Interplay of Glycemic Index, Glycemic Load, and Dietary Antioxidant Capacity with Insulin Resistance in Subjects with a Cardiometabolic Risk Profile
Abstract
1. Introduction
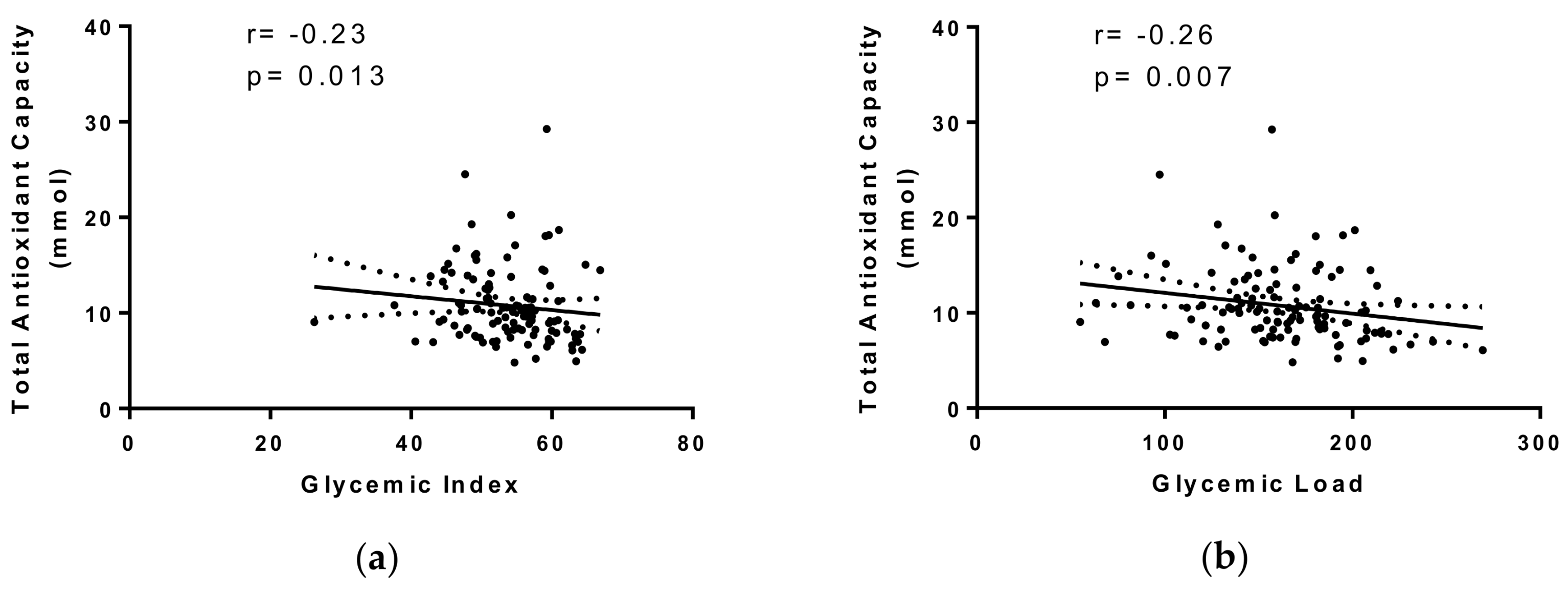
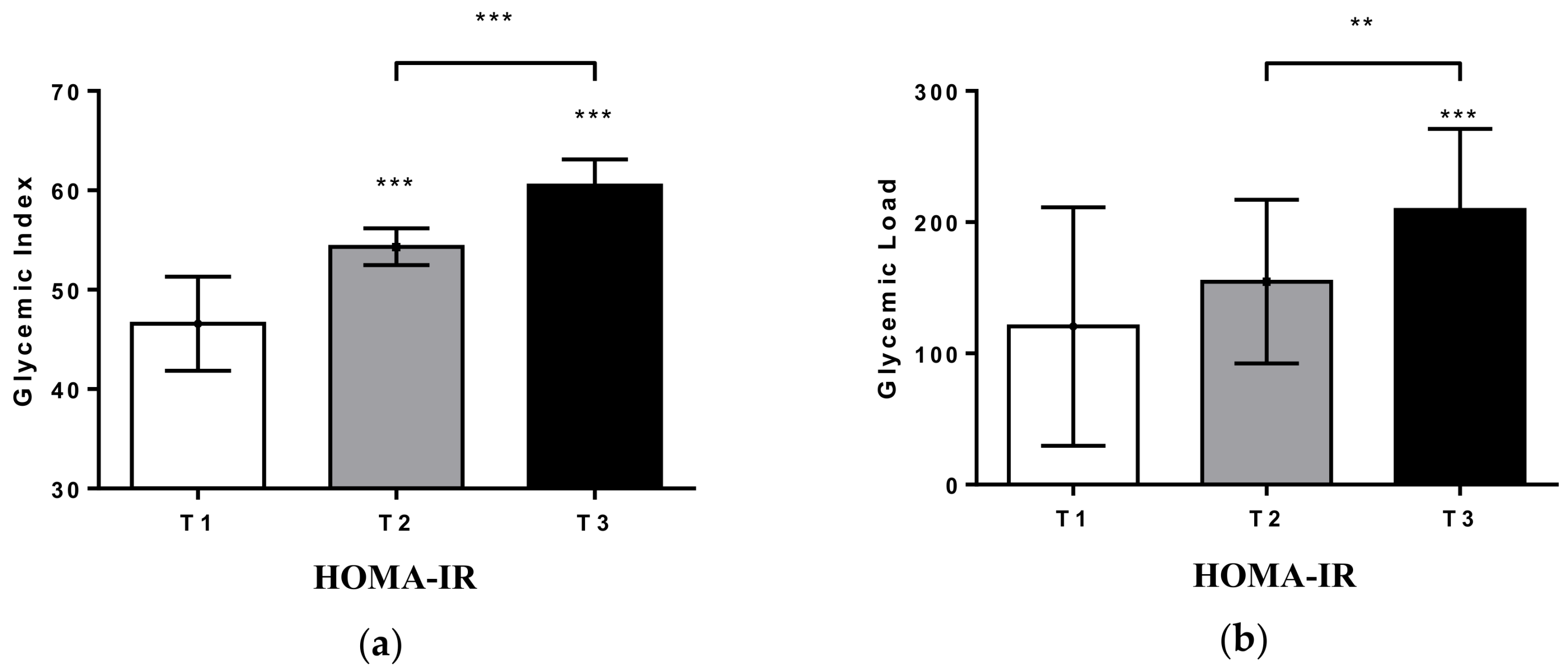
2. Results
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Anthropometric and Biochemical Measurements
4.3. Dietary Data
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TAC | total antioxidant capacity |
| GI | glycemic index |
| GL | glycemic load |
| IR | insulin resistance |
| FFQ | food frequency questionnaire |
| DXA | dual-energy X-ray absorptiometry |
| CRP | C-reactive protein |
| MRI | magnetic resonance imaging |
| OS | oxidative stress |
| NAFLD | nonalcoholic fatty liver disease |
| BMI | body mass index |
| HbA1c | glycosidic hemoglobin |
| TG | triglycerides |
| TC | total cholesterol |
| LDL-c | high-density lipoprotein |
| HDL-c | low-density lipoprotein |
| AST | aspartate aminotransferase |
| ALT | alanine aminotransferase |
| FLiO | fatty liver in obesity—Spain |
References
- Hermsdorff, H.H.; Puchau, B.; Volp, A.C.; Barbosa, K.B.; Bressan, J.; Zulet, M.A.; Martínez, J.A. Dietary total antioxidant capacity is inversely related to central adiposity as well as to metabolic and oxidative stress markers in healthy young adults. Nutr. Metab. (Lond.) 2011, 22, 59. [Google Scholar] [CrossRef] [PubMed]
- Betteridge, D.J. What is oxidative stress? Metabolism 2000, 49, 3–8. [Google Scholar] [CrossRef]
- Cefalu, W.T. Insulin resistance: Cellular and clinical concepts. Exp. Biol. Med. (Maywood) 2001, 226, 13–26. [Google Scholar] [CrossRef] [PubMed]
- De la Iglesia, R.; Loria-Kohen, V.; Zulet, M.A.; Martínez, J.A.; Reglero, G.; Ramírez de Molina, A. Dietary Strategies Implicated in the Prevention and Treatment of Metabolic Syndrome. Int. J. Mol. Sci. 2016, 17, 1877. [Google Scholar] [CrossRef] [PubMed]
- Puchau, B.; Zulet, M.A.; de Echavarri, A.G.; Hermsdorff, H.H.; Martinez, J.A. Dietary total antioxidant capacity: A novel indicator of diet quality in healthy young adults. J. Am. Coll. Nutr. 2009, 28, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Puchau, B.; Zulet, M.A.; de Echavarri, A.G.; Hermsdorff, H.H.; Martinez, J.A. Dietary total antioxidant capacity is negatively associated with some metabolic syndrome features in healthy young adults. Nutrition 2010, 26, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Arikawa, A.Y.; Jakits, H.E.; Flood, A.; Thomas, W.; Gross, M.; Schmitz, K.H.; Kurzer, M.S. Consumption of a high glycemic load but not a high glycemic index diet is marginally associated with oxidative stress in young women. Nutr. Res. 2015, 35, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Griffith, J.A.; Ma, Y.; Chasan-Taber, L.; Olendzki, B.C.; Chiriboga, D.E.; Stanek, E.J., III; Merriam, P.A.; Ockene, I.S. Association between dietary glycemic index, glycemic load, and high-sensitivity C-reactive protein. Nutrition 2008, 24, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B.; Liu, S.; Rimm, E.B.; Manson, J.E.; Willett, W.C.; Hu, F.B. Glycemic index, glycemic load, and dietary fiber intake and incidence of type 2 diabetes in younger and middle-aged women. Am. J. Clin. Nutr. 2004, 80, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Willett, W.C.; Stampfer, M.J.; Hu, F.B.; Franz, M.; Sampson, L.; Hennekens, C.H.; Manson, J.E. A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in US women. Am. J. Clin. Nutr. 2000, 71, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Wolever, T.M.; Taylor, R.H.; Barker, H.; Fielden, H.; Baldwin, J.M.; Bowling, A.C.; Newman, H.C.; Jenkins, A.L.; Goff, D.V. Glycemic index of foods: A physiological basis for carbohydrate exchange. Am. J. Clin. Nutr. 1981, 34, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Bhupathiraju, S.N.; Tobias, D.K.; Malik, V.S.; Pan, A.; Hruby, A.; Manson, J.E.; Willett, W.C.; Hu, F.B. Glycemic index, glycemic load, and risk of type 2 diabetes: Results from 3 large US cohorts and an updated meta-analysis. Am. J. Clin. Nutr. 2014, 100, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Perez-Cornago, A.; Lopez-Legarrea, P.; de la Iglesia, R.; Lahortiga, F.; Martinez, J.A.; Zulet, M.A. Longitudinal relationship of diet and oxidative stress with depressive symptoms in patients with metabolic síndrome after following a weight loss treatment: The resmena project. Clin. Nutr. 2014, 33, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Gastaldelli, A.; Nauck, M.A.; Balena, R. Eight weeks of treatment with long-acting GLP-1 analog taspoglutide improves postprandial insulin secretion and sensitivity in metformin-treated patients with type 2 diabetes. Metabolism 2013, 62, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Marsh, S.; Hu, J.; Feng, W.; Wu, C. The Pathogenesis of Nonalcoholic Fatty Liver Disease: Interplay between Diet, Gut Microbiota, and Genetic Background. Gastroenterol. Res. Pract. 2016, 2016, 2862173. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Matute, P.; Zulet, M.A.; Martinez, J.A. Reactive species and diabetes: Counteracting oxidative stress to improve health. Curr. Opin. Pharmacol. 2009, 9, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Hassimotto, N.M.; Pinto, M.D.; Lajolo, F.M. Antioxidant status in humans after consumption of blackberry (Rubus fruticosus L.) juices with and without defatted milk. J. Agric. Food Chem. 2008, 56, 11727–11733. [Google Scholar] [CrossRef] [PubMed]
- Pitsavos, C.; Panagiotakos, D.B.; Tzima, N.; Chrysohoou, C.; Economou, M.; Zampelas, A.; Stefanadis, C. Adherence to the Mediterranean diet is associated with total antioxidant capacity in healthy adults: The ATTICA study. Am. J. Clin. Nutr. 2005, 82, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Serafini, M.; Del Rio, D. Understanding the association between dietary antioxidants, redox status and disease: Is the Total Antioxidant Capacity the right tool? Redox Rep. 2004, 9, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Valtuena, S.; Pellegrini, N.; Franzini, L.; Bianchi, M.A.; Ardigo, D.; Del Rio, D.; Piatti, P.; Scazzina, F.; Zavaroni, I.; Brighenti, F. Food selection based on total antioxidant capacity can modify antioxidant intake, systemic inflammation, and liver function without altering markers of oxidative stress. Am. J. Clin. Nutr. 2008, 87, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Rautiainen, S.; Serafini, M.; Morgenstern, R.; Prior, R.L.; Wolk, A. The validity and reproducibility of food-frequency questionnaire-based total antioxidant capacity estimates in Swedish women. Am. J. Clin. Nutr. 2008, 87, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- De la Iglesia, R.; Lopez-Legarrea, P.; Celada, P.; Sánchez-Muniz, F.J.; Martinez, J.A.; Zulet, M.A. Beneficial effects of the RESMENA dietary pattern on oxidative stress in patients suffering from metabolic syndrome with hyperglycemia are associated to dietary TAC and fruit consumption. Int. J. Mol. Sci. 2013, 14, 6903–6919. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Hamer, M.; Chida, Y. Intake of fruit, vegetables, and antioxidants and risk of type 2 diabetes: Systematic review and metaanalysis. J. Hypertens. 2007, 25, 2361–2369. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, R.; Nikfar, S.; Larijani, B.; Abdollahi, M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed. Pharmacother. 2005, 59, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Montonen, J.; Knekt, P.; Järvinen, R.; Reunanen, A. Dietary antioxidant intake and risk of type 2 diabetes. Diabetes Care 2004, 27, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Franzini, L.; Ardigo, D.; Zavaroni, I. Dietary antioxidants and glucose metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Abete, I.; Goyenechea, E.; Zulet, M.A.; Martinez, J.A. Obesity and metabolic syndrome: Potential benefit from specific nutritional components. Nutr. Metab. Cardiovasc. Dis. 2011, 21, B1–B15. [Google Scholar] [CrossRef] [PubMed]
- Rösen, P.; Nawroth, P.P.; King, G.; Möller, W.; Tritschler, H.J.; Packer, L. The role of oxidative stress in the onset and progression of diabetes and its complications: A summary of a congress series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab. Res. Rev. 2001, 17, 189–212. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Legarrea, P.; de la Iglesia, R.; Abete, I.; Navas-Carretero, S.; Martínez, J.A.; Zulet, M.A. The protein type within a hypocaloric diet affects obesity-related inflammation: The RESMENA project. Nutrition 2014, 30, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Gögebakan, O.; Kohl, A.; Osterhoff, M.A.; van Baak, M.A.; Jebb, S.A.; Papadaki, A.; Martinez, J.A.; Handjieva-Darlenska, T.; Hlavaty, P.; Weickert, M.O.; et al. Effects of weight loss and long-term weight maintenance with diets varying in protein and glycemic index on cardiovascular risk factors: The diet, obesity, and genes (DiOGenes) study: A randomized, controlled trial. Circulation 2011, 124, 2829–2838. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, S.; Pellegrini, N.; Brenna, O.V.; Del Rio, D.; Frasca, G.; Brighenti, F.; Tumino, R. Antioxidant characterization of some Sicilian edible wild greens. J. Agric. Food Chem. 2005, 53, 9465–9471. [Google Scholar] [CrossRef] [PubMed]
- Zulet, M.A.; Bondia-Pons, I.; Abete, I.; de la Iglesia, R.; López-Legarrea, P.; Forga, L.; Navas-Carretero, S.; Martínez, J.A. The reduction of the metabolyc syndrome in navarra-spain (resmena s) study: A multidisciplinary strategy based on chrononutrition and nutritional education, together with dietetic and psychological control. Nutr. Hosp. 2011, 26, 16–26. [Google Scholar] [PubMed]
- Acosta, A.M.; Escalona, M.; Maiz, A.; Pollak, F.; Leighton, F. Determinación del índice de resistencia insulínica mediante HOMA en una población de la Región Metropolitana de Chile. Rev. Méd. Chile 2002, 130, 17. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [PubMed]
- Martin-Moreno, J.M.; Boyle, P.; Gorgojo, L.; Maisonneuve, P.; Fernandez-Rodriguez, J.C.; Salvini, S. Development and validation of a food frequency questionnaire in Spain. Int. J. Epidemiol. 1993, 22, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Moreiras, O.; Carvajal, A.; Cabrera, L. Tablas de Composición de Alimentos Food Composition Tables; Pirámide: Madrid, Spain, 2009. [Google Scholar]
- Halvorsen, B.L.; Carlsen, M.H.; Phillips, K.M.; Bohn, S.K.; Holte, K.; Jacobs, D.R.; Blomhoff, R. Content of redox-active compounds (i.e., antioxidants) in foods consumed in the United States. Am. J. Clin. Nutr. 2006, 84, 95–135. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, B.L.; Holte, K.; Myhrstad, M.C.; Barikmo, I.; Hvattum, E.; Remberg, S.F.; Wold, A.B.; Haffner, K.; Baugerod, H.; Andersen, L.F.; et al. A systematic screening of total antioxidants in dietary plants. J. Nutr. 2002, 132, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total antioxidant capacity of plant foods, beverages, and oils consumed in Italy assessed by three different in vitro assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, N.; Serafini, M.; Salvatore, S.; Del Rio, D.; Bianchi, M.; Brighenti, F. Total antioxidant capacity of spices, dried fruits, nuts, pulses, cereals, and sweets consumed in Italy assessed by three different in vitro assays. Mol. Nutr. Food Res. 2006, 50, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International Tables of Glycemic Index and Glycemic Load Values: 2008. Diabetes Care 2008, 31, 12. [Google Scholar] [CrossRef] [PubMed]
- University of Sydney Glycemic Index. The Official Website of the Glycemic Index and GI Database, 2006 Sydney. Available online: http://www.glycemicindex.com/ (accessed on 27 July 2018).
- Neuhouser, M.L.; Tinker, L.F.; Thomson, C.; Caan, B.; Horn, L.V.; Snetselaar, L.; Parker, L.M.; Patterson, R.E.; Robinson-O’Brien, R.; Beresford, S.A.; Shikany, J.M. Development of a Glycemic Index Database for Food Frequency Questionnaires Used in Epidemiologic Studies. J. Nutr. 2006, 136, 1604–1609. [Google Scholar] [CrossRef] [PubMed]
| n = 112 | All Participants | T1 (<8.6 mmol) (n = 38) | T2 (8.6–11.36 mmol) (n = 37) | T3 (>11.36 mmol) (n = 37) | p-Value |
|---|---|---|---|---|---|
| Sex (men/women) | 65/47 | 22/16 | 22/15 | 20/17 | 0.863 |
| Age (years) | 50.8 (9) | 48.1 (10) | 54.2 (9) # | 50.3 (8) | 0.017 |
| BMI (kg/m2) | 33.9 (4) | 33.6 (4) | 34.6 (4) | 33.1 (3) | 0.241 |
| Cardiometabolic risk factors | |||||
| Waist circumference (cm) | 109.8 (8) | 109.4 (11) | 111.6 (10) | 108.6 (9) | 0.400 |
| Total fat mass (%) | 42.9 (6) | 43.7 (6) | 43.0 (7) | 42.8 (6) | 0.674 |
| Visceral fat mass (g) | 2374 (1051) | 2530 (1293) | 2371 (873) | 2211 (1010) | 0.455 |
| Hepatic fat by MRI (%) | 9.4 (9) | 10.75 (13) | 11.5 (9) † | 5.7 (4.8) | 0.023 |
| Blood pressure levels (mmHg) | |||||
| Systolic | 131 (17) | 130 (14) | 131 (15) | 130 (21) | 0.984 |
| Diastolic | 87 (10) | 86 (10) | 87 (7) | 88 (10) | 0.521 |
| Diabetes mellitus (%) | 8.6 | 8.1 | 10.8 | 8.1 | 0.898 |
| Metabolic syndrome (%) | 68.5 | 67.6 | 70.3 | 70.3 | 0.960 |
| Glucose (mg/dL) | 108.0 (30) | 114.2 (45) | 112.7 (25) | 99.3 (15) | 0.081 |
| HbA1c (%) | 5.9 (11) | 6.2 (2) | 5.9 (1) | 5.7 (0.5) | 0.134 |
| Insulin (U/L) | 19.0 (12) | 18.1 (10) | 22.3 (14) | 14.8 (7) * | 0.010 |
| HOMA-IR | 5.4 (5) | 5.7 (6) | 6.5 (5) | 3.6 (2) * | 0.031 |
| TG (mg/dL) | 137.0 (77) | 137.9 (91) | 139.1 (80) | 137.6 (69) | 0.996 |
| TC (mg/dL) | 195.3 (38) | 193.2 (36) | 193.7 (42.4) | 203.0 (39.2) | 0.485 |
| LDL-c/HDL-c ratio | 2.4 (1) | 2.4 (1) | 2.4 (0.8) | 2.4 (0.9) | 0.965 |
| TyG index | 1.3 (0.7) | 1.2 (0.6) | 1.3 (0.7) | 1.4 (0.8) | 0.404 |
| Homocysteine (µmol/L) | 15.6 (6) | 15.6 (8) | 15.8 (5) | 14.9 (5) | 0.790 |
| CRP (mg/dL) | 0.5 (1.2) | 0.5 (0.8) | 0.4 (0.5) | 0.7 (2.1) | 0.739 |
| AST/ALT ratio | 0.8 | 0.8 (0.3) | 0.8 (0.2) | 0.86 (0.3) | 0.406 |
| Cholesterol-lowering drugs (no/yes) | 95/17 | 30/7 | 30/7 | 34/3 | 0.329 |
| Blood pressure medications (no/yes) | 86/26 | 28/9 | 29/8 | 29/8 | 0.950 |
| Lifestyle factors | |||||
| Smoking habit (n) | 0.946 | ||||
| Never | 32 | 11 | 9 | 11 | 0.966 |
| Former smoker | 43 | 15 | 13 | 15 | 0.946 |
| Sporadically | 5 | 2 | 2 | 1 | 0.871 |
| Current smoker | 19 | 5 | 5 | 9 | 0.871 |
| Physical activity (n) | 0.579 | ||||
| Never | 46 | 17 | 15 | 14 | 0.417 |
| Mild | 26 | 9 | 6 | 10 | 0.579 |
| Moderated | 26 | 5 | 11 | 10 | 0.669 |
| Elevated | 14 | 6 | 5 | 3 | 0.508 |
| n = 112 | All | T1 (<8.6 mmol) (n = 38) | T2 (8.6–11.36) (n = 37) | T3 (>11.36) (n = 37) | p-Value |
|---|---|---|---|---|---|
| Energy and macronutrients | |||||
| Energy intake (kcal/day) | 2691 (1010) | 2210 (570) | 2701 (744) | 3169 (1343) * | <0.001 |
| Carbohydrates (%E) | 43.0 (7) | 42.6 (7) | 44.3 (7) | 42.2 (8) | 0.405 |
| Dietary fiber (g/day) | 24.8 (9) | 20.3 (6) | 26.4 (18) # | 27.1 (9) * | <0.001 |
| Total protein (%E) | 17.4 (4) | 18.1 (4) | 17.7 (4) | 16.5 (3) | 0.140 |
| Total lipid (%E) | 37.1 (7) | 38.1 (7) | 36.4 (7) | 36.9 (7) | 0.558 |
| Micronutrients | |||||
| Vitamin A (µg/day) | 1100.2 (820) | 908.9 (531) | 1089.3 (627) | 1309.3 (1151) | 0.108 |
| Vitamin C (mg/day) | 200.8 (115) | 157.9 (68.6) | 198.7 (84) | 245.3 (159) * | 0.004 |
| Vitamin D (µg/day) | 6.2 (4) | 5.5 (3) | 6.2 (4) | 6.8 (4) | 0.306 |
| Vitamin E (mg/day) | 10.5 (4) | 8.9 (3) | 10.6 (4) | 12.0 (5) * | 0.009 |
| Folic acid (µg/day) | 362.8 (140) | 290.4 (75) | 389.8 (159) | 408.9 (147) * | <0.001 |
| Phenolic compounds rich fermented beverages (g) | 8.7 (11) | 3.8 (5) | 6.1 (7) | 16.7 (14) * | <0.001 |
| Marine Ω3 (g/day) | 0.64 (0.4) | 0.62 (0.4) | 0.62 (0.4) | 0.69 (0.3) | 0.611 |
| Model 1 | ||
|---|---|---|
| Variables | β (95% CI) | p-Value |
| GI | 0.12 (0.03; 0.21) | 0.012 |
| GL | 0.02 (0.001; 0.03) | 0.037 |
| TAC (mmol/day) | ||
| TAC < p50 | 1 | |
| TAC ≥ p50 | −1.33 (2.63; −0.04) | 0.044 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galarregui, C.; Zulet, M.Á.; Cantero, I.; Marín-Alejandre, B.A.; Monreal, J.I.; Elorz, M.; Benito-Boillos, A.; Herrero, J.I.; Tur, J.A.; Abete, I.; et al. Interplay of Glycemic Index, Glycemic Load, and Dietary Antioxidant Capacity with Insulin Resistance in Subjects with a Cardiometabolic Risk Profile. Int. J. Mol. Sci. 2018, 19, 3662. https://doi.org/10.3390/ijms19113662
Galarregui C, Zulet MÁ, Cantero I, Marín-Alejandre BA, Monreal JI, Elorz M, Benito-Boillos A, Herrero JI, Tur JA, Abete I, et al. Interplay of Glycemic Index, Glycemic Load, and Dietary Antioxidant Capacity with Insulin Resistance in Subjects with a Cardiometabolic Risk Profile. International Journal of Molecular Sciences. 2018; 19(11):3662. https://doi.org/10.3390/ijms19113662
Chicago/Turabian StyleGalarregui, Cristina, María Ángeles Zulet, Irene Cantero, Bertha Araceli Marín-Alejandre, José Ignacio Monreal, Mariana Elorz, Alberto Benito-Boillos, José Ignacio Herrero, Josep Antoni Tur, Itziar Abete, and et al. 2018. "Interplay of Glycemic Index, Glycemic Load, and Dietary Antioxidant Capacity with Insulin Resistance in Subjects with a Cardiometabolic Risk Profile" International Journal of Molecular Sciences 19, no. 11: 3662. https://doi.org/10.3390/ijms19113662
APA StyleGalarregui, C., Zulet, M. Á., Cantero, I., Marín-Alejandre, B. A., Monreal, J. I., Elorz, M., Benito-Boillos, A., Herrero, J. I., Tur, J. A., Abete, I., & Martínez, J. A. (2018). Interplay of Glycemic Index, Glycemic Load, and Dietary Antioxidant Capacity with Insulin Resistance in Subjects with a Cardiometabolic Risk Profile. International Journal of Molecular Sciences, 19(11), 3662. https://doi.org/10.3390/ijms19113662







