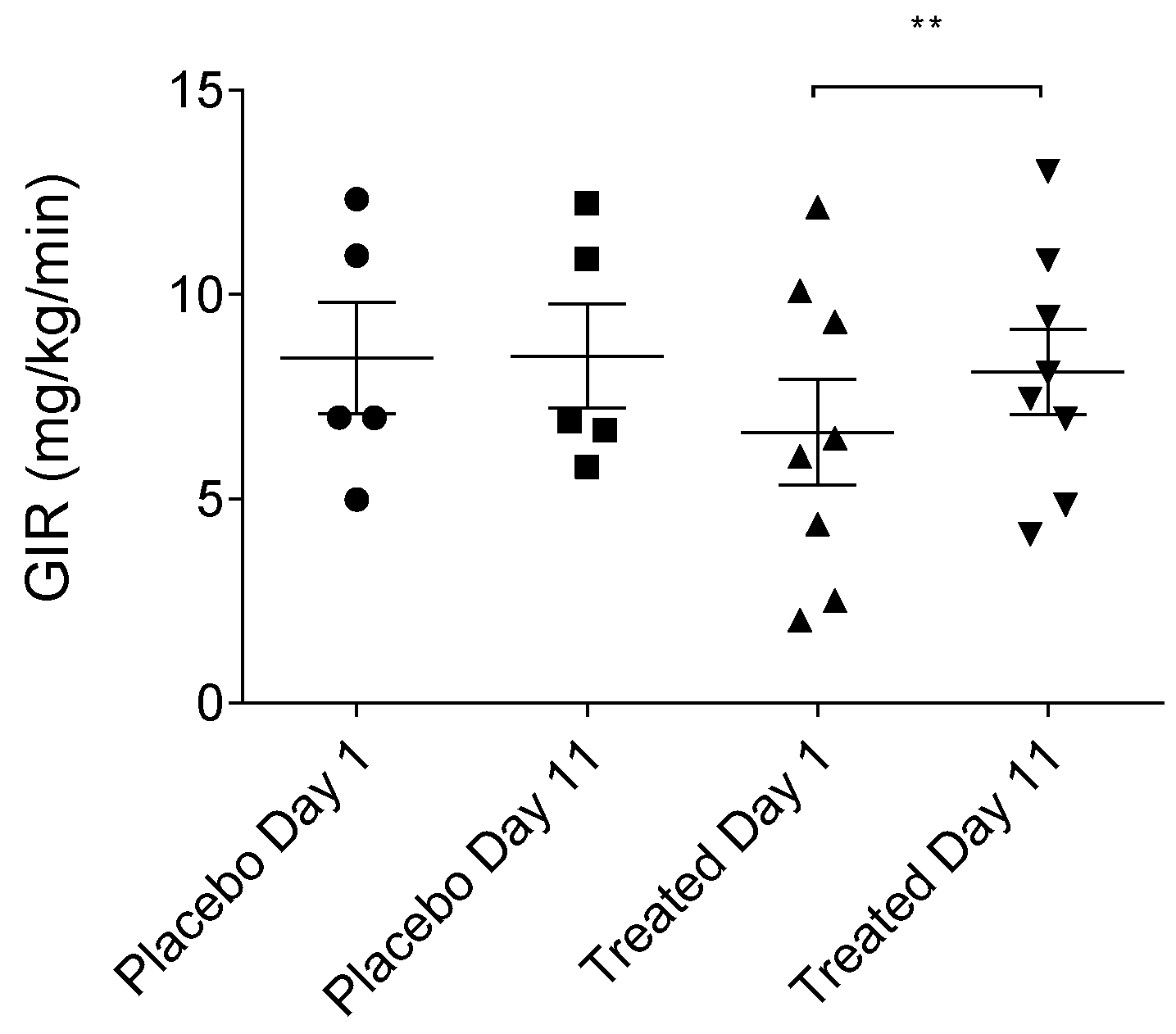
2.3. Effects of TFG on Glucose Infusion Rate (GIR)
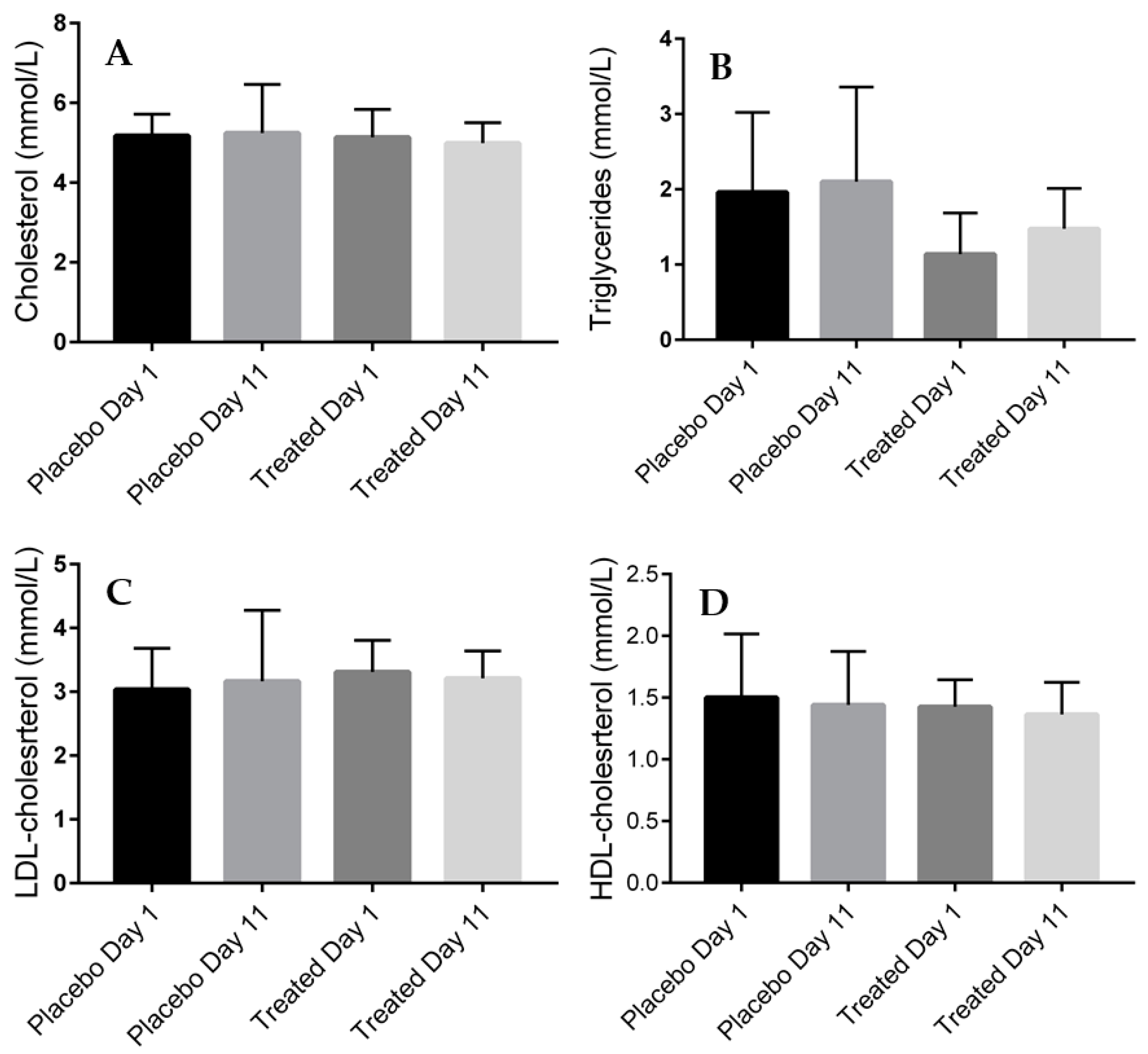
2.4. Serum Lipid Levels of the Placebo- and Fenugreek-Treated Subjects
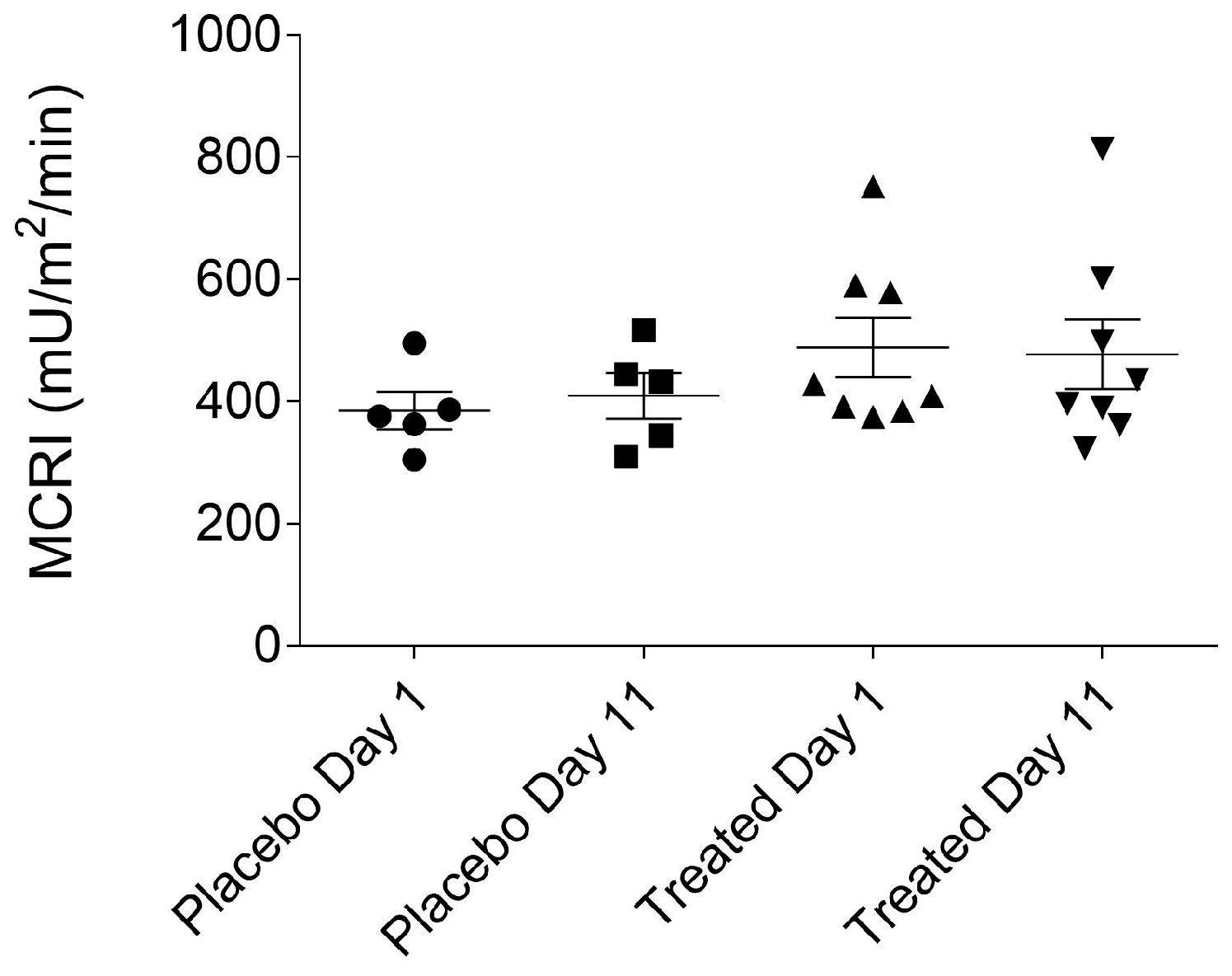
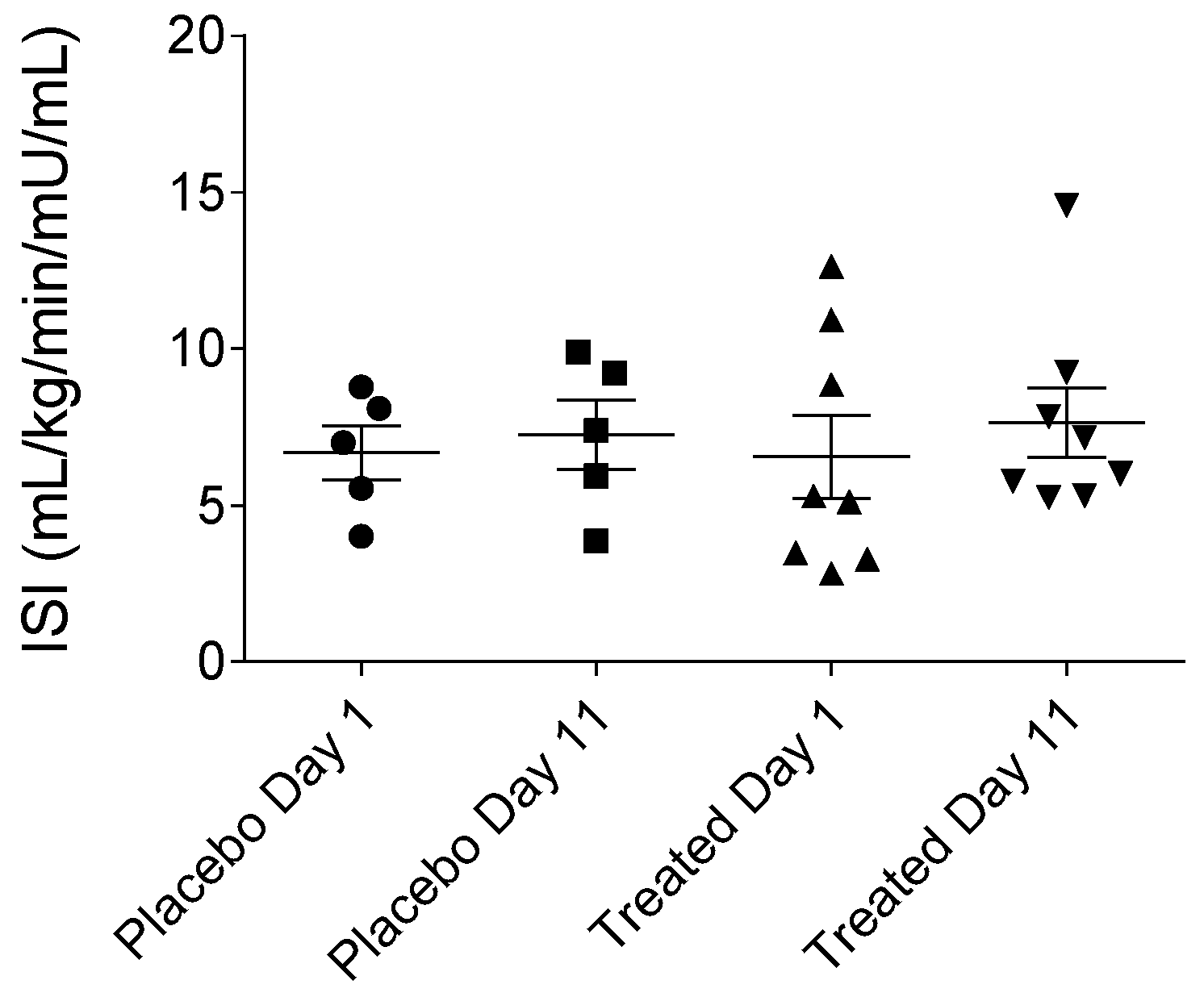
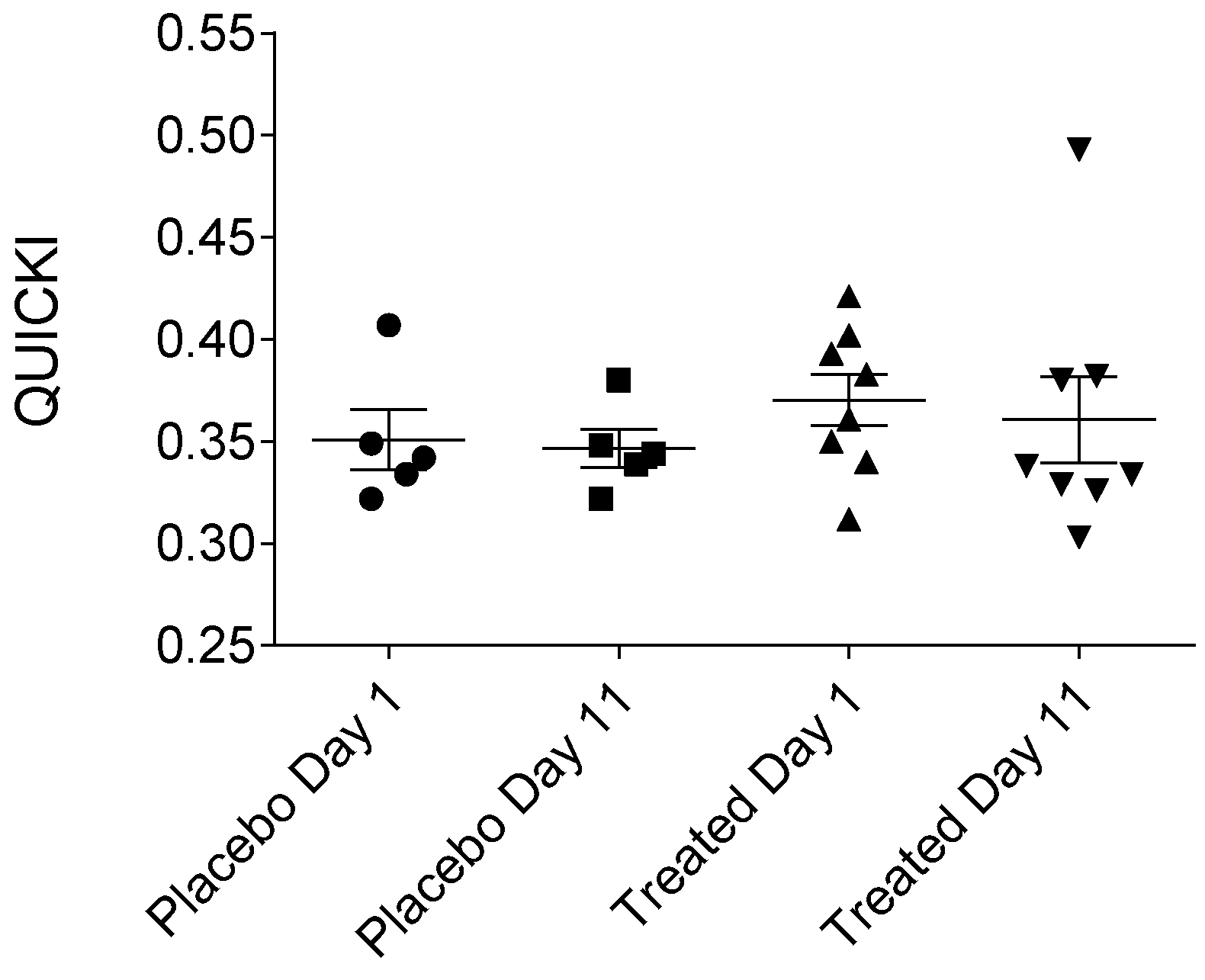
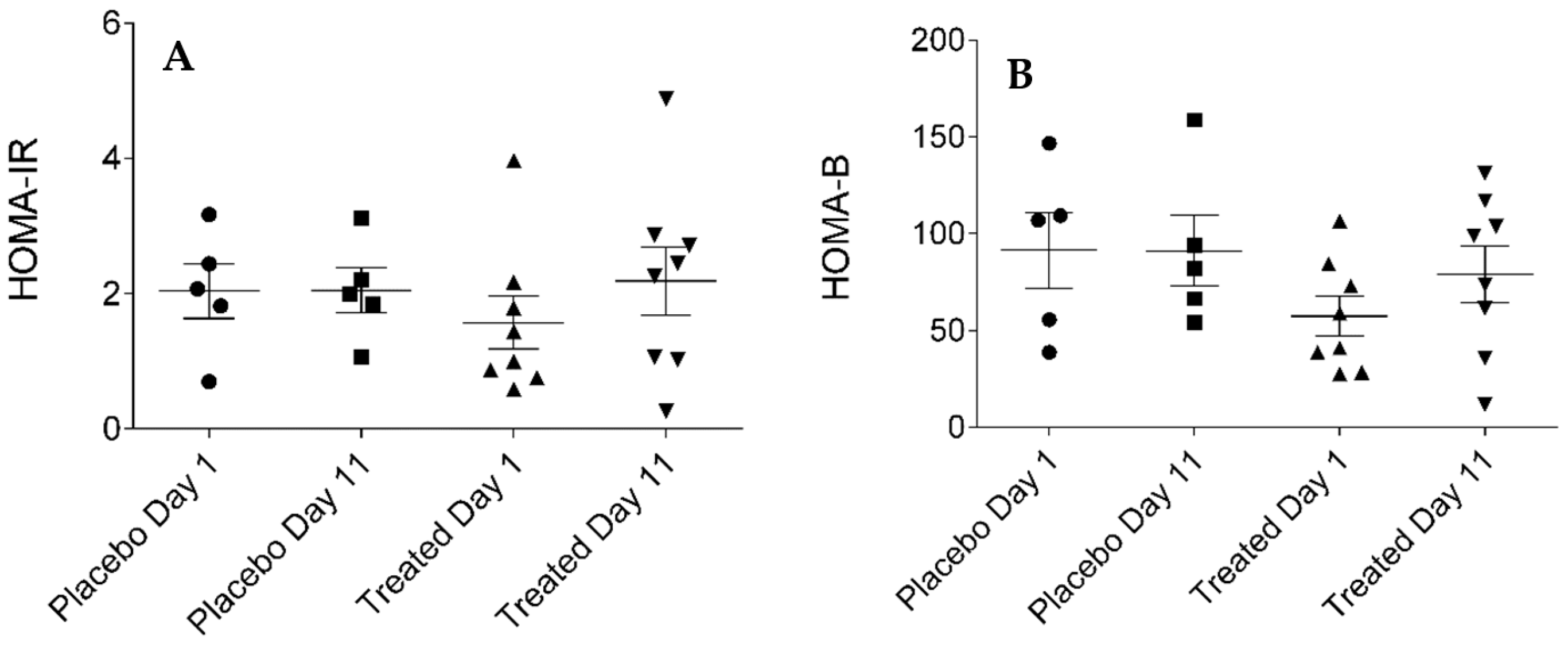
2.5. Calculated Parameters of Insulin Sensitivity Assessment (MCRI, ISI, QUICKI, HOMA-IR and HOMA-%β) in the Placebo- and Fenugreek-Treated Subjects
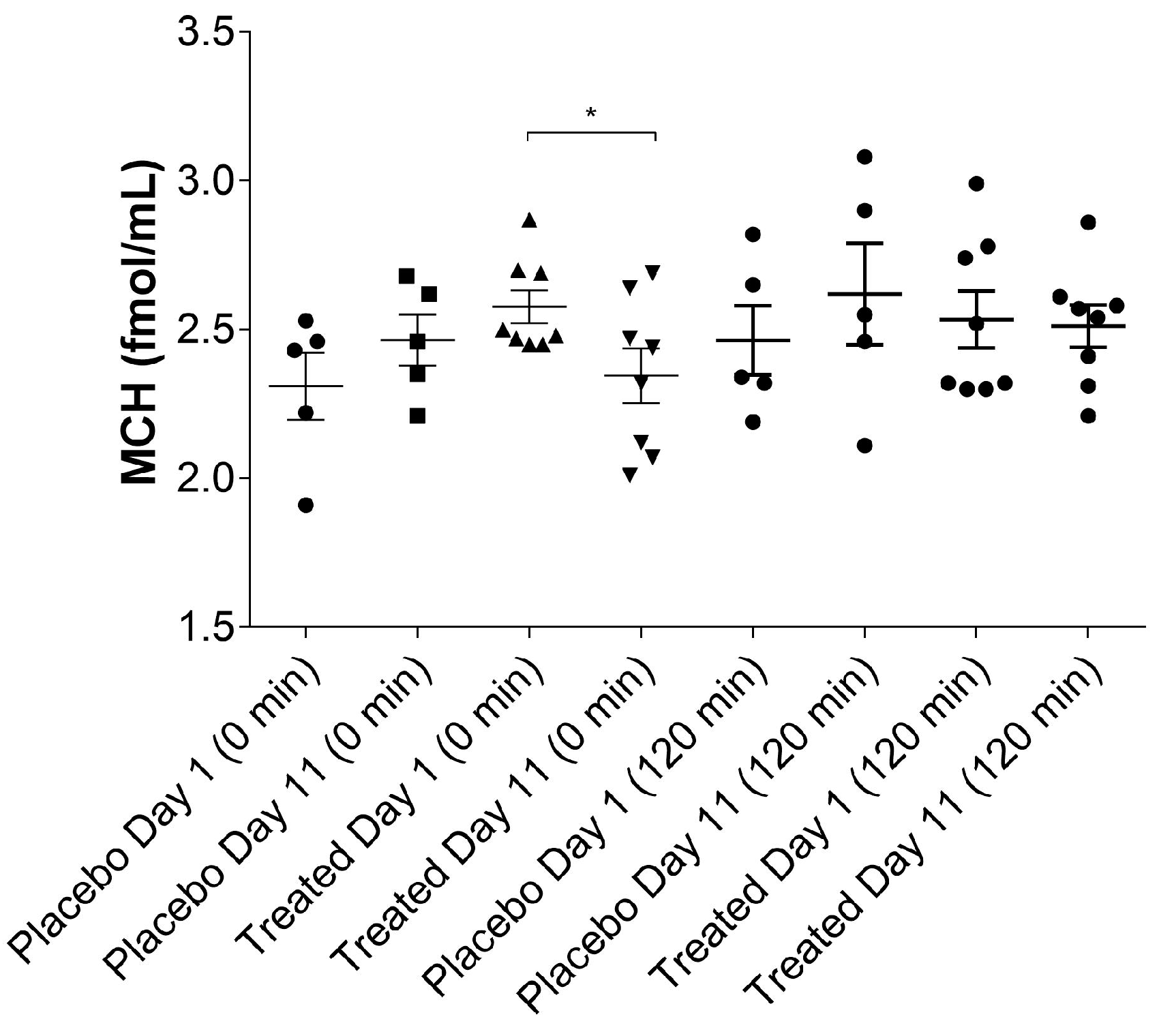
2.6. Effect of TFG on Plasma MCH Levels
2.7. Correlation between MCH and Serum Lipid Levels
2.8. Correlation between MCH and Serum Glucose Levels
2.9. Correlation between MCH and Plasma Insulin Levels
3. Discussion
4. Experimental Section
4.1. Study Design
4.2. Ethical Considerations
4.3. Subject Recruitment
4.4. Inclusion and Exclusion Criteria
4.5. Used Products
4.6. Hyperinsulinemic Euglycemic Glucose Clamp (HEGC)
4.7. Analytical Methods
4.8. Measurement of Melanin-Concentrating Hormone
4.9. Assesment of Insulin-Sensitivity
- GIR = glucose infusion rate [(µL/min)/BW (body weight in kg) × 100] × (20 × 10)
- MCRI = (insulin infusion rate/steady state plasma insulin concentration − basal plasma insulin concentration) × 1000, expressed in mU/m2/min.
- ISI = (glucose infusion rate/steady state plasma insulin concentration) × 100, expressed in mg/kg/min/mU/mL.
- QUICKI = 1/logInsuline(0 min) (µIU/mL) + logGlucose(0 min) (mg/dL).
4.10. Data Analysis and Statistics
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tseung, J. Robbins and Cotran Pathologic Basis of Disease: 7th Edition. Pathology 2005, 37, 190. [Google Scholar] [CrossRef]
- Olokoba, A.B.; Obateru, O.A.; Olokoba, L.B. Type 2 Diabetes Mellitus: A Review of Current Trends. Oman Med. J. 2012, 27, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, L.; Mollica, M.P.; Sica, R.; Donizzetti, I.; Gifuni, G.; Pignalosa, A.; Cavaliere, G.; Putti, R. Differential Effects of High-Fish Oil and High-Lard Diets on Cells and Cytokines Involved in the Inflammatory Process in Rat Insulin-Sensitive Tissues. Int. J. Mol. Sci. 2014, 15, 3040–3063. [Google Scholar] [CrossRef] [PubMed]
- Lowell, B.B.; Shulman, G.I. Mitochondrial Dysfunction and Type 2 Diabetes. Science 2005, 307, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Tubbs, E.; Chanon, S.; Robert, M.; Bendridi, N.; Bidaux, G.; Chauvin, M.-A.; Ji-Cao, J.; Durand, C.; Gauvrit-Ramette, D.; Vidal, H.; et al. Disruption of Mitochondria-Associated Endoplasmic Reticulum Membranes (MAMs) Integrity Contributes to Muscle Insulin Resistance in Mice and Humans. Diabetes 2018. [Google Scholar] [CrossRef] [PubMed]
- Engin, A.B.; Tsatsakis, A.M.; Tsoukalas, D.; Engin, A. Do flavanols-rich natural products relieve obesity-related insulin resistance? Food Chem. Toxicol. 2018, 112, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Kloppel, G.; Lohr, M.; Habich, K.; Oberholzer, M.; Heitz, P.U. Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv. Syn. Pathol. Res. 1985, 4, 110–125. [Google Scholar]
- Kassaian, N.; Azadbakht, L.; Forghani, B.; Amini, M. Effect of fenugreek seeds on blood glucose and lipid profiles in type 2 diabetic patients. Int. J. Vitam. Nutr. Res. 2009, 79, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Tabish, S.A. Is Diabetes Becoming the Biggest Epidemic of the Twenty-first Century? Int. J. Health Sci. 2007, 1, 5–8. [Google Scholar]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Guariguata, L.; Whiting, D.R.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Chiasson, J.-L.; Josse, R.G.; Gomis, R.; Hanefeld, M.; Karasik, A.; Laakso, M. Acarbose for prevention of type 2 diabetes mellitus: The STOP-NIDDM randomised trial. Lancet 2002, 359, 2072–2077. [Google Scholar] [CrossRef]
- Eriksson, K.F.; Lindgarde, F. Prevention of type 2 (non-insulin-dependent) diabetes mellitus by diet and physical exercise. The 6-year Malmo feasibility study. Diabetologia 1991, 34, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C.; Yusuf, S.; Bosch, J.; Pogue, J.; Sheridan, P.; Dinccag, N.; Hanefeld, M.; Hoogwerf, B.; Laakso, M.; Mohan, V.; et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: A randomised controlled trial. Lancet 2006, 368, 1096–1105. [Google Scholar] [PubMed]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [PubMed]
- Basch, E.; Ulbricht, C.; Kuo, G.; Szapary, P.; Smith, M. Therapeutic applications of fenugreek. Altern. Med. Rev. 2003, 8, 20–27. [Google Scholar] [PubMed]
- Al-Asadi, J. Therapeutic Uses of Fenugreek (Trigonella foenum-graecum L.). Am. J. Soc. Issues Hum. 2014. Special issue. [Google Scholar]
- Verma, N.; Usman, K.; Patel, N.; Jain, A.; Dhakre, S.; Swaroop, A.; Bagchi, M.; Kumar, P.; Preuss, H.G.; Bagchi, D. A multicenter clinical study to determine the efficacy of a novel fenugreek seed (Trigonella foenum-graecum) extract (Fenfuro™) in patients with type 2 diabetes. Food Nutr. Res. 2016, 60. [Google Scholar] [CrossRef] [PubMed]
- Losso, J.N.; Holliday, D.L.; Finley, J.W.; Martin, R.J.; Rood, J.C.; Yu, Y.; Greenway, F.L. Fenugreek bread: A treatment for diabetes mellitus. J. Med. Food 2009, 12, 1046–1049. [Google Scholar] [CrossRef] [PubMed]
- Grover, J.K.; Yadav, S.; Vats, V. Medicinal plants of India with anti-diabetic potential. J. Ethnopharmacol. 2002, 81, 81–100. [Google Scholar] [CrossRef]
- Mathern, J.R.; Raatz, S.K.; Thomas, W.; Slavin, J.L. Effect of Fenugreek Fiber on Satiety, Blood Glucose and Insulin Response and Energy Intake in Obese Subjects. Phytother. Res. 2009, 23, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.A.; Kumar, P. Fenugreek: A review on its nutraceutical properties and utilization in various food products. J. Saudi Soc. Agric. Sci. 2016. [Google Scholar] [CrossRef]
- Sulieman, A.M.; Ahmed, H.E.; Abdelrahim, A.M. The Chemical Composition of Fenugreek (Trigonella foenum graceum L) and the Antimicrobial Properties of its Seed Oil. Gezira J. Eng. Appl. Sci. 2008, 3, 52–71. [Google Scholar]
- Uemura, T.; Goto, T.; Kang, M.S.; Mizoguchi, N.; Hirai, S.; Lee, J.Y.; Nakano, Y.; Shono, J.; Hoshino, S.; Taketani, K.; et al. Diosgenin, the main aglycon of fenugreek, inhibits LXRalpha activity in HepG2 cells and decreases plasma and hepatic triglycerides in obese diabetic mice. J. Nutr. 2011, 141, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Chevassus, H.; Molinier, N.; Costa, F.; Galtier, F.; Renard, E.; Petit, P. A fenugreek seed extract selectively reduces spontaneous fat consumption in healthy volunteers. Eur. J. Clin. Pharmacol. 2009, 65, 1175–1178. [Google Scholar] [CrossRef] [PubMed]
- Tharaheswari, M.; Jayachandra Reddy, N.; Kumar, R.; Varshney, K.C.; Kannan, M.; Sudha Rani, S. Trigonelline and diosgenin attenuate ER stress, oxidative stress-mediated damage in pancreas and enhance adipose tissue PPARγ activity in type 2 diabetic rats. Mol. Cell. Biochem. 2014, 396, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Neelakantan, N.; Narayanan, M.; de Souza, R.J.; van Dam, R.M. Effect of fenugreek (Trigonella foenum-graecum L.) intake on glycemia: A meta-analysis of clinical trials. Nutr. J. 2014, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Kawauchi, H. Chemistry of proopiocortin-related peptides in the salmon pituitary. Arch. Biochem. Biophys. 1983, 227, 343–350. [Google Scholar] [CrossRef]
- Presse, F.; Nahon, J.-L. Mch. In Handbook of Biologically Active Peptides; Academic Press: Cambridge, MA, USA, 2013; pp. 828–837. [Google Scholar]
- David, D.J.; Klemenhagen, K.C.; Holick, K.A.; Saxe, M.D.; Mendez, I.; Santarelli, L.; Craig, D.A.; Zhong, H.; Swanson, C.J.; Hegde, L.G.; et al. Efficacy of the MCHR1 antagonist N-[3-(1-{[4-(3,4-difluorophenoxy)phenyl]methyl}(4-piperidyl))-4-methylphenyl]-2-m ethylpropanamide (SNAP 94847) in mouse models of anxiety and depression following acute and chronic administration is independent of hippocampal neurogenesis. J. Pharmacol. Exp. Ther. 2007, 321, 237–248. [Google Scholar] [PubMed]
- Gavrila, A.; Chan, J.L.; Miller, L.C.; Heist, K.; Yiannakouris, N.; Mantzoros, C.S. Circulating melanin-concentrating hormone, agouti-related protein, and alpha-melanocyte-stimulating hormone levels in relation to body composition: Alterations in response to food deprivation and recombinant human leptin administration. J. Clin. Endocrinol. Metab. 2005, 90, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Philippe, C.; Nics, L.; Zeilinger, M.; Kuntner, C.; Wanek, T.; Mairinger, S.; Shanab, K.; Spreitzer, H.; Viernstein, H.; Wadsak, W.; et al. Preclinical in vitro & in vivo evaluation of [11C]SNAP-7941—The first PET tracer for the melanin-concentrating hormone receptor 1. Nucl. Med. Biol. 2013, 40, 919–925. [Google Scholar] [PubMed]
- Imbernon, M.; Beiroa, D.; Vázquez, M.J.; Morgan, D.A.; Veyrat-Durebex, C.; Porteiro, B.; Díaz-Arteaga, A.; Senra, A.; Busquets, S.; Velásquez, D.A.; et al. Central Melanin-Concentrating Hormone Influences Liver and Adipose Metabolism Via Specific Hypothalamic Nuclei and Efferent Autonomic/JNK1 Pathways. Gastroenterology 2013, 144, 636–649. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.I. Melanin-concentration hormone updated functional considerations. Trends Endocrinol. Metab. 1994, 5, 120–126. [Google Scholar] [CrossRef]
- Lelesz, B.; Szilvássy, Z.; Tóth, G.K.; Tóth, A.; Enyedi, A.; Felszeghy, E.; Varga, A.; Juhász, B.; Németh, J. Radioanalytical methods for the measurement of melanin-concentrating hormone (MCH) and detection its receptor in rat tissues. J. Radioanal. Nucl. Chem. 2016, 310, 1325–1333. [Google Scholar] [CrossRef]
- Naufahu, J.; Alzaid, F.; Fiuza Brito, M.; Doslikova, B.; Valencia, T.; Cunliffe, A.; Murray, J.F. Melanin-concentrating hormone in peripheral circulation in the human. J. Endocrinol. 2017, 232, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Nahon, J.-L. The melanocortins and melanin-concentrating hormone in the central regulation of feeding behavior and energy homeostasis. C. R. Biol. 2006, 329, 623–638. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.K.; Cheon, H.G. Promising strategies for obesity pharmacotherapy: Melanocortin-4 (MC-4) receptor agonists and melanin-concentrating hormone (MCH) receptor-1 antagonists. Curr. Top. Med. Chem. 2009, 9, 504–538. [Google Scholar] [CrossRef] [PubMed]
- MacNeil, D.J. The Role of Melanin-Concentrating Hormone and Its Receptors in Energy Homeostasis. Front. Endocrinol. 2013, 4, 49. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Li, Y.; Fu, X.; Wang, J.; Zhang, S.; Yang, L. Profiling the Interaction Mechanism of Quinoline/Quinazoline Derivatives as MCHR1 Antagonists: An in Silico Method. Int. J. Mol. Sci. 2014, 15, 15475–15502. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Behan, J.; O′Neill, K.; Weig, B.; Fried, S.; Laz, T.; Bayne, M.; Gustafson, E.; Hawes, B.E. Identification and Pharmacological Characterization of a Novel Human Melanin-concentrating Hormone Receptor, MCH-R2. J. Biol. Chem. 2001, 276, 34664–34670. [Google Scholar] [CrossRef] [PubMed]
- Kawata, Y.; Okuda, S.; Hotta, N.; Igawa, H.; Takahashi, M.; Ikoma, M.; Kasai, S.; Ando, A.; Satomi, Y.; Nishida, M.; et al. A novel and selective melanin-concentrating hormone receptor 1 antagonist ameliorates obesity and hepatic steatosis in diet-induced obese rodent models. Eur. J. Pharmacol. 2017, 796, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Tadayyon, M.; Welters, H.J.; Haynes, A.C.; Cluderay, J.E.; Hervieu, G. Expression of melanin-concentrating hormone receptors in insulin-producing cells: MCH stimulates insulin release in RINm5F and CRI-G1 cell-lines. Biochem. Biophys. Res. Commun. 2000, 275, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Buijs, R.M.; Chun, S.J.; Niijima, A.; Romijn, H.J.; Nagai, K. Parasympathetic and sympathetic control of the pancreas: A role for the suprachiasmatic nucleus and other hypothalamic centers that are involved in the regulation of food intake. J. Comp. Neurol. 2001, 431, 405–423. [Google Scholar] [CrossRef]
- Pereira-da-Silva, M.; De Souza, C.T.; Gasparetti, A.L.; Saad, M.J.; Velloso, L.A. Melanin-concentrating hormone induces insulin resistance through a mechanism independent of body weight gain. J. Endocrinol. 2005, 186, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Sasmal, P.K.; Sasmal, S.; Abbineni, C.; Venkatesham, B.; Rao, P.T.; Roshaiah, M.; Khanna, I.; Sebastian, V.J.; Suresh, J.; Singh, M.P.; et al. Synthesis and SAR studies of benzimidazole derivatives as melanin-concentrating hormone receptor 1 (MCHR1) antagonists: Focus to detune hERG inhibition. MedChemComm 2011, 2, 385–389. [Google Scholar] [CrossRef]
- Bradley, R.L.; Kokkotou, E.G.; Maratos-Flier, E.; Cheatham, B. Melanin-concentrating hormone regulates leptin synthesis and secretion in rat adipocytes. Diabetes 2000, 49, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Haines, D.D.; Bak, I.; Ferdinandy, P.; Mahmoud, F.F.; Al-Harbi, S.A.; Blasig, I.E.; Tosaki, A. Cardioprotective effects of the calcineurin inhibitor FK506 and the PAF receptor antagonist and free radical scavenger, EGb 761, in isolated ischemic/reperfused rat hearts. J. Cardiovasc. Pharmacol. 2000, 35, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Gomori, A.; Ishihara, A.; Ito, M.; Mashiko, S.; Matsushita, H.; Yumoto, M.; Ito, M.; Tanaka, T.; Tokita, S.; Moriya, M.; et al. Chronic intracerebroventricular infusion of MCH causes obesity in mice. Melanin-concentrating hormone. Am. J. Pathophysiol. Endocrinol. Metab. 2003, 284, E583–E588. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Ludwig, D.S.; Gammeltoft, S.; Piper, M.; Pelleymounter, M.A.; Cullen, M.J.; Mathes, W.F.; Przypek, J.; Kanarek, R.; Maratos-Flier, E. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature 1996, 380, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Della-Zuana, O.; Presse, F.; Ortola, C.; Duhault, J.; Nahon, J.L.; Levens, N. Acute and chronic administration of melanin-concentrating hormone enhances food intake and body weight in Wistar and Sprague–Dawley rats. Int. J. Obes. 2002, 26, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S.; Tritos, N.A.; Mastaitis, J.W.; Kulkarni, R.; Kokkotou, E.; Elmquist, J.; Lowell, B.; Flier, J.S.; Maratos-Flier, E. Melanin-concentrating hormone overexpression in transgenic mice leads to obesity and insulin resistance. J. Clin. Investig. 2001, 107, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Marsh, D.J.; Weingarth, D.T.; Novi, D.E.; Chen, H.Y.; Trumbauer, M.E.; Chen, A.S.; Guan, X.-M.; Jiang, M.M.; Feng, Y.; Camacho, R.E.; et al. Melanin-concentrating hormone 1 receptor-deficient mice are lean, hyperactive, and hyperphagic and have altered metabolism. Proc. Nat. Acad. Sci. USA 2002, 99, 3240–3245. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, C.; Hsu, C.-K.; Zhang, Q.; Bi, C.; Asnicar, M.; Hsiung, H.M.; Fox, N.; Slieker, L.J.; Yang, D.D.; et al. Targeted Disruption of the Melanin-Concentrating Hormone Receptor-1 Results in Hyperphagia and Resistance to Diet-Induced Obesity. Endocrinology 2002, 143, 2469–2477. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jeon, J.Y.; Bradley, R.L.; Kokkotou, E.G.; Marino, F.E.; Wang, X.; Pissios, P.; Maratos-Flier, E. MCH-/- mice are resistant to aging-associated increases in body weight and insulin resistance. Diabetes 2006, 55, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Hervieu, G.; Volant, K.; Grishina, O.; Descroix-Vagne, M.; Nahon, J.-L. Similarities in Cellular Expression and Functions of Melanin-Concentrating Hormone and Atrial Natriuretic Factor in the Rat Digestive Tract. Endocrinology 1996, 137, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Pissios, P.; Ozcan, U.; Kokkotou, E.; Okada, T.; Liew, C.W.; Liu, S.; Peters, J.N.; Dahlgren, G.; Karamchandani, J.; Kudva, Y.C.; et al. Melanin-concentrating hormone Is a Novel Regulator of Islet Function and Growth. Diabetes 2007, 56, 311–319. [Google Scholar] [CrossRef] [PubMed]
- W Schwartz, M.; Gelling, R. Rats lighten up with MCH antagonist. Nat. Med. 2002, 8, 779–781. [Google Scholar] [CrossRef] [PubMed]
- Konadhode, R.R.; Pelluru, D.; Shiromani, P.J. Neurons containing orexin or melanin-concentrating hormone reciprocally regulate wake and sleep. Front. Syst. Neurosci. 2015, 8, 244. [Google Scholar] [CrossRef] [PubMed]
- Hausen, A.C.; Ruud, J.; Jiang, H.; Hess, S.; Varbanov, H.; Kloppenburg, P.; Brüning, J.C. Insulin-Dependent Activation of MCH Neurons Impairs Locomotor Activity and Insulin Sensitivity in Obesity. Cell Rep. 2016, 17, 2512–2521. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yuan, C.; Wei, L.; Yi, F.; Song, F. Melanin-concentrating hormone receptor 2 affects 3T3-L1 preadipocyte differentiation. Mol. Cell. Endocrinol. 2009, 311, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Gaddam, A.; Galla, C.; Thummisetti, S.; Marikanty, R.K.; Palanisamy, U.D.; Rao, P.V. Role of Fenugreek in the prevention of type 2 diabetes mellitus in prediabetes. J. Diabetes Metab. Disord. 2015, 14, 74. [Google Scholar] [CrossRef] [PubMed]
- Rafraf, M.; Malekiyan, M.; Asghari Jafarabadi, M.; Asgarzadeh, A.A.; Pourmoradian, S. Effect of Fenugreek Deeds on Serum Metabolic Factors and ICAM-1 (Intercellular adhesion molecule-1) levels in type 2 Diabetic Patients. ZUMS 2015, 23, 11–21. [Google Scholar]
- Sauvaire, Y.; Petit, P.; Broca, C.; Manteghetti, M.; Baissac, Y.; Fernandez-Alvarez, J.; Gross, R.; Roye, M.; Leconte, A.; Gomis, R.; et al. 4-Hydroxyisoleucine: A Novel Amino Acid Potentiator of Insulin Secretion. Diabetes 1998, 47, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Nagulapalli Venkata, K.C.; Swaroop, A.; Bagchi, D.; Bishayee, A. A small plant with big benefits: Fenugreek (Trigonella foenum-graecum Linn.) for disease prevention and health promotion. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Fuller, S.; Stephens, J.M. Diosgenin, 4-Hydroxyisoleucine, and Fiber from Fenugreek: Mechanisms of Actions and Potential Effects on Metabolic Syndrome. Adv. Nutr. 2015, 6, 189–197. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Tobin, J.D.; Andres, R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am. J. Physiol. 1979, 237, E214–E223. [Google Scholar] [CrossRef] [PubMed]
- Radikova, Z. Assessment of insulin sensitivity/resistance in epidemiological studies. Endocr. Regul. 2003, 37, 189–194. [Google Scholar] [PubMed]
- Muniyappa, R.; Madan, R.; Quon, M.J. Assessing Insulin Sensitivity and Resistance in Humans. In Endotext; De Groot, L.J., Chrousos, G., Dungan, K., Feingold, K.R., Grossman, A., Hershman, J.M., Koch, C., Korbonits, M., McLachlan, R., New, M., et al., Eds.; Endotext: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Gutch, M.; Kumar, S.; Razi, S.; Gupta, K.; Gupta, A. Assessment of insulin sensitivity/resistance. Indian J. Endocrinol. Metab. 2015, 19, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Bradley, R.; Oberg, E.B.; Calabrese, C.; Standish, L.J. Algorithm for complementary and alternative medicine practice and research in type 2 diabetes. J. Altern. Complement. Med. 2007, 13, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Manson, J.E.; Tinker, L.; Howard, B.V.; Kuller, L.H.; Nathan, L.; Rifai, N.; Liu, S. Insulin sensitivity and insulin secretion determined by homeostasis model assessment and risk of diabetes in a multiethnic cohort of women: The Women’s Health Initiative Observational Study. Diabetes Care 2007, 30, 1747–1752. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
| (A) | ||||
| Summary | GIR (mg/kg/min) | GIR (mg/kg/min) | GIR (mg/kg/min) | GIR (mg/kg/min) |
| Day 1 (Placebo, n = 5) | Day 11 (Placebo, n = 5) | Day 1 (TFG, n = 8) | Day 11 (TFG, n = 8) | |
| Mean | 8.45 | 8.5 | 6.63 | 8.13 ** |
| S.D. | 3.07 | 2.86 | 3.65 | 3.01 |
| (B) | ||||
| Individual GIR | Day 1 (Placebo, n = 5) | Day 11 (Placebo, n = 5) | Day 1 (TFG, n = 8) | Day 11 (TFG, n = 8) |
| 1. | 4.99 | 5.79 | 6.49 | 7.46 |
| 2. | 6.99 | 6.69 | 2.04 | 4.86 |
| 3. | 12.34 | 12.23 | 6.05 | 8.09 |
| 4. | 7.0 | 6.9 | 4.39 | 6.96 |
| 5. | 10.95 | 10.87 | 2.52 | 4.14 |
| 6. | 10.09 | 10.84 | ||
| 7. | 12.14 | 13.02 | ||
| 8. | 9.34 | 9.45 | ||
| Mean | 8.454 | 8.496 | 6.633 | 8.125 |
| S.D. | 3.065 | 2.86 | 3.645 | 3.005 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiss, R.; Szabó, K.; Gesztelyi, R.; Somodi, S.; Kovács, P.; Szabó, Z.; Németh, J.; Priksz, D.; Kurucz, A.; Juhász, B.; et al. Insulin-Sensitizer Effects of Fenugreek Seeds in Parallel with Changes in Plasma MCH Levels in Healthy Volunteers. Int. J. Mol. Sci. 2018, 19, 771. https://doi.org/10.3390/ijms19030771
Kiss R, Szabó K, Gesztelyi R, Somodi S, Kovács P, Szabó Z, Németh J, Priksz D, Kurucz A, Juhász B, et al. Insulin-Sensitizer Effects of Fenugreek Seeds in Parallel with Changes in Plasma MCH Levels in Healthy Volunteers. International Journal of Molecular Sciences. 2018; 19(3):771. https://doi.org/10.3390/ijms19030771
Chicago/Turabian StyleKiss, Rita, Katalin Szabó, Rudolf Gesztelyi, Sándor Somodi, Péter Kovács, Zoltán Szabó, József Németh, Dániel Priksz, Andrea Kurucz, Béla Juhász, and et al. 2018. "Insulin-Sensitizer Effects of Fenugreek Seeds in Parallel with Changes in Plasma MCH Levels in Healthy Volunteers" International Journal of Molecular Sciences 19, no. 3: 771. https://doi.org/10.3390/ijms19030771
APA StyleKiss, R., Szabó, K., Gesztelyi, R., Somodi, S., Kovács, P., Szabó, Z., Németh, J., Priksz, D., Kurucz, A., Juhász, B., & Szilvássy, Z. (2018). Insulin-Sensitizer Effects of Fenugreek Seeds in Parallel with Changes in Plasma MCH Levels in Healthy Volunteers. International Journal of Molecular Sciences, 19(3), 771. https://doi.org/10.3390/ijms19030771