Association between Plasma HMGB-1 and Silicosis: A Case-Control Study
Abstract
1. Introduction
2. Results
2.1. Basic Characteristics of All Subjects
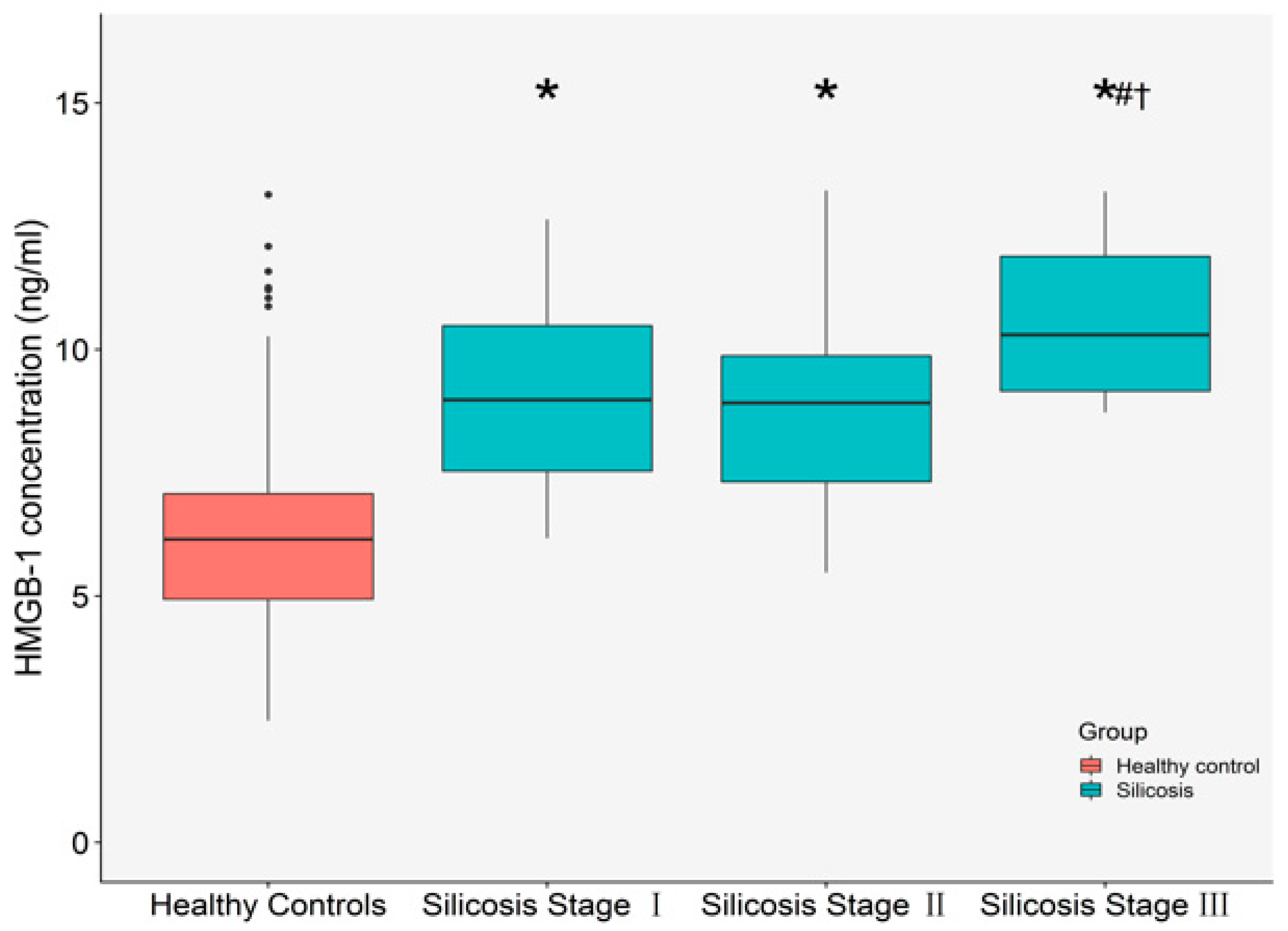
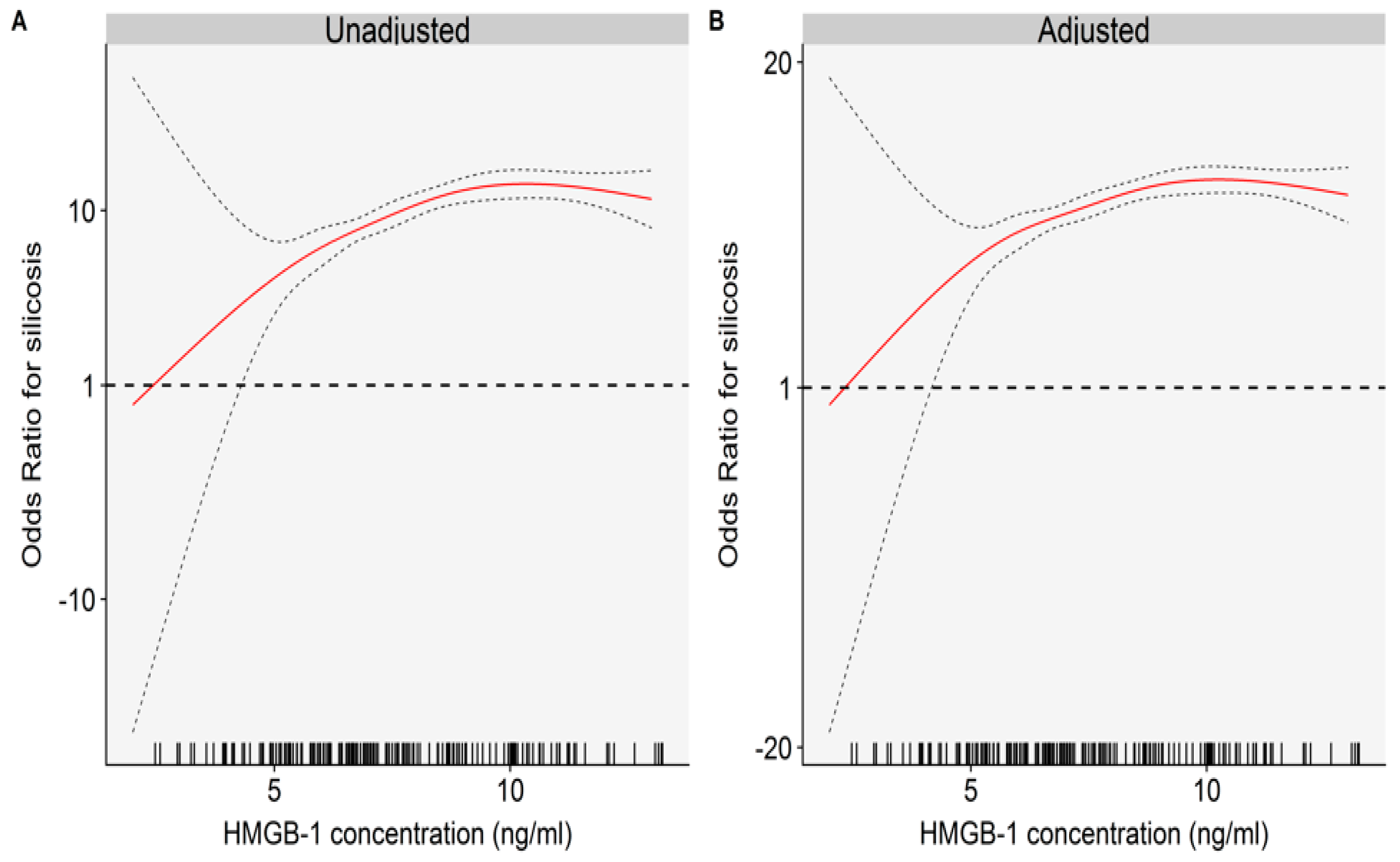
2.2. The Relationships between Plasma HMGB-1 Concentrations and Silicosis
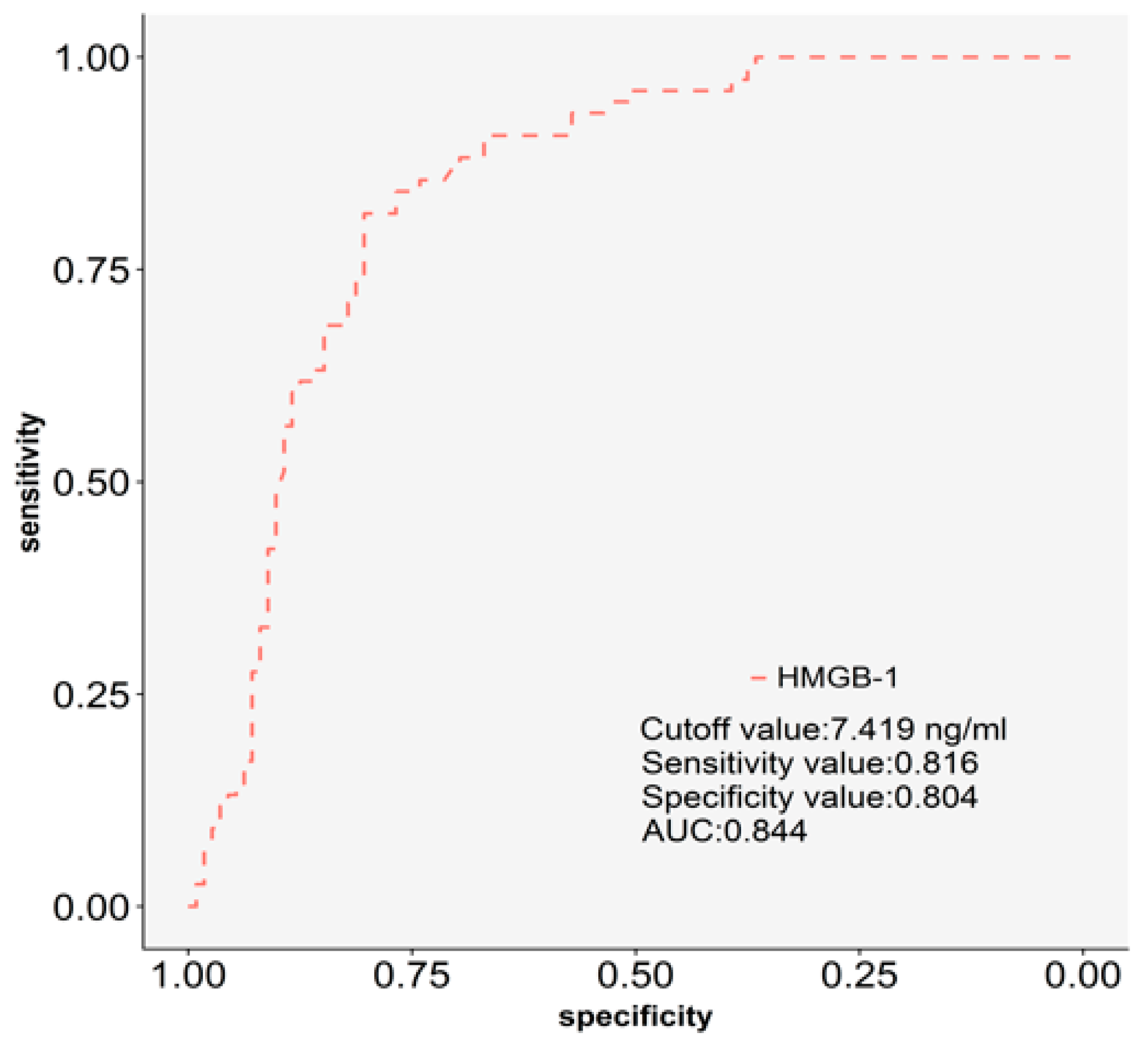
2.3. The Discriminatory Power of Plasma HMGB-1 for Silicosis.
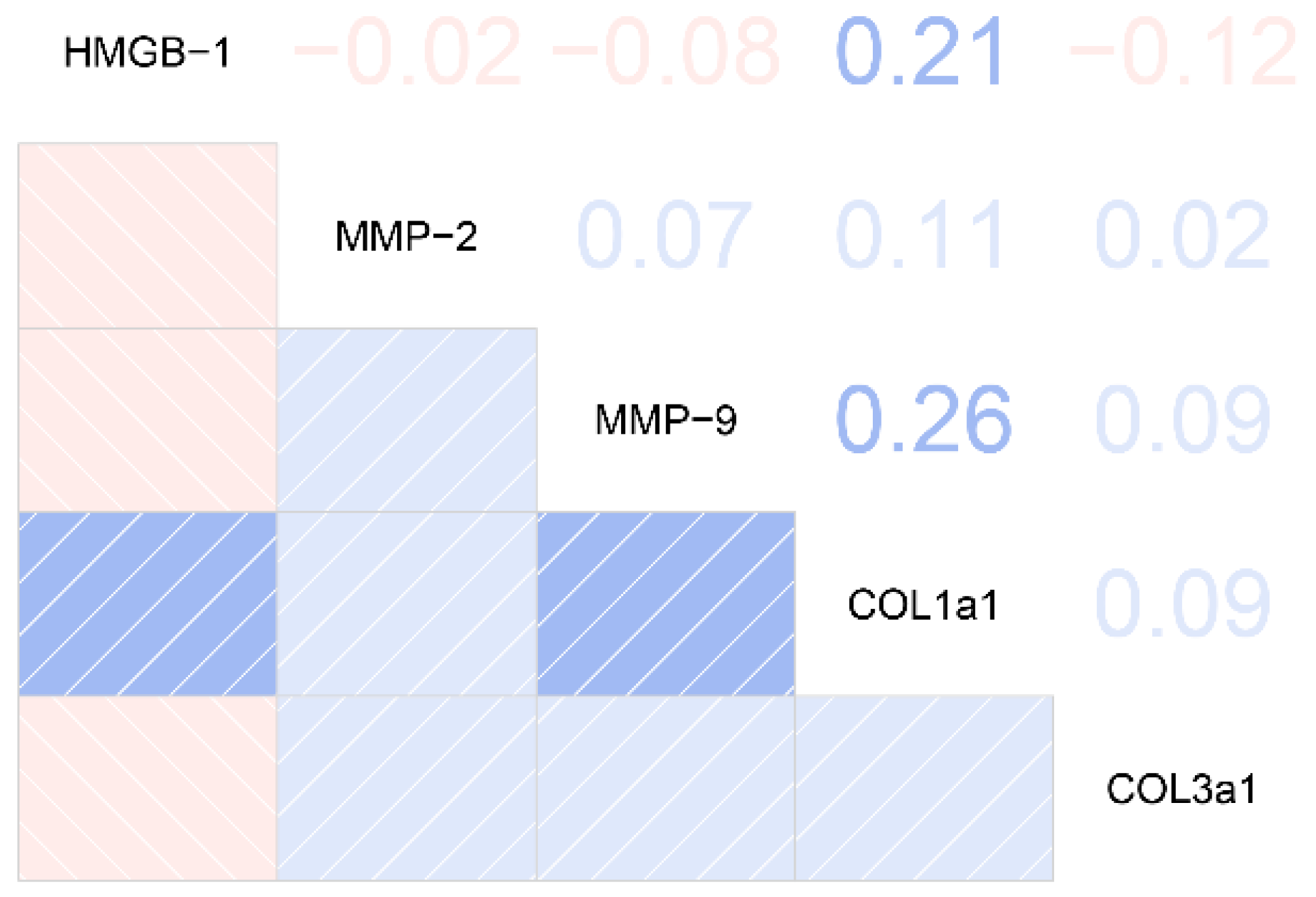
2.4. The Relationships between Plasma HMGB-1 Concentrations and Clinical/Biological Features in Silicosis Patients
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Data and Blood Sample Collection
4.3. Covariates
4.4. Enzyme-Linked Immunosorbent Assay for Plasma Measurements (ELISA)
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HMGB-1 | High-mobility group box-1 |
| ELISA | Enzyme-linked immunosorbent assay |
| ROC | Receiver operating characteristic curve |
| OR | Odds ratio |
| AUC | Area under the curve |
| COPD | Chronic obstructive pulmonary disease |
| BMI | Body mass index |
| SD | Standardized deviation |
| MMP-2 | Matrix metalloproteinase-2 |
| MMP-9 | Matrix metalloproteinase-9 |
| COL1A1 | Collagen alpha-1(I) protein |
| COL3A1 | Collagen alpha-1(III) protein |
| CI | Confidence interval |
| BALF | Bronchoalveolar lavage fluid |
| RAGE | Receptor for advanced glycation end products |
| PF | Pulmonary fibrosis |
References
- Leung, C.C.; Yu, I.T.; Chen, W. Silicosis. Lancet 2012, 379, 2008–2018. [Google Scholar] [CrossRef]
- Pollard, K.M. Silica, Silicosis, and Autoimmunity. Front. Immunol. 2016, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Castranova, V.; Vallyathan, V. Silicosis and coal workers’ pneumoconiosis. Environ. Health Perspect. 2000, 108 (Suppl.4), 675–684. [Google Scholar] [PubMed]
- Chen, W.; Liu, Y.; Wang, H.; Hnizdo, E.; Sun, Y.; Su, L.; Zhang, X.; Weng, S.; Bochmann, F.; Hearl, F.J.; et al. Long-term exposure to silica dust and risk of total and cause-specific mortality in Chinese workers: A cohort study. PLoS Med. 2012, 9, e1001206. [Google Scholar] [CrossRef] [PubMed]
- Dumitriu, I.E.; Baruah, P.; Manfredi, A.A.; Bianchi, M.E.; Rovere-Querini, P. HMGB1: Guiding immunity from within. Trends Immunol. 2005, 26, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H.; Ban, T.; Taniguchi, T. Essential role of high-mobility group box proteins in nucleic acid-mediated innate immune responses. J. Int. Med. 2011, 270, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Lotze, M.T.; Tracey, K.J. High-mobility group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 2005, 5, 331–342. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, J.; Zhang, X.; Liu, Z.; Yang, Y.; Gong, Q.; Ren, B. The Role of HMGB1 in the Pathogenesis of Type 2 Diabetes. J. Diabetes Res. 2016, 2016, 2543268. [Google Scholar] [CrossRef]
- Boteanu, R.M.; Suica, V.I.; Uyy, E.; Ivan, L.; Dima, S.O.; Popescu, I.; Simionescu, M.; Antohe, F. Alarmins in chronic noncommunicable diseases: Atherosclerosis, diabetes and cancer. J. Proteom. 2017, 153, 21–29. [Google Scholar] [CrossRef]
- Imbalzano, E.; Quartuccio, S.; Di Salvo, E.; Crea, T.; Casciaro, M.; Gangemi, S. Association between HMGB1 and asthma: A literature review. Clin. Mol. Allergy 2017, 15, 12. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, Y.Q.; Wang, W.X.; Zhou, Z.X.; Wang, Y.G.; Yang, L.; Ji, Y.L. HMGB1 and RAGE levels in induced sputum correlate with asthma severity and neutrophil percentage. Hum. Immunol. 2012, 73, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Pouwels, S.D.; Nawijn, M.C.; Bathoorn, E.; Riezebos-Brilman, A.; van Oosterhout, A.J.; Kerstjens, H.A.; Heijink, I.H. Increased serum levels of LL37, HMGB1 and S100A9 during exacerbation in COPD patients. Eur. Respir. J. 2015, 45, 1482–1485. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Xin, X.; Fang, L.; Jiang, H.; Xu, X.; Su, X.; Shi, Y. HMGB1 mediates Aspergillus fumigatus-induced inflammatory response in alveolar macrophages of COPD mice via activating MyD88/NF-κB and syk/PI3K signalings. Int. Immunopharmacol. 2017, 53, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Joseph, L.B.; Cervelli, J.A.; Bremer, N.M.; Kim, Y.-K.; Elzind, D.A.; Castranova, V.; Gow, A.J.; Laskin, J.D.; Laskin, D.L. Role of HMGB1 signaling in silica-induced lung inflammation and fibrosis. In Proceedings of the American Thoracic Society 2010 International Conference, New Orleans, LA, USA, 16 May 2010. A2334. [Google Scholar]
- Peeters, P.M.; Perkins, T.N.; Wouters, E.F.; Mossman, B.T.; Reynaert, N.L. Silica induces NLRP3 inflammasome activation in human lung epithelial cells. Part. Fibre Toxicol. 2013, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, M.; Wang, Z.; Cheng, Y.; Liu, H.; Zhou, Z.; Han, B.; Chen, B.; Yao, H.; Chao, J. Neogambogic acid prevents silica-induced fibrosis via inhibition of high-mobility group box 1 and MCP-1-induced protein 1. Toxicol. Appl. Pharmacol. 2016, 309, 129–140. [Google Scholar] [CrossRef]
- Hamada, N.; Maeyama, T.; Kawaguchi, T.; Yoshimi, M.; Fukumoto, J.; Yamada, M.; Yamada, S.; Kuwano, K.; Nakanishi, Y. The role of high mobility group box1 in pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2008, 39, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.; Hayashi, H.; Seo, Y.; Matsuda, K.; Kamio, K.; Saito, Y.; Usuki, J.; Azuma, A.; Kudo, S.; Gemma, A. Reduction in serum high mobility group box-1 level by polymyxin B-immobilized fiber column in patients with idiopathic pulmonary fibrosis with acute exacerbation. Blood Purif. 2011, 32, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Hori, O.; Brett, J.; Slattery, T.; Cao, R.; Zhang, J.; Chen, J.X.; Nagashima, M.; Lundh, E.R.; Vijay, S.; Nitecki, D.; et al. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J. Biol. Chem. 1995, 270, 25752–25761. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, R.; Galvez, B.G.; Pusterla, T.; De Marchis, F.; Cossu, G.; Marcu, K.B.; Bianchi, M.E. Cells migrating to sites of tissue damage in response to the danger signal HMGB1 require NF-κB activation. J. Cell Biol. 2007, 179, 33–40. [Google Scholar] [CrossRef]
- Park, J.S.; Arcaroli, J.; Yum, H.K.; Yang, H.; Wang, H.; Yang, K.Y.; Choe, K.H.; Strassheim, D.; Pitts, T.M.; Tracey, K.J.; Abraham, E. Activation of gene expression in human neutrophils by high mobility group box 1 protein. Am. J. Physiol. Cell Physiol. 2003, 284, C870–C879. [Google Scholar] [CrossRef]
- He, M.; Kubo, H.; Ishizawa, K.; Hegab, A.E.; Yamamoto, Y.; Yamamoto, H.; Yamaya, M. The role of the receptor for advanced glycation end-products in lung fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 293, L1427–L1436. [Google Scholar] [CrossRef] [PubMed]
- Chirico, V.; Lacquaniti, A.; Leonardi, S.; Grasso, L.; Rotolo, N.; Romano, C.; Di Dio, G.; Lionetti, E.; David, A.; Arrigo, T.; et al. Acute pulmonary exacerbation and lung function decline in patients with cystic fibrosis: High-mobility group box 1 (HMGB1) between inflammation and infection. Clin. Microbiol. Infect. 2015, 21, 368.e1–368.e19. [Google Scholar] [CrossRef] [PubMed]
- Guiot, J.; Struman, I.; Chavez, V.; Henket, M.; Herzog, M.; Scoubeau, K.; Hardat, N.; Bondue, B.; Corhay, J.L.; Moermans, C.; Louis, R. Altered epigenetic features in circulating nucleosomes in idiopathic pulmonary fibrosis. Clin. Epigenet. 2017, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Tabata, C.; Shibata, E.; Tabata, R.; Kanemura, S.; Mikami, K.; Nogi, Y.; Masachika, E.; Nishizaki, T.; Nakano, T. Serum HMGB1 as a prognostic marker for malignant pleural mesothelioma. BMC Cancer 2013, 13, 205. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Healthy Subjects (n = 107) | Silicosis (n = 74) | p |
|---|---|---|---|
| Age, years, mean (SD) | 59.67 (9.10) | 59.48 (9.20) | 0.65 |
| BMI, kg/m2, mean (SD) | 24.69 (3.19) | 22.93 (3.16) | <0.05 |
| Current smoking status (n, %) | <0.05 | ||
| Smoker | 37 (34.58) | 13 (17.57) | |
| Non-smoker | 70 (65.42) | 61 (82.43) | |
| Current drinking status (n, %) | 0.68 | ||
| drinker | 66 (61.68) | 26 (35.14) | |
| non-drinker | 41 (38.32) | 48 (64.86) | |
| Fibrosis-related cytokines, mean (SD) | |||
| MMP-2 (ng/mL) | 153.88 (19.35) | 203.99 (9.87) | <0.05 |
| MMP-9 (ng/mL) | 73.43 (24.02) | 124.01 (12.07) | <0.05 |
| COL1A1 (ng/mL) | 28.71 (1.42) | 34.10 (1.30) | <0.05 |
| COL3A1 (ng/mL) | 68.90 (8.50) | 112.14 (9.80) | <0.05 |
| Stage of silicosis (n, %) | - | ||
| Stage I | - | 42 (56.75) | |
| Stage II | - | 22 (29.73) | |
| Stage III | - | 10 (13.52) |
| Characteristics | HMGB-1 Concentrations (ng/mL) | |||
|---|---|---|---|---|
| Per 1 ng/mL Increase of HMGB-1 | Low Concentration Group | High Concentration Group | p | |
| No. of Case/Control Subjects | 11/79 | 63/28 | ||
| Model 1 | 1.91 (1.57, 2.32) | 1.00 (ref.) | 16.16 (7.47, 34.97) | <0.05 |
| Model 2 | 1.89 (1.55, 2.30) | 1.00 (ref.) | 15.52 (7.10, 33.94) | <0.05 |
| Model 3 | 1.85 (1.52, 2.26) | 1.00 (ref.) | 14.52 (6.54, 32.25) | <0.05 |
| Model 4 | 1.85 (1.52, 2.26) | 1.00 (ref.) | 14.45 (6.44, 32.43) | <0.05 |
| Model 5 | 1.86 (1.52, 2.27) | 1.00 (ref.) | 15.33 (6.70, 35.10) | <0.05 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Zhou, Y.; Li, W.; Xiao, L.; Yang, M.; Tan, Q.; Xu, Y.; Chen, W. Association between Plasma HMGB-1 and Silicosis: A Case-Control Study. Int. J. Mol. Sci. 2018, 19, 4043. https://doi.org/10.3390/ijms19124043
Ma J, Zhou Y, Li W, Xiao L, Yang M, Tan Q, Xu Y, Chen W. Association between Plasma HMGB-1 and Silicosis: A Case-Control Study. International Journal of Molecular Sciences. 2018; 19(12):4043. https://doi.org/10.3390/ijms19124043
Chicago/Turabian StyleMa, Jixuan, Yun Zhou, Wei Li, Lili Xiao, Meng Yang, Qiyou Tan, Yiju Xu, and Weihong Chen. 2018. "Association between Plasma HMGB-1 and Silicosis: A Case-Control Study" International Journal of Molecular Sciences 19, no. 12: 4043. https://doi.org/10.3390/ijms19124043
APA StyleMa, J., Zhou, Y., Li, W., Xiao, L., Yang, M., Tan, Q., Xu, Y., & Chen, W. (2018). Association between Plasma HMGB-1 and Silicosis: A Case-Control Study. International Journal of Molecular Sciences, 19(12), 4043. https://doi.org/10.3390/ijms19124043