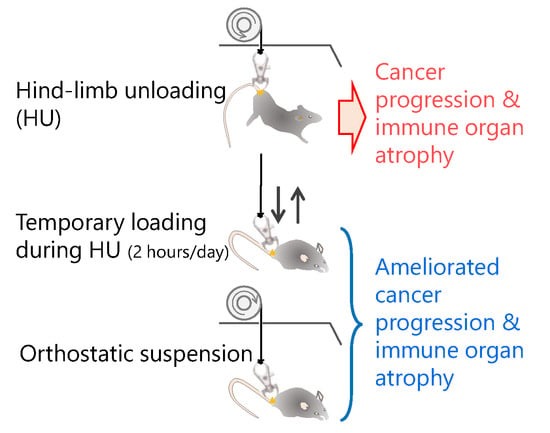
Temporary Loading Prevents Cancer Progression and Immune Organ Atrophy Induced by Hind-Limb Unloading in Mice
Abstract
1. Introduction
2. Results
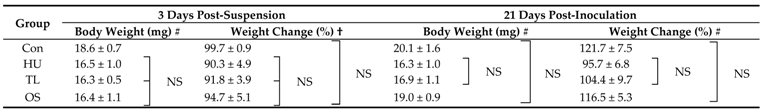
2.1. Change of Body Weight by Four Suspension Conditions
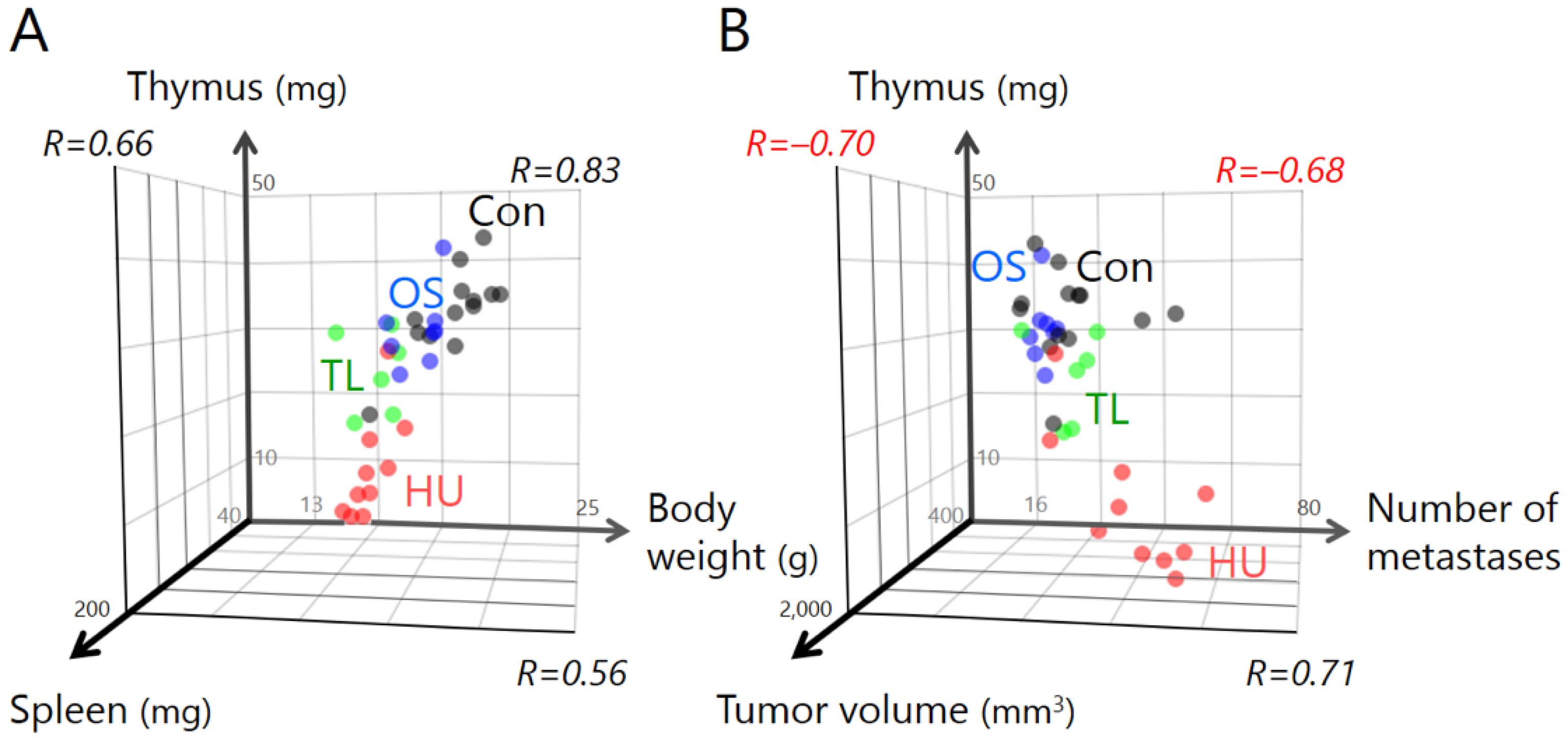
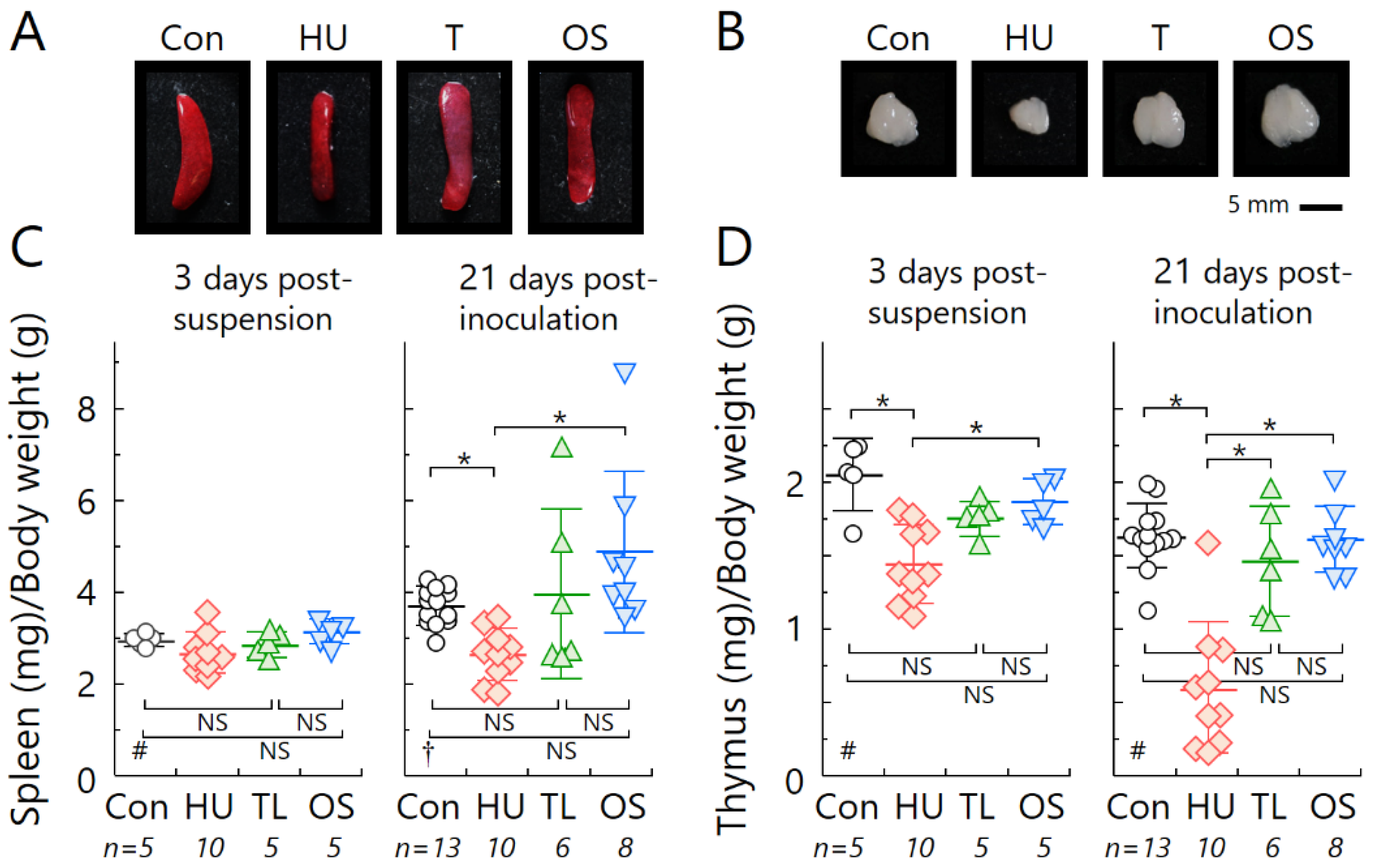
2.2. Temporary Loading Prevents Immune Organ Atrophy by Hind-Limb Unloading
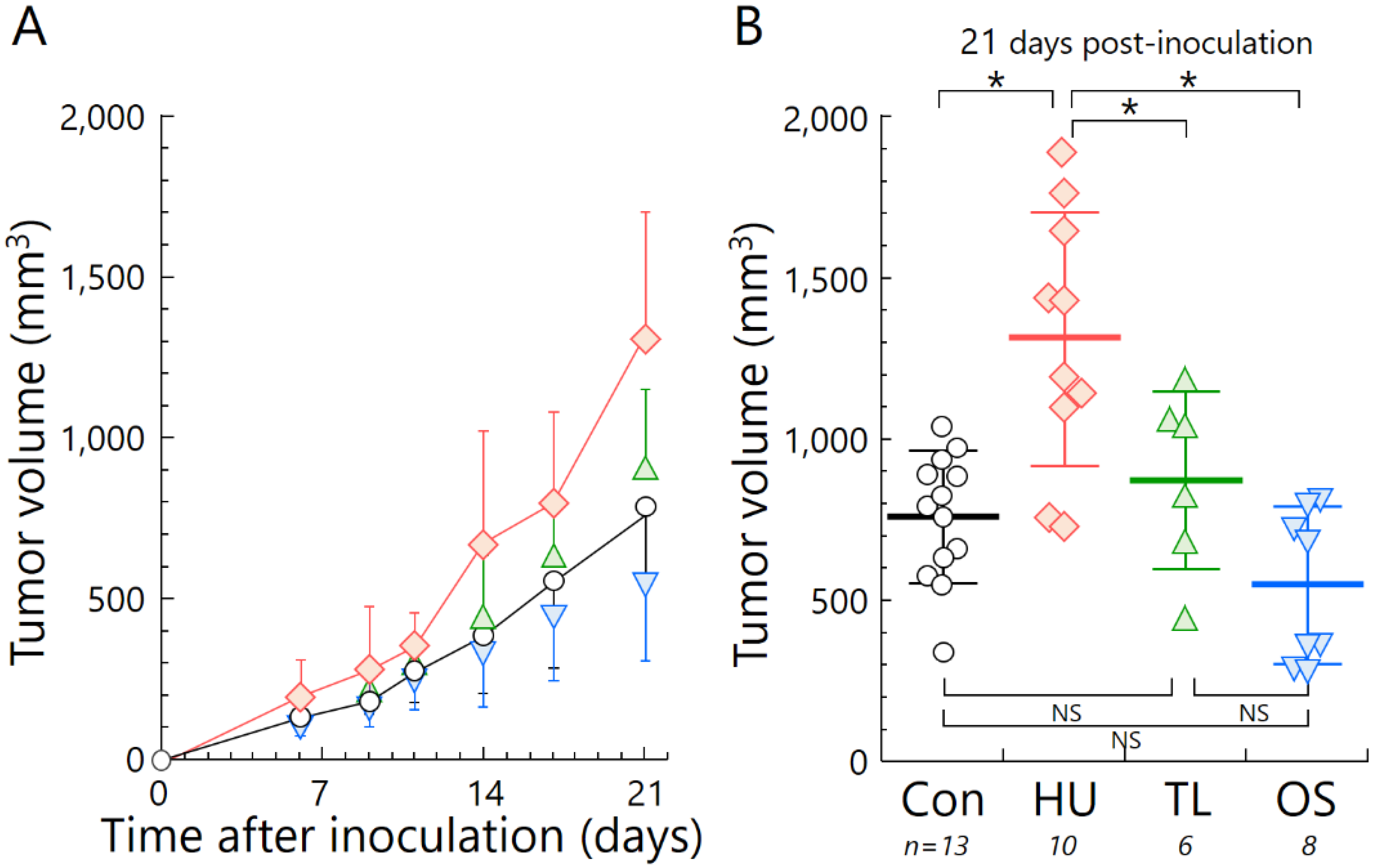
2.3. Temporary Loading Prevents Acceleration of Tumor Growth by Hind-Limb Unloading
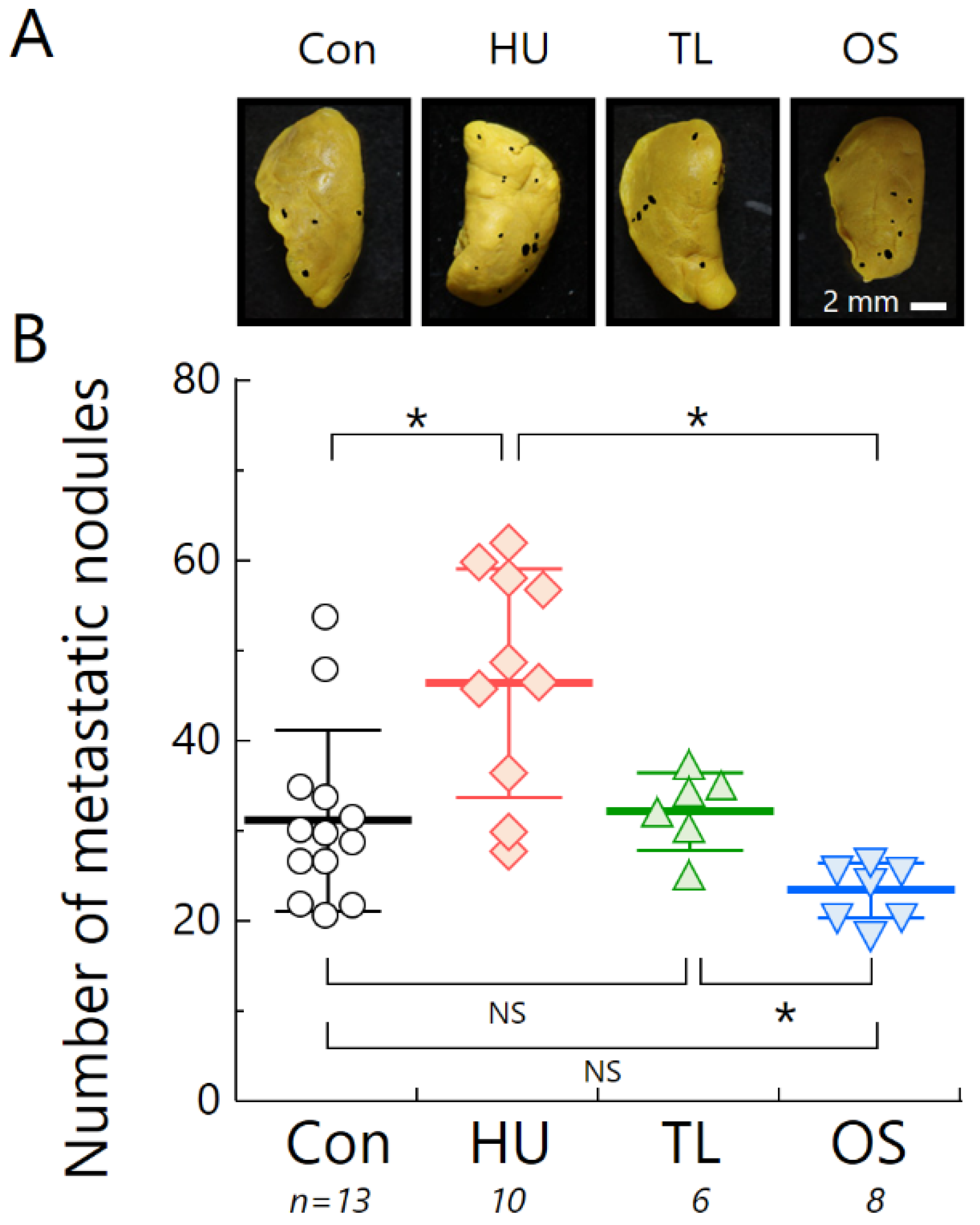
2.4. Temporary Loading Prevents Acceleration of Metastasis by Hind-Limb Unloading
3. Discussion
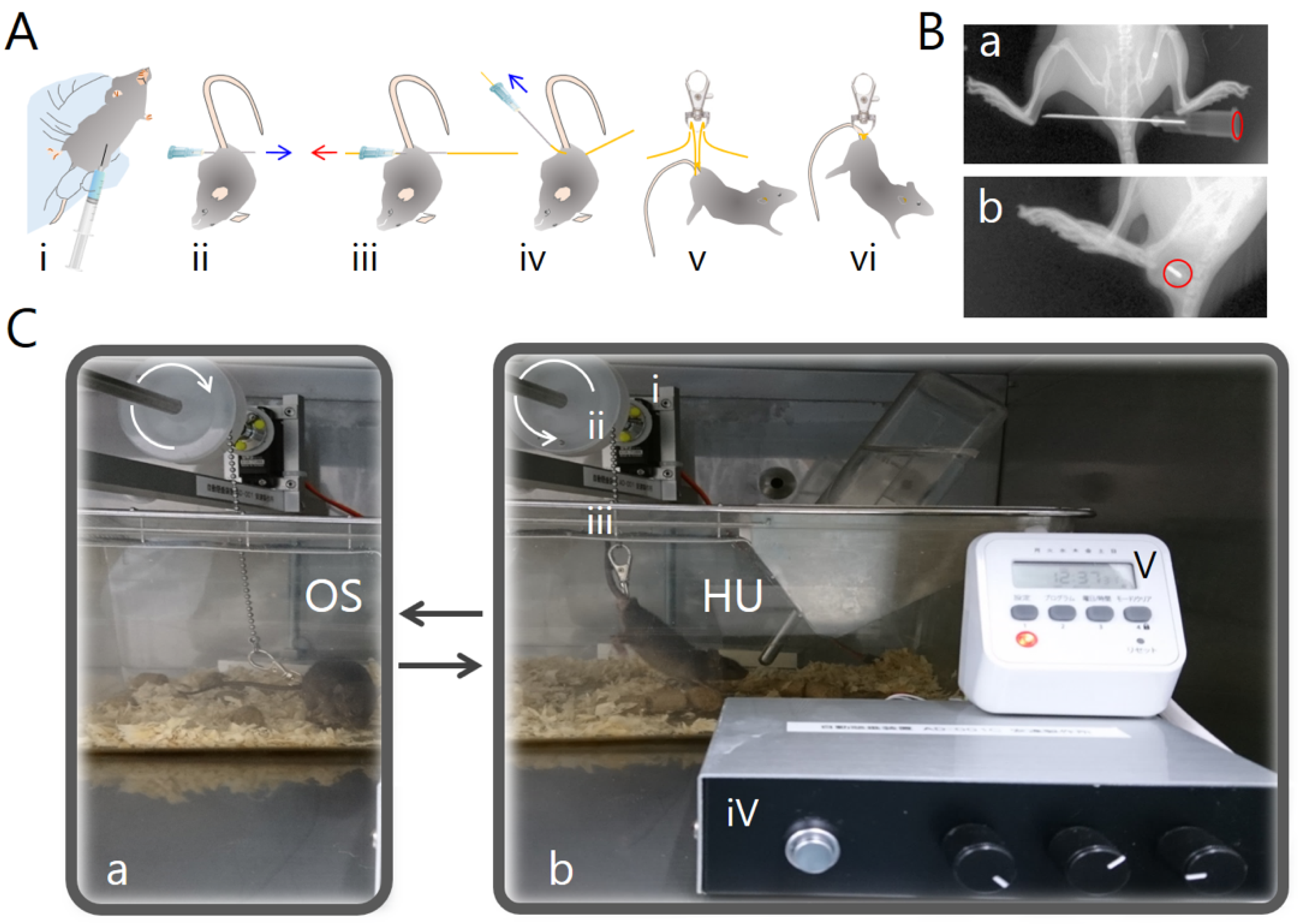
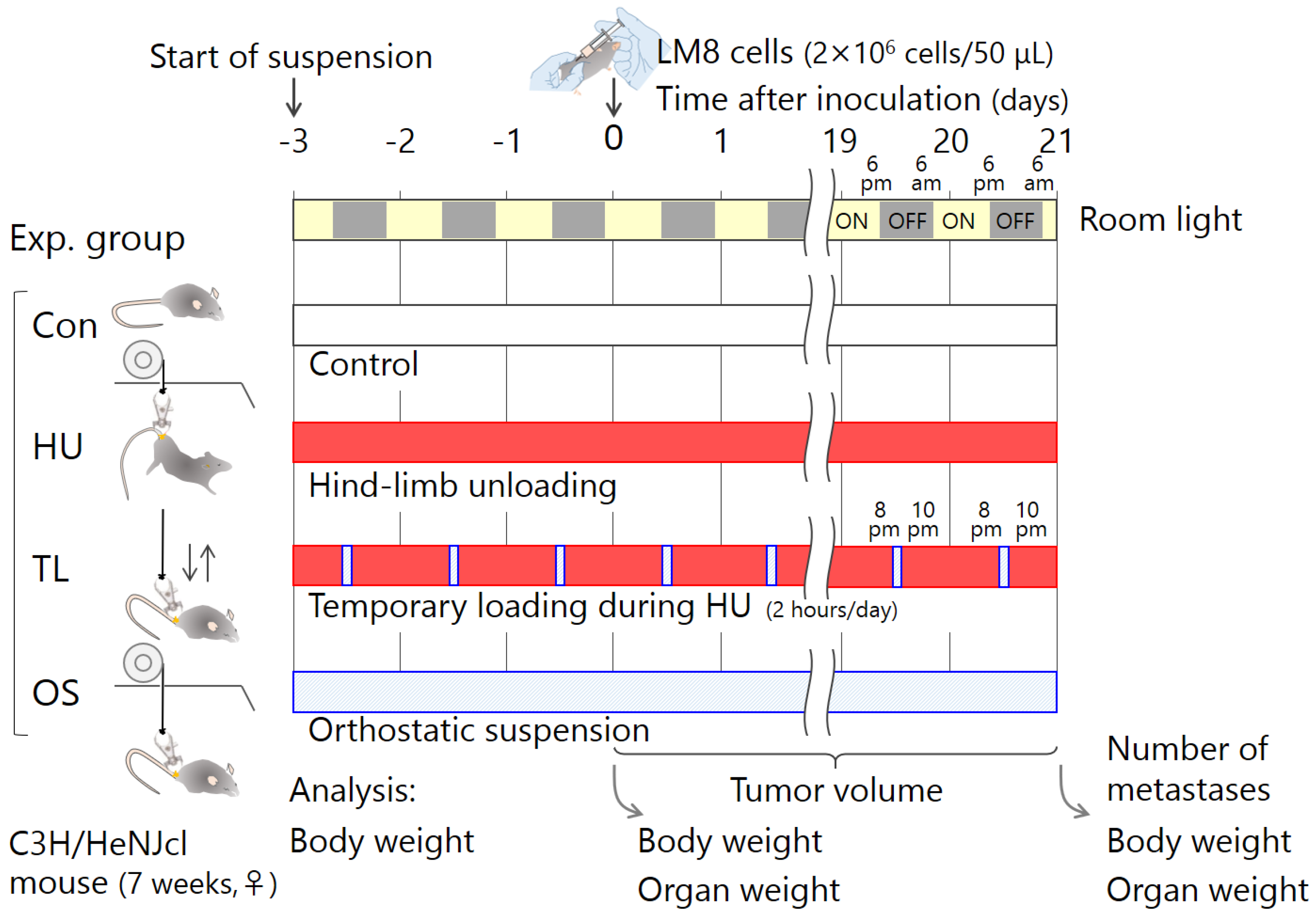
4. Materials and Methods
4.1. Mice
4.2. Cell Culture
4.3. Tail Suspension
4.4. Experimental Schedule
4.5. Measurement of Tumor Growth
4.6. Measurement of Lung Metastatic Nodules
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Con | No suspension control |
| FBS | Fetal bovine serum |
| GCR | Galactic cosmic rays |
| GHMC | Gunma University Heavy Ion Medical Center |
| HU | Hind-limb unloading |
| ISS | International Space Station |
| OS | Orthostatic suspension |
| TL | Temporary loading during hind-limb unloading |
| μG | Microgravity |
Appendix A
References
- Cucinotta, F.A. Review of NASA approach to space radiation risk assessments for Mars exploration. Health Phys. 2015, 108, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Barcellos-Hoff, M.H.; Blakely, E.A.; Burma, S.; Fornace, A.J., Jr.; Gerson, S.; Hlatky, L.; Kirsch, D.G.; Luderer, U.; Shay, J.; Wang, Y.; et al. Concepts and challenges in cancer risk prediction for the space radiation environment. Life Sci. Space Res. 2015, 6, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, F.A.; Manuel, F.K.; Jones, J.; Iszard, G.; Murrey, J.; Djojonegro, B.; Wear, M. Space radiation and cataracts in astronauts. Radiat. Res. 2001, 156, 460–466. [Google Scholar] [CrossRef]
- Jones, J.A.; McCarten, M.; Manuel, K.; Djojonegoro, B.; Murray, J.; Feiversen, A.; Wear, M. Cataract formation mechanisms and risk in aviation and space crews. Aviat. Space Environ. Med. 2007, 78, A56–A66. [Google Scholar] [PubMed]
- Cherry, J.D.; Liu, B.; Frost, J.L.; Lemere, C.A.; Williams, J.P.; Olschowka, J.A.; O’Banion, M.K. Galactic cosmic radiation leads to cognitive impairment and increased Aβ plaque accumulation in a mouse model of Alzheimer’s disease. PLoS ONE 2012, 7, e53275. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, F.A.; Alp, M.; Sulzman, F.M.; Wang, M. Space radiation risks to the central nervous system. Life Sci. Space Res. 2014, 2, 54–69. [Google Scholar] [CrossRef]
- Parihar, V.K.; Allen, B.; Tran, K.K.; Macaraeg, T.G.; Chu, E.M.; Kwok, S.F.; Chmielewski, N.N.; Craver, B.M.; Baulch, J.E.; Acharya, M.M.; et al. What happens to your brain on the way to Mars. Sci. Adv. 2015, 1, e1400256. [Google Scholar] [CrossRef] [PubMed]
- Delp, M.D.; Charvat, J.M.; Limoli, C.L.; Globus, R.K.; Ghosh, P. Apollo lunar astronauts show higher cardiovascular disease mortality: Possible deep space radiation effects on the vascular endothelium. Sci. Rep. 2016, 6, 29901. [Google Scholar] [CrossRef]
- Fernandez-Gonzalo, R.; Baatout, S.; Moreels, M. Impact of particle irradiation on the immune system: From the clinic to Mars. Front. Immunol. 2017, 8, 177. [Google Scholar] [CrossRef]
- Durante, M.; Cucinotta, F.A. Heavy ion carcinogenesis and human space exploration. Nat. Rev. Cancer 2008, 8, 465–472. [Google Scholar] [CrossRef]
- Shiba, D.; Mizuno, H.; Yumoto, A.; Shimomura, M.; Kobayashi, H.; Morita, H.; Shimbo, M.; Hamada, M.; Kudo, T.; Shinohara, M.; et al. Development of new experimental platform ‘MARS’-multiple artificial-gravity research system-to elucidate the impacts of micro/partial gravity on mice. Sci. Rep. 2017, 7, 10837. [Google Scholar] [CrossRef] [PubMed]
- Fitts, R.H.; Riley, D.R.; Widrick, J.J. Physiology of a microgravity environment; Microgravity and skeletal muscle. J. Appl. Physiol. (1985) 2000, 89, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Eckberg, D.L.; Halliwill, J.R.; Beightol, L.A.; Brown, T.E.; Taylor, J.A.; Goble, R. Human vagal baroreflex mechanisms in space. J. Physiol. 2010, 588, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Cogoli, A. The effect of space flight on human cellular immunity. Environ. Med. 1993, 37, 107–116. [Google Scholar] [PubMed]
- Konstantinova, I.V.; Rykova, M.P.; Lesnyak, A.T.; Antropova, E.A. Immune changes during long-duration missions. J. Leukoc. Biol. 1993, 54, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Sonnenfeld, G. Effect of space flight on cytokine production. Acta Astronaut. 1994, 33, 143–147. [Google Scholar] [CrossRef]
- Guéguinou, N.; Huin-Schohn, C.; Bascove, M.; Bueb, J.-L.; Tschirhart, E.; Legrand-Frossi, C.; Frippiat, J.P. Could spaceflight-associated immune system weakening preclude the expansion of human presence beyond Earth’s orbit? J. Leukoc. Biol. 2009, 86, 1027–1038. [Google Scholar] [CrossRef]
- Williams, D.; Kuipers, A.; Mukai, C.; Thirsk, R. Acclimation during space flight: Effects on human physiology. CMAJ 2009, 180, 1317–1323. [Google Scholar] [CrossRef]
- Crucian, B.E.; Choukèr, A.; Simpson, R.J.; Mehta, S.; Marshall, G.; Smith, S.M.; Zwart, S.R.; Heer, M.; Ponomarev, S.; Whitmire, A.; et al. Immune system dysregulation during spaceflight: Potential countermeasures for deep space exploration missions. Front. Immunol. 2018, 9, 1437. [Google Scholar] [CrossRef]
- Gridley, D.S.; Mao, X.W.; Stodieck, L.S.; Ferguson, V.L.; Bateman, T.A.; Moldovan, M.; Cunningham, C.E.; Jones, T.A.; Slater, J.M.; Pecaut, M.J. Changes in mouse thymus and spleen after return from the STS-135 mission in space. PLoS ONE 2013, 8, e75097. [Google Scholar] [CrossRef]
- Benjamin, C.L.; Stowe, R.P.; St John, L.; Sams, C.F.; Mehta, S.K.; Crucian, B.E.; Pierson, D.L.; Komanduri, K.V. Decreases in thymopoiesis of astronauts returning from space flight. JCI Insight. 2016, 1, e88787. [Google Scholar] [CrossRef] [PubMed]
- Crucian, B.; Stowe, R.P.; Mehta, S.; Quiriarte, H.; Pierson, D.; Sams, C. Alterations in adaptive immunity persist during long-duration spaceflight. NPJ Microgravity 2015, 1, 15013. [Google Scholar] [CrossRef]
- Mehta, S.K.; Laudenslager, M.L.; Stowe, R.P.; Crucian, B.E.; Feiveson, A.H.; Sams, C.F.; Pierson, D.L. Latent virus reactivation in astronauts on the international space station. NPJ Microgravity 2017, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.K.; Nelman-Gonzalez, M.; Tyring, S.K.; Tong, Y.; Beitman, A.; Crucian, B.E.; Renner, A.N.; Pierson, D.L. Localization of VZV in saliva of zoster patients. J. Med. Virol. 2017, 89, 1686–1689. [Google Scholar] [CrossRef] [PubMed]
- Sonnenfeld, G. Use of animal models for space flight physiology studies, with special focus on the immune system. Gravit. Space Biol. Bull. 2005, 18, 31–35. [Google Scholar]
- Wang, K.X.; Shi, Y.; Denhardt, D.T. Osteopontin regulates hindlimb-unloading-induced lymphoid organ atrophy and weight loss by modulating corticosteroid production. Proc. Natl. Acad. Sci. USA 2007, 104, 14777–14782. [Google Scholar] [CrossRef] [PubMed]
- Globus, R.K.; Morey-Holton, E. Hindlimb unloading: Rodent analog for microgravity. J. Appl. Physiol. (1985) 2016, 120, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Horie, K.; Kudo, T.; Yoshinaga, R.; Akiyama, N.; Sasanuma, H.; Kobayashi, T.J.; Shimbo, M.; Jeon, H.; Miyao, T.; Miyauchi, M.; et al. Long-term hindlimb unloading causes a preferential reduction of medullary thymic epithelial cells expressing autoimmune regulator (Aire). Biochem. Biophys. Res. Commun. 2018, 501, 745–750. [Google Scholar] [CrossRef]
- Lee, E.H.; Ding, W.; Kulkarni, A.D.; Granstein, R.D. Tumor growth and immune function in mice during hind-limb unloading. Aviat. Space Environ. Med. 2005, 76, 536–540. [Google Scholar]
- Asai, T.; Ueda, T.; Itoh, K.; Yoshioka, K.; Aoki, Y.; Mori, S.; Yoshikawa, H. Establishment and characterization of a murine osteosarcoma cell line (LM8) with high metastatic potential to the lung. Int. J. Cancer 1998, 76, 418–422. [Google Scholar] [CrossRef]
- Terashima, A.; Okamoto, K.; Nakashima, T.; Akira, S.; Ikuta, K.; Takayanagi, H. Sepsis-induced osteoblast ablation causes immunodeficiency. Immunity 2016, 44, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- Gould, C.L.; Sonnenfeld, G. Enhancement of viral pathogenesis in mice maintained in an antiorthostatic suspension model: Coordination with effects on interferon production. J. Biol. Regul. Homeost. Agents 1987, 1, 33–36. [Google Scholar] [PubMed]
- Fuse, A.; Sato, T. Effect of microgravity changes on virus infection in mice. J. Gravit. Physiol. 2004, J11, 65–66. [Google Scholar]
- Brinley, A.A.; Theriot, C.A.; Nelman-Gonzalez, M.; Crucian, B.; Stowe, R.P.; Barrett, A.D.; Pierson, D.L. Characterization of Epstein-Barr virus reactivation in a modeled spaceflight system. J. Cell Biochem. 2013, 114, 616–624. [Google Scholar] [CrossRef]
- Jhala, D.V.; Kale, R.K.; Singh, R.P. Microgravity alters cancer growth and progression. Curr. Cancer Drug Targets 2014, 14, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef]
- Cucinotta, F.A.; Durante, M. Cancer risk from exposure to galactic cosmic rays: Implications for space exploration by human beings. Lancet Oncol. 2006, 7, 431–435. [Google Scholar] [CrossRef]
- Simpson, R.J.; Kunz, H.; Agha, N.; Graff, R. Exercise and the regulation of immune functions. Prog. Mol. Biol. Transl. Sci. 2015, 135, 355–380. [Google Scholar] [CrossRef]
- Fomina, E.V.; Uskov, K.V.; Rykova, M.P.; Antropova, E.N.; Ponomarev, S.A.; Kalinin, S.A.; Berendeeva, T.A.; Smoleevsky, A.E. Adaptive immunity as an indicator of optimum physical loads during 520-day isolation. Hum. Physiol. 2017, 43, 301–311. [Google Scholar] [CrossRef]
- Wang, X.D.; Kawano, F.; Matsuoka, Y.; Fukunaga, K.; Terada, M.; Sudoh, M.; Ishihara, A.; Ohira, Y. Mechanical load-dependent regulation of satellite cell and fiber size in rat soleus muscle. Am. J. Physiol. Cell Physiol. 2006, 290, C981–C989. [Google Scholar] [CrossRef]
- Ohira, T.; Terada, M.; Kawano, F.; Nakai, N.; Ogura, A.; Ohira, Y. Region-specific responses of adductor longus muscle to gravitational load-dependent activity in Wistar Hannover rats. PLoS ONE 2011, 6, e21044. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, A.; Wakihata, S.; Ma, L.; Adachi, T.; Hirose, H.; Yoshida, Y.; Ohira, Y. Temporary Loading Prevents Cancer Progression and Immune Organ Atrophy Induced by Hind-Limb Unloading in Mice. Int. J. Mol. Sci. 2018, 19, 3959. https://doi.org/10.3390/ijms19123959
Takahashi A, Wakihata S, Ma L, Adachi T, Hirose H, Yoshida Y, Ohira Y. Temporary Loading Prevents Cancer Progression and Immune Organ Atrophy Induced by Hind-Limb Unloading in Mice. International Journal of Molecular Sciences. 2018; 19(12):3959. https://doi.org/10.3390/ijms19123959
Chicago/Turabian StyleTakahashi, Akihisa, Shoto Wakihata, Liqiu Ma, Takuya Adachi, Hiroki Hirose, Yukari Yoshida, and Yoshinobu Ohira. 2018. "Temporary Loading Prevents Cancer Progression and Immune Organ Atrophy Induced by Hind-Limb Unloading in Mice" International Journal of Molecular Sciences 19, no. 12: 3959. https://doi.org/10.3390/ijms19123959
APA StyleTakahashi, A., Wakihata, S., Ma, L., Adachi, T., Hirose, H., Yoshida, Y., & Ohira, Y. (2018). Temporary Loading Prevents Cancer Progression and Immune Organ Atrophy Induced by Hind-Limb Unloading in Mice. International Journal of Molecular Sciences, 19(12), 3959. https://doi.org/10.3390/ijms19123959