Biological Aggressiveness of Subclinical No-Mass Ductal Carcinoma In Situ (DCIS) Can Be Reflected by the Expression Profiles of Epithelial-Mesenchymal Transition Triggers
Abstract
1. Introduction
2. Results
2.1. Clinical Features
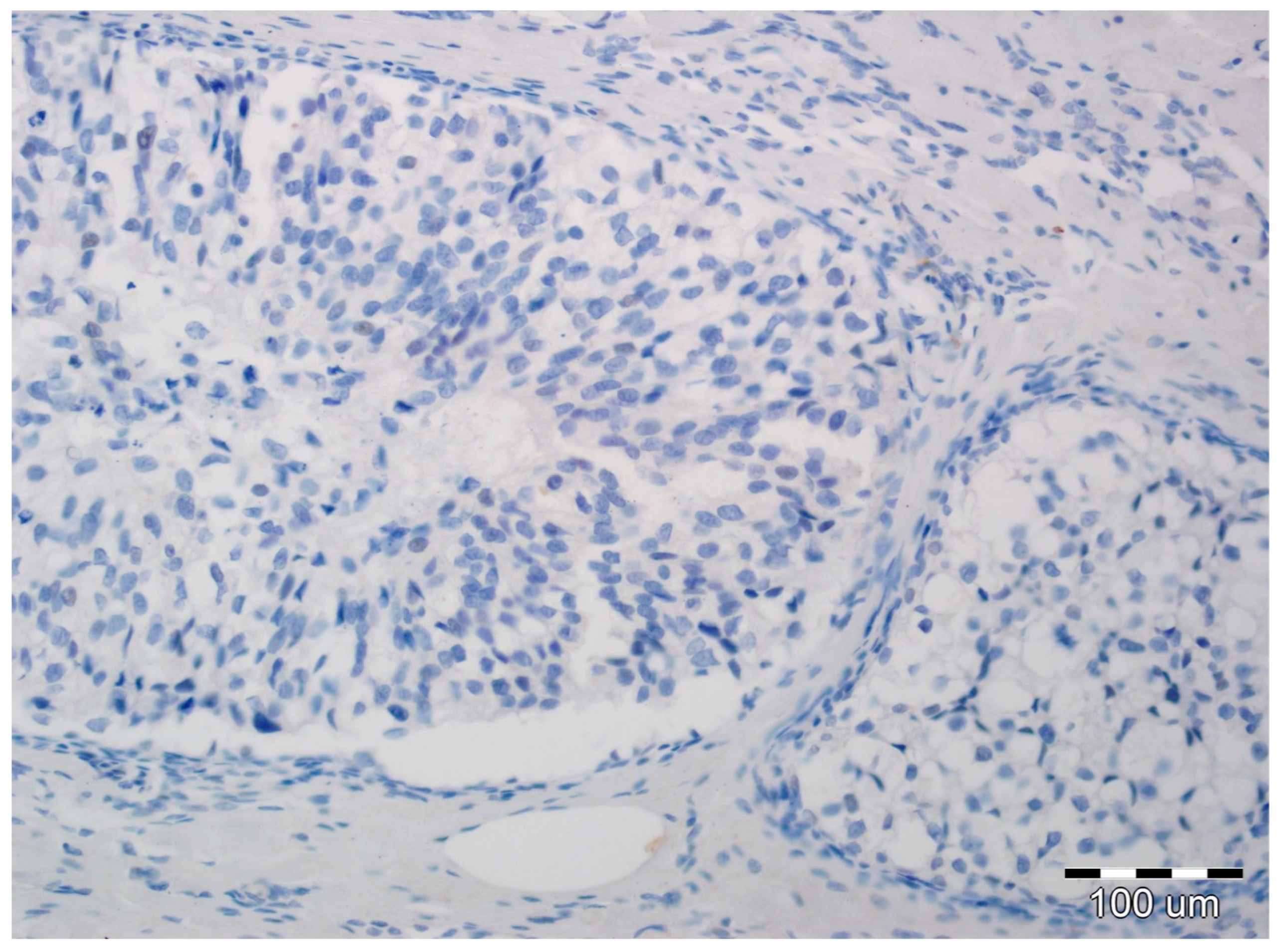
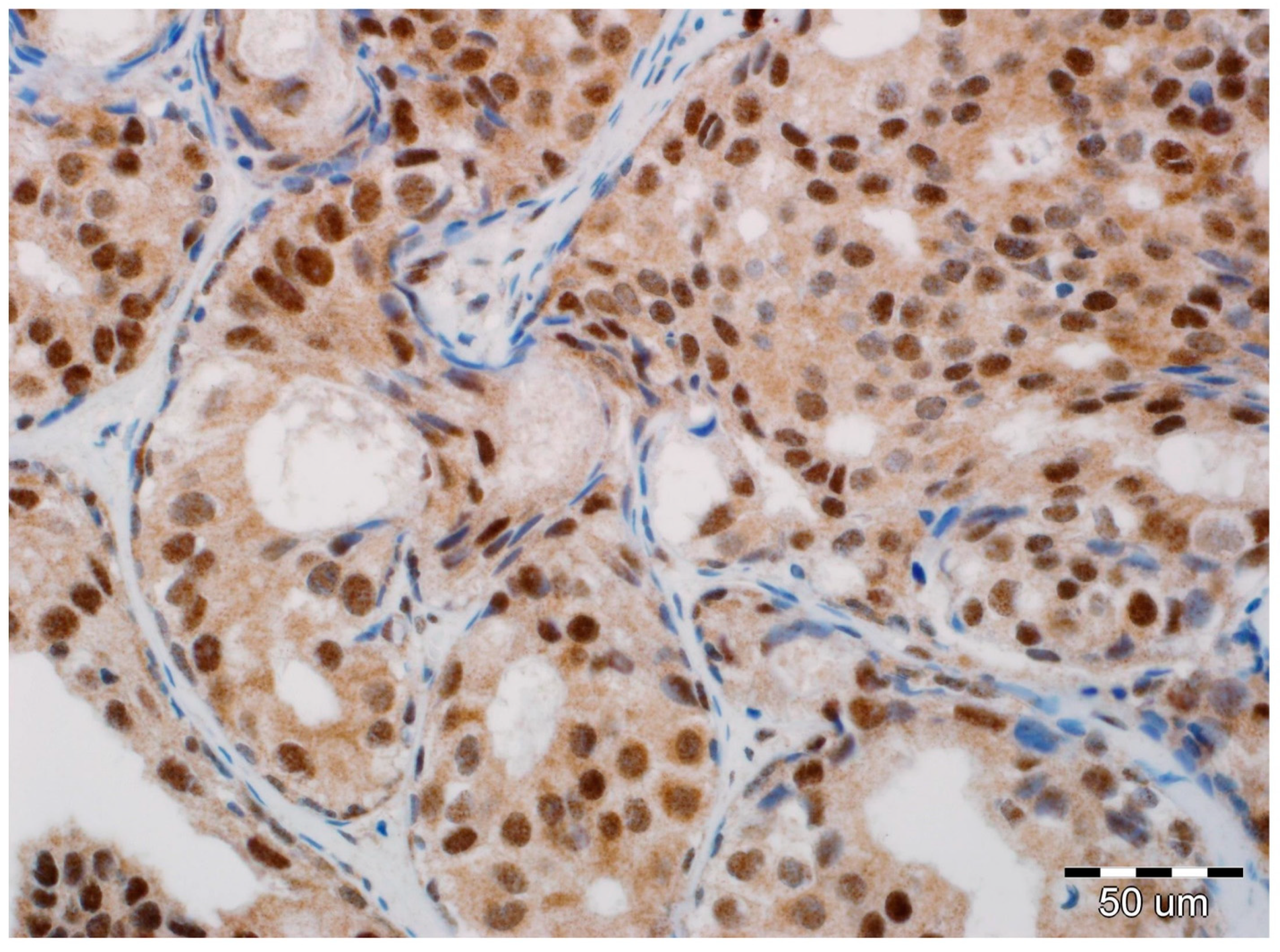
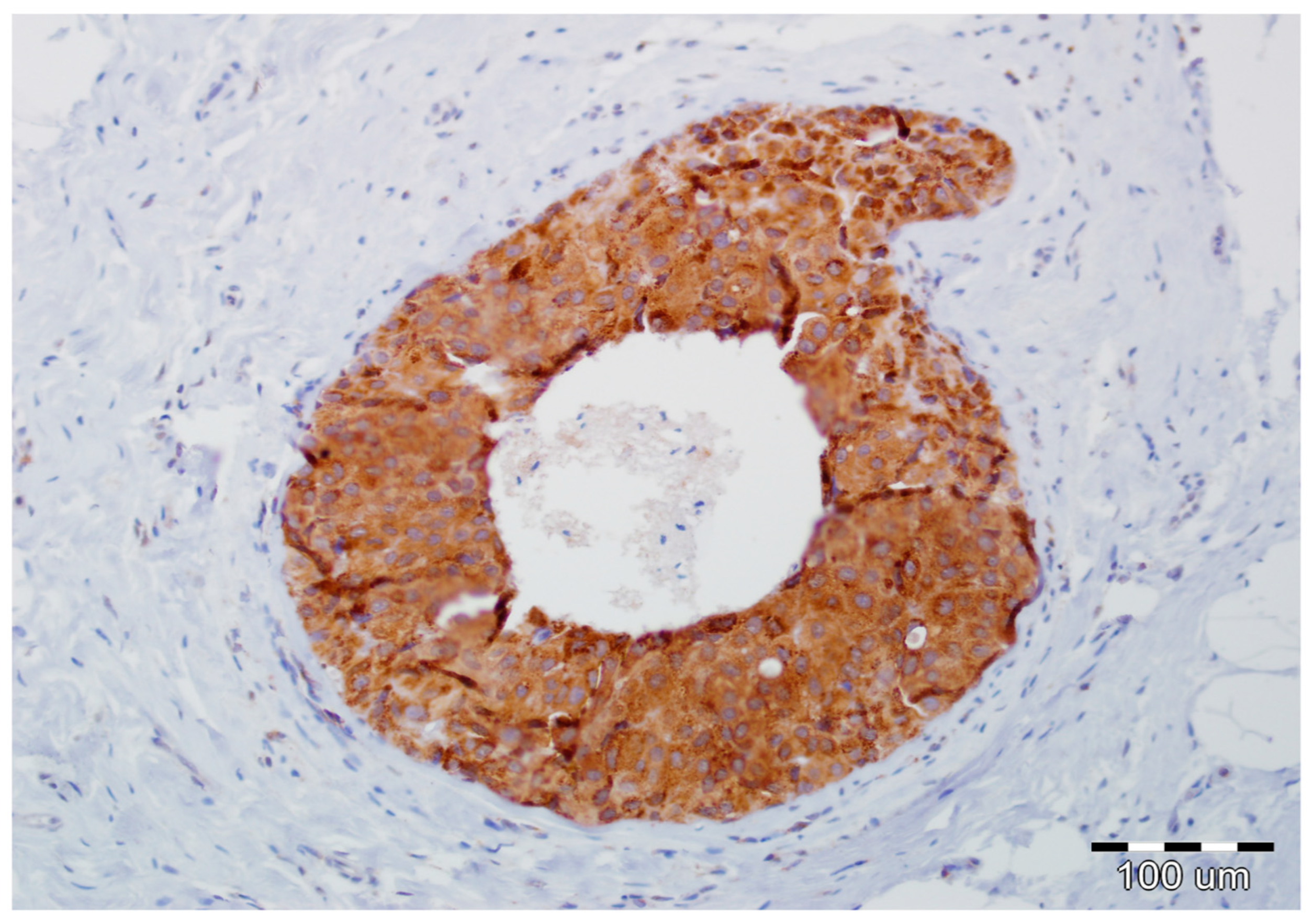
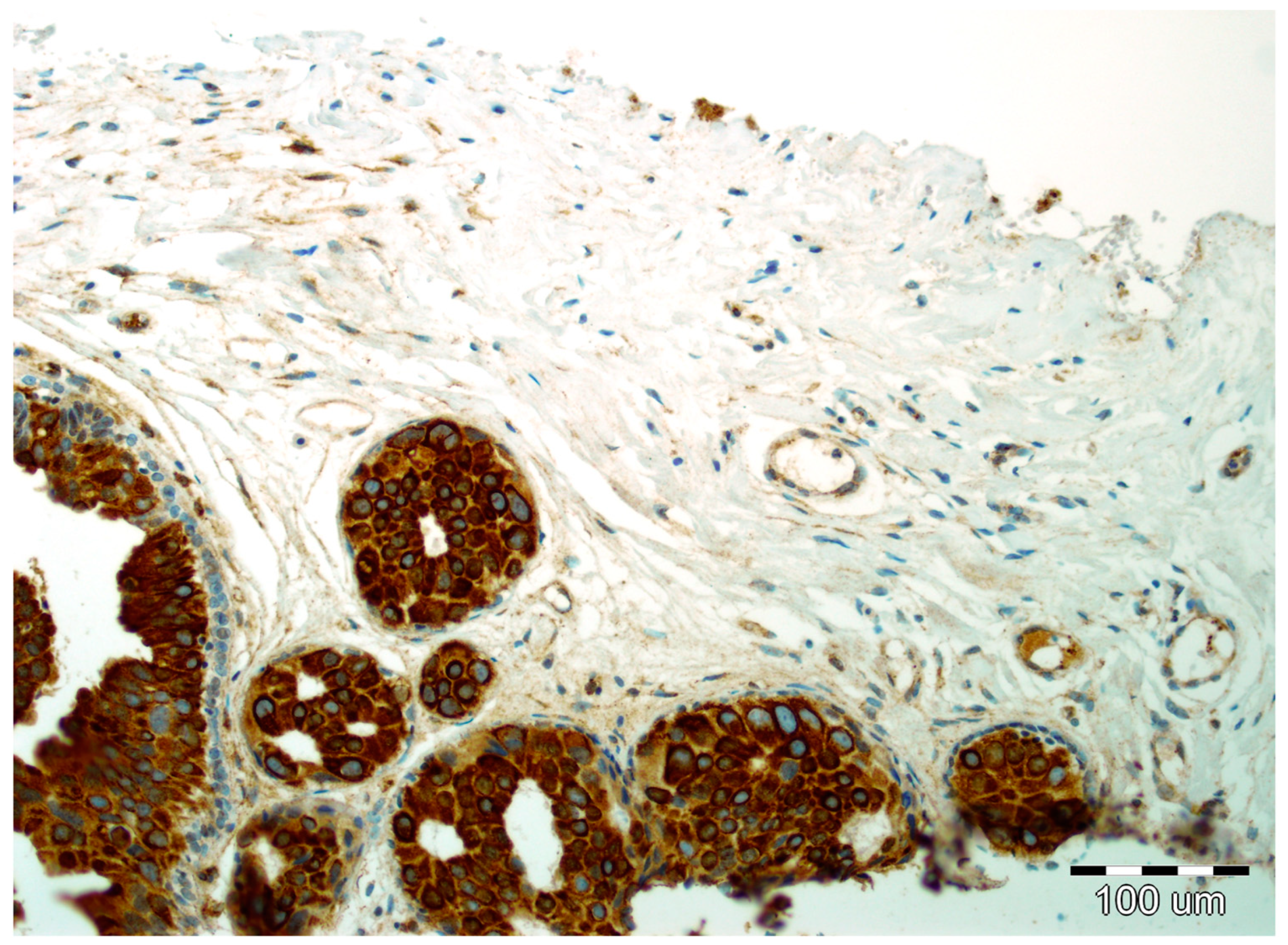
2.2. EMT Biomarkers in Preoperative Pathology (Biopsy Specimens)
2.3. Final Invasion in Postoperative Pathology (Surgical Specimens)
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Immunohistochemistry
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DCIS | Ductal carcinoma in situ |
| EMT | Epithelial-mesenchymal transition |
| SPARC | Secreted protein acidic and rich in cysteine |
| BIRADS | Breast imaging reporting and data system |
| ER | Estrogen receptor |
| PR | Progesterone receptor |
| EGFR | Epidermal growth factor receptor |
| HER2 | Human epidermal growth factor receptor 2 |
References
- Sagara, Y.; Julia, W.; Golshan, M.; Toi, M. Paradigm Shift toward Reducing Overtreatment of Ductal Carcinoma in Situ of Breast. Front. Oncol. 2017, 7, 192. [Google Scholar] [CrossRef] [PubMed]
- Virnig, B.A.; Shamliyan, T.; Tuttle, T.M.; Kane, R.L.; Wilt, T.J. Diagnosis and management of ductal carcinoma in situ (DCIS). Evid. Rep. Technol. Assess. 2009, 185, 1–549. [Google Scholar]
- Virnig, B.A.; Tuttle, T.M.; Shamliyan, T.; Kane, R.L. Ductal carcinoma in situ of the breast: A systematic review of incidence, treatment, and outcomes. J. Natl. Cancer Instit. 2010, 102, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Page, D.L.; Dupont, W.D.; Rogers, L.W.; Landenberger, M. Intraductal carcinoma of the breast: Follow-up after biopsy only. Cancer 1982, 49, 751–758. [Google Scholar] [CrossRef]
- Page, D.L.; Dupont, W.D.; Rogers, L.W.; Jensen, R.A.; Schuyler, P.A. Continued local recurrence of carcinoma 15–25 years after a diagnosis of low grade ductal carcinoma in situ of the breast treated only by biopsy. Cancer 1995, 76, 1197–1200. [Google Scholar] [CrossRef]
- Sanders, M.E.; Schuyler, P.A.; Dupont, W.D.; Page, D.L. The natural history of low-grade ductal carcinoma in situ of the breast in women treated by biopsy only revealed over 30 years of long-term follow-up. Cancer 2005, 103, 2481–2484. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Xing, T.; Yang, Z.; Dudek, R.; Lu, Q.; Chen, Y.H. Epithelial Mesenchymal Transition in Embryonic Development, Tissue Repair and Cancer: A Comprehensive Overview. J. Clin. Med. 2017, 7, 1. [Google Scholar] [CrossRef]
- Brabletz, T.; Kalluri, R.; Nieto, M.A.; Weinberg, R.A. EMT in cancer. Nat. Rev. Cancer 2018, 18, 128–134. [Google Scholar] [CrossRef]
- Jie, X.X.; Zhang, X.Y.; Xu, C.J. Epithelial-to-mesenchymal transition, circulating tumor cells and cancer metastasis: Mechanisms and clinical applications. Oncotarget 2017, 8, 81558–81571. [Google Scholar] [CrossRef]
- Ashaie, M.A.; Chowdhury, E.H. Cadherins: The superfamily critically involved in breast cancer. Curr. Pharm. Des. 2016, 22, 616–638. [Google Scholar] [CrossRef] [PubMed]
- Szynglarewicz, B.; Kasprzak, P.; Donizy, P.; Biecek, P.; Halon, A.; Matkowski, R. Ductal carcinoma in situ on stereotactic biopsy of suspicious breast microcalcifications: Expression of SPARC (Secreted Protein, Acidic and Rich in Cysteine) can predict postoperative invasion. J. Surg. Oncol. 2016, 114, 548–556. [Google Scholar] [CrossRef]
- Szynglarewicz, B.; Kasprzak, P.; Donizy, P.; Biecek, P.; Halon, A.; Matkowski, R. Epithelial-mesenchymal transition inducer Snail1 and invasive potential of intraductal breast cancer. J. Surg. Oncol. 2017, 116, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Kourtidis, A.; Lu, R.; Pence, L.J.; Anastasiadis, P.Z. A central role for E-cadherin signaling in cancer. Exp. Cell. Res. 2017, 358, 78–85. [Google Scholar] [CrossRef]
- ElMoneim, H.M.A.; Zaghloul, N.M. Expression of e-cadherin, n-cadherin and snail and their correlation with clinicopathological variants: an immunohistochemical study of 132 invasive ductal breast carcinomas in Egypt. Clinics 2011, 66, 1765–1771. [Google Scholar] [PubMed]
- Kovacs, A.; Dhillon, J.; Walker, R.A. Expression of P-cadherin, but not E-cadherin or N-cadherin, relates to pathological and functional differentiation of breast carcinomas. Mol. Pathol. 2003, 56, 318–322. [Google Scholar] [CrossRef]
- Aleskandarany, M.A.; Negm, O.H.; Green, A.R.; Ahmed, M.A.; Nolan, C.C.; Tighe, P.J.; Ellis, I.O.; Rakha, E.A. Epithelial mesenchymal transition in early invasive breast cancer: An immunohistochemical and reverse phase protein array study. Breast Cancer Res. Treat. 2014, 145, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Nagi, C.; Guttman, M.; Jaffer, S.; Qiao, R.; Keren, R.; Triana, A.; Li, M.; Godbold, J.; Bleiweiss, I.J.; Hazan, R.B. N-cadherin expression in breast cancer: correlation with an aggressive histologic variant—Invasive micropapillary carcinoma. Breast Cancer Res. Treat. 2005, 94, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Lee, H.J.; Jang, M.H.; Gwak, J.M.; Lee, K.S.; Kim, E.J.; Kim, H.J.; Lee, H.E.; Park, S.Y. Epithelial-mesenchymal transition increases during the progression of in situ to invasive basal-like breast cancer. Hum. Pathol. 2013, 44, 2581–2589. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yu, T.; Zhang, Q.; Fu, Q.; Hu, Y.; Xiang, M.; Peng, H.; Zheng, T.; Lu, L.; Shi, H. Upregulated N-cadherin expression is associated with poor prognosis in epithelial-derived solid tumours: A meta-analysis. Eur. J. Clin. Investig. 2018, 48, e12903. [Google Scholar] [CrossRef]
- Szynglarewicz, B.; Kasprzak, P.; Halon, A.; Matkowski, R. Preoperatively diagnosed ductal cancers in situ of the breast presenting as even small masses are of high risk for the invasive cancer foci in postoperative specimen. World J. Surg. Oncol. 2015, 13, 218. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, B.P. Snail. More than EMT. Cell Adh. Migr. 2010, 4, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.A. The snail superfamily of zinc-finger transcription factors. Nat. Rev. Mol. Cell Biol. 2002, 3, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Natrajan, R.; Weigelt, B.; Mackay, A.; Geyer, F.C.; Grigoriadis, A.; Tan, D.S.; Jones, C.; Lord, C.J.; Vatcheva, R.; Rodriguez-Pinilla, S.M.; et al. An integrative genomic and transcriptomic analysis reveals molecular pathways and networks regulated by copy number aberrations in basal-like, HER2 and luminal cancers. Breast Cancer Res Treat. 2010, 121, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Tam, W.L.; Shibue, T.; Kaygusuz, Y.; Reinhardt, F.; Eaton, E.; Weinberg, R.A. Distinct EMT programs control mammary stem cells and tumour-initiating cells. Nature 2015, 525, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajj, M.; Clarke, M.H. Self-renewal and solid tumor stem cells. Oncogene 2004, 23, 7274–7282. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.A.; Kreso, A.; Dick, J.E. Cancer stem cells in solid tumors: an overview. Semin. Radiat. Oncol. 2009, 19, 71–77. [Google Scholar] [CrossRef]
- Stefania, D.; Vergara, D. The many-faced program of epithelial-mesenchymal transition: A system biology-based view. Front. Oncol. 2017, 7, 274. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef] [PubMed]
- De Craene, B.; Berx, G. Regulatory networks defining EMT during cancer initiation and progression. Nat. Rev. Cancer 2013, 13, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, D.M.; Medici, D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci. Signal. 2014, 7, re8. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.J.; Sage, E.H. A prototypic matricellular protein in the tumor microenvironment—Where there’s SPARC, there’s fire. J. Cell Biochem. 2008, 104, 721–732. [Google Scholar] [CrossRef]
- Nagaraju, G.P.; Dontula, R.; El-Rayes, B.F.; Lakka, S.S. Molecular mechanisms underlying the divergent roles of SPARC in human carcinogenesis. Carcinogenesis 2014, 35, 967–973. [Google Scholar] [CrossRef]
- Witkiewicz, A.K.; Freydin, B.; Chervoneva, I.; Potoczek, M.; Rizzo, W.; Rui, H.; Brody, J.R.; Schwartz, G.F.; Lisanti, M.P. Stromal CD10 and SPARC expression in ductal carcinoma in situ (DCIS) patients predicts disease recurrence. Cancer Biol. Ther. 2010, 10, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Hu, P.; Shen, H.; Yu, J.; Liu, Q.; Du, J. Prognostic role of Twist and Snail in various carcinomas: a systematic review and meta-analysis. Eur. J. Clin. Investig. 2014, 44, 1072–1094. [Google Scholar] [CrossRef] [PubMed]
- Narod, S.A.; Sopik, V. Is invasion a necessary step for metastases in breast cancer? Breast Cancer Res. Treat. 2018, 169, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Cunha, P.O.; Ornstein, M.; Jones, J.L. Progression of Ductal Carcinoma in Situ form the Pathological Perspective. Breast Care 2010, 5, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Geradts, J.; de Herreros, A.G.; Su, Z.; Burchette, J.; Broadwater, G.; Bachelder, R.E. Nuclear Snail1 and nuclear ZEB1 protein expression in invasive and intraductal human breast carcinomas. Hum. Pathol. 2011, 42, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Muenst, S.; Daster, S.; Obermann, E.C.; Droeser, R.A.; Weber, W.P.; von Holzen, U.; Gao, F.; Viehl, C.; Oertli, D.; Soysal, S.D. Nuclear expression of Snail is an independent negative prognostic factor in human breast cancer. Dis. Markers 2013, 35, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, K.; Nordenskjold, B.; Landberg, G. Hypoxia, Snail and incomplete epithelial-mesenchymal transition in breast cancer. Br. J. Cancer 2009, 101, 1769–1781. [Google Scholar] [CrossRef]
- Logullo, A.F.; Nonogaki, S.; Pasini, F.S.; Osorio, C.A.; Soares, F.A.; Brentani, M.M. Concomitant expression of epithelial-mesenchymal transition biomarkers in breast ductal carcinoma: association with progression. Oncol. Rep. 2010, 23, 313–320. [Google Scholar] [PubMed]
- Gwin, K.; Contreras, A.; Tretiakova, M.; Montag, A. Expression of the epithelial mesenchymal transition regulator Snail in metaplastic breast carcinomas with chondroid differentiation. In Proceedings of the United States & Canadian Academy of Pathology 98th Annual Meeting, Breast Pathology Session, Boston, MA, USA, 3–7 March 2009. [Google Scholar]
- Bezdekova, M.; Brychtova, S.; Sedlakova, E.; Langova, K.; Brychta, T.; Belej, K. Analysis of Snail-1, E-cadherin and claudin-1 expression in colorectal adenomas and carcinomas. Int. J. Mol. Sci. 2012, 13, 1632–1643. [Google Scholar] [CrossRef] [PubMed]
- Gorringe, K.L.; Fox, S.B. Ductal Carcinoma in Situ Biology, Biomarkers, and Diagnosis. Front. Oncol. 2017, 7, 248. [Google Scholar] [CrossRef] [PubMed]
- Groen, E.J.; Elshof, L.E.; Visser, L.L.; Rutgers, E.J.T.; Winter-Warnars, H.A.O.; Lips, E.H.; Wesseling, J. Finding the balance between over- and under-treatment of ductal carcinoma in situ (DCIS). Breast 2017, 31, 274–283. [Google Scholar] [CrossRef] [PubMed]
| Variable | Results |
|---|---|
| Patient age | |
| Median/range | 59/41–88 |
| Mean ± SD | 58.4 ± 7.9 |
| Microcalcification type, n (%) | |
| Powdery | 70 (34) |
| Crushed stone-like | 107 (51) |
| Casting-type | 32 (15) |
| Microcalcification distribution, n (%) | |
| Cluster | 145 (69) |
| Group | 44 (21) |
| Regional | 20 (10) |
| Nuclear grade, n (%) | |
| Low | 110 (52) |
| Intermediate | 66 (32) |
| High | 33 (16) |
| Comedonecrosis, n (%) | |
| Absent | 93 (45) |
| Present | 116 (55) |
| N-cadherin expression, n (%) | |
| Negative | 192 (92) |
| Positive | 17 (8) |
| Snail1 expression, n (%) | |
| Negative | 80 (38) |
| Positive | 129 (62) |
| Weak | 57 (27) |
| Intermediate | 43 (21) |
| Strong | 29 (14) |
| SPARC expression, n (%) | |
| Negative | 130 (62) |
| Positive | 79 (38) |
| Weak | 33 (16) |
| Intermediate | 20 (10) |
| Strong | 26 (12) |
| N-Cadherin | SPARC | Snail1 | ||||
|---|---|---|---|---|---|---|
| Negative | Positive | Negative | Positive | Negative | Positive | |
| N-cadherin | ||||||
| Negative | - | - | 124 (65) | 68 (35) | 78 (41) | 114 (59) |
| Positive | - | - | 6 (35) | 1 (65) | 2 (12) | 15 (88) |
| SPARC | ||||||
| Negative | 124 (95) | 6 (5) | - | - | 66 (51) | 64 (49) |
| Positive | 68 (86) | 11 (14) | - | - | 14 (18) | 65 (82) |
| Snail1 | ||||||
| Negative | 78 (97) | 2 (3) | 66 (82) | 14 (18) | - | - |
| Positive | 114 (88) | 15 (12) | 64 (49) | 65 (51) | - | - |
| Variable | Postoperative Invasion | Univariate Analysis p | Multivariate Analysis p | |
|---|---|---|---|---|
| Absent n (%) | Present n (%) | |||
| Microcalcifications type | ||||
| Powdery | 59 (84) | 11 (16) | 0.645 | - |
| Crushed stone-like | 91 (85) | 16 (15) | ||
| Casting-type | 25 (78) | 7 (22) | ||
| Microcalcifications distribution | ||||
| Cluster | 123 (85) | 22 (15) | 0.771 | - |
| Group | 36 (76) | 8 (24) | ||
| Regional | 16 (80) | 4 (20) | ||
| Nuclear grade | ||||
| Low | 95 (86) | 15 (14) | 0.417 | - |
| Intermediate | 52 (79) | 14 (21) | ||
| High | 28 (85) | 5 (15) | ||
| Comedonecrosis | ||||
| Absent | 80 (86) | 13 (14) | 0.520 | - |
| Present | 95 (82) | 21 (18) | ||
| N-cadherin expression | ||||
| Negative | 162 (84) | 30 (16) | 0.061 | - |
| Positive | 13 (76) | 4 (24) | ||
| Snail1 expression | ||||
| Negative | 78 (97) | 2 (3) | <0.0001 | 0.007 |
| Positive | 97 (75) | 32 (25) | ||
| Weak | 52 (91) | 5 (9) | ||
| Intermediate | 32 (74) | 11 (26) | ||
| Strong | 13 (45) | 16 (55) | ||
| SPARC expression | ||||
| Negative | 126 (97) | 4 (3) | <0.0001 | <0.0001 |
| Positive | 49 (62) | 30 (38) | ||
| Weak | 32 (97) | 1 (3) | ||
| Intermediate | 10 (50) | 10 (50) | ||
| Strong | 7 (27) | 19 (73) | ||
| Patient age | ||||
| Median (range) | 59 (41–88) | 58.5 (43–75) | 0.980 | - |
| SPARC Expression n (%) | Snail1 Expression n (%) | |||
|---|---|---|---|---|
| Negative | Weak | Intermediate | Strong | |
| Negative | 0 (0/66) | 3% (1/38) | 10% (2/21) | 20% (1/5) |
| Weak | 0 (0/9) | 0 (0/9) | 0 (0/9) | 17% (1/6) |
| Intermediate | 0 (0/3) | 33% (3/9) | 75% (3/4) | 100% (4/4) |
| Strong | 100% (2/2) | 100% (1/1) | 66% (6/9) | 71% (10/14) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szynglarewicz, B.; Kasprzak, P.; Donizy, P.; Biecek, P.; Halon, A.; Matkowski, R. Biological Aggressiveness of Subclinical No-Mass Ductal Carcinoma In Situ (DCIS) Can Be Reflected by the Expression Profiles of Epithelial-Mesenchymal Transition Triggers. Int. J. Mol. Sci. 2018, 19, 3941. https://doi.org/10.3390/ijms19123941
Szynglarewicz B, Kasprzak P, Donizy P, Biecek P, Halon A, Matkowski R. Biological Aggressiveness of Subclinical No-Mass Ductal Carcinoma In Situ (DCIS) Can Be Reflected by the Expression Profiles of Epithelial-Mesenchymal Transition Triggers. International Journal of Molecular Sciences. 2018; 19(12):3941. https://doi.org/10.3390/ijms19123941
Chicago/Turabian StyleSzynglarewicz, Bartlomiej, Piotr Kasprzak, Piotr Donizy, Przemyslaw Biecek, Agnieszka Halon, and Rafal Matkowski. 2018. "Biological Aggressiveness of Subclinical No-Mass Ductal Carcinoma In Situ (DCIS) Can Be Reflected by the Expression Profiles of Epithelial-Mesenchymal Transition Triggers" International Journal of Molecular Sciences 19, no. 12: 3941. https://doi.org/10.3390/ijms19123941
APA StyleSzynglarewicz, B., Kasprzak, P., Donizy, P., Biecek, P., Halon, A., & Matkowski, R. (2018). Biological Aggressiveness of Subclinical No-Mass Ductal Carcinoma In Situ (DCIS) Can Be Reflected by the Expression Profiles of Epithelial-Mesenchymal Transition Triggers. International Journal of Molecular Sciences, 19(12), 3941. https://doi.org/10.3390/ijms19123941






