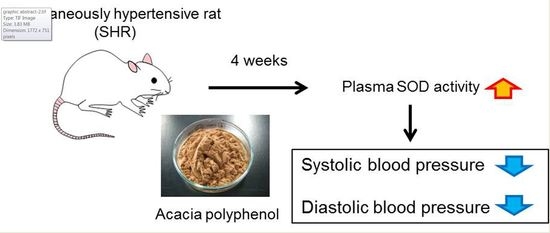
Anti-Hypertensive Effects of Acacia Polyphenol in Spontaneously Hypertensive Rats
Abstract
1. Introduction
2. Results
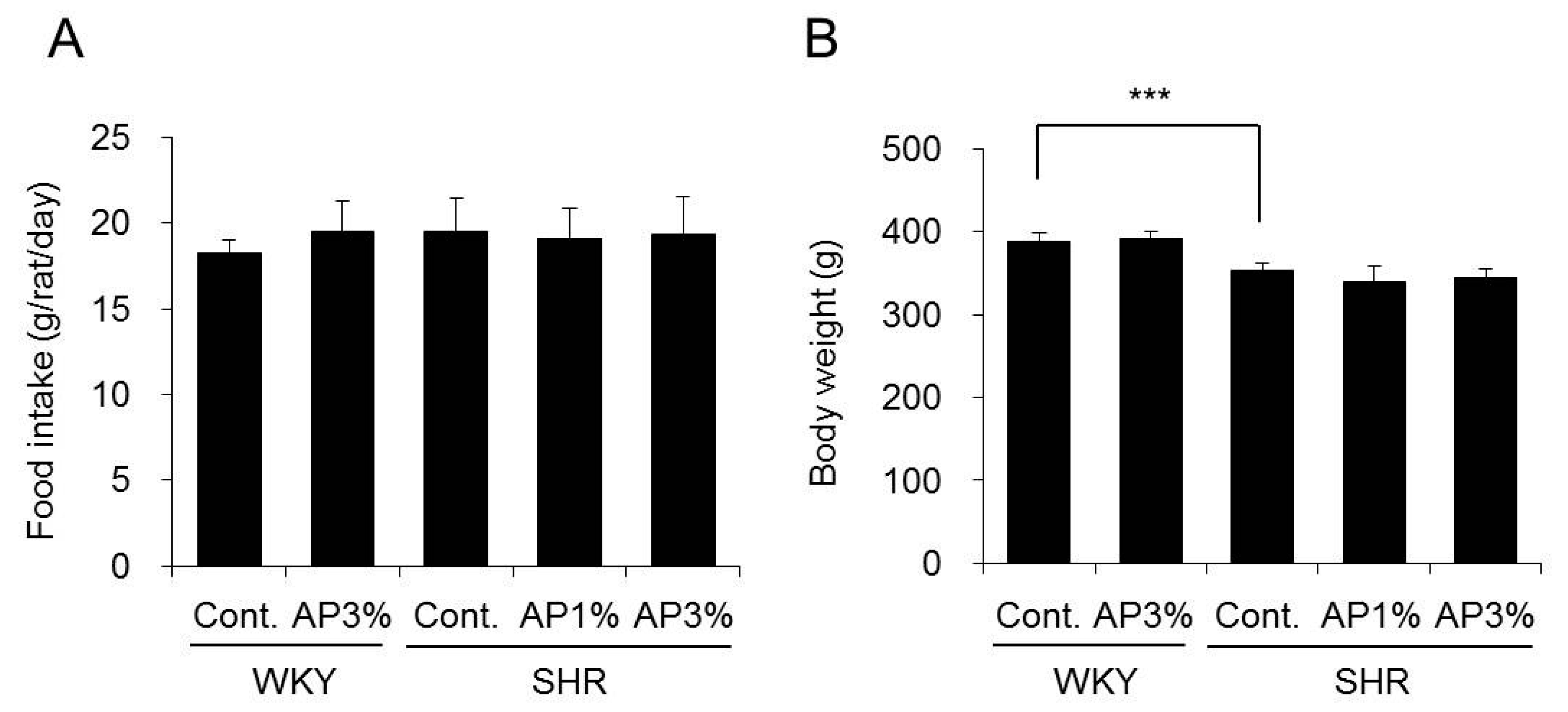
2.1. Food Intake and Body Weight
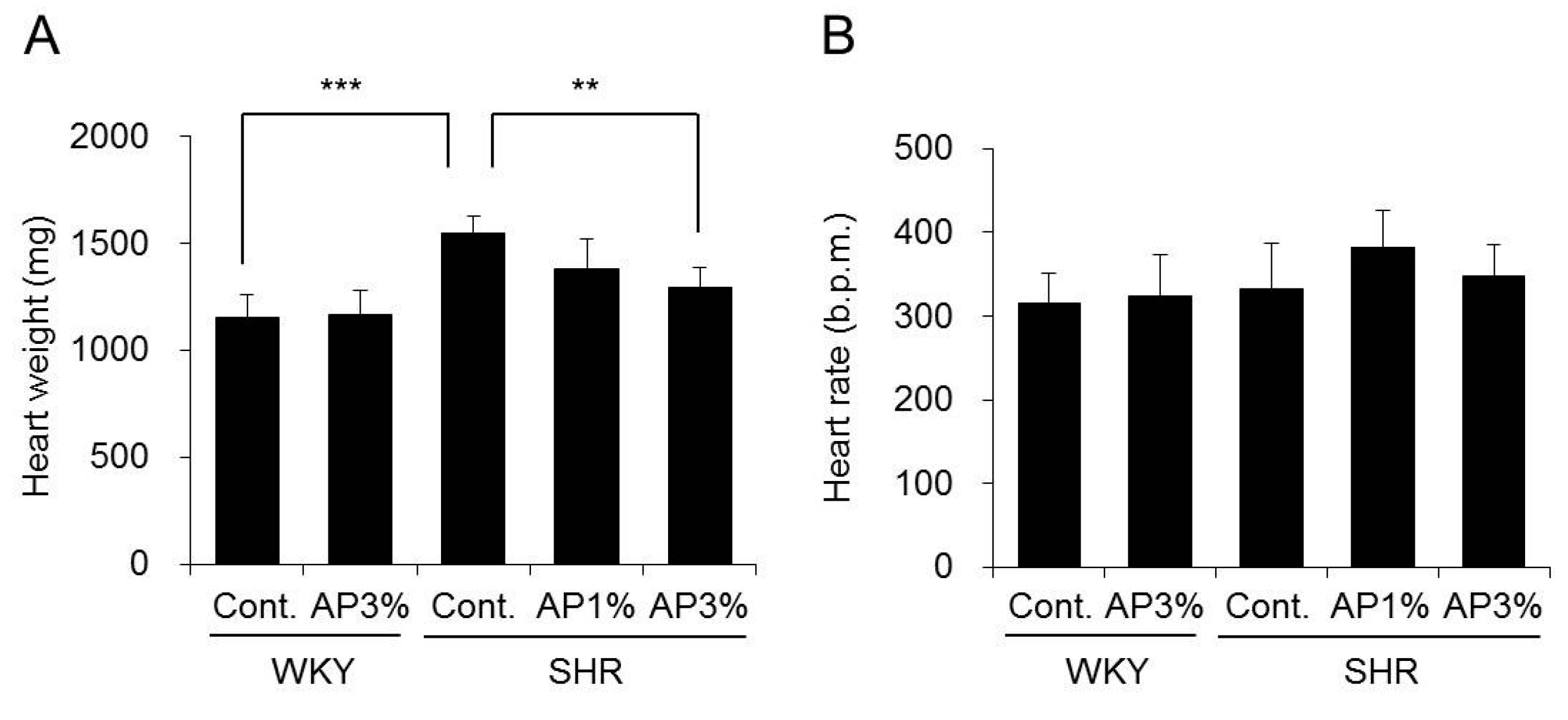
2.2. Heart Weight and Heart Rate
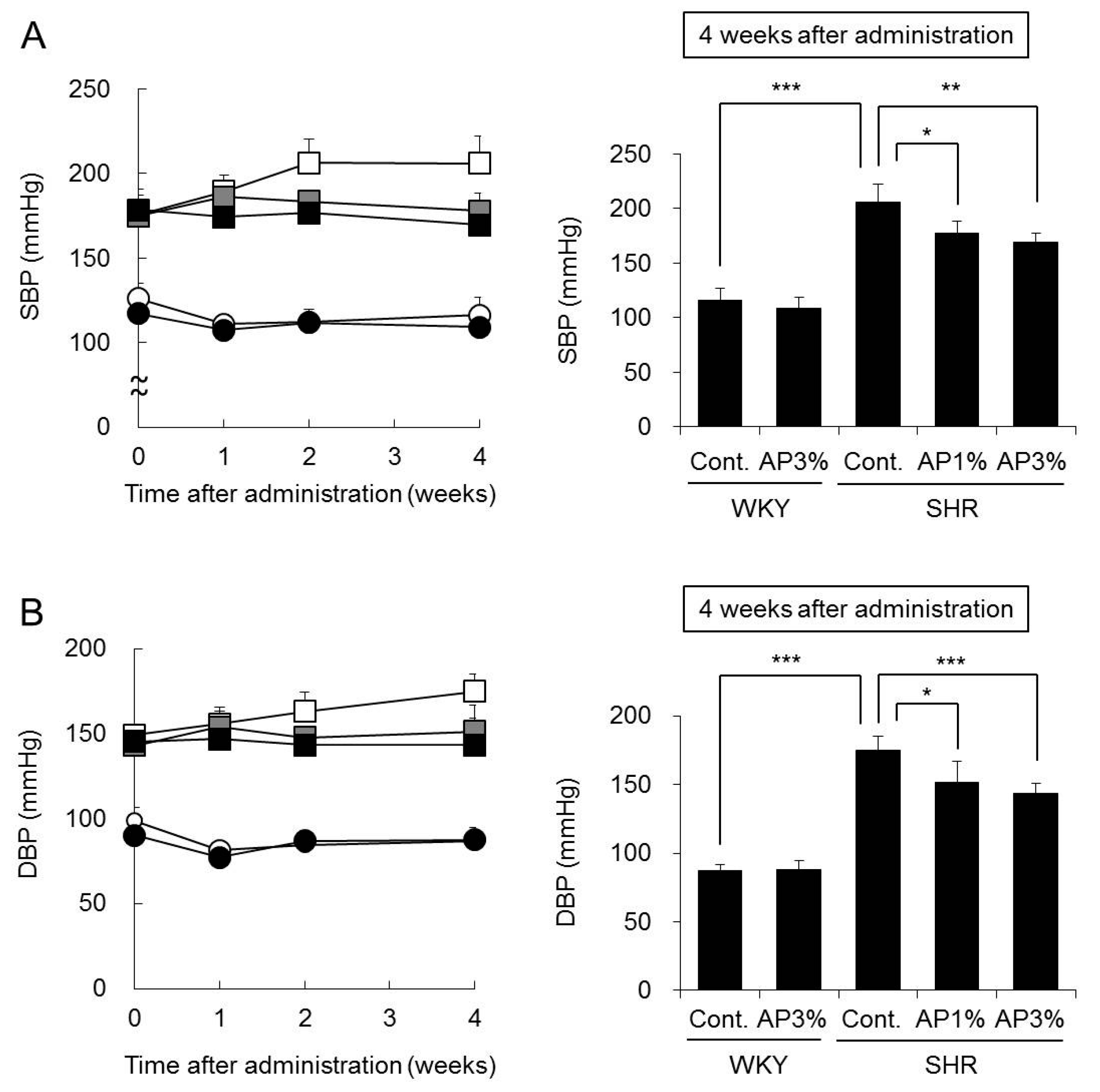
2.3. Systolic Blood Pressure (SBP) and Diastolic Blood Pressure (DBP)
2.4. Angiotensin-Converting Enzyme (ACE) Activity in the Kidney
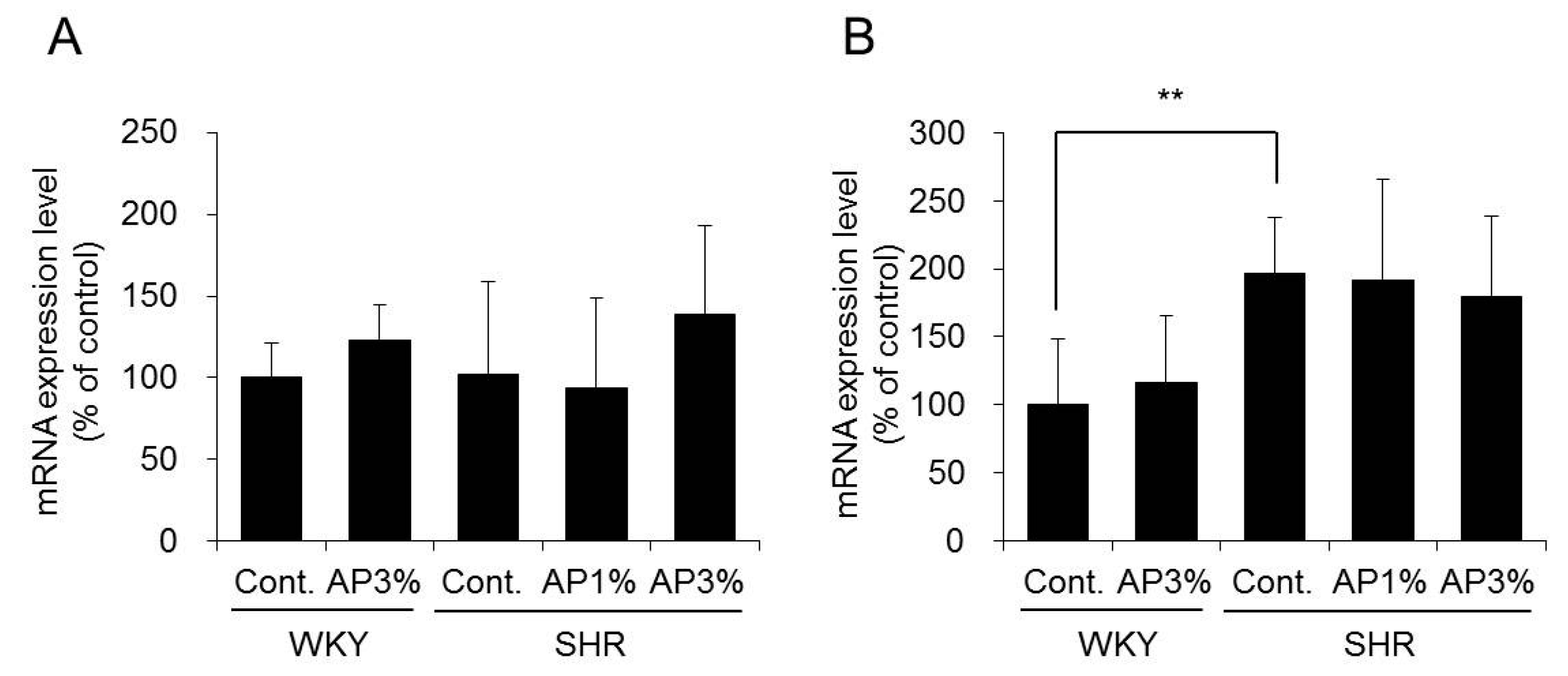
2.5. Expression of Nicotinamide Adenine Dinucleotide Phosphate (NADPH) Oxidase in the Kidney
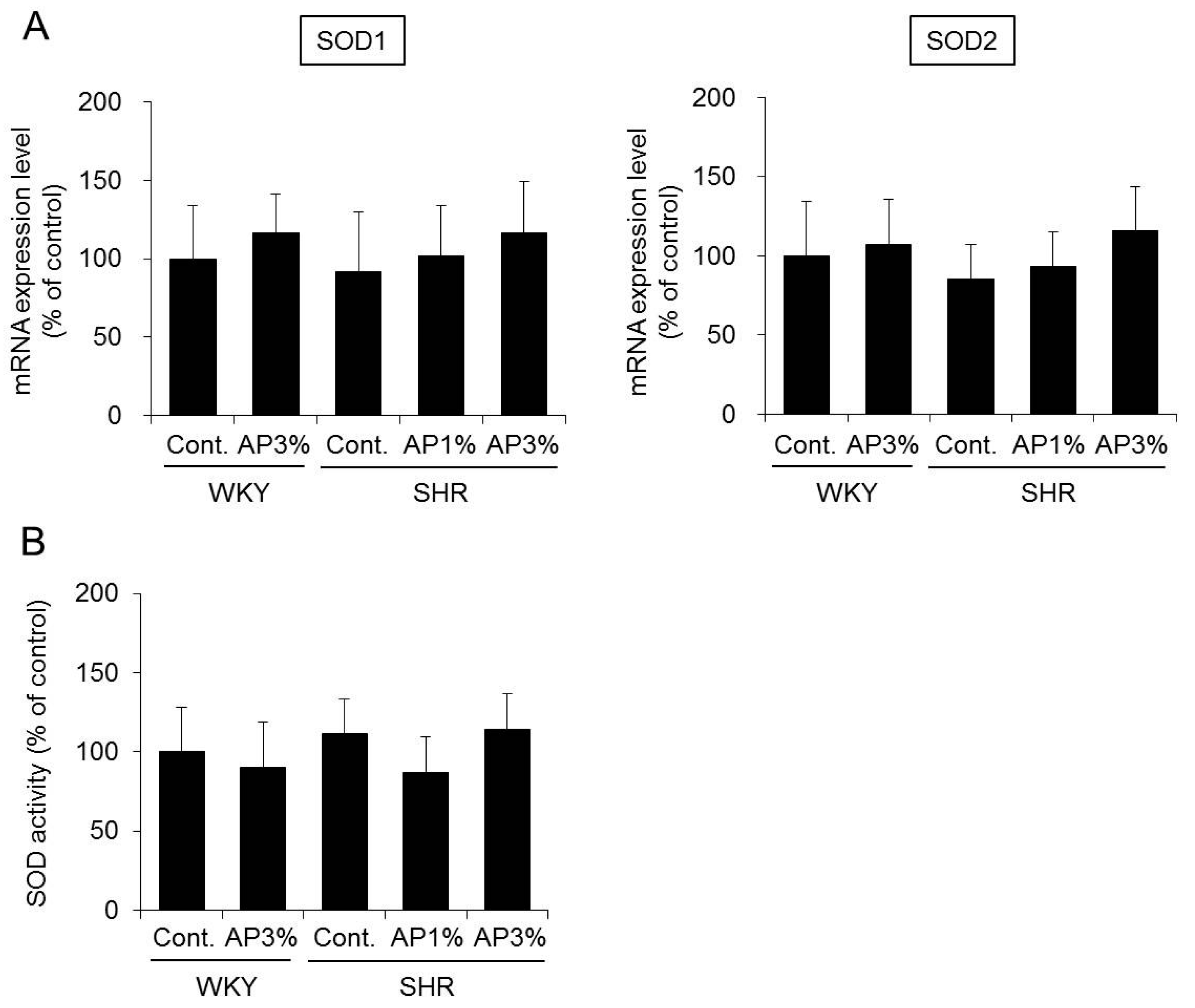
2.6. Superoxide Dismutase (SOD) Expression and Activity in the Kidney
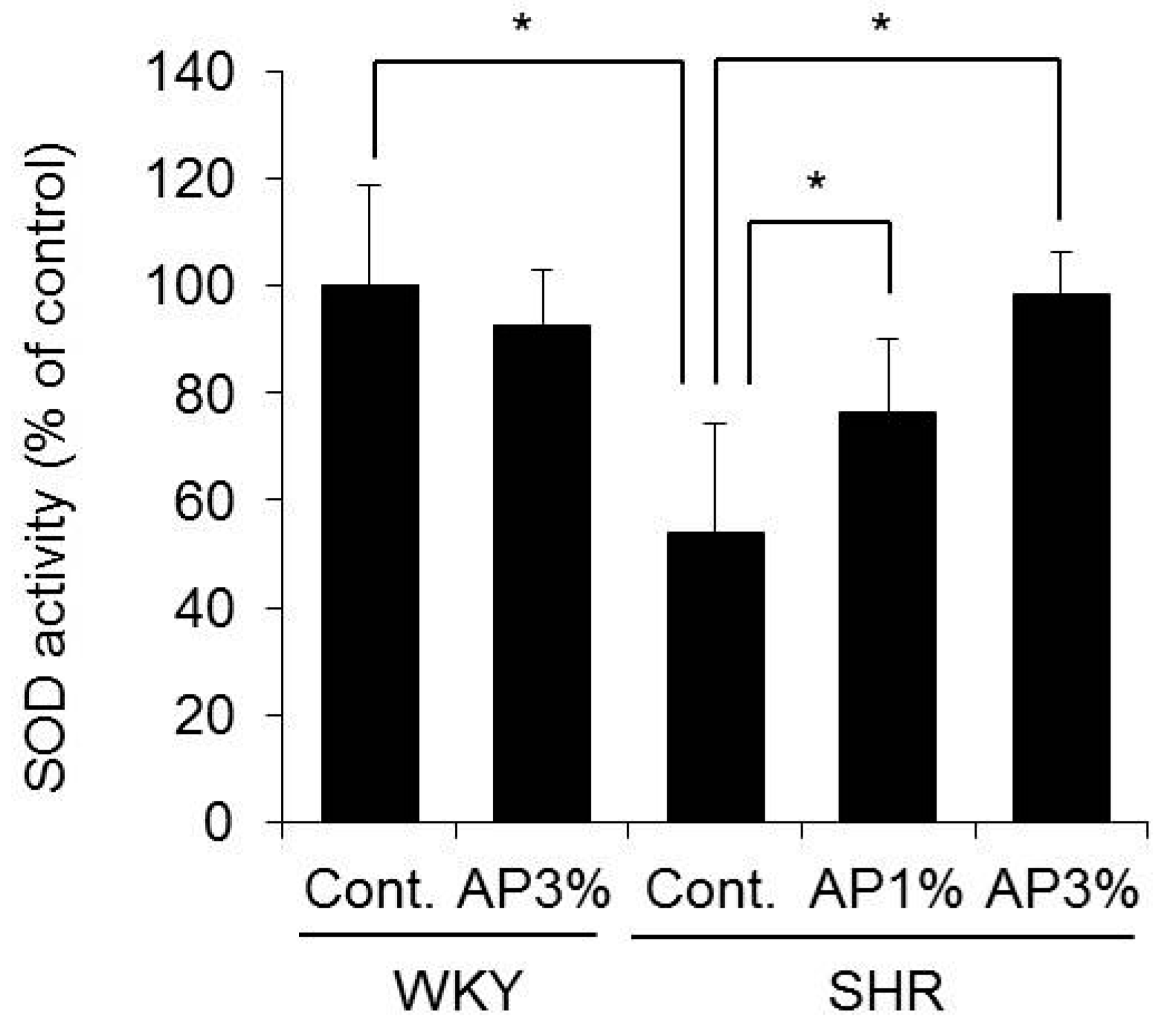
2.7. SOD Activity in the Blood
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Hot Water Extraction from Acacia Bark
4.3. Animals and Treatments
4.4. Measurement of ACE Activity
4.5. Measurement of SOD Activity
4.6. Real-Time RT-PCR
4.7. Statistical Analysis
Author Contributions
Conflicts of Interest
Abbreviations
| ACE | angiotensin-converting enzyme |
| AP | acacia polyphenol |
| DBP | diastolic blood pressure |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| SBP | systolic blood pressure |
| SHR | spontaneously hypertensive rats |
| SOD | superoxide dismutase |
| ROS | reactive oxygen species |
| WKY | Wistar Kyoto rats |
References
- Ikarashi, N.; Toda, T.; Okaniwa, T.; Ito, K.; Ochiai, W.; Sugiyama, K. Anti-obesity and anti-diabetic effects of acacia polyphenol in obese diabetic KKAy mice fed high-fat diet. Evid. Based Complement. Altern. Med. 2011, 2011, 952031. [Google Scholar] [CrossRef] [PubMed]
- Ikarashi, N.; Takeda, R.; Ito, K.; Ochiai, W.; Sugiyama, K. The inhibition of lipase and glucosidase activities by acacia polyphenol. Evid. Based Complement. Altern. Med. 2011, 2011, 272075. [Google Scholar] [CrossRef] [PubMed]
- Ikarashi, N.; Sato, W.; Toda, T.; Ishii, M.; Ochiai, W.; Sugiyama, K. Inhibitory Effect of Polyphenol-Rich Fraction from the Bark of Acacia mearnsii on Itching Associated with Allergic Dermatitis. Evid. Based Complement. Altern. Med. 2012, 2012, 120389. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.R.; Lokhandwala, M.F.; Banday, A.A. Resveratrol prevents endothelial nitric oxide synthase uncoupling and attenuates development of hypertension in spontaneously hypertensive rats. Eur. J. Pharmacol. 2011, 667, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Luna, R.; Munoz-Hernandez, R.; Miranda, M.L.; Costa, A.F.; Jimenez-Jimenez, L.; Vallejo-Vaz, A.J.; Muriana, F.J.; Villar, J.; Stiefel, P. Olive oil polyphenols decrease blood pressure and improve endothelial function in young women with mild hypertension. Am. J. Hypertens. 2012, 25, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Kusano, R.; Ogawa, S.; Matsuo, Y.; Tanaka, T.; Yazaki, Y.; Kouno, I. alpha-Amylase and lipase inhibitory activity and structural characterization of acacia bark proanthocyanidins. J. Nat. Prod. 2011, 74, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Corvol, P.; Williams, T.A.; Soubrier, F. Peptidyl dipeptidase A: Angiotensin I-converting enzyme. Methods Enzymol. 1995, 248, 283–305. [Google Scholar] [PubMed]
- Babior, B.M. NADPH oxidase: An update. Blood 1999, 93, 1464–1476. [Google Scholar] [PubMed]
- Lassegue, B.; Griendling, K.K. Reactive oxygen species in hypertension; An update. Am. J. Hypertens. 2004, 17, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Mukai, Y.; Yamate, J.; Kato, J.; Kurasaki, M.; Hatai, A.; Sagai, M. Effect of polyphenol-containing azuki bean (Vigna angularis) extract on blood pressure elevation and macrophage infiltration in the heart and kidney of spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2008, 35, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Chabrashvili, T.; Kitiyakara, C.; Blau, J.; Karber, A.; Aslam, S.; Welch, W.J.; Wilcox, C.S. Effects of ANG II type 1 and 2 receptors on oxidative stress, renal NADPH oxidase, and SOD expression. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R117–R124. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Dicus, M.; Ho, N.D.; Boroujerdi-Rad, L.; Sindhu, R.K. Oxidative stress and dysregulation of superoxide dismutase and NADPH oxidase in renal insufficiency. Kidney Int. 2003, 63, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Caraceni, P.; Ryu, H.S.; van Thiel, D.H.; Borle, A.B. Source of oxygen free radicals produced by rat hepatocytes during postanoxic reoxygenation. Biochim. Biophys. Acta 1995, 1268, 249–254. [Google Scholar] [CrossRef][Green Version]
- Ookawara, T.; Imazeki, N.; Matsubara, O.; Kizaki, T.; Oh-Ishi, S.; Nakao, C.; Sato, Y.; Ohno, H. Tissue distribution of immunoreactive mouse extracellular superoxide dismutase. Am. J. Physiol. 1998, 275, C840–C847. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Liu, J.W.; Ufur, H.; He, G.S.; Liqian, H.; Chen, P. The antihypertensive effect of ethyl acetate extract from red raspberry fruit in hypertensive rats. Pharmacogn. Mag. 2011, 7, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Cienfuegos-Jovellanos, E.; Quinones Mdel, M.; Muguerza, B.; Moulay, L.; Miguel, M.; Aleixandre, A. Antihypertensive effect of a polyphenol-rich cocoa powder industrially processed to preserve the original flavonoids of the cocoa beans. J. Agric. Food Chem. 2009, 57, 6156–6162. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Aoki, K. Development of a strain of spontaneously hypertensive rats. Jpn. Circ. J. 1963, 27, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yu, J.; Jia, B.; Zhao, F.; Tang, M.; Hu, L.; Lin, F. Effect of losartan with folic acid on plasma homocysteine and vascular ultrastructural changes in spontaneously hypertensive rats. Int. J. Clin. Exp. Pathol. 2015, 8, 12908–12914. [Google Scholar] [PubMed]
- Camilion de Hurtado, M.C.; Portiansky, E.L.; Perez, N.G.; Rebolledo, O.R.; Cingolani, H.E. Regression of cardiomyocyte hypertrophy in SHR following chronic inhibition of the Na(+)/H(+) exchanger. Cardiovasc. Res. 2002, 53, 862–868. [Google Scholar] [CrossRef]
- Hermida, N.; Lopez, B.; Gonzalez, A.; Dotor, J.; Lasarte, J.J.; Sarobe, P.; Borras-Cuesta, F.; Diez, J. A synthetic peptide from transforming growth factor-beta1 type III receptor prevents myocardial fibrosis in spontaneously hypertensive rats. Cardiovasc. Res. 2009, 81, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Actis-Goretta, L.; Ottaviani, J.I.; Fraga, C.G. Inhibition of angiotensin converting enzyme activity by flavanol-rich foods. J. Agric. Food Chem. 2006, 54, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Asaad, M.M.; Antonaccio, M.J. Vascular wall renin in spontaneously hypertensive rats. Potential relevance to hypertension maintenance and antihypertensive effect of captopril. Hypertension 1982, 4, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.M.; Berecek, K.H.; Tsoporis, J.; McKenzie, R.; Triggle, C.R. Prevention of hypertension and vascular changes by captopril treatment. Hypertension 1991, 17, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Fujii, K.; Kansui, Y.; Iida, M. Changes in endothelium-derived hyperpolarizing factor in hypertension and ageing: Response to chronic treatment with renin-angiotensin system inhibitors. Clin. Exp. Pharmacol. Physiol. 2004, 31, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Vaja, V.; Ochodnicky, P.; Krenek, P.; Klimas, J.; Bajuszova, Z.; Kyselovic, J. Rapid large artery remodeling following the administration and withdrawal of calcium channel blockers in spontaneously hypertensive rats. Eur. J. Pharmacol. 2009, 619, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Ni, Z.; Oveisi, F.; Trnavsky-Hobbs, D.L. Effect of antioxidant therapy on blood pressure and NO synthase expression in hypertensive rats. Hypertension 2000, 36, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Kagawa, D.; Ochiai, R.; Tokimitsu, I.; Saito, I. Green coffee bean extract and its metabolites have a hypotensive effect in spontaneously hypertensive rats. Hypertens. Res. 2002, 25, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Gill, P.S.; Wilcox, C.S. NADPH oxidases in the kidney. Antioxid. Redox Signal. 2006, 8, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Sepulveda, R.; Jimenez, R.; Romero, M.; Zarzuelo, M.J.; Sanchez, M.; Gomez-Guzman, M.; Vargas, F.; O’Valle, F.; Zarzuelo, A.; Perez-Vizcaino, F.; et al. Wine polyphenols improve endothelial function in large vessels of female spontaneously hypertensive rats. Hypertension 2008, 51, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Shao, W.; Xiao, R.; Xu, K.; Ma, Z.; Johnstone, B.H.; Du, Y. Pu-erh tea aqueous extracts lower atherosclerotic risk factors in a rat hyperlipidemia model. Exp. Gerontol. 2009, 44, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Mukai, Y.; Sato, S. Polyphenol-containing azuki bean (Vigna angularis) extract attenuates blood pressure elevation and modulates nitric oxide synthase and caveolin-1 expressions in rats with hypertension. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Cutting, N.J. The development and application of speciality wattle extracts. J. Soc. Leather Technol. Chem. 1997, 81, 89–93. [Google Scholar]
- Masuda, O.; Nakamura, Y.; Takano, T. Antihypertensive peptides are present in aorta after oral administration of sour milk containing these peptides to spontaneously hypertensive rats. J. Nutr. 1996, 126, 3063–3068. [Google Scholar] [CrossRef] [PubMed]
- Nakano, D.; Ogura, K.; Miyakoshi, M.; Ishii, F.; Kawanishi, H.; Kurumazuka, D.; Kwak, C.J.; Ikemura, K.; Takaoka, M.; Moriguchi, S.; et al. Antihypertensive effect of angiotensin I-converting enzyme inhibitory peptides from a sesame protein hydrolysate in spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2006, 70, 1118–1126. [Google Scholar] [CrossRef] [PubMed]

| Target | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| SOD1 | TGTACCAGTGCAGGACCTC | ACACATTGGCCACACCGTC |
| SOD2 | GACTGTGTTCCTGTGCACTG | GGATGACAGGAAGATGGTGAG |
| p22 | GGCCTGATCCTCATCACAG | CAGATGAGCACTCCTGCAAC |
| p47 | CAGGTGAAGAAGCCAGAGAC | CCCGATAGGTCTGAAGGATG |
| 18S rRNA | GTCTGTGATGCCCTTAGATG | AGCTTATGACCCGCACTTAC |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikarashi, N.; Toda, T.; Hatakeyama, Y.; Kusunoki, Y.; Kon, R.; Mizukami, N.; Kaneko, M.; Ogawa, S.; Sugiyama, K. Anti-Hypertensive Effects of Acacia Polyphenol in Spontaneously Hypertensive Rats. Int. J. Mol. Sci. 2018, 19, 700. https://doi.org/10.3390/ijms19030700
Ikarashi N, Toda T, Hatakeyama Y, Kusunoki Y, Kon R, Mizukami N, Kaneko M, Ogawa S, Sugiyama K. Anti-Hypertensive Effects of Acacia Polyphenol in Spontaneously Hypertensive Rats. International Journal of Molecular Sciences. 2018; 19(3):700. https://doi.org/10.3390/ijms19030700
Chicago/Turabian StyleIkarashi, Nobutomo, Takahiro Toda, Yusuke Hatakeyama, Yoshiki Kusunoki, Risako Kon, Nanaho Mizukami, Miho Kaneko, Sosuke Ogawa, and Kiyoshi Sugiyama. 2018. "Anti-Hypertensive Effects of Acacia Polyphenol in Spontaneously Hypertensive Rats" International Journal of Molecular Sciences 19, no. 3: 700. https://doi.org/10.3390/ijms19030700
APA StyleIkarashi, N., Toda, T., Hatakeyama, Y., Kusunoki, Y., Kon, R., Mizukami, N., Kaneko, M., Ogawa, S., & Sugiyama, K. (2018). Anti-Hypertensive Effects of Acacia Polyphenol in Spontaneously Hypertensive Rats. International Journal of Molecular Sciences, 19(3), 700. https://doi.org/10.3390/ijms19030700