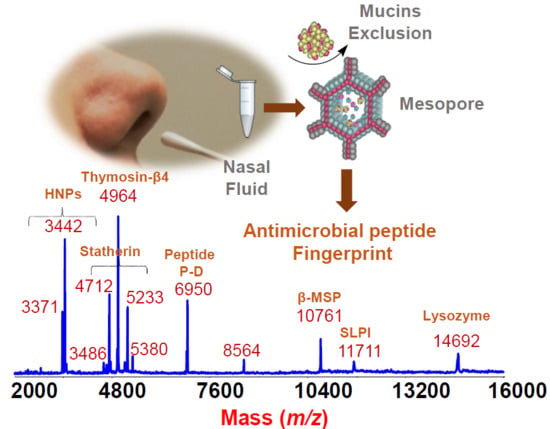
Rapid Detection and Identification of Antimicrobial Peptide Fingerprints of Nasal Fluid by Mesoporous Silica Particles and MALDI-TOF/TOF Mass Spectrometry: From the Analytical Approach to the Diagnostic Applicability in Precision Medicine
Abstract
1. Introduction
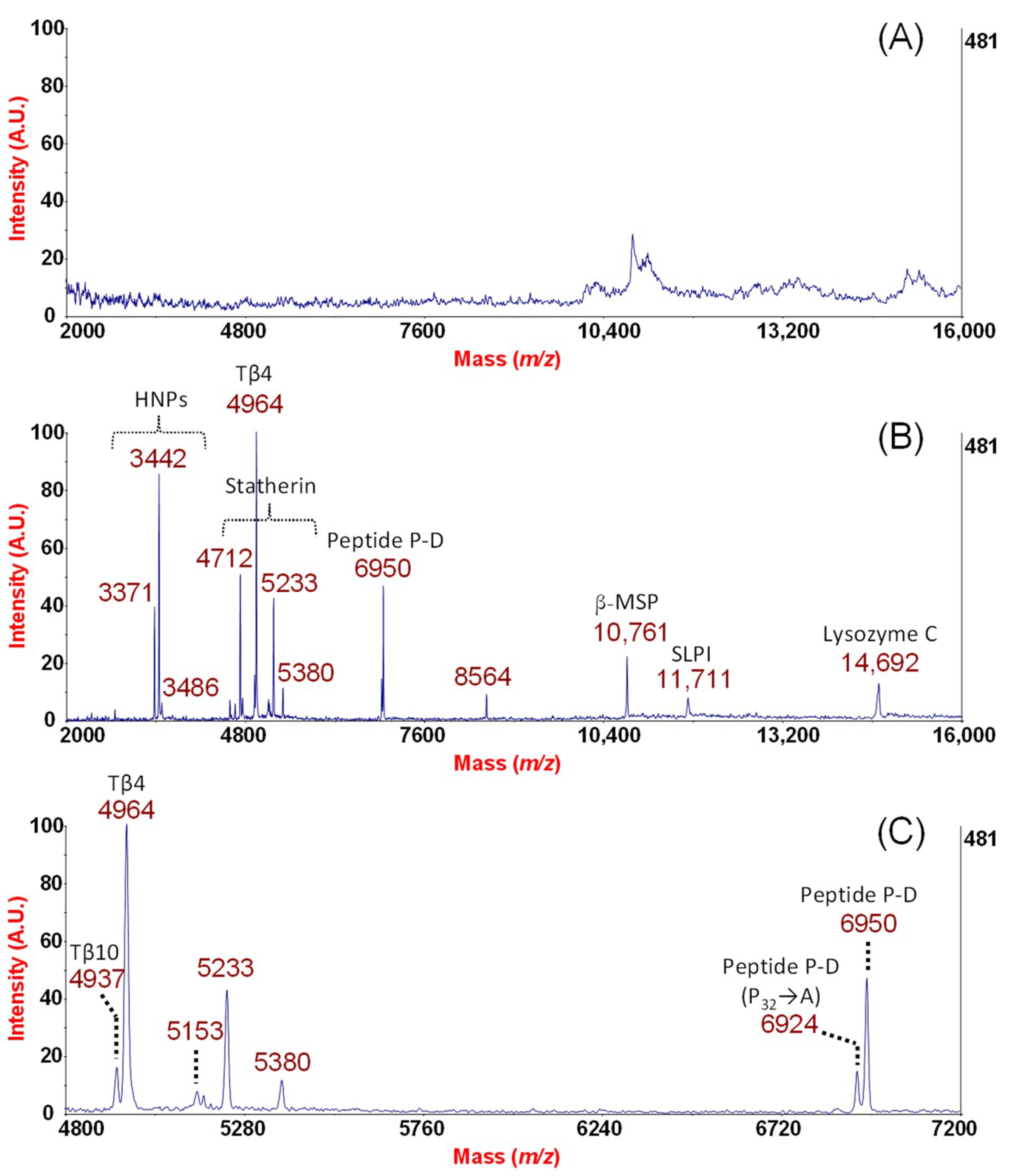
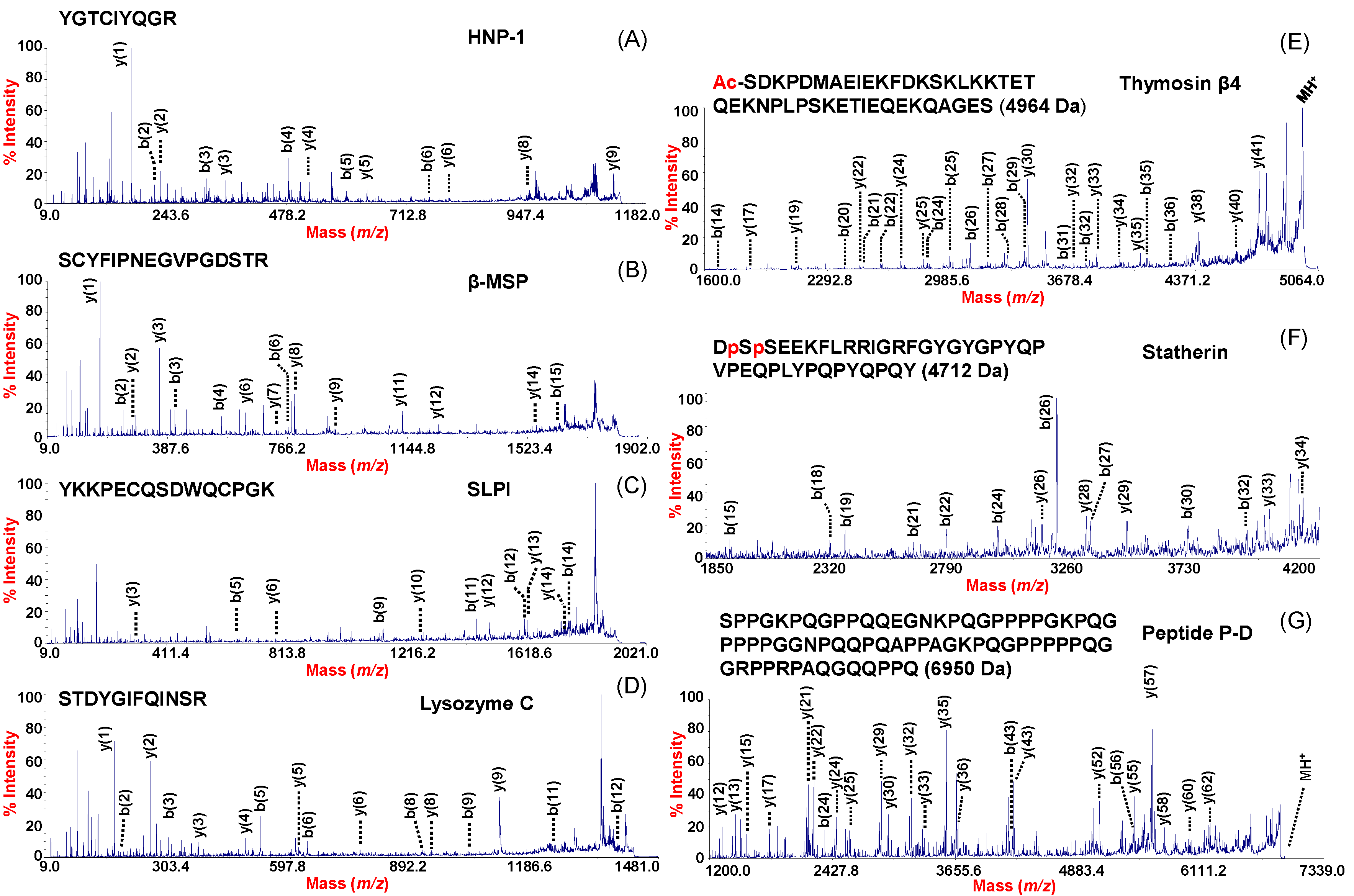
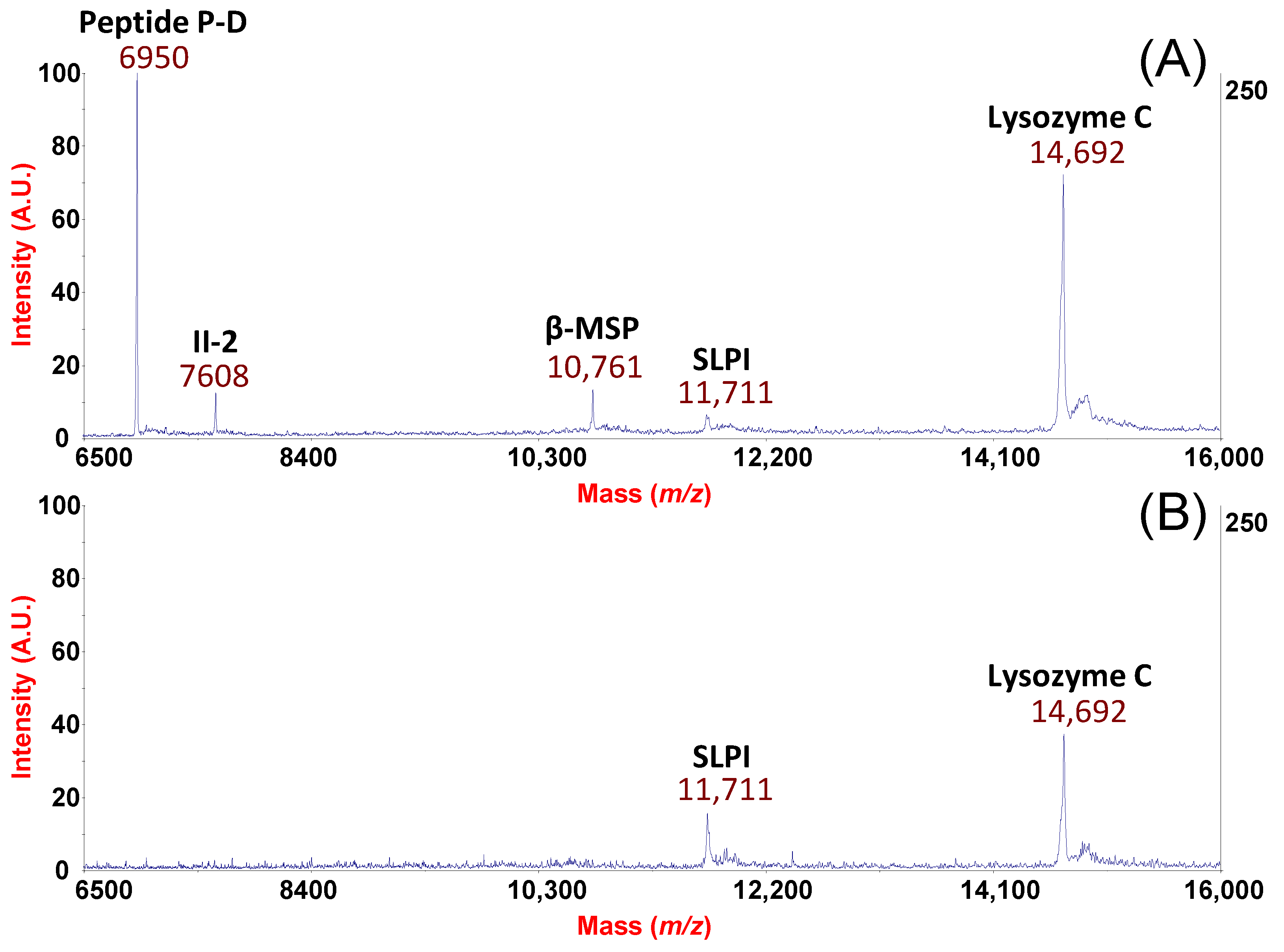
2. Results and Discussion
From the Analytical Approach to Diagnostic Application
3. Materials and Methods
3.1. Reagents
3.2. Patient Recruitment
3.3. NF Collection by Nasal Swab
3.4. NF Recovery from Nasal Swabs
3.5. NF Samples Normalization
3.6. NF Processing by MPS-C
3.7. MALDI-TOF MS Analysis
3.8. Identification of NF AMP
3.8.1. MALDI-TOF/TOF AMP Sequencing in MPS Processed NF
3.8.2. HNPs Identification
3.8.3. NF Proteins Identification by 1D Gel Electrophoresis and MALDI TOF MS
3.9. PCA
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Calzetta, L.; Rogliani, P.; Cazzola, M.; Matera, M.G. Advances in asthma drug discovery: Evaluating the potential of nasal cell sampling and beyond. Expert Opin. Drug Discov. 2014, 9, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Gaga, M.; Vignola, A.M.; Chanez, P. Upper and lower airways: Similarities and differences. Eur. Respir. Monogr. 2001, 6, 1–15. [Google Scholar]
- Cole, A.M.; Dewan, P.; Ganz, T. Innate Antimicrobial Activity of Nasal Secretions. Infect. Immun. 1999, 67, 3267–3275. [Google Scholar] [PubMed]
- Epanda, R.M.; Vogel, H.J. Diversity of antimicrobial peptides and their mechanisms of action. BBA Biomembr. 1999, 1462, 11–28. [Google Scholar] [CrossRef]
- Ganz, T. Antimicrobial polypeptides. J. Leukoc. Biol. 2004, 75, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Lü, F.X.; Esch, R.E. Novel nasal secretion collection method for the analysis of allergen specific antibodies and inflammatory biomarkers. J. Immunol. Methods 2010, 356, 6–17. [Google Scholar] [CrossRef]
- Lombardo, N.; Preianò, M.; Maggisano, G.; Murfuni, M.S.; Messina, L.; Pelaia, G.; Savino, R.; Terracciano, R. A rapid differential display analysis of nasal swab fingerprints to distinguish allergic from non-allergic rhinitis subjects by mesoporous silica particles and MALDI-TOF mass spectrometry. Proteomics 2017, 17. [Google Scholar] [CrossRef]
- Casado, B.; Pannell, L.K.; Iadarola, P.; Baraniuk, J.N. Identification of human nasal mucous proteins using proteomics. Proteomics 2005, 5, 2949–2959. [Google Scholar] [CrossRef]
- Débat, H.; Eloit, C.; Blon, F.; Sarazin, B.; Henry, C.; Huet, J.C.; Trotier, D.; Pernollet, J.C. Identification of Human Olfactory Cleft Mucus Proteins Using Proteomic Analysis. J. Proteome Res. 2007, 6, 1985–1996. [Google Scholar] [CrossRef]
- Lee, J.Y.; Byun, J.Y.; Lee, S.H. Proteomic analysis of normal human nasal mucosa: Establishment of a two-dimensional electrophoresis reference map. Clin. Biochem. 2009, 42, 692–700. [Google Scholar] [CrossRef]
- Ali, M.; Lillehoj, E.P.; Park, Y.; Kyo, Y.; Kim, K.C. Analysis of the proteome of human airway epithelial secretions. Proteome Sci. 2011, 9. [Google Scholar] [CrossRef] [PubMed]
- Simões, T.; Charro, N.; Blonder, J.; Faria, D.; Couto, F.M.; Chan, K.C.; Waybright, T.; Isaaq, H.J.; Veenstra, T.D.; Penque, D. Molecular profiling of the human nasal epithelium: A proteomics approach. J. Proteomics 2011, 75, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Gelardi, M.; Siciliano, R.A.; Papa, F.; Mazzeo, M.F.; De Nitto, E.; Quaranta, N.; Lippolis, R. Proteomic analysis of human nasal mucosa: Different expression profile in rhino-pathologic states. Eur. Ann. Allergy Clin. Immunol. 2014, 46, 164–171. [Google Scholar] [PubMed]
- Bryborn, M.; Adner, M.; Cardell, L.O. Psoriasin, one of several new proteins identified in nasal lavage fluid from allergic and nonallergic individuals using 2-dimensional gel electrophoresis and mass spectrometry. Respir. Res. 2005, 6, 118. [Google Scholar] [CrossRef]
- Ghafouri, B.; Irander, K.; Lindbom, J.; Tagesson, C.; Lindahl, M. Comparative Proteomics of Nasal Fluid in Seasonal Allergic Rhinitis. J. Proteome Res. 2006, 5, 330–338. [Google Scholar] [CrossRef]
- Benson, L.M.; Mason, C.J.; Friedman, O.; Kita, H.; Bergen, H.R., 3rd; Plager, D.A. Extensive fractionation and identification of proteins within nasal lavage fluids from allergic rhinitis and asthmatic chronic rhinosinusitis patients. J. Sep. Sci. 2009, 32, 44–56. [Google Scholar] [CrossRef]
- Tomazic, P.V.; Birner-Gruenberger, R.; Leitner, A.; Obrist, B.; Spoerk, S.; Lang-Loidolt, D. Nasal mucus proteomic changes reflect altered immune responses and epithelial permeability in patients with allergic rhinitis. J. Allergy Clin. Immunol. 2014, 133, 741–750. [Google Scholar] [CrossRef]
- Tomazic, P.V.; Birner-Gruenberger, R.; Leitner, A.; Darnhofer, B.; Spoerk, S.; Lang-Loidolt, D. Apolipoproteins have a potential role in nasal mucus of allergic rhinitis patients: A proteomic study. Laryngoscope 2015, 125, E91–E96. [Google Scholar] [CrossRef]
- Sande, C.J.; Mutunga, M.; Muteti, J.; Berkley, J.A.; Nokes, D.; Njunge, J. Untargeted analysis of the airway proteomes of children with using mass spectrometry based proteomics. Sci. Rep. 2018, 8, 13814. [Google Scholar] [CrossRef]
- Roxo-Rosa, M.; da Costa, G.; Luider, T.M.; Scholte, B.J.; Coelho, A.V.; Amaral, M.D.; Penque, D. Proteomic analysis of nasal cells from cystic fibrosis patients and non-cystic fibrosis control individuals: Search for novel biomarkers of cystic fibrosis lung disease. Proteomics 2006, 6, 2314–2325. [Google Scholar] [CrossRef]
- Jeanson, L.; Guerrera, I.C.; Papon, J.F.; Chhuon, C.; Zadigue, P.; Prulière-Escabasse, V.; Amselem, S.; Escudier, E.; Coste, A.; Edelman, A. Proteomic analysis of nasal epithelial cells from cystic fibrosis patients. PLoS ONE 2014, 9, e108671. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Sun, H.; Xiao, Z.; Feng, X.; Li, C.; Li, D. Differential proteomic analysis of nasal polyps, chronic sinusitis, and normal nasal mucosa tissues. Otolaryngol. Head Neck Surg. 2009, 141, 364–368. [Google Scholar]
- Saieg, A.; Brown, K.J.; Pena, M.T.; Rose, M.C.; Preciado, D. Proteomic analysis of pediatric sinonasal secretions shows increased MUC5B mucin in CRS. Pediatr. Res. 2015, 77, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Savino, R.; Paduano, S.; Preianò, M.; Terracciano, R. The proteomics big challenge for biomarkers and new drug-targets discovery. Int. J. Mol. Sci. 2012, 13, 13926–13948. [Google Scholar] [CrossRef] [PubMed]
- Trindade, F.; Amado, F.; Oliveira-Silva, R.P.; Daniel-da-Silva, A.L.; Ferreira, R.; Klein, J.; Faria-Almeida, R.; Gomes, P.S.; Vitorino, R. Toward the definition of a peptidome signature and protease profile in chronic periodontitis. Proteomics Clin. Appl. 2015, 9, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef]
- Terracciano, R.; Pasqua, L.; Casadonte, F.; Frascà, S.; Preianò, M.; Falcone, D.; Savino, R. Derivatized mesoporous silica beads for MALDI-TOF MS profiling of human plasma and urine. Bioconjug. Chem. 2009, 20, 913–923. [Google Scholar] [CrossRef]
- Linzmeier, R.M.; Ganz, T. Human defensin gene copy number polymorphisms: Comprehensive analysis of independent variation in alpha- and beta-defensin regions at 8p22-p23. Genomics 2005, 86, 423–430. [Google Scholar] [CrossRef]
- Castagnola, M.; Cabras, T.; Iavarone, F.; Vincenzoni, F.; Vitali, A.; Pisano, E.; Nemolato, S.; Scarano, E.; Fiorita, A.; Vento, G.; et al. Top-down platform for deciphering the human salivary proteome. J. Matern. Fetal Neonatal. Med. 2012, 25, 27–43. [Google Scholar] [CrossRef]
- Schenkels, L.C.; Veerman, E.C.; Nieuw Amerongen, A.V. Biochemical composition of human saliva in relation to other mucosal fluids. Crit. Rev. Oral Biol. Med. 1995, 6, 161–175. [Google Scholar] [CrossRef]
- Melis, M.; Aragoni, M.C.; Arca, M.; Cabras, T.; Caltagirone, C.; Castagnola, M.; Crnjar, R.; Messana, I.; Tepper, B.J.; Barbarossa, I.T. Marked increase in PROP taste responsiveness following oral supplementation with selected salivary proteins or their related free amino acids. PLoS ONE 2013, 8, e59810. [Google Scholar] [CrossRef] [PubMed]
- Canon, F.; Paté, F.; Cheynier, V.; Sarni-Manchado, P.; Giuliani, A.; Pérez, J.; Durand, D.; Li, J.; Cabane, B. Aggregation of the salivary proline-rich protein IB5 in the presence of the tannin EgCG. Langmuir 2013, 29, 1926–1937. [Google Scholar] [CrossRef] [PubMed]
- Manconi, B.; Castagnola, M.; Cabras, T.; Olianas, A.; Vitali, A.; Desiderio, C.; Sanna, M.T.; Messana, I. The intriguing heterogeneity of human salivary proline-rich proteins: Short title: Salivary proline-rich protein species. J. Proteomics 2016, 134, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Preianò, M.; Maggisano, G.; Murfuni, M.S.; Villella, C.; Pelaia, C.; Montalcini, T.; Lombardo, N.; Pelaia, G.; Savino, R.; Terracciano, R. An Analytical Method for Assessing Optimal Storage Conditions of Gingival Crevicular Fluid and Disclosing a Peptide Biomarker Signature of Gingivitis by MALDI-TOF MS. Proteomics Clin. Appl. 2018, 12, e1800005. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.J.; Kita, H. Pathogenesis of chronic rhinosinusitis: Role of airborne fungi and bacteria. Immunol. Allergy Clin. N. Am. 2004, 24, 59–73. [Google Scholar] [CrossRef]
- Proud, D.; Riker, D.K.; Togias, A. Reproducibility of nasal allergen challenge in evaluating the efficacy of intranasal corticosteroid treatment. Clin. Exp. Allergy 2010, 40, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Pipkorn, U.; Proud, D.; Lichtenstein, L.M.; Kagey-Sobotka, A.; Norman, P.S.; Naclerio, R.M. Inhibition of mediator release in allergic rhinitis by pretreatment with topical glucocorticosteroids. N. Engl. J. Med. 1987, 316, 1506–1510. [Google Scholar] [CrossRef] [PubMed]
- Gelardi, M.; Iannuzzi, L.; Tafuri, S.; Passalacqua, G.; Quaranta, N. Allergic and non-allergic rhinitis: Relationship with nasal polyposis, asthma and family history. Acta Otorhinolaryngol. Ital. 2014, 34, 36–41. [Google Scholar]
- Ngo, L.H.; Veith, P.D.; Chen, Y.Y.; Chen, D.; Darby, I.B.; Reynolds, E.C. Mass spectrometric analyses of peptides and proteins in human gingival crevicular fluid. J. Proteome Res. 2010, 9, 1683–1693. [Google Scholar] [CrossRef]


| Swiss Prot Number | Protein Name | Sequence | PI d | Identification Method | Mascot Score f | Peptide Peak Frequency g |
|---|---|---|---|---|---|---|
| P59665/6 | HNPs | R.YGTCIYQGR.L a | 8.67 | NF samples reduction with DTT e; 1DE-MALDI-TOF/TOF | 68 | 5/8 (HNP-1) 5/8 (HNP-2) 3/8 (HNP-3) |
| P02808 | Statherin (di-phosph.) Des39–43 | DSSEEKFLRRIGRFGYGYGPYQPVPEQPLYPQPYQPQY + Phospho (S) b | 4.41 | MALDI–TOF/TOF | De Novo Sequencing | 8/8 |
| P63313 | Thymosin β10 | ADKPDMGEIASFDKAKLKKTETQEKNTLPTKETIEQEKRSEIS + Acetyl (N-Term) c | 5.32 | MALDI–TOF/TOF | 97 | 4/8 |
| P62328 | Thymosin β4 | SDKPDMAEIEKFDKSKLKKTETQEKNPLPSKETIEQEKQAGES + Acetyl (N-Term) c | 5.02 | MALDI–TOF/TOF | 100 | 8/8 |
| P10163 | Peptide P-D (P32→A) | SPPGKPQGPPQQEGNKPQGPPPPGKPQGPPPAGGNPQQPQAPPAGKPQGPPPPPQGGRPPRPAQGQQPPQ c | 11.17 | MALDI–TOF/TOF | 96 | 5/8 |
| P10163 | Peptide P-D | SPPGKPQGPPQQEGNKPQGPPPPGKPQGPPPPGGNPQQPQAPPAGKPQGPPPPPQGGRPPRPAQGQQPPQ c | 11.17 | MALDI–TOF/TOF | 89 | 8/8 |
| P04280 | II-2 | QNLNEDVSQEESPSLIAGNPQGPSPQGGNKPQGPPPPPGKPQGPPPQGGNKPQGPPPPGKPQGPPPQGDKSRSPR + Pyro-glu c | 9.40 | MALDI–TOF/TOF | 100 | 5/8 |
| P04280 | II-2 | QNLNEDVSQEESPSLIAGNPQGPSPQGGNKPQGPPPPPGKPQGPPPQGGNKPQGPPPPGKPQGPPPQGDKSRSPR + Phospho (S) + Pyro-glu c | 7.35 | MALDI–TOF/TOF | 100 | 7/8 |
| P08118 | β-MSP | SCYFIPNEGVPGDSTR.K a | 5.36 | 1DE-MALDI-TOF/TOF | 76 | 7/8 |
| P03973 | SLPI | R.YKKPECQSDWQCPGK.K a | 9.11 | 1DE-MALDI-TOF/TOF | 68 | 8/8 |
| P61626 | Lysozyme C | R.STDYGIFQINSR.Y a | 9.28 | 1DE-MALDI-TOF/TOF | 68 | 8/8 |
| Subject | Gender | Age | Nasal Cytology a |
|---|---|---|---|
| Healthy 1 | F | 19 | Negative |
| Healthy 2 | F | 23 | Negative |
| Healthy 3 | M | 38 | Negative |
| Healthy 4 | F | 22 | Negative |
| Healthy 5 | F | 39 | Negative |
| Healthy 6 | F | 28 | Negative |
| Healthy 7 | F | 30 | Negative |
| Healthy 8 | F | 19 | Negative |
| AR | M | 22 | M+ |
| NARMA CRS UNP | M | 60 | M+++ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Preianò, M.; Maggisano, G.; Murfuni, M.S.; Villella, C.; Colica, C.; Fregola, A.; Pelaia, C.; Lombardo, N.; Pelaia, G.; Savino, R.; et al. Rapid Detection and Identification of Antimicrobial Peptide Fingerprints of Nasal Fluid by Mesoporous Silica Particles and MALDI-TOF/TOF Mass Spectrometry: From the Analytical Approach to the Diagnostic Applicability in Precision Medicine. Int. J. Mol. Sci. 2018, 19, 4005. https://doi.org/10.3390/ijms19124005
Preianò M, Maggisano G, Murfuni MS, Villella C, Colica C, Fregola A, Pelaia C, Lombardo N, Pelaia G, Savino R, et al. Rapid Detection and Identification of Antimicrobial Peptide Fingerprints of Nasal Fluid by Mesoporous Silica Particles and MALDI-TOF/TOF Mass Spectrometry: From the Analytical Approach to the Diagnostic Applicability in Precision Medicine. International Journal of Molecular Sciences. 2018; 19(12):4005. https://doi.org/10.3390/ijms19124005
Chicago/Turabian StylePreianò, Mariaimmacolata, Giuseppina Maggisano, Maria Stella Murfuni, Chiara Villella, Carmela Colica, Annalisa Fregola, Corrado Pelaia, Nicola Lombardo, Girolamo Pelaia, Rocco Savino, and et al. 2018. "Rapid Detection and Identification of Antimicrobial Peptide Fingerprints of Nasal Fluid by Mesoporous Silica Particles and MALDI-TOF/TOF Mass Spectrometry: From the Analytical Approach to the Diagnostic Applicability in Precision Medicine" International Journal of Molecular Sciences 19, no. 12: 4005. https://doi.org/10.3390/ijms19124005
APA StylePreianò, M., Maggisano, G., Murfuni, M. S., Villella, C., Colica, C., Fregola, A., Pelaia, C., Lombardo, N., Pelaia, G., Savino, R., & Terracciano, R. (2018). Rapid Detection and Identification of Antimicrobial Peptide Fingerprints of Nasal Fluid by Mesoporous Silica Particles and MALDI-TOF/TOF Mass Spectrometry: From the Analytical Approach to the Diagnostic Applicability in Precision Medicine. International Journal of Molecular Sciences, 19(12), 4005. https://doi.org/10.3390/ijms19124005