Applications of Noble Metal-Based Nanoparticles in Medicine
Abstract
1. Nanoparticles—General Information
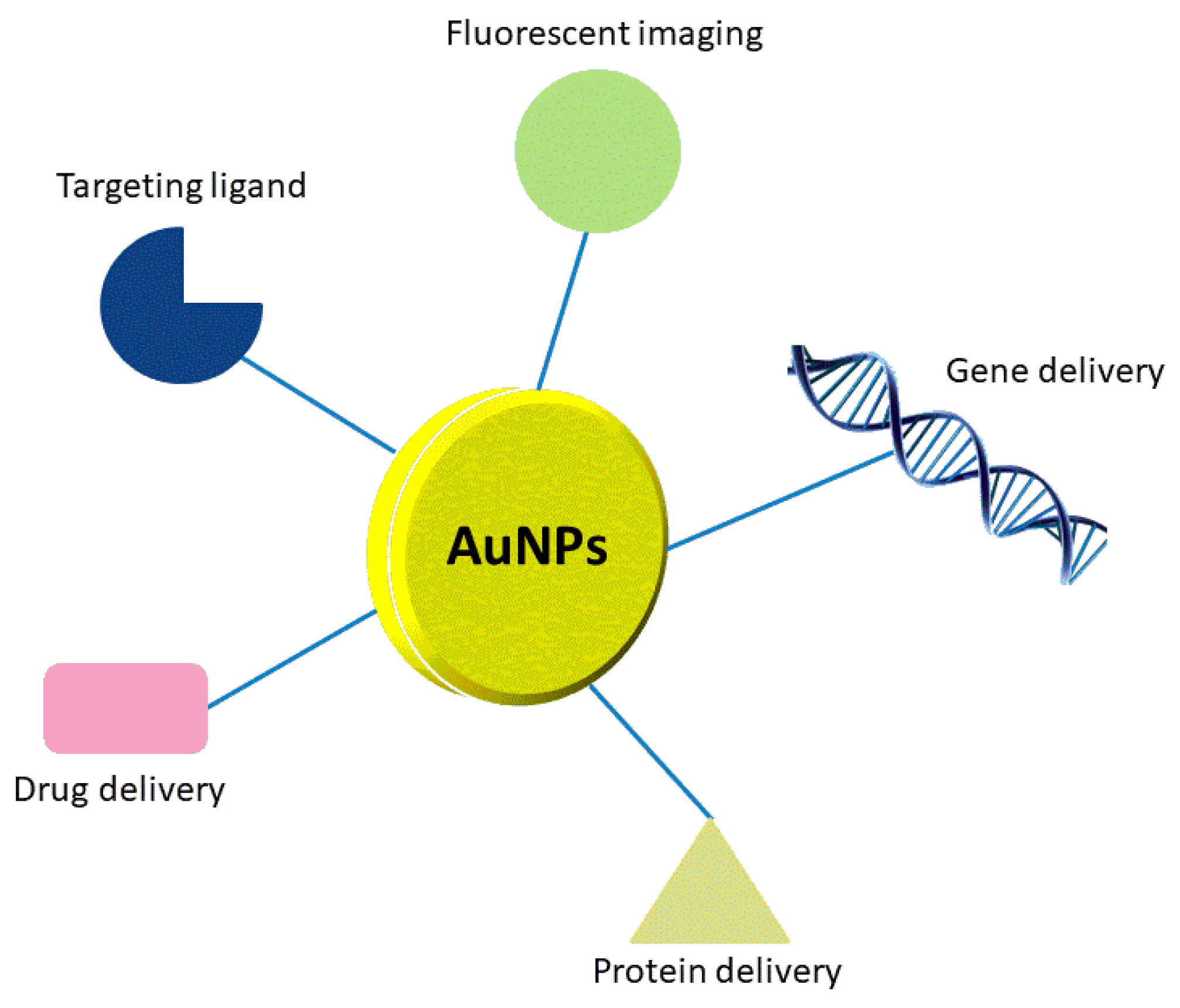
2. Drug Delivery Carriers
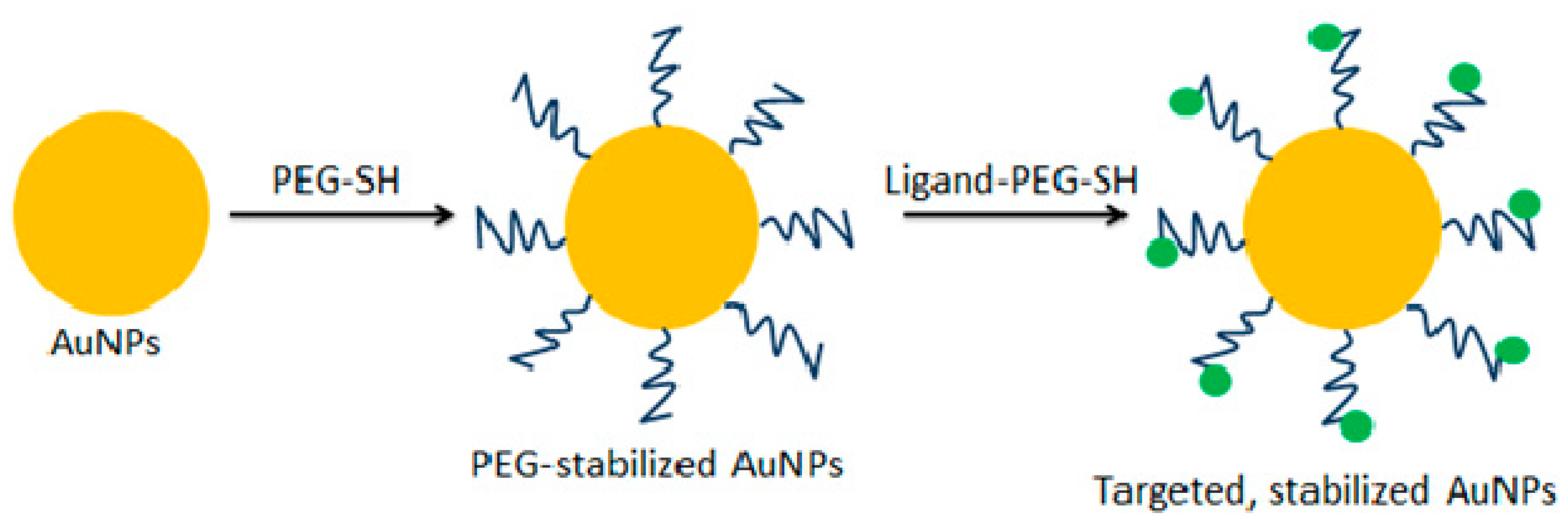
2.1. Nanoparticles for Drug Delivery
2.2. Nanoparticles for Gene Delivery
2.3. Nanoparticles for Protein Delivery
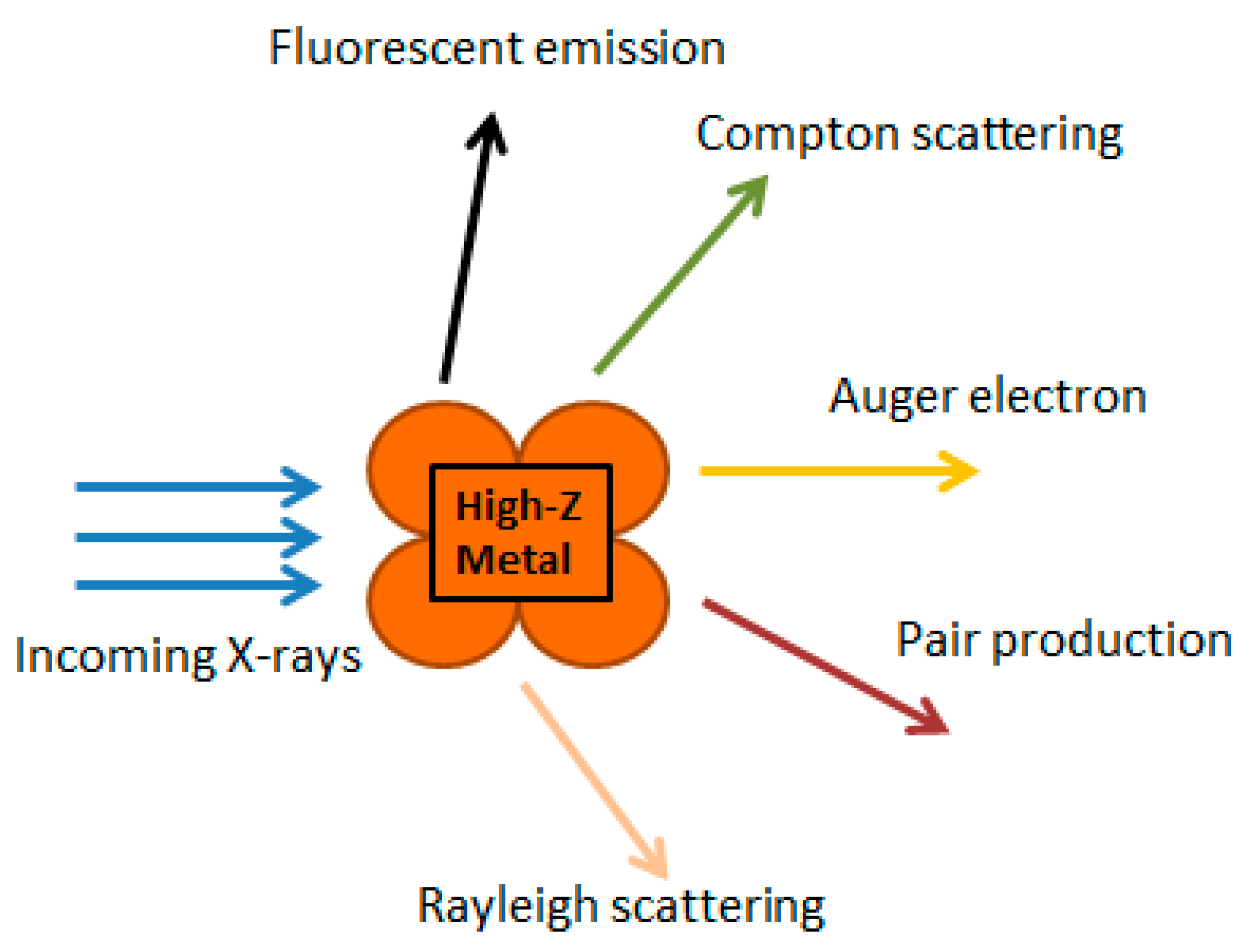
3. Nanoparticles in Radiation-Based Anticancer Therapy
4. Bioimaging and Molecular Diagnosis
5. Antibacterial, Antiviral, and Antifungal Agents
6. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Ag NPs | Silver nanoparticles |
| AMR | Antimicrobial resistant bacteria |
| Anti-EGFR | Anti-epidermal growth factor receptor |
| ATP | Adenosine triphosphate |
| Au NPs | Gold nanoparticles |
| CPP | Cell penetrating peptides |
| CT | Computer tomography |
| DBDT | Biphenyl-4,4′-dithiol |
| DDS | Drug delivery systems |
| DNA | Deoxyribonucleic acid |
| DOX | Doxorubicin |
| Gd NPs | Gadolinium nanoparticles |
| ID | Injected dose |
| MR | Magnetic resonance |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| MTX | Methotrexate |
| NIR | Near-infrared |
| PC | Phthalocyanine |
| Pd NPs | Palladium nanoparticles |
| PDT | Photodynamic therapy |
| PEG | Polyethylene glycol |
| PT | Proton therapy |
| Pt NPs | Platinum nanoparticles |
| PTT | Photothermal therapy |
| RBE | Relative biological effectiveness |
| SERS | Surface enhanced Raman spectroscopy |
| SPR | Surface plasmon resonance |
| RNA | Ribonucleic acid |
| ROS | Reactive oxygen species |
| RT | Radiation therapy |
| TS-1 | Titanium silicate-1 |
| VEGF | Vascular endothelial growth factor |
| TPL | Two-photon-induced photoluminescence |
References
- McNamara, K.; Tofail, A.M. Nanoparticles in biomedical application. Adv. Phys. X 2007, 2, 54–88. [Google Scholar] [CrossRef]
- Moreno-Vega, A.I.; Gomez-Quintero, T.; Nunez-Anita, R.E.; Acosta-Torres, L.S.; Castano, V. Polymeric and ceramic nanoparticles in biomedical applications. J. Nanotechnol. 2012, 2012, 936041. [Google Scholar] [CrossRef]
- Chatterjee, K.; Sarkar, S.; Rao, K.J.; Paria, S. Core/shell nanoparticles in biomedical applications. Adv. Colloid Interface Sci. 2014, 209, 8–39. [Google Scholar] [CrossRef] [PubMed]
- Brun, E.; Sicard-Roselli, C. Actual question raised by nanoparticle radiosensitization. Radiat. Phys. Chem. 2016, 128, 134–142. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Chiang, L.Y.; Lakshmanan, S.; Huang, Y.Y.; Garcia-Diaz, M.; Karimi, M.; de Souza Rastelli, A.N.; Chandran, R. Nanotechnology for photodynamic therapy: A perspective from the laboratory of Dr. Michael, R. Ramblin in the Wellman Center for Photomedicine at Massachusetts General Hospital and Harvard Medical School. Nanotechnol. Rev. 2015, 4, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Alaqad, K.; Saleh, T.A. Gold and silver nanoparticles: Synthesis methods, characterization routes and applications towards drugs. J. Environ. Anal. Toxicol. 2016, 6. [Google Scholar] [CrossRef]
- Kudr, J.; Haddad, Y.; Richtera, L.; Heger, Z.; Cemak, M.; Adam, V.; Zitka, O. Magnetic nanoparticles: From design and synthesis to real world applications. Nanomaterials 2017, 7, 243. [Google Scholar] [CrossRef]
- Zhang, I.; Gu, F.X.; Chan, J.M.; Wang, A.Z.; Langer, R.S.; Farokhzad, O.C. Nanoparticles in medicine: Therapeutic applications and developments. Clin. Pharmacol. Ther. 2008, 83, 761–769. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Mokkapati, V.R.S.S.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold nanoparticles in diagnostics and therapeutics for human cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich method for gold nanoparticle synthesis revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef]
- Garcia-Barrasa, J.; Lopez-de-Luzuriaga, J.M.; Monge, M. Silver nanoparticles: Synthesis through chemical methods in solution and biomedical applications. Cent. Eur. J. Chem. 2011, 9, 7–19. [Google Scholar] [CrossRef]
- Yang, M.; Xia, J. Preparation and characterization of platinum nanorods using ascorbic acid as the reducing agent. Adv. Mater. Res. 2013, 774–776, 577–580. [Google Scholar] [CrossRef]
- An, K.; Samorjai, G.A. Size and shape control of metal nanoparticles for reaction selectivity in catalysis. ChemCatChem 2012, 4, 1512–1524. [Google Scholar] [CrossRef]
- Yin, X.; Chen, S.; Wu, A. Green chemistry synthesis of gold nanoparticles using lactic acid as a reducing agent. IET Micro Nano Lett. 2010, 5, 270–273. [Google Scholar] [CrossRef]
- Sujitha, M.V.; Kannan, S. Green synthesis of gold nanoparticles using citrus fruits (Citrus limon, Citrus reticulate, Citrus sinensis) aqueous extract and its characterization. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 102, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Bogireddy, N.K.R.; Pal, U.; Martinez Gomez, L.; Agarwal, V. Size controlled green synthesis of gold nanoparticles using Coffea arabica seed extract and their catalytic performance in 4-nitrophenol reduction. RSC Adv. 2018, 8, 412. [Google Scholar] [CrossRef]
- Okitsu, K.; Ashokkumar, M.; Grieser, F. Sonochemical synthesis of gold nanoparticles: Effects of ultrasound frequency. J. Phys. Chem. B 2005, 109, 20673–20675. [Google Scholar] [CrossRef]
- Sahoo, G.P.; Basu, S.; Samanta, S.; Misra, A. Microwave-assisted synthesis of anisotropic gold nanocrystals in polymer matrix and their catalytic activities. J. Exp. Nanosci. 2015, 10, 690–702. [Google Scholar] [CrossRef]
- Roldan, M.V.; Pellegri, N.; de Sanctis, O. Electrochemical method for Ag-PEG nanoparticles synthesis. J. Nanopart. 2013, 2013, 524150. [Google Scholar] [CrossRef]
- Martinho, N.; Damgé, C.; Reis, C. Recent advances in drug delivery systems. J. Biomater. Nanobiotechnol. 2011, 2, 510–526. [Google Scholar] [CrossRef]
- Rigon, R.B.; Oyafuso, M.H.; Fujimura, A.T.; Goncalez, M.L.; do Prado, A.H.; Daflon-Gremiao, M.P.; Chorilli, M. Nanotechnology-based drug delivery systems for melanoma antitumoral therapy: A review. BioMed Res. Int. 2015, 2015, 841817. [Google Scholar] [CrossRef] [PubMed]
- Anajafi, T.; Mallik, S. Polymersome-based drug-delivery strategies for cancer therapeutics. Ther. Deliv. 2015, 6, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Vashist, A.; Vashist, A.; Gupta, Y.K.; Ahmad, S. Recent advances in hydrogel based drug delivery systems for the human body. J. Mater. Chem. B 2014, 2, 147–166. [Google Scholar] [CrossRef]
- Yokoyama, M. Polymeric micelles as drug carriers: Their lights and shadows. J. Drug Target. 2014, 22, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.Y.; Zhang, J.W.; Li, R.F.; Wang, Z.X.; Wang, W.J.; Wang, W. Unique roles of gold nanoparticles in drug delivery, targeting and imaging applications. Molecules 2017, 22, 1445. [Google Scholar] [CrossRef] [PubMed]
- Ajnai, G.; Chiu, A.; Kan, T.; Cheng, C.C.; Tsai, T.H.; Chang, J. Trends of gold nanoparticle-based drug delivery system in cancer therapy. J. Exp. Clin. Med. 2014, 6, 172–178. [Google Scholar] [CrossRef]
- Gu, Y.J.; Cheng, J.; Lin, C.C.; Lam, Y.W.; Cheng, S.H.; Wong, W.T. Nuclear penetration of surface functionalized gold nanoparticles. Toxicol. Appl. Pharmacol. 2009, 237, 196–204. [Google Scholar] [CrossRef]
- Wójcik, M.; Lewandowski, W.; Król, M.; Pawłowski, K.; Mieczkowski, J.; Lechowski, R.; Zabielska, K. Enhancing anti-tumor efficacy of doxorubicin by non-covalent conjugation to gold nanoparticles—In vitro studies on feline fibrosarcoma cell lines. PLoS ONE 2015, 10, e0124955. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Patra, C.R.; Earl, A.; Wang, S.; Katarya, A.; Lu, L.; Kizhakkedathu, J.N.; Yaszemski, M.J.; Greipp, P.R.; Mukhopadhyay, D.; et al. Attaching folic acid on gold nanoparticles using noncovalent interaction via different polyethylene glycol backbones and targeting of cancer cells. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 224–238. [Google Scholar] [CrossRef]
- Tran, N.T.T.; Wang, T.H.; Lin, C.Y.; Tai, Y. Synthesis of methotrexate-conjugated gold nanoparticles with enhanced cancer therapeutic effect. Biochem. Eng. J. 2013, 78, 175–180. [Google Scholar] [CrossRef]
- Cui, T.; Liang, J.J.; Chen, H.; Geng, D.D.; Jiao, L.; Yang, J.Y.; Qian, H.; Zhang, C.; Ding, Y. The performance of doxorubicin-conjugated gold nanoparticles: Regulation of drug location. ACS Appl. Mater. Interfaces 2017, 9, 8569–8580. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhou, Y.Y.; Chen, H.; Geng, D.D.; Wu, D.Y.; Hong, J.; Shen, W.B.; Hang, T.J.; Zhang, C. The performance of thiol-terminated PEG-paclitaxel-conjugated gold nanoparticles. Biomaterials 2013, 34, 10217–10227. [Google Scholar] [CrossRef] [PubMed]
- Bergen, J.M.; von Recum, H.A.; Goodman, T.T.; Massey, A.P.; Pun, S.H. Gold nanoparticles as a versatile platform for optimizing physicochemical parameters for targeted drug delivery. Macromol. Biosci. 2006, 6, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.U.; Novosad, V.; Rozhkova, E.A.; Wali, H.; Ali, A.; Fateh, A.A.; Neogi, P.B.; Neogi, A.; Wang, Z. Gold nanoparticles-enabled efficient dual delivery of anticancer therapeutics to HeLa cells. Sci. Rep. 2018, 8, 2907. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Dar, M.Y.; Joshi, B.; Sharma, B.; Shrivastava, S.; Shukla, S. Phytofabrication of silver nanoparticles: Novel drug to overcome hepatocellular ailments. Toxicol. Rep. 2018, 5, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Molano, V.; Hollering, M.; Pöthig, A.; Casini, A.; Kühn, F.E. Evaluation of new palladium cages as potential delivery systems for the anticancer drug cisplatin. Chem. Eur. J. 2016, 22, 2253–2256. [Google Scholar] [CrossRef] [PubMed]
- Riley, M.K.; Vermerris, W. Recent advances in nanomaterials for gene delivery—A review. Nanomaterials 2017, 7, 94. [Google Scholar] [CrossRef]
- Ding, Y.; Jiang, Z.; Saha, K.; Kim, C.S.; Kim, S.T.; Landis, R.F.; Rotello, V.M. Gold nanoparticles for nucleic acid delivery. Mol. Ther. 2014, 22, 1075–1083. [Google Scholar] [CrossRef]
- Kim, E.Y.; Schulz, R.; Swantek, P.; Kunstman, K.; Malim, M.H.; Wolinsky, S.M. Gold nanoparticle-mediated gene delivery induces widespread changes in the expression of innate immunity genes. Gene Ther. 2012, 19, 347–353. [Google Scholar] [CrossRef]
- Son, S.; Nam, J.; Kim, J.; Kim, S.; Kim, W.J. i-motif-driven Au nanomachines in programmed siRNA delivery for gene-silencing and photothermal ablation. ACS Nano 2014, 8, 5574–5584. [Google Scholar] [CrossRef]
- Peng, L.H.; Huang, Y.F.; Zhang, C.Z.; Niu, J.; Chen, Y.; Chu, Y.; Jiang, Z.H.; Gao, J.Q.; Mao, Z.W. Integration of antimicrobial peptides with gold nanoparticles as unique non-viral vectors for gene delivery to mesenchymal stem cells with antibacterial activity. Biomaterials 2016, 103, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Joshi, H.M.; Bhumkar, D.R.; Joshi, K.; Pokharkar, V.; Sastry, M. Gold nanoparticles as carriers for efficient transmucosal insulin delivery. Langmuir 2006, 22, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Schäffler, M.; Sousa, F.; Wenk, A.; Sitia, L.; Hirn, S.; Schleh, C.; Haberl, N.; Violatto, M.; Canovi, M.; Andreozzi, P.; et al. Blood protein coating of gold nanoparticles as potential tool for organ targeting. Biomaterials 2014, 35, 3455–3466. [Google Scholar] [CrossRef] [PubMed]
- Rathinaraj, P.; Al-Jumaily, A.M.; Huh, D.S. Internalization: Acute apoptosis of breast cancer cells using herceptin-immobilized gold nanoparticles. Breast Cancer Targets Ther. 2015, 7, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Farkhani, S.A.; Fard, A.A.; Zakeri-Milani, P.; Mojarrad, J.S.; Valizadeh, H. Enhancing antitumor activity of silver nanoparticles by modification with cell-penetrating peptides. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, P.; Zaccaro, L.; Comegna, D.; Del Gatto, A.; Saviano, M.; Snyders, R.; Cossement, D.; Satriano, C.; Rizzarelli, E. Silver nanoparticles functionalized with a fluorescent cyclic RGD peptide: A versatile integrin targeting platform for cells and bacteria. RSC Adv. 2016, 6, 112381–112392. [Google Scholar] [CrossRef]
- Wang, H.; Mu, X.; He, H.; Zhang, X.D. Cancer radiosensitizers. Trends Pharmacol. Sci. 2018, 39, 24–48. [Google Scholar] [CrossRef]
- Retif, P.; Pinel, S.; Toussaint, M.; Frochot, C.; Chouikrat, R.; Bastogne, T.; Barberi-Heyob, M. Nanoparticles for radiation therapy enhancement: The key parameters. Theranostics 2015, 5, 1030–1044. [Google Scholar] [CrossRef]
- Kwatra, D.; Venugopal, A.; Anant, S. Nanoparticles in radiation therapy: A summary of various approaches to enhance radiosensitization in cancer. Transl. Cancer Res. 2013, 2, 330–342. [Google Scholar] [CrossRef]
- Jeynes, J.C.G.; Merchant, M.J.; Spindler, A.; Wera, A.C.; Kirkby, K.J. Investigation of gold nanoparticle radiosensitization mechanisms using a free radical scavenger and protons of different energies. Phys. Med. Biol. 2014, 59, 6431–6443. [Google Scholar] [CrossRef]
- Butterworth, K.T.; McMahon, S.J.; Taggart, L.E.; Prise, K.M. Radiosensitization by gold nanoparticles: Effective at megavoltage energies and potential role of oxidative stress. Transl. Cancer Res. 2013, 2, 269–279. [Google Scholar] [CrossRef]
- Saberi, A.; Shahbazi-Gahrouei, D.; Abbasian, M.; Fesharaki, M.; Baharlouei, A.; Arab-Bafrani, Z. Gold nanoparticles in combination with megavoltage radiation energy increased radiosensitization and apoptosis in colon cancer HT-29 cells. Int. J. Radiat. Biol. 2017, 93, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Akhter, S.; Ahmad, M.Z.; Ahmad, F.J.; Storm, G.; Kok, R.J. Gold nanoparticles in theranostic oncology: Current state-of-the-art. Expert Opin. Drug Deliv. 2012, 9, 1225–1243. [Google Scholar] [CrossRef] [PubMed]
- Haume, K.; Rosa, S.; Grellet, S.; Śmiałek, M.A.; Butterworth, K.T.; Solov’yov, A.V.; Prise, K.M.; Golding, J.; Mason, N.J. Gold nanoparticles for cancer radiotherapy: A review. Cancer Nanotechnol. 2016, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Beddoes, C.M.; Case, C.P.; Briscoe, W.H. Understanding nanoparticle cellular entry: A physicochemical perspective. Adv. Colloid Interface Sci. 2015, 218, 48–68. [Google Scholar] [CrossRef] [PubMed]
- Stylianpoulos, T.; Soteriou, K.; Fukumura, D.; Jain, R.K. Cationic nanoparticles have superior transvascular flux into solid tumors: Insights from a mathematical model. Ann. Biomed. Eng. 2013, 41, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Su, X.Y.; Liu, P.D.; Wu, H.; Gu, N. Enhancement of radiosensitization by metal-based nanoparticles in cancer radiation therapy. Cancer Biol. Med. 2014, 11, 86–91. [Google Scholar] [CrossRef]
- Liu, P.; Jin, H.; Ma, J.; Zhao, J.; Li, D.; Wu, H.; Gu, N. Silver nanoparticles outperform gold nanoparticles in radiosensitizing U25I cells in vitro and in an intracranial mouse model of glioma. Int. J. Nanomed. 2016, 11, 5003–5014. [Google Scholar] [CrossRef]
- Torrisi, L. Gold nanoparticles enhancing protontherapy efficiency. Recent Pat. Nanotechnol. 2015, 9, 51–60. [Google Scholar] [CrossRef]
- Levin, W.P.; Kooy, H.; Loeffler, J.S.; De Laney, T.F. Proton beam therapy. Br. J. Cancer 2005, 93, 849–854. [Google Scholar] [CrossRef]
- Sotiropoulos, M.; Henthorn, N.T.; Warmenhoven, J.W.; Mackay, R.I.; Kirkby, K.J.; Merchant, M.J. Modelling direct DNA damage for gold nanoparticle enhanced proton therapy. Nanoscale 2017, 9, 18413–18422. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Royle, G.; Lourenco, A.; Schwarz, M.; Fracchiolla, F.; Ricketts, K. Investigation into the effects of high-Z nanomaterials in proton therapy. Phys. Med. Biol. 2016, 61, 4537–4550. [Google Scholar] [CrossRef] [PubMed]
- Porcel, E.; Liehn, S.; Remita, H.; Usami, N.; Kobayashi, K.; Furusawa, Y.; Le Sech, C.; Lacombe, S. Platinum nanoparticles: A promising material for future cancer therapy? Nanotechnology 2010, 21, 85103. [Google Scholar] [CrossRef] [PubMed]
- Schlathölter, T.; Eustache, P.; Porcel, E.; Salado, D.; Stefancikova, L.; Tillement, O.; Lux, F.; Mowat, P.; Biegun, A.K.; van Goethem, M.J.; et al. Improving proton therapy by metal-containing nanoparticles: Nanoscale insights. Int. J. Nanomed. 2016, 11, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
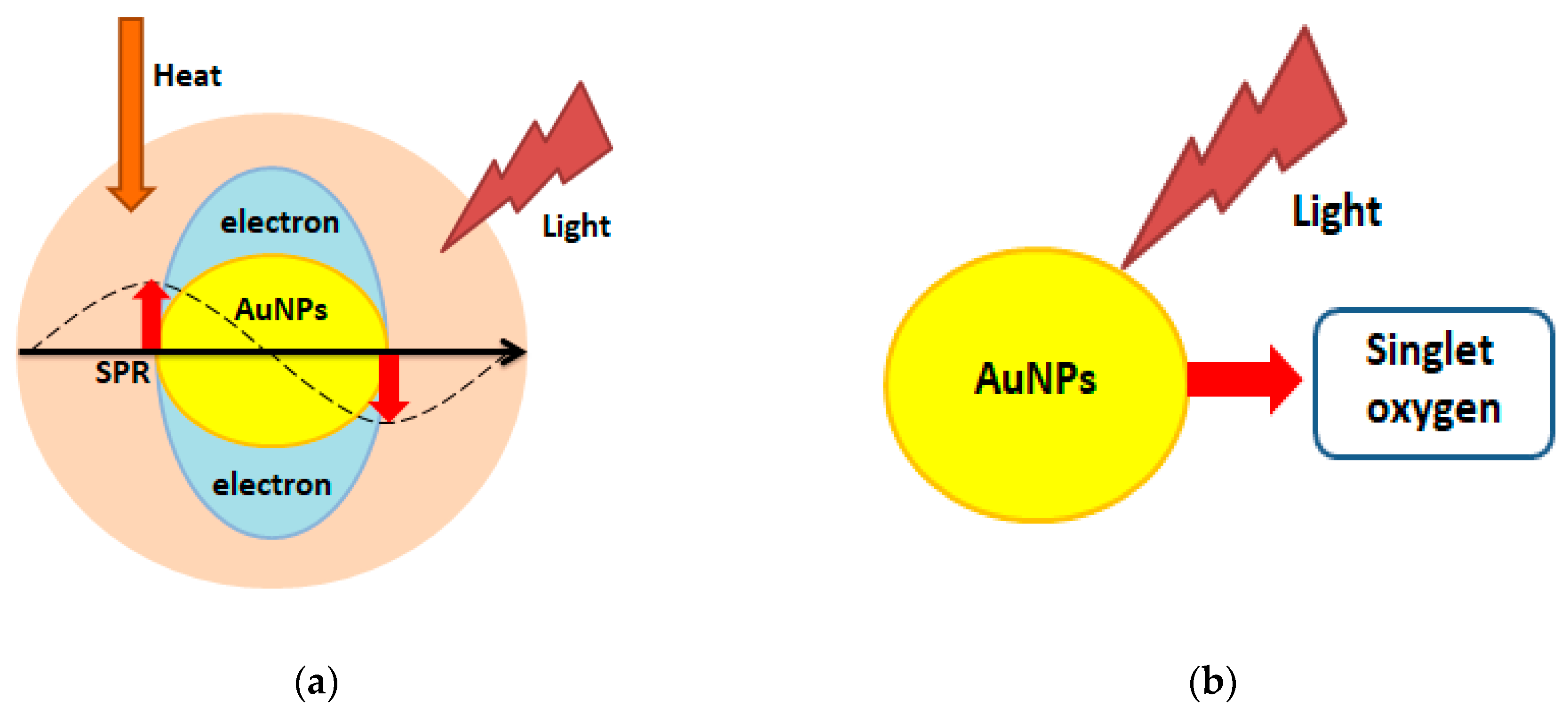
- Zou, L.; Wang, H.; He, B.; Zeng, L.; Tan, T.; Cao, H.; He, X.; Zhang, Z.; Guo, S.; Li, Y. Current approaches of photothermal therapy in treating cancer metastasis with nanotherapeutics. Theranostics 2016, 6, 762–772. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Yao, C.; Zhang, L.; Wang, J.; He, Y.; Xin, J.; Wang, S.; Xu, H.; Zhang, Z. Gold nanoparticle mediated phototherapy for cancer. J. Nanomater. 2016, 4, 5497136. [Google Scholar] [CrossRef]
- Riley, R.S.; Day, E.S. Gold nanoparticle-mediated photothermal therapy: Applications and opportunities for multimodal cancer treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9. [Google Scholar] [CrossRef]
- Camerin, M.; Magaraggia, M.; Soncin, M.; Jori, G.; Moreno, M.; Chambrier, I.; Cook, M.J.; Russell, D.A. The in vivo efficacy of phthalocyanine-nanoparticle conjugates for the photodynamic therapy of amelanotic melanoma. Eur. J. Cancer 2010, 46, 1910–1918. [Google Scholar] [CrossRef]
- Meyers, J.D.; Cheng, Y.; Broome, A.M.; Agnes, R.S.; Schluchter, M.D.; Margevicius, S.; Wang, X.; Kenney, M.E.; Burda, C.; Basilion, J.P. Peptide-targeted gold nanoparticles for photodynamic therapy of brain cancer. Part. Part. Syst. Charact. 2015, 32, 448–457. [Google Scholar] [CrossRef]
- El-Hussein, A. Study DNA damage after photodynamic therapy using silver nanoparticles with A549 cell line. J. Nanomed. Nanotechnol. 2016, 7, 346. [Google Scholar] [CrossRef]
- Thapa, R.K.; Soe, Z.C.; Ou, W.; Poudel, K.; Jeong, J.H.; Jin, S.G.; Ku, S.K.; Choi, H.G.; Lee, Y.M.; Yong, C.S.; et al. Palladium nanoparticle-decorated 2-D graphene oxide for effective photodynamic and photothermal therapy of prostate sold tumors. Colloids Surf. B Biointerfaces 2018, 169, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Cormode, D.P.; Naha, P.C.; Fayad, Z.A. Nanoparticle contrast agents for computed tomography: A focus on micelles. Contrast Media Mol. Imaging 2014, 9, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Cheheltani, R.; Ezzibdeh, R.M.; Chhour, P.; Pulaparthi, K.; Kim, J.; Jurcova, M.; Hsu, J.C.; Blundell, C.; Litt, H.I.; Ferrari, V.A.; et al. Tunable, biodegradable gold nanoparticles as contrast agents for computed tomography and photoacoustic imaging. Biomaterials 2016, 102, 87–97. [Google Scholar] [CrossRef]
- Popovtzer, R.; Agrawal, A.; Kotov, N.A.; Popovtzer, A.; Balter, J.; Carey, T.E.; Kopelman, R. Targeted gold nanoparticles enable molecular CT imaging of cancer. Nano Lett. 2008, 8, 4593–4596. [Google Scholar] [CrossRef] [PubMed]
- Hainfeld, J.F.; Slatkin, D.N.; Focella, T.M.; Smilowitz, H.M. Gold nanoparticles: A new X-ray contrast agent. Br. J. Radiol. 2006, 79, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Karunamuni, R.; Tsourkas, A.; Maidment, A.D.A. Exploring silver as a contrast agent for contrast-enhanced dual-energy X-ray breast imaging. Br. J. Radiol. 2014, 87, 20140081. [Google Scholar] [CrossRef]
- Rayavarapu, R.G.; Petersen, W.; Ungureanu, C.; Post, J.N.; van Leeuwen, T.G.; Monohar, S. Synthesis and bioconjugation of gold nanoparticles as potential molecular probes for light-based imaging techniques. Int. J. Biomed. Imaging 2007, 2007, 29817. [Google Scholar] [CrossRef]
- El-Sayed, I.H.; Huang, X.; El-Sayed, M.A. Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: Applications in oral cancer. Nano Lett. 2005, 5, 829–834. [Google Scholar] [CrossRef]
- Nicholls, F.J.; Rotz, M.W.; Ghuman, H.; MacRenaris, K.W.; Meade, T.J.; Modo, M. DNA-gadolinium-gold nanoparticles for in vitro T1 MR imaging of transplanted human neural stem cells. Biomaterials 2016, 77, 291–306. [Google Scholar] [CrossRef]
- Blythe, K.L.; Willets, K.A. Super-resolution imaging of fluorophore-labeled DNA bound to gold nanoparticles: A single molecule, single particle approach. J. Phys. Chem. C 2016, 120, 803–815. [Google Scholar] [CrossRef]
- Hutter, E.; Maysinger, D. Gold nanoparticles and quantum dots for bioimaging. Microsc. Res. Tech. 2011, 74, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Polat, O.; Karagoz, A.; Isik, S.; Ozturk, R. Influence of gold nanoparticle architecture on in vitro bioimaging and cellular uptake. J. Nanopart. Res. 2014, 16, 2725. [Google Scholar] [CrossRef]
- Shah, N.B.; Dong, J.; Bischof, J.C. Cellular uptake and nanoscale localization of gold nanoparticles in cancer using label-free confocal Raman microscopy. Mol. Pharm. 2011, 8, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Seferos, D.S.; Giljohann, D.A.; Patel, P.C.; Mirkin, C.A. Aptamer nano-flares for molecular detection in living cells. Nano Lett. 2009, 9, 3258–3261. [Google Scholar] [CrossRef] [PubMed]
- Indrasekara, A.S.; Paladini, B.J.; Naczynski, D.J.; Starovoytov, V.; Moghe, P.V.; Fabris, L. Dimeric gold nanoparticle assemblies as tags for SERS-based cancer detection. Adv. Health Mater. 2013, 2, 1370–1376. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Hwang, K.; Lan, T.; Lu, Y. A DNAzyme-gold nanoparticle probe for uranyl ion in living cells. J. Am. Chem. Soc. 2013, 135, 5254–5257. [Google Scholar] [CrossRef]
- Liu, Y.; Ashton, J.R.; Moding, E.J.; Yuan, H.; Register, J.K.; Fales, A.M.; Choi, J.; Whitley, M.J.; Zhao, X.; Qi, Y.; et al. A plasmonic gold nanostar theranostic probe for in vivo tumor imaging and photothermal therapy. Theranostics 2015, 5, 946–960. [Google Scholar] [CrossRef]
- Gopinath, P.M.; Ranjani, A.; Dhanasekaran, D.; Thajuddin, N.; Archunan, G.; Akbarsha, M.A.; Gulyas, B.; Padmanabhan, P. Multi-functional nano silver: A novel disruptive and theranostic agent for pathogenic organism in real-time. Sci. Rep. 2016, 6, 34058. [Google Scholar] [CrossRef]
- Yue, Y.; Wagner, S.; Medina-Kauwe, L.; Cui, X.; Zhang, G.; Shiao, S.; Sandler, H.; Fraass, B. WE-FG-BRA-11: Theranostic platinum nanoparticles for radiation sensitization in breast cancer radiotherapy. Med. Phys. 2016, 43, 3826. [Google Scholar] [CrossRef]
- Chandra, P.; Das, D.; Abdelwahab, A.A. Gold nanoparticles in molecular diagnostics and therapeutics. Dig. J. Nanomater. Biostruct. 2010, 5, 363–367. [Google Scholar]
- Sintubin, L.; Verstraete, W.; Boon, N. Biologically produced nanosilver: Current state and future perspectives. Biotechnol. Bioeng. 2012, 109, 2422–2436. [Google Scholar] [CrossRef] [PubMed]
- De Kraker, M.E.A.; Stewardson, A.J.; Harbarth, S. Will 10 million people die a year due to antimicrobial resistance by 2050? PLoS Med. 2016, 13, e1002184. [Google Scholar] [CrossRef] [PubMed]
- Santajit, S.; Indrawattana, N. Mechanism of antimicrobial resistance in ESKAPE pathogens. BioMed Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef] [PubMed]
- Lutgring, J.D.; Limbago, B.M. The problem of carbapenemase-producing-carbapenem-resistant Enterobacteriaceae detection. J. Clin. Microbiol. 2016, 54, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, M.; Hara, K.; Kudo, J. Bactericidal actions of a silver ion solution on Escherichia coli, studied by energy-filtering transmission electron microscopy and proteomic analysis. Appl. Environ. Microbiol. 2005, 71, 7589–7593. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, J.Y.; Kim, J.; Lee, J.H.; Hahn, J.S.; Gu, M.B.; Yoon, J. Silver-on-mediated reactive oxygen species generation affecting bactericidal activity. Water Res. 2009, 43, 1027–1032. [Google Scholar] [CrossRef]
- Yang, W.; Shen, C.; Ji, Q.; An, H.; Wang, J.; Liu, Q.; Zhang, Z. Food storage material silver nanoparticles interfere with DNA replication fidelity and bind with DNA. Nanotechnology 2009, 20, 085102. [Google Scholar] [CrossRef]
- Shrivastava, S.; Bera, T.; Roy, A.; Singh, G.; Ramachandrarao, P.; Dash, D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 2007, 18, 225103. [Google Scholar] [CrossRef]
- Parlinska-Wojtan, M.; Kus-Liskiewicz, M.; Depciuch, J.; Sadik, O. Green synthesis and antibacterial effects of aqueous colloidal solutions of silver nanoparticles using chamomile terpenoids as a combined reducing and capping agent. Bioprocess Biosyst. Eng. 2016, 39, 1213–1223. [Google Scholar] [CrossRef]
- Parlinska-Wojtan, M.; Depciuch, J.; Fryc, B.; Kus-Liskiewicz, M. Green synthesis and antibacterial of aqueous colloidal solution of silver nanoparticles using clove eugenol. Appl. Organomet. Chem. 2018, 32, e4276. [Google Scholar] [CrossRef]
- Senthil, B.; Devasena, T.; Prakash, B.; Rajesekar, A. Non-cytotoxic effect of green synthesized silver nanoparticles and its antibacterial activity. J. Photochem. Photobiol. B 2017, 177, 1–7. [Google Scholar] [CrossRef]
- Holubnycha, V.; Kalinkevich, O.; Ivashchenko, O.; Pogorielov, M. Antibacterial activity of in situ prepared chitosan/silver nanoparticles solution against methicillin-resistant strains of Staphylococcus aureus. Nanoscale Res. Lett. 2018, 13, 71. [Google Scholar] [CrossRef] [PubMed]
- Panacek, A.; Kvitek, L.; Smekalova, M.; Vecerowa, R.; Kolar, M.; Roderova, M.; Dycka, F.; Sebela, M.; Prucek, R.; Tomanec, O.; et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nat. Nanotechnol. 2018, 13, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Shamaila, S.; Zafar, N.; Riaz, S.; Sharif, R.; Nazir, J.; Naseem, S. Gold nanoparticles: An efficient antimicrobial agent against enteric bacterial human pathogen. Nanomaterials 2016, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.M.; Fouad, S.A.; Elshoky, H.A.; Mohammed, G.M.; Salaheldin, T.A. Antibacterial effect of gold nanoparticles against Corynebacterium pseudotuberculosis. Int. J. Vet. Med. 2017, 5, 23–29. [Google Scholar] [CrossRef]
- Guerra, R.; Lima, E.; Guzman, A. Antimicrobial supported nanoparticles: Gold versus silver for the cases of Escherichia coli and Salmonella typhi. Microporous Mesoporous Mater. 2013, 170, 62–66. [Google Scholar] [CrossRef]
- Ahmed, K.B.A.; Raman, T.; Anbazhagan, V. Platinum nanoparticles inhibits bacteria proliferation and rescue zebrafish from bacterial infection. RSC Adv. 2016, 50, 44415–44424. [Google Scholar] [CrossRef]
- Xia, Z.K.; Ma, Q.H.; Li, S.Y.; Zhang, D.Q.; Cong, L.; Tian, Y.L.; Yang, R.Y. The antifungal effect of silver nanoparticles on Trichosporon asahii. J. Microbiol. Immunol. Infect. 2016, 49, 182–188. [Google Scholar] [CrossRef]
- Elgorban, A.M.; El-Samawaty, A.E.R.; Yassin, M.A.; Sayed, S.R.; Adil, S.F.; Elhindi, K.M.; Makri, M.; Khan, M. Antifungal silver nanoparticles: Synthesis, characterization and biological evaluation. Biotechnol. Biotechnol. Equip. 2016, 30, 56–62. [Google Scholar] [CrossRef]
- Khatami, M.; Mortazavi, S.M.; Kishani-Farahani, Z.; Amini, A.; Amini, E.; Heli, H. Biosynthesis of silver nanoparticles using pine pollen and evaluation of the antifungal efficiency. Iran. J. Biotechnol. 2017, 15, e1436. [Google Scholar] [CrossRef] [PubMed]
- Babu, B.; Nair, R.S.; Angelo, J.M.C.; Mathai, V.; Vineet, R.V. Evaluation of efficacy of chitosan-silver nanocomposite on Candida albicans when compared to three different antifungal agents in combination with standard irrigation protocol: An ex vivo study. Saudi Endod. J. 2017, 7, 87–91. [Google Scholar] [CrossRef]
- Wani, I.A.; Ahmad, T. Size and shape dependent antifungal activity of gold nanoparticles: A case study of Candida. Colloids Surf. B Biointerfaces 2013, 101, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Sun, R.W.Y.; Chen, R.; Hui, C.K.; Ho, C.M.; Luk, J.M.; Lau, G.K.K.; Che, C.M. Silver nanoparticles inhibit hepatitis B virus replication. Antivir. Ther. 2008, 13, 253–262. [Google Scholar] [PubMed]
- Speshock, J.L.; Murdock, R.C.; Braydich-Stolle, L.K.; Schrand, A.M.; Hussain, S.M. Interaction of silver nanoparticles with Tacaribe virus. J. Nanobiotechnol. 2010, 8, 19. [Google Scholar] [CrossRef]
- Sun, R.W.Y.; Chen, R.; Chung, N.P.Y.; Ho, C.M.; Lin, C.L.S.; Che, C.M. Silver nanoparticles fabricated in Hepes buffer exhibit cytoprotective activities toward HIV-1 infected cells. Chem. Commun. 2005, 40, 5059–5061. [Google Scholar] [CrossRef] [PubMed]
- Baram-Pinto, D.; Shukla, S.; Perkas, N.; Gedanken, A.; Sarid, R. Inhibition of herpes simplex virus type 1 infection by silver nanoparticles capped with mercaptoethane sulfonate. Bioconjug. Chem. 2009, 20, 1497–1502. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klębowski, B.; Depciuch, J.; Parlińska-Wojtan, M.; Baran, J. Applications of Noble Metal-Based Nanoparticles in Medicine. Int. J. Mol. Sci. 2018, 19, 4031. https://doi.org/10.3390/ijms19124031
Klębowski B, Depciuch J, Parlińska-Wojtan M, Baran J. Applications of Noble Metal-Based Nanoparticles in Medicine. International Journal of Molecular Sciences. 2018; 19(12):4031. https://doi.org/10.3390/ijms19124031
Chicago/Turabian StyleKlębowski, Bartosz, Joanna Depciuch, Magdalena Parlińska-Wojtan, and Jarek Baran. 2018. "Applications of Noble Metal-Based Nanoparticles in Medicine" International Journal of Molecular Sciences 19, no. 12: 4031. https://doi.org/10.3390/ijms19124031
APA StyleKlębowski, B., Depciuch, J., Parlińska-Wojtan, M., & Baran, J. (2018). Applications of Noble Metal-Based Nanoparticles in Medicine. International Journal of Molecular Sciences, 19(12), 4031. https://doi.org/10.3390/ijms19124031





