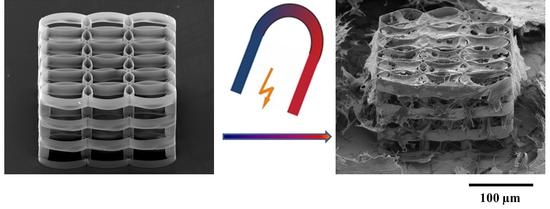
3D Biomimetic Magnetic Structures for Static Magnetic Field Stimulation of Osteogenesis
Abstract
1. Introduction
2. Results and Discussion
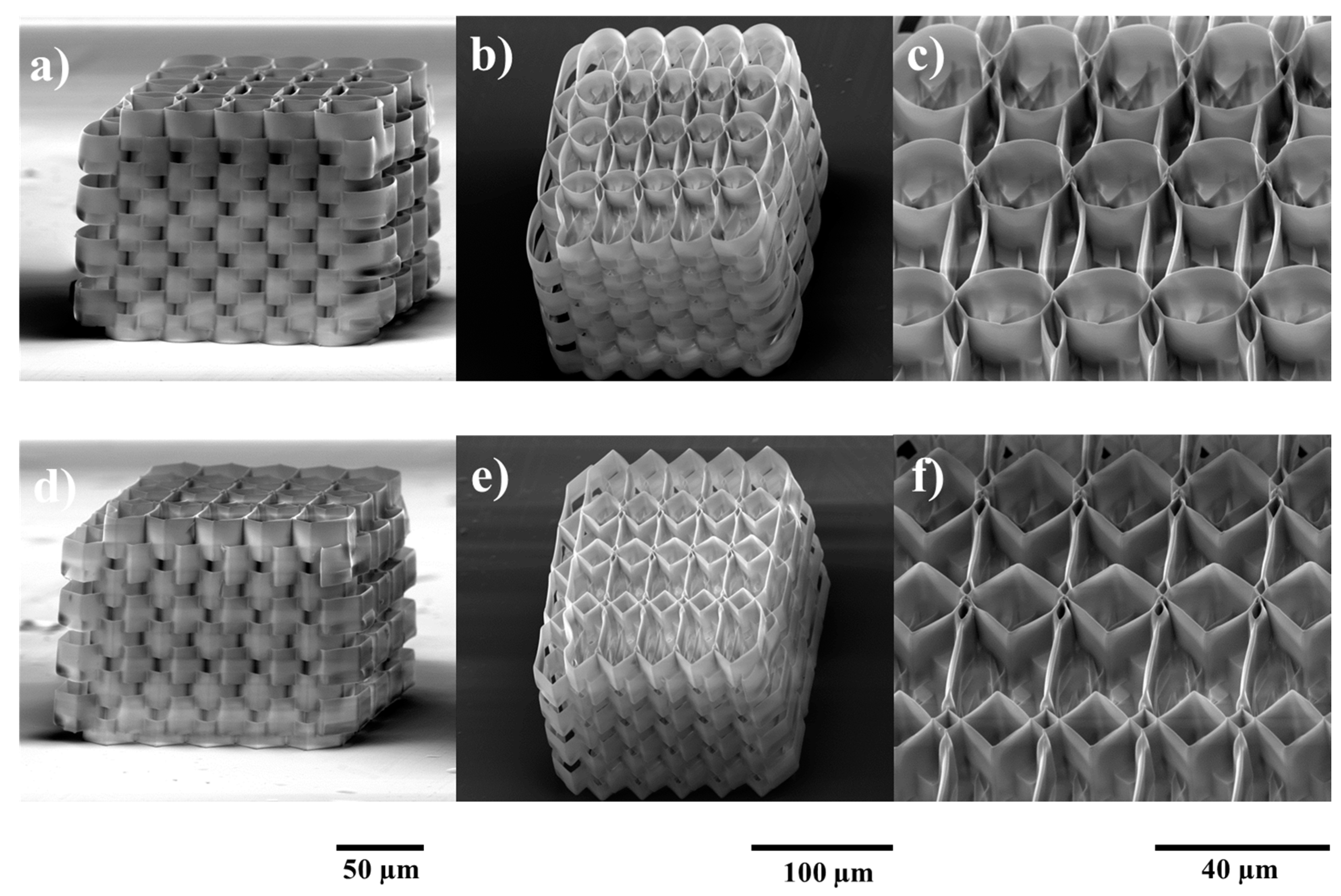
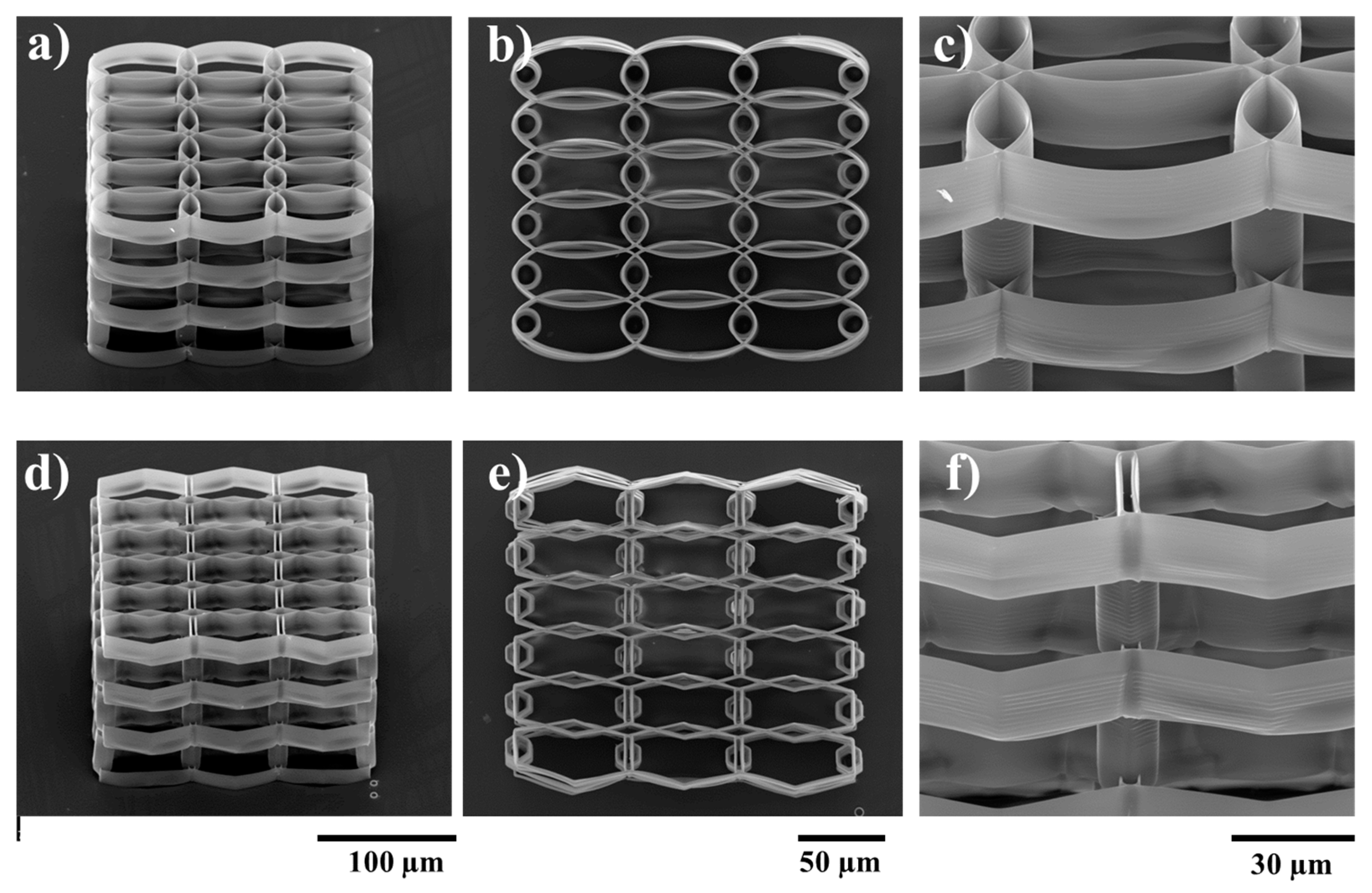
2.1. Structures Optimization
2.2. Structure Functionalization
2.3. Static Magnetic Field Stimulation
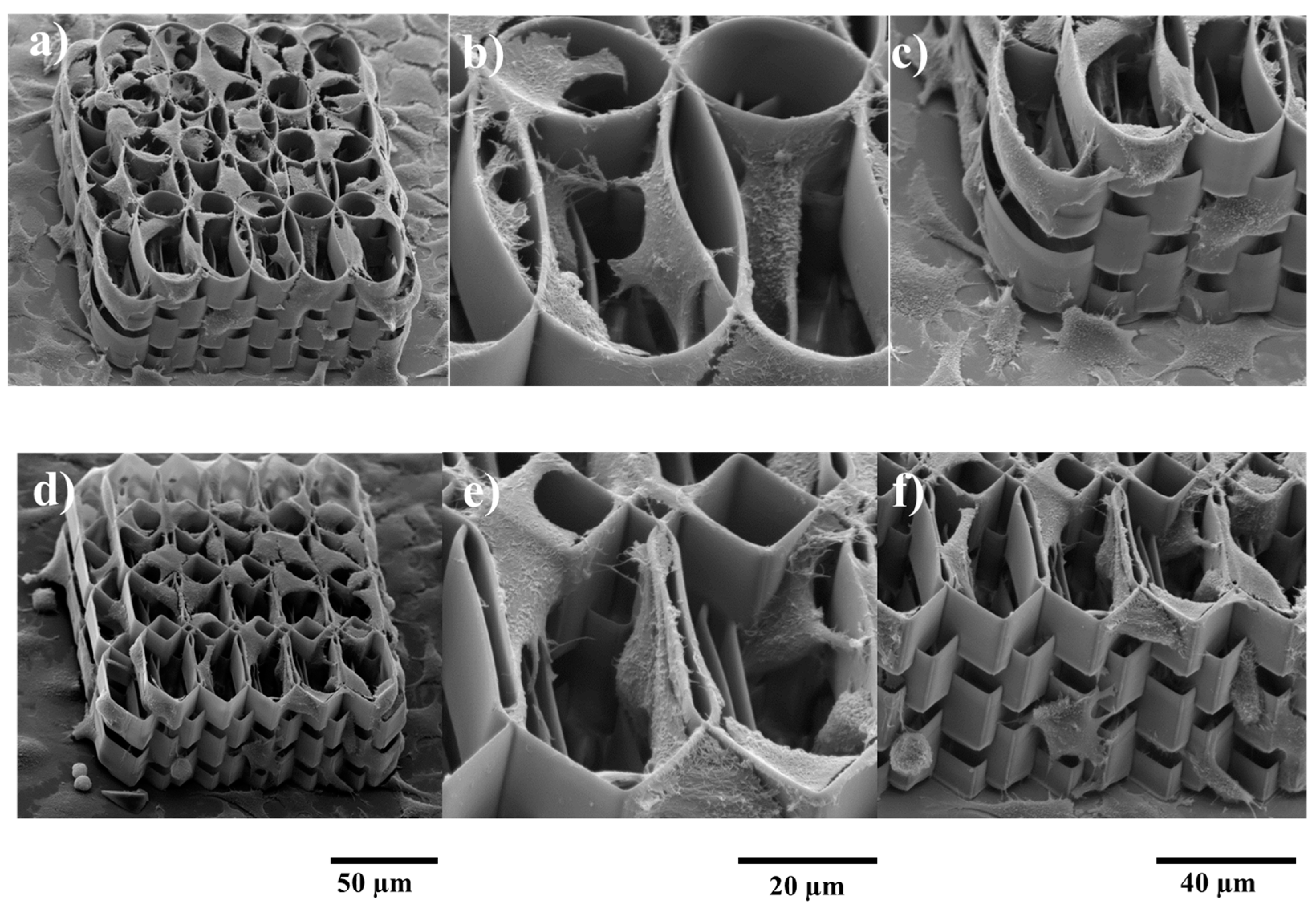
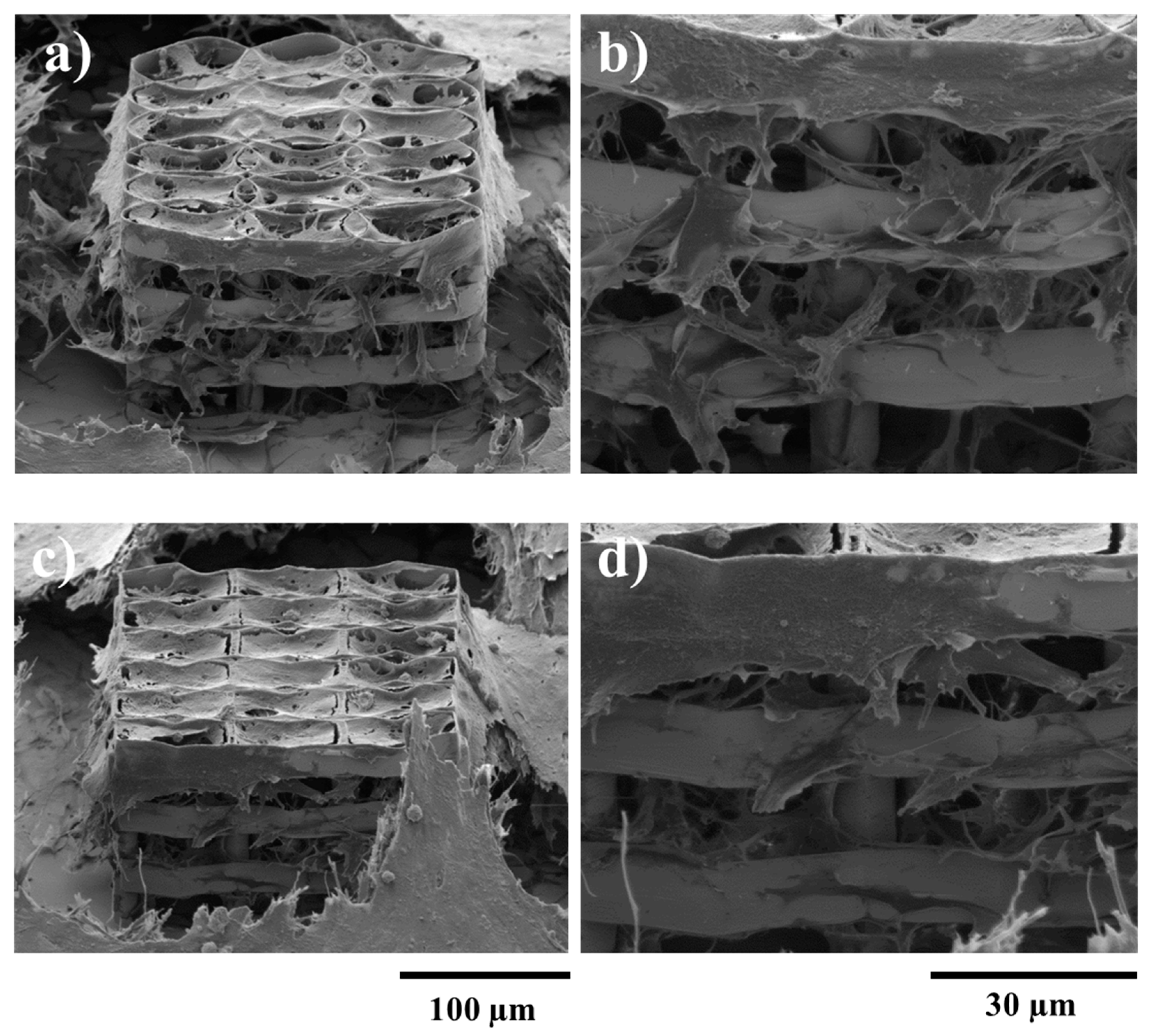
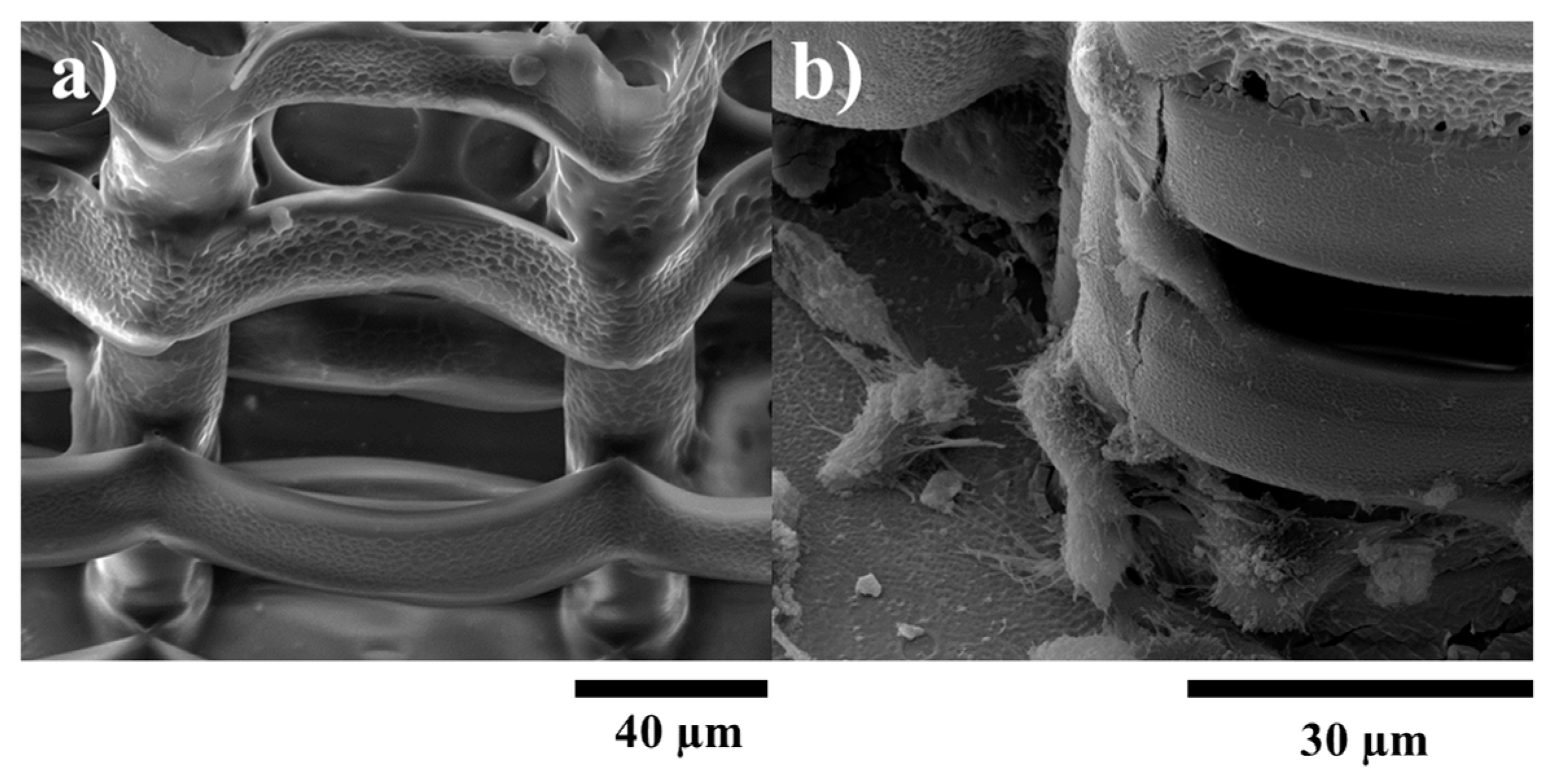
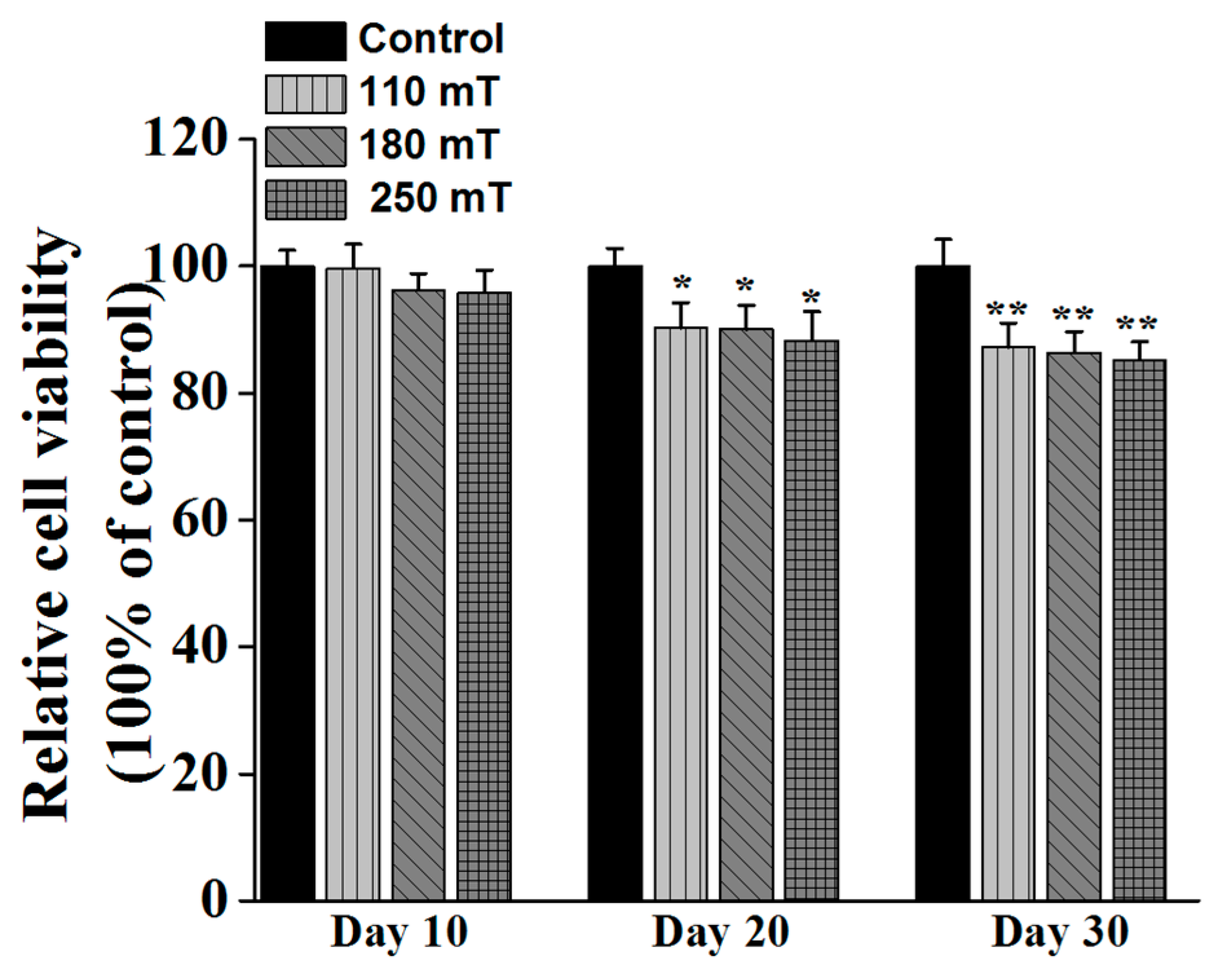
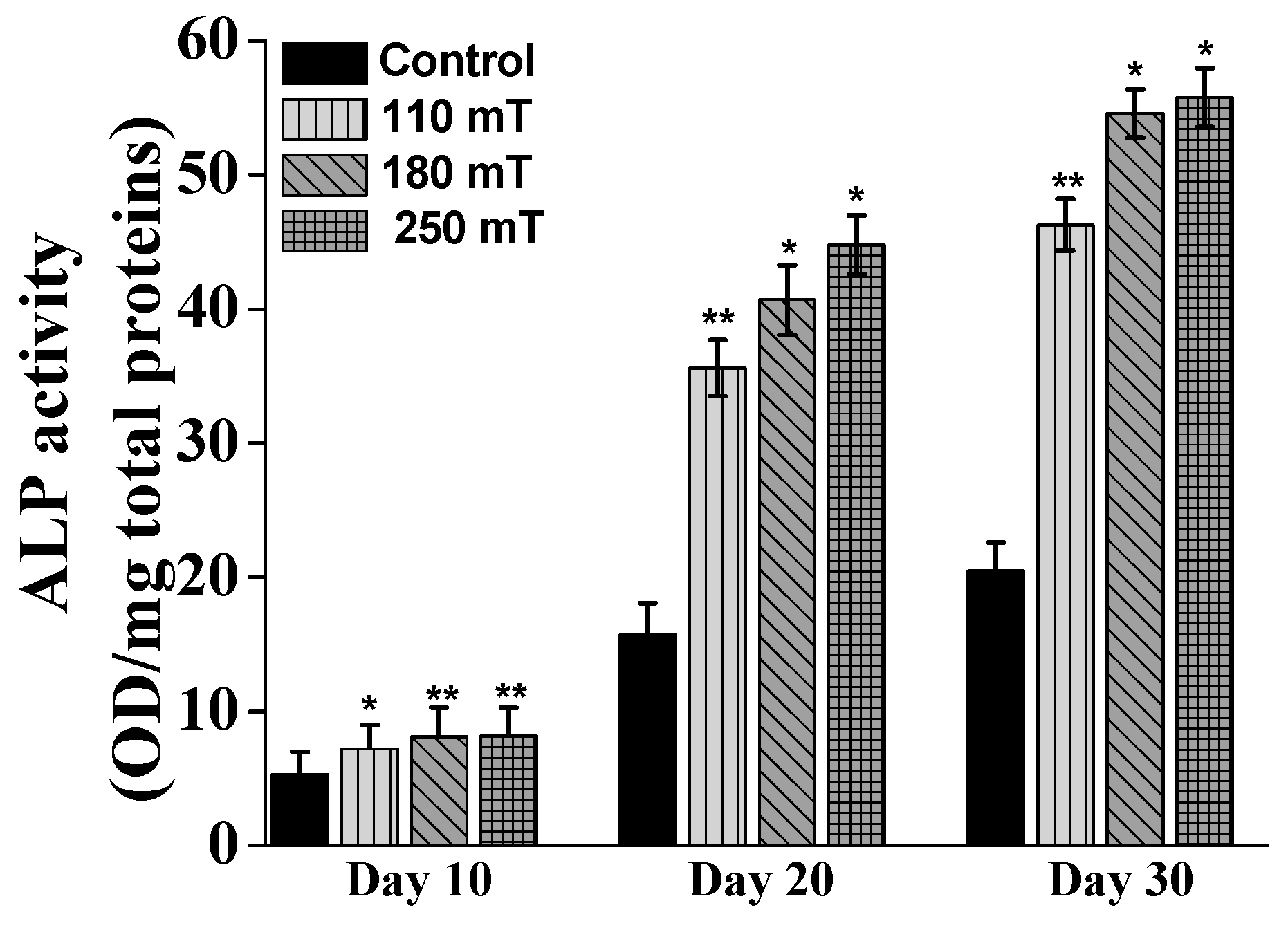
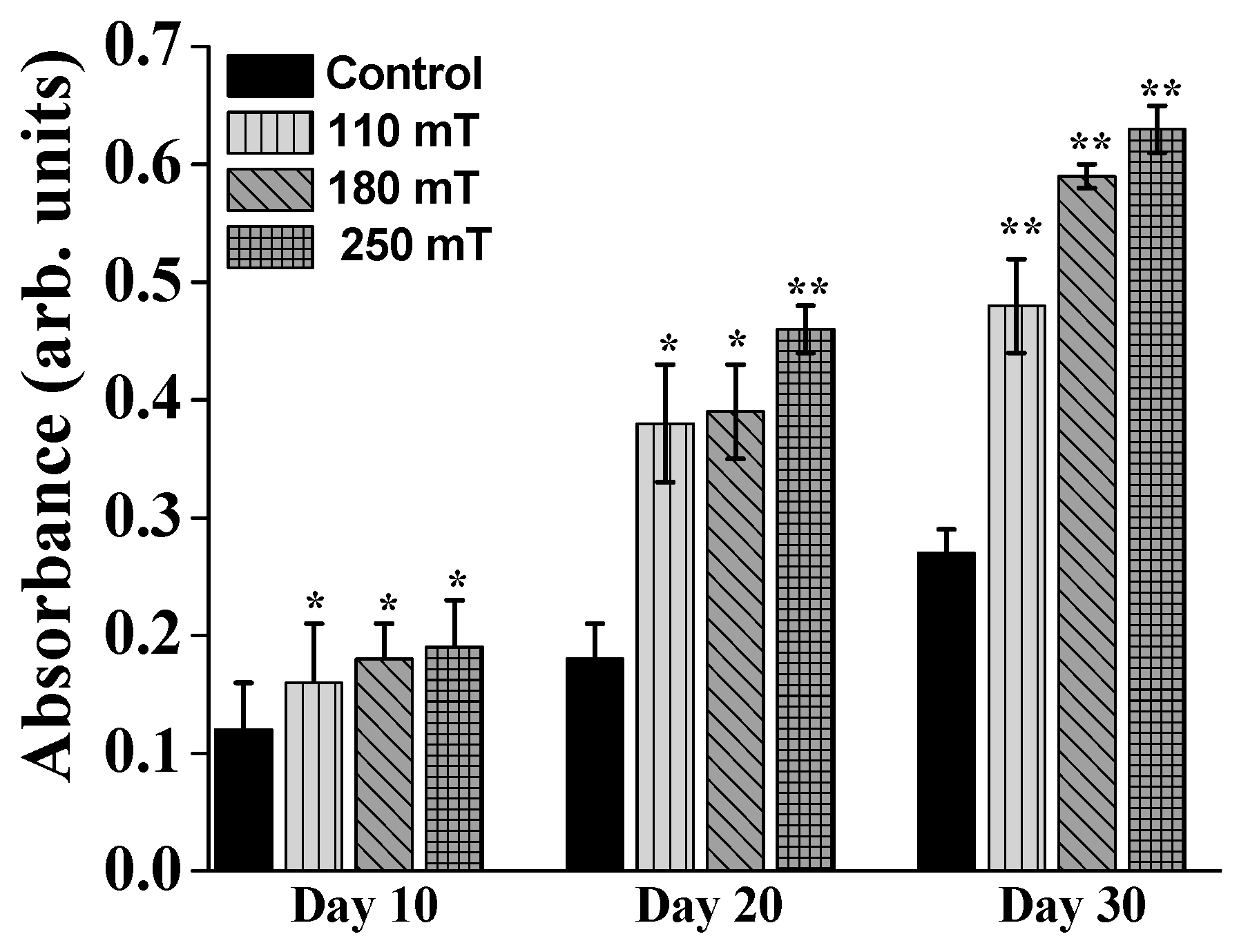
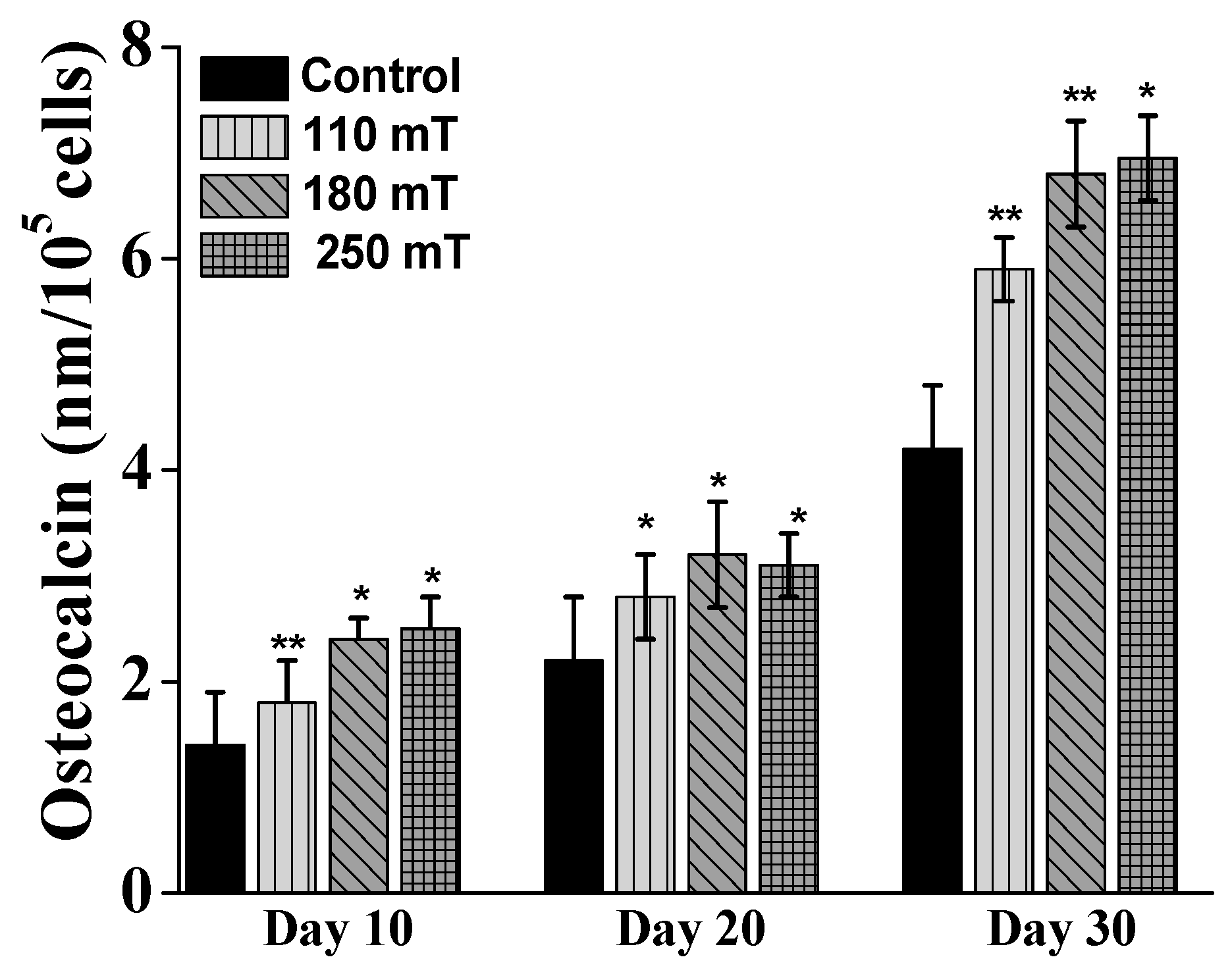
2.4. Biological Assessments
3. Materials and Methods
3.1. Structures Design
3.2. Structure Fabrication and Characterization
SEM
3.3. Biological Assessments
3.3.1. Cell Cultures
3.3.2. Cells Morphological Investigations by SEM
3.3.3. MTS
3.3.4. ALP
3.3.5. Osteocalcin
3.3.6. Alizarin Red (ARS) Assay
3.3.7. Statistical Analysis
3.3.8. Static Magnetic Fields Stimulation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.M.; Ahn, S.J.; Park, K.R.; Kim, M.J.; Kim, J.J.; Jin, G.Z.; Kim, H.W.; Kim, E.C. Magnetic nanocomposite scaffolds combined with static magnetic field in the simulation of osteoblastic differentiation and bone formation. Biomaterials 2016, 85, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Fini, M.; Cadossi, R.; Canè, V.; Cavani, F.; Giavaresi, G.; Krajewski, A.; Martini, L.; Aldini, N.N.; Ravaglioli, A.; Rimondini, L.; et al. The effect of pulsed electromagnetic fields on the osteointegration of hydroxyapatite implants in cancellous bone: A morphologic and microstructural in vivo study. J. Orthop. Res. 2002, 20, 756–763. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Ohsaki, Y.; Goto, T.; Nakashima, A.; Iijima, T. Effects of static magnetic fields on bone formation in rat osteoblast cultures. J. Dent. Res. 2003, 82, 926–966. [Google Scholar] [CrossRef] [PubMed]
- Ba, X.; Hadjiargyrou, M.; DiMasi, E.; Meng, Y.; Simon, M.; Tan, Z.; Rafailovich, M.H. The role of moderate static magnetic fields on biomineralization of osteoblasts on suflonated polystyrene films. Biomaterials 2011, 32, 7831–7838. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.W.; Lo, Y.J.; Chang, W.J.; Lin, C.T.; Lee, S.Y.; Abiko, Y.; Huang, H.M. Static magnetic field exposure promotes differentiation of osteoblastic cells grown on the surface of a poly-l-lactide substrate. Med. Biol. Eng. Comput. 2010, 48, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.H.; Ou, K.L.; Lee, S.Y.; Lin, C.T.; Chang, W.J.; Chen, C.C.; Huang, H.M. Static magnetic fields promote osteoblast-like cells differentiation via increasing the membrane rigidity. Ann. Biomed. Eng. 2007, 35, 1932–1939. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.; Panseri, S.; Marcacci, M.; Tampieri, A. Evaluation of the Effects of a Moderate Intensity Static Magnetic Field Application on Human Osteoblast-like Cells. Am. J. Biomed. Eng. 2012, 2, 263–268. [Google Scholar] [CrossRef]
- Lin, S.L.; Chang, W.J.; Hsieh, S.C.; Lin, C.T.; Chen, C.C.; Huang, H.M. Mechanobiology of MG63 osteoblast-like cells adaptation to static magnetic forces. Electromagn. Biol. Med. 2008, 27, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.C.; Leesunbok, R.; Lee, S.W.; Park, S.H.; Mah, S.J.; Ahn, S.J. Effects of moderate intensity static magnetic fields on human bone marrow-derived mesenchymal stem cells. Bioelectromagnetics 2015, 36, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, R.; Bantleon, R.; Kehlbach, R.; Siegel, G.; Wiskirchen, J.; Wolburg, H.; Kluba, T.; Eibofner, F.; Northoff, H.; Claussen, C.D.; et al. Functional investigations on human mesenchymal stem cells exposed to magnetic fields and labeled with clinically approved iron nanoparticles. BMC Cell Biol. 2010, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, D.; Chen, J.; Liu, W.; Duan, L.; You, W.; Xiong, J.; Wang, D. Osteogenic differentiation of bone marrow mesenchymal stem cells by magnetic nanoparticle composite scaffolds under a pulsed electromagnetic field. Saudi Pharm. J. 2017, 25, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Rosen, A.D. Mechanism of action of moderate-intensity static magnetic fields on biological systems. Cell Biochem. Biophys. 2003, 39, 163–173. [Google Scholar] [CrossRef]
- Rosen, A.D. Membrane response to static magnetic fields: Effect of exposure duration. Biochim. Biophys. Acta 1993, 1148, 317–320. [Google Scholar] [CrossRef]
- Aoki, H.; Yamazaki, H.; Yoshino, T.; Akagi, T. Effects of static magnetic fields on membrane permeability of a cultured cell line. Res. Commun. Chem. Pathol. Pharmacol. 1990, 69, 103–106. [Google Scholar] [PubMed]
- Kotani, H.; Iwasaka, M.; Ueno, S. Magnetic orientation of collagen and bone mixture. J. Appl. Phys. 2000, 87, 6191–6193. [Google Scholar] [CrossRef]
- Bañobre-López, M.; Piñeiro-Redondo, Y.; De Santis, R.; Gloria, A.; Ambrosio, L.; Tampieri, A.; Dediu, V.; Rivas, J. Poly(caprolactone) based magnetic scaffolds for bone tissue engineering. J. Appl. Phys. 2011, 109, 07B313. [Google Scholar] [CrossRef]
- Bock, N.; Riminucci, A.; Dionigi, C.; Russo, A.; Tampieri, A.; Landi, E.; Goranov, V.A.; Marcacci, M.; Dediu, V. A novel route in bone tissue engineering: Magnetic biomimetic scaffolds. Acta Biomater. 2010, 6, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Xiao, B.; Zhang, Y.; Liu, J.; Xue, H.; Lei, J.; Kong, H.; Huang, Y.; Jin, Z.; Gu, N.; et al. Super-paramagnetic responsive nanofibrous scaffolds under static magnetic field enhance osteogenesis for bone repair in vivo. Sci. Rep. 2013, 3, 2655. [Google Scholar] [CrossRef] [PubMed]
- De Santis, R.; Gloria, A.; Russo, T.; D’Amora, U.; Zeppetelli, S.; Dionigi, C.; Sytcheva, A.; Herrmannsdörfer, T.; Dediu, V.; Ambrosio, L. A basic approach toward the development of nanoscomposite magnetic scaffolds for advanced bone tissue engineering. J. Appl. Polym. Sci. 2011, 122, 3599–3605. [Google Scholar] [CrossRef]
- Tampieri, A.; D’Alessandro, T.; Sandri, M.; Sprio, S.; Landi, E.; Bertinetti, L.; Panseri, S.; Pepponi, G.; Goettlicher, J.; Bañobre-López, M.; et al. Intrinsic magnetism and hyperthermia in bioactive Fe-doped hydroxyapatite. Acta Biomater. 2012, 8, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Tampieri, A.; Landi, E.; Valentini, F.; Sandri, M.; D’Alessandro, T.; Dediu, V.; Marcacci, M. A conceptually new type of bio-hybrid scaffold for bone regeneration. Nanotechnology 2011, 22, 015104. [Google Scholar] [CrossRef] [PubMed]
- Gloria, A.; Russo, T.; D’Amora, U.; Zeppetelli, S.; D’Alessandro, T.; Sandri, M.; Bañobre-López, M.; Piñeiro-Redondo, Y.; Uhlarz, M.; Tampieri, A.; et al. Magnetic poly(ε-caprolactone)/iron-doped hydroxyapatite nanocomposite substrates for advanced bone tissue engineering. J. R. Soc. Interface 2013, 10, 20120833. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Patel, K.D.; Lee, J.H.; Lee, E.J.; Kim, T.H.; Kim, H.W. Potential of magnetic nanofiber scaffolds with mechanical and biological properties applicable for bone regeneration. PLoS ONE 2014, 9, e91584. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jiang, W.; Wen, X.; He, B.; Zeng, X.; Wang, G.; Gu, Z. A novel calcium phosphate ceramic-magnetic nanoparticle composite as a potential bone substitute. Biomed. Mater. 2010, 5, 015001. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhang, X.; Song, Y.; Han, B.; Hu, X.; Wang, X.; Lin, Y.; Deng, X. Magnetic biodegradable Fe3O4/CS/PVA nanofibrous membranes for bone regeneration. Biomed. Mater. 2011, 6, 055008. [Google Scholar] [CrossRef] [PubMed]
- Panseri, S.; Russo, A.; Giavaresi, G.; Sartori, M.; Veronesi, F.; Fini, M.; Salter, D.M.; Ortolani, A.; Strazzari, A.; Visani, A.; et al. Inovative magnetic scaffolds for orthopedic tissue engineering. J. Biomed. Mater. Res. 2012, 100, 2278–2286. [Google Scholar] [CrossRef]
- Zhou, X.; Hou, Y.; Lin, J. A review on the processing accuracy of two-photon polymerization. AIP Adv. 2015, 5, 030701. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-Dimensional scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Li, Y.; Yang, S.T.; Kniss, D.A. Effects of pore size in 3-D fibrous matrix on human trophoblast tissue development. Biotechnol. Bioeng. 2000, 70, 606–618. [Google Scholar] [CrossRef]
- Mandal, B.B.; Kundu, S.C. Cell proliferation and migration in silk fibroin 3D scaffolds. Biomaterials 2009, 30, 2956–2965. [Google Scholar] [CrossRef] [PubMed]
- Mata, A.; Kim, E.J.; Boehm, C.A.; Fleischman, A.J.; Muschler, G.F.; Roy, S. A three-dimensional scaffold with precise micro-architecture and surface micro-textures. Biomaterials 2009, 30, 4610–4617. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Larsen, L.B.; Trifol, J.; Szabo, P.; Burri, H.V.; Canali, C.; Dufva, M.; Emnéus, J.; Wolff, A. Fabrication of scalable and structured tissue engineering scaffolds using water dissolvable sacrificial 3D printed moulds. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 55, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.M.; Hausman, R.E. The Cell: A Molecular Approach, 4th ed.; Sinauer Associates: Sunderland, MA, USA, 2007; pp. 599–648. ISBN 0-87893-219-4. [Google Scholar]
- Noda, M. Cellular and Molecular Biology of the Bone; Academic Press: London, UK, 1993; pp. 49–63. ISBN 0-12-520225-3. [Google Scholar]
- Zhang, N.; Ying, M.-D.; Wu, Y.-P.; Zhou, Z.-H.; Ye, Z.-M.; Li, H.; Lin, D.-S. Hyperoside, a flavonoid compound, inhibits proliferation and stimulates osteogenic differentiation of human osteosarcoma cells. PLoS ONE 2014, 9, e98973. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-H.; Guo, Z.-Y.; Shi, S.-L.; Li, Q.-F. Effect of cinnamic acid on proliferation and differentiation of human osteosarcoma MG-63 cells. Chin. Pharm. Bull. 2012, 9, 1262–1266. [Google Scholar] [CrossRef]
- Panseri, S.; Cunha, C.; D’Alessandro, T.; Sandri, M.; Russo, A.; Giavaresi, G.; Marcacci, M.; Hung, C.T.; Tampieri, A. Magnetic hydroxyapatite bone substitutes to enhance tissue regeneration: Evaluation in vitro using osteoblast-like cells and in vivo in a bone defect. PLoS ONE 2012, 7, e38710. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, G.; Li, J.; Ding, S.; Zhou, S. Cell behaviors on magnetic electrospun poly-d, l-lactide nanofibers. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 34, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.B.; Hu, H.; Xie, L.Q.; Lan, F.; Jiang, W.; Wu, Y.; Gu, Z.W. Magnetic responsive hydroxyapatite composite scaffolds construction for bone defect reparation. Int. J. Nanomed. 2012, 7, 3365–3378. [Google Scholar] [CrossRef] [PubMed]
- Pautke, C.; Schieker, M.; Tischer, T.; Kolk, A.; Neth, P.; Mutschler, W.; Milz, S. Characterization of osteosarcoma cell lines MG-63, Saos-2 and U-2 OS in comparison to human osteoblasts. Anticancer Res. 2004, 24, 3743–3748. [Google Scholar] [PubMed]
- Tsai, S.W.; Liou, H.M.; Lin, C.J.; Kuo, K.L.; Hung, Y.S.; Weng, R.C.; Hsu, F.Y. MG63 osteoblast-like cells exhibit different behaviour when grown on electrospun collagen matrix versus electrospun gelatin matrix. PLoS ONE 2012, 7, e31200. [Google Scholar] [CrossRef]
- Popescu, R.C.; Andronescu, E.; Vasile, B.Ş.; Truşcă, R.; Boldeiu, A.; Mogoantă, L.; Mogoşanu, G.D.; Temelie, M.; Radu, M.; Grumezescu, A.M.; Savu, D. Fabrication and Cytotoxicity of Gemcitabine-Functionalized Magnetite Nanoparticles. Molecules 2017, 22, 1080. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paun, I.A.; Popescu, R.C.; Calin, B.S.; Mustaciosu, C.C.; Dinescu, M.; Luculescu, C.R. 3D Biomimetic Magnetic Structures for Static Magnetic Field Stimulation of Osteogenesis. Int. J. Mol. Sci. 2018, 19, 495. https://doi.org/10.3390/ijms19020495
Paun IA, Popescu RC, Calin BS, Mustaciosu CC, Dinescu M, Luculescu CR. 3D Biomimetic Magnetic Structures for Static Magnetic Field Stimulation of Osteogenesis. International Journal of Molecular Sciences. 2018; 19(2):495. https://doi.org/10.3390/ijms19020495
Chicago/Turabian StylePaun, Irina Alexandra, Roxana Cristina Popescu, Bogdan Stefanita Calin, Cosmin Catalin Mustaciosu, Maria Dinescu, and Catalin Romeo Luculescu. 2018. "3D Biomimetic Magnetic Structures for Static Magnetic Field Stimulation of Osteogenesis" International Journal of Molecular Sciences 19, no. 2: 495. https://doi.org/10.3390/ijms19020495
APA StylePaun, I. A., Popescu, R. C., Calin, B. S., Mustaciosu, C. C., Dinescu, M., & Luculescu, C. R. (2018). 3D Biomimetic Magnetic Structures for Static Magnetic Field Stimulation of Osteogenesis. International Journal of Molecular Sciences, 19(2), 495. https://doi.org/10.3390/ijms19020495