Anticancer Action and Mechanism of Ergosterol Peroxide from Paecilomyces cicadae Fermentation Broth
Abstract
1. Introduction
2. Results
2.1. Identification of Ergosterol Peroxide (EP) Products
2.2. EP Inhibited In Vitro RCC Cell Growth
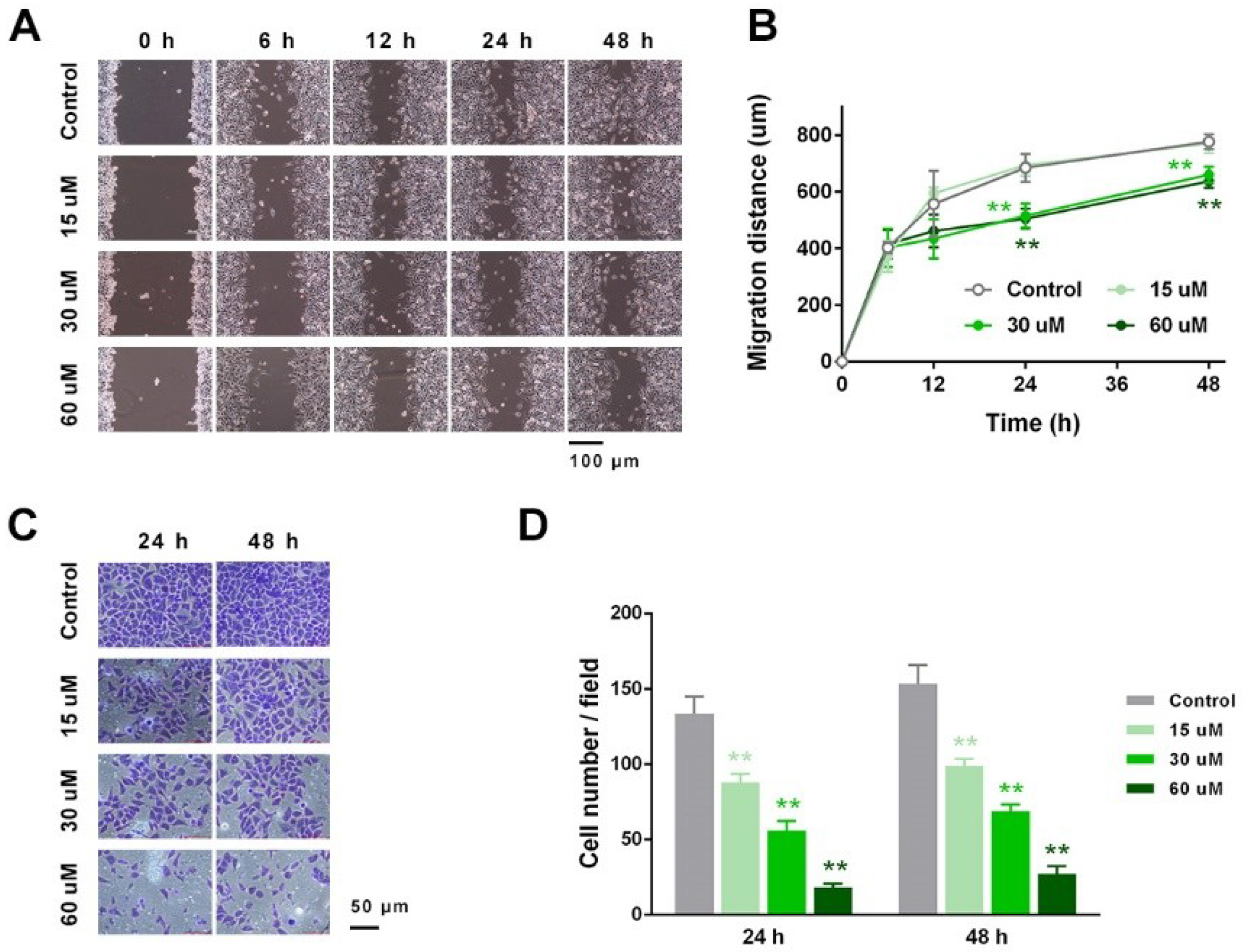
2.3. EP Suppressed RCC Cell Migration and Invasion In Vitro
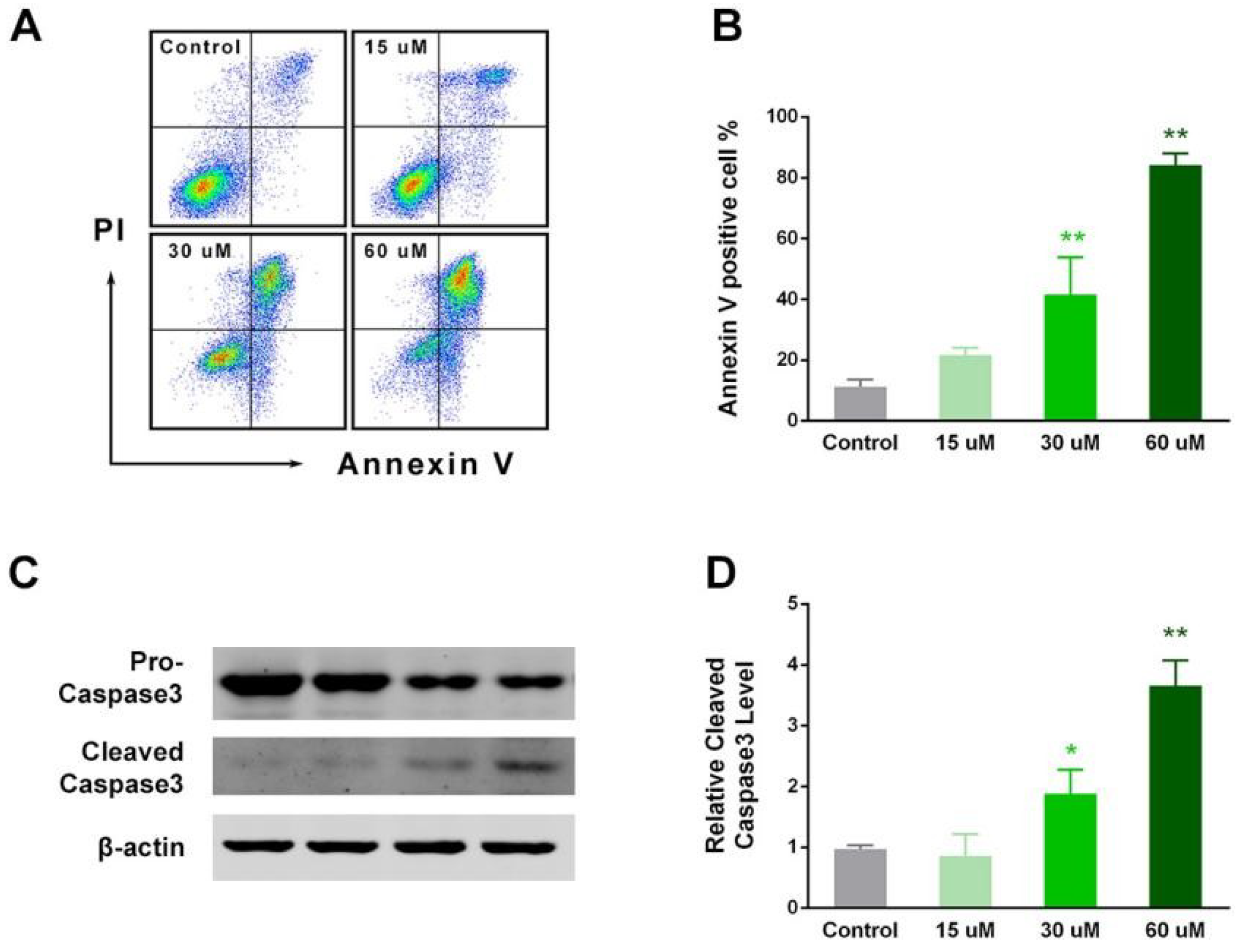
2.4. EP Enhanced RCC Cell Apoptosis In Vitro
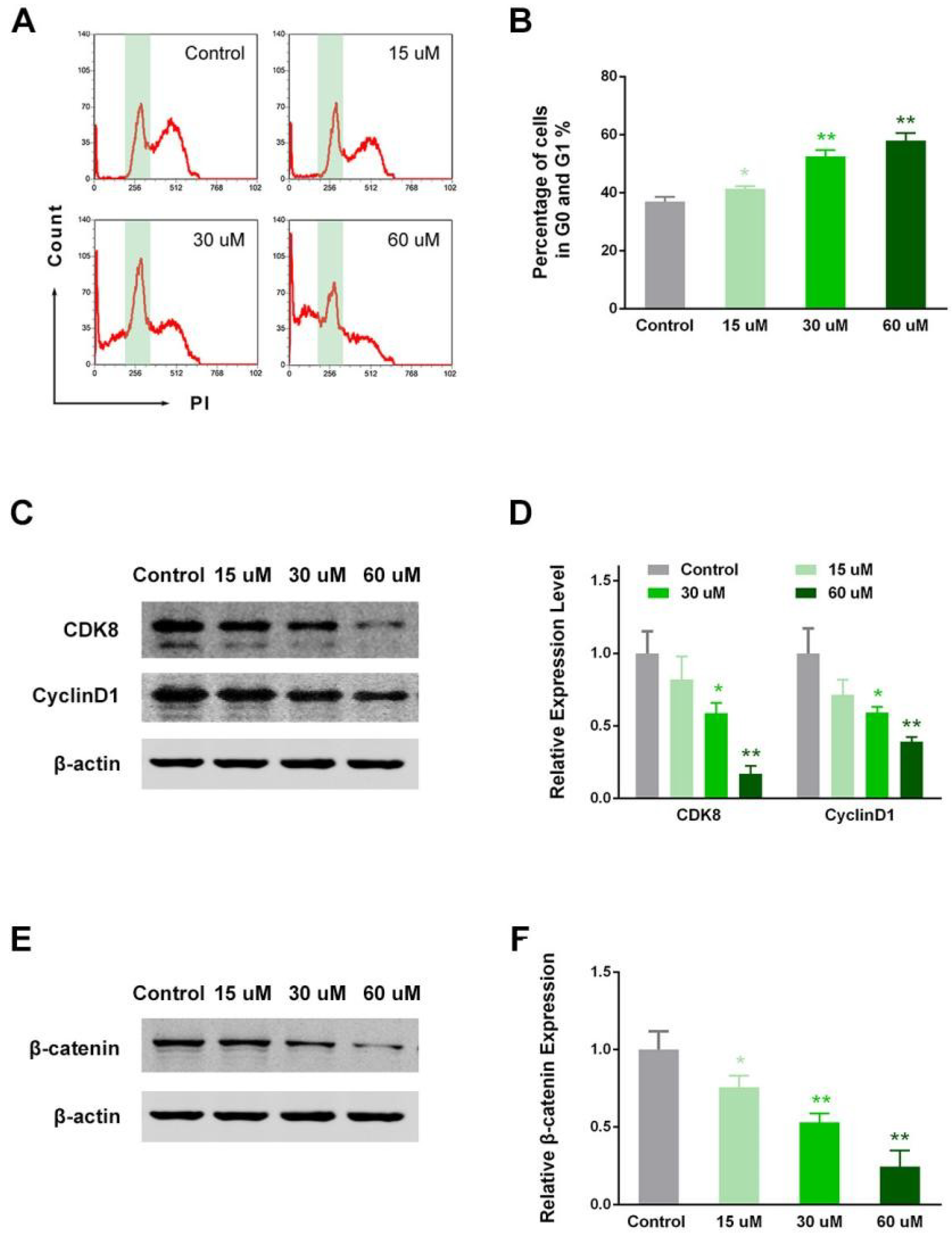
2.5. EP Modulated RCC Cell Cycle in Vitro
3. Discussion
4. Materials and Methods
4.1. EP Preparation
4.2. IC50 Analysis of EP Inhibition on Human RCC Cells
4.3. Colony Formation Assay
4.4. Cell Migration Assay
4.5. Cell Invasion Assay
4.6. Flow Cytometry for Apoptosis and Cell Cycle Analysis
4.7. Western Blotting
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| EP | ergosterol peroxide |
| RCC | renal cell carcinoma |
| P. cicadae | Paecilomyces cicadae |
| TCM | traditional Chinese medicine |
| PI | propidium iodide |
References
- Cai, W.; Huang, J.; Yuan, Y.; Hu, X.; Li, M.; Kong, W.; Zhang, J.; Guo, J.; Chen, Y.; Huang, Y. Sunitinib or Sorafenib as Neoadjuvant Therapy May not Improve the Survival Outcomes of Renal Cell Carcinoma with Tumor Thrombus. Urol. Int. 2018, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Breen, D.J.; King, A.J.; Patel, N.; Lockyer, R.; Hayes, M. Image-guided Cryoablation for Sporadic Renal Cell Carcinoma: Three- and 5-year Outcomes in 220 Patients with Biopsy-proven Renal Cell Carcinoma. Radiology 2018, 180249. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Zhu, W.R.; Zhou, W.C.; Ying, H.F.; Zheng, L.; Guo, Y.B.; Chen, J.X.; Shen, X.H. Traditional Chinese medicinal herbs combined with epidermal growth factor receptor tyrosine kinase inhibitor for advanced non-small cell lung cancer: A systematic review and meta-analysis. J. Integr. Med. 2014, 12, 346–358. [Google Scholar] [CrossRef]
- Jia, L.; Ma, S.; Hou, X.; Wang, X.; Qased, A.B.; Sun, X.; Liang, N.; Li, H.; Yi, H.; Kong, D.; et al. The synergistic effects of traditional Chinese herbs and radiotherapy for cancer treatment. Oncol. Lett. 2013, 5, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Hao, J.; Niu, Y.; Liu, D.; Chen, D.; Wu, X. Molecular targets of Chinese herbs: A clinical study of metastatic colorectal cancer based on network pharmacology. Sci. Rep. 2018, 8, 7238. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Qiang, W.W.; Tan, W.; Zhang, H.; Wang, S.; Wang, C.; Qiang, W.; Wang, Y. Chinese Herbs Interfering with Cancer Reprogramming Metabolism. Evid. Based Complement. Altern. Med. 2016, 2016, 9282813. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Wang, N.; Tan, H.Y.; Tsao, S.W.; Feng, Y. MicroRNAs and Chinese Medicinal Herbs: New Possibilities in Cancer Therapy. Cancers 2015, 7, 1643–1657. [Google Scholar] [CrossRef]
- Sun, Y.F.; Sun, Y.; Wang, Z.A.; Han, R.L.; Lu, H.F.; Zhang, J.L.; Liu, H.T.; Wang, S.X.; Wang, P.; Dian, L.L.; et al. Isaria cicadae conidia possess antiproliferative and inducing apoptosis properties in gynaecological carcinoma cells. Mycology 2017, 8, 327–334. [Google Scholar] [CrossRef]
- Xu, Z.; Yan, X.; Song, Z.; Li, W.; Zhao, W.; Ma, H.; Du, J.; Li, S.; Zhang, D. Two heteropolysaccharides from Isaria cicadae Miquel differ in composition and potentially immunomodulatory activity. Int. J. Biol. Macromol. 2018, 117, 610–616. [Google Scholar] [CrossRef]
- Matsueda, S.; Shimoyama, M.; Imaizumi, T.; Tsushima, Y. Studies on fungal products. VI. Biological effects of ergosterol-5,8-peroxide. Yakugaku Zasshi J. Pharm. Soc. Jpn. 1982, 102, 347–351. [Google Scholar] [CrossRef]
- Lindequist, U.; Lesnau, A.; Teuscher, E.; Pilgrim, H. The antiviral action of ergosterol peroxide. Die Pharm. 1989, 44, 579–580. [Google Scholar]
- Kahlos, K.; Kangas, L.; Hiltunen, R. Ergosterol peroxide, an active compound from Inonotus radiatus. Planta Med. 1989, 55, 389–390. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.K.; Kuo, Y.H.; Chiang, B.H.; Lo, J.M.; Sheen, L.Y. Cytotoxic activities of 9,11-dehydroergosterol peroxide and ergosterol peroxide from the fermentation mycelia of Ganoderma lucidum cultivated in the medium containing leguminous plants on Hep 3B cells. J. Agric. Food Chem. 2009, 57, 5713–5719. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Cardile, V.; Piovano, M.; Caggia, S.; Espinoza, C.L.; Garbarino, J.A. Pro-apoptotic activity of ergosterol peroxide and (22E)-ergosta-7,22-dien-5α-hydroxy-3,6-dione in human prostate cancer cells. Chem. Biol. Interactions 2010, 184, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, Q.; Bu, M.; Hu, L.; Du, W.W.; Jiao, C.; Pan, H.; Sdiri, M.; Wu, N.; Xie, Y.; et al. Ergosterol peroxide activates Foxo3-mediated cell death signaling by inhibiting AKT and c-Myc in human hepatocellular carcinoma cells. Oncotarget 2016, 7, 33948–33959. [Google Scholar] [CrossRef] [PubMed]
- Kobori, M.; Yoshida, M.; Ohnishi-Kameyama, M.; Shinmoto, H. Ergosterol peroxide from an edible mushroom suppresses inflammatory responses in RAW264.7 macrophages and growth of HT29 colon adenocarcinoma cells. Br. J. Pharmacol. 2007, 150, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.S.; Jo, Y.S.; Kim, Y.H.; Hyun, J.W.; Kim, H.W. Cytotoxic activities of acetoxyscirpenediol and ergosterol peroxide from Paecilomyces tenuipes. Life Sci. 2001, 69, 229–237. [Google Scholar] [CrossRef]
- Bauslaugh, G.; Just, G.; Blank, F. Isolation of Ergosterol Peroxide from Trichophyton Schoenleini. Nature 1964, 202, 1218. [Google Scholar] [CrossRef]
- Takei, T.; Yoshida, M.; Ohnishi-Kameyama, M.; Kobori, M. Ergosterol peroxide, an apoptosis-inducing component isolated from Sarcodon aspratus (Berk.) S. Ito. Biosci. Biotechnol. Biochem. 2005, 69, 212–215. [Google Scholar] [CrossRef]
- Zhu, R.; Zheng, R.; Deng, Y.; Chen, Y.; Zhang, S. Ergosterol peroxide from Cordyceps cicadae ameliorates TGF-β1-induced activation of kidney fibroblasts. Phytomed. Int. J. Phytother. Phytopharm. 2014, 21, 372–378. [Google Scholar] [CrossRef]
- Bok, J.W.; Lermer, L.; Chilton, J.; Klingeman, H.G.; Towers, G.H. Antitumor sterols from the mycelia of Cordyceps sinensis. Phytochemistry 1999, 51, 891–898. [Google Scholar] [CrossRef]
- Lee, J.S.; Ma, C.M.; Park, D.K.; Yoshimi, Y.; Hatanaka, M.; Hattori, M. Transformation of ergosterol peroxide to cytotoxic substances by rat intestinal bacteria. Biol. Pharm. Bull. 2008, 31, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Jang, J.E.; Mishra, S.K.; Lee, H.J.; Nho, C.W.; Shin, D.; Jin, M.; Kim, M.K.; Choi, C.; Oh, S.H. Ergosterol peroxide from Chaga mushroom (Inonotus obliquus) exhibits anti-cancer activity by down-regulation of the β-catenin pathway in colorectal cancer. J. Ethnopharmacol. 2015, 173, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Pan, M.; Liu, H.; Tian, H.; Ye, Q.; Liu, H. Ergosterol peroxide inhibits ovarian cancer cell growth through multiple pathways. Oncol. Targets Ther. 2017, 10, 3467–3474. [Google Scholar] [CrossRef] [PubMed]
- Rhee, Y.H.; Jeong, S.J.; Lee, H.J.; Lee, H.J.; Koh, W.; Jung, J.H.; Kim, S.H.; Sung-Hoon, K. Inhibition of STAT3 signaling and induction of SHP1 mediate antiangiogenic and antitumor activities of ergosterol peroxide in U266 multiple myeloma cells. BMC Cancer 2012, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Choi, S.Y.; Hong, S.M.; Hwang, S.G.; Park, D.K. The ethyl acetate extract of Phellinus linteus grown on germinated brown rice induces G0/G1 cell cycle arrest and apoptosis in human colon carcinoma HT29 cells. Phytother. Res. 2010, 24, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Lee, S.J.; Kwak, H.Y.; Jung, L.; Heo, J.; Hong, S.; Kim, G.W.; Baek, N.I. Sterols isolated from Nuruk (Rhizopus oryzae KSD-815) inhibit the migration of cancer cells. J. Microbiol. Biotechnol. 2009, 19, 1328–1332. [Google Scholar] [CrossRef]
- Wen, H.; Wu, Z.; Hu, H.; Wu, Y.; Yang, G.; Lu, J.; Yang, G.; Guo, G.; Dong, Q. The anti-tumor effect of pachymic acid on osteosarcoma cells by inducing PTEN and Caspase 3/7-dependent apoptosis. J. Nat. Med. 2018, 72, 57–63. [Google Scholar] [CrossRef]
- Zhu, Y.T.; Li, F.; Han, B.; Tighe, S.; Zhang, S.; Chen, S.Y.; Liu, X.; Tseng, S.C. Activation of RhoA-ROCK-BMP signaling reprograms adult human corneal endothelial cells. J. Cell Biol. 2014, 206, 799–811. [Google Scholar] [CrossRef]
- Hagenbuchner, J.; Rupp, M.; Salvador, C.; Meister, B.; Kiechl-Kohlendorfer, U.; Muller, T.; Geiger, K.; Sergi, C.; Obexer, P.; Ausserlechner, M.J. Nuclear FOXO3 predicts adverse clinical outcome and promotes tumor angiogenesis in neuroblastoma. Oncotarget 2016, 7, 77591–77606. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.H. Tumor-suppressive activity of 1,25-dihydroxyvitamin D3 against kidney cancer cells via up-regulation of FOXO3. Biosci. Biotechnol. Biochem. 2016, 80, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Barker, B.M.; Kroll, K.; Vodisch, M.; Mazurie, A.; Kniemeyer, O.; Cramer, R.A. Transcriptomic and proteomic analyses of the Aspergillus fumigatus hypoxia response using an oxygen-controlled fermenter. BMC Genom. 2012, 13, 62. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, L.; Shi, W.; Liu, X.; Zhao, X.; Zhang, Z. Anticancer Action and Mechanism of Ergosterol Peroxide from Paecilomyces cicadae Fermentation Broth. Int. J. Mol. Sci. 2018, 19, 3935. https://doi.org/10.3390/ijms19123935
He L, Shi W, Liu X, Zhao X, Zhang Z. Anticancer Action and Mechanism of Ergosterol Peroxide from Paecilomyces cicadae Fermentation Broth. International Journal of Molecular Sciences. 2018; 19(12):3935. https://doi.org/10.3390/ijms19123935
Chicago/Turabian StyleHe, Linfu, Wenjing Shi, Xiaocui Liu, Xiaohuan Zhao, and Zhicai Zhang. 2018. "Anticancer Action and Mechanism of Ergosterol Peroxide from Paecilomyces cicadae Fermentation Broth" International Journal of Molecular Sciences 19, no. 12: 3935. https://doi.org/10.3390/ijms19123935
APA StyleHe, L., Shi, W., Liu, X., Zhao, X., & Zhang, Z. (2018). Anticancer Action and Mechanism of Ergosterol Peroxide from Paecilomyces cicadae Fermentation Broth. International Journal of Molecular Sciences, 19(12), 3935. https://doi.org/10.3390/ijms19123935




